Recombinant human erythropoietin (rHu-EPO) is used to treat anemia by activating the erythropoietin receptor (EPOR) in erythroid progenitor cells, leading to proliferation and differentiation into mature red blood cells. To allow less frequent dosing, a hyperglycosylated version of EPO has been developed with a longer half-life. In principle, an agonistic antibody targeting EPOR would offer an even longer half-life, support robust monthly dosing, and, unlike EPO products, reduce the risk of pure red cell aplasia. The efficiency of signaling and corresponding potency of previously reported antibody mimics are generally suboptimal compared with EPO and not suitable for clinical use. Here we describe a potent, fully human, agonistic antibody (ABT007) targeting EPOR that supports potent, more sustained, and less pulsatile elevation of hematocrit in a human EPOR–expressing transgenic mouse model compared with standard doses of rHu-EPO while requiring less frequent dosing. Resolution of the crystal structure of the EPOR extracellular domain (ECD) complexed to the ABT007 Fab fragment, determined at 0.32 nm, identifies a binding site that is consistent with a novel mechanism of receptor activation based on a unique antibody-imposed conformational change. These results demonstrate that a symmetric molecule can serve as a potent activator of the EPOR.
Introduction
EPO, a naturally occurring hematopoietic growth factor produced by the kidney, is the primary regulator of erythropoiesis.1 Recombinant human EPO (rHu-EPO) has important clinical uses in patients with anemia associated with renal disease and cancer. Analogs rHu-EPO with extended serum half-lives have been developed and of shown to provide a clinical advantage by allowing maintenance of stable hemoglobin levels with less frequent dosing.2,3 A full-length human agonistic antibody targeting the EPO receptor (EPOR) would offer a longer serum half-life and may support even less frequent dosing regimens that could better match with many chemotherapy regimens and may provide better convenience for both predialysis and peritoneal dialysis patients who need to attend the clinic only infrequently. In addition, an antibody EPO mimic is unlikely to induce pure red cell aplasia, a condition associated with some forms of rHu-EPO due to the formation of rHu-EPO–induced neutralizing antibodies.4
Mouse monoclonal antibodies (mAbs) that are raised to the soluble extracellular domain (ECD) of the human EPOR and that mimic EPO activation by inducing ligand-dependent cell proliferation and differentiation have been described.5,6 These mAbs, however, activate the EPOR less efficiently than the natural hormone does and consequently are less potent agonists and unsuitable for clinical use. Crystal structure of the EPO-(EPOR)2 complex reveals that EPO binds 2 distinct sites of the 2 cell-surface EPORs and that asymmetric molecules may therefore be required for optimal signaling.7
This report describes a fully human agonistic antibody, ABT-007, that effectively stimulates both proliferation and erythroid differentiation. Since ABT007 exhibits a high degree of selectivity and does not recognize rodent EPOR, mice expressing the human EPOR transgene were used to establish its in vivo efficacy. Surprisingly, the activation signal achieved with the symmetric molecule ABT007 is sufficient to support potent and more sustained erythropoiesis in animal models compared with standard doses of rHu-EPO. We examined the crystal structure of human monomeric EPOR ECD complexed with a single antibody fragment of ABT007 (Fab-EPOR) to better understand the molecular basis for the erythropoietic potency of ABT007. Resolution of the resulting crystal structure identified a unique EPOR nonlinear epitope distinct from the EPO binding site, resulting in a receptor conformation that supports activation. In vivo properties of ABT007 may be further enhanced by extended serum half-life of the human antibody and its fast off rate from the receptor, which permits continuous stimulation.
Materials and methods
Mice and cell lines
Mouse EPOR−/−/human EPOR+ transgenic mice were obtained from Dr Constance Noguchi of the National Institutes of Health (NIH). F36E cell line8 was purchased from Cell Bank (RIKEN BioResourse Center, Ibaraki, Japan). Fresh human bone marrow cells were obtained from Cambrex (East Rutherford, NJ). All animal studies were conducted in accordance with the guidelines established by the Abbott Laboratories Institutional Animal Care and Use Committee.
Generation of ABT007
XenoMouse mice (XenoMouse XG2; Amgen Fremont, Fremont, CA) were immunized with soluble EPOR9 coupled to a universal T-cell epitope.10 The specific titers obtained from XenoMouse animals were determined by enzyme-linked immunosorbent assay (ELISA) with immobilized biotinylated EPOR on streptavidin plates (Sigma-Aldrich, St Louis, MO). B cells from the harvested animals were cultured and those secreting EPOR-specific antibodies were isolated using the XenoMax approach as described in Babcook et al.11 EPOR-specific wells were identified by ELISA. Supernatants from several thousand wells were tested. Those wells testing positive for binding were screened in cell proliferation assays using EPO-dependent human cell lines (see “In vitro assays”). Single plasma cells producing HuMabs of the desired specificity were isolated by a plaque-forming assay, mRNA was extracted, and reverse transcriptase polymerase chain reaction (PCR) was conducted to generate cDNA. Recombinant antibody, harvested as cell culture supernatant from transfected cells, was purified over protein A Sepharose columns. ABT007 was one of several recombinant antibodies identified based on its ability to stimulate the proliferation of EPO-responsive cells. It was engineered for improved potency using yeast display technology.12
In vitro assays
F36E cells were maintained in RPMI 1640 media with 10% FBS and 5 U/mL of rHu-EPO (Epogen; Amgen). Prior to assays, cells were cultured overnight at a density of 4.0 × 105 to 5.0 × 105 cells/mL in growth medium without EPO. Cells were recovered, washed, and resuspended at a density of 106 cells/mL in assay medium (RPMI 1640 + 10% FBS), and 50 μL cells was added to wells of a 96-well microtiter plate. Fifty microliters each of ABT007, isotype control antibody, or EPO standards in assay medium were added to wells and the plates were incubated in a humidified incubator at 37°C with a 5% CO2 atmosphere. After 72 hours, 20 μL Promega Cell Titer 96 Aqueous MTS reagent (Madison, WI) was added to all wells. Plates were incubated at 37°C with a 5% CO2 atmosphere for 4 hours, and the optical density at 490 nm was determined using a microplate reader. To measure formation of erythroid colonies, fresh human bone marrow cells or bone marrow harvested from mouse EPOR−/−/human EPOR+ transgenic mice were resuspended at 106 cells/mL in IMDM–2% FBS. Cells (0.3 mL) were added to 12-mL tubes containing 2.6 mL Methocult (Stem Cell Technologies, Vancouver, BC, Canada); 66 μL stem cell growth factor (Sigma; 1 μg/mL); and EPO, novel erythropoiesis stimulating protein (NESP; Amgen), ABT007, or isotype control at the concentrations shown. EPO (50 ng/mL), 50 ng/mL NESP, and 1 μg/mL ABT007 correspond to 1.66 nM, 1.35 nM, and 6.6 nM, respectively. After mixing, 1.1 mL of the Methocult suspension was added to a 35-mm non–tissue culture–treated sterile Petri dish and incubated at 37°C, 5% CO2 for 2 weeks. Images were acquired with an Olympus IX50 inverted microscope equipped with a 4×/0.10 objective lens and mounted DP12 camera and associated image acquisition software (Olympus, Melville, NY), and were processed using Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA).
Transgenic mouse model for erythropoiesis
Male mouse EPOR−/−/human EPOR+ transgenic mice were dosed subcutaneously (4-6 mice per treatment group) with NESP (Amgen) at 3, 12, and 20 μg/kg on days 0 and 14 or with ABT007 on day 0 only, at 0.2, 0.8, and 1.6 mg/kg. A human IgG2 isotype control antibody was dosed at 1.6 mg/kg on day 0 only. Twenty-five microliters of blood was collected weekly via orbital bleed from each animal, and hematocrit was measured using a HESKA Vet ABC-Diff hematology analyzer (Heska, Loveland, CO).
Protein preparation and crystallization
A soluble form of mature EPOR ECD, representing residues 1 to 225, was expressed in Escherichia coli and refolded and purified as described.9 In order to facilitate the generation of Fab fragments, ABT007 was re-engineered as an IgG1 human antibody and subjected to papain cleavage.13 Samples for crystallization contained 1:1 complexes of EPOR ECD and ABT007 Fab fragments at a concentration of 14 mg/mL in 20 mM HEPES, 150 mM NaCl, 1 mM NaN3 (pH 7.5). Crystallization was carried out using the hanging drop vapor diffusion method at 17°C combining 2 μL protein with 2 μL of reservoir solution consisting of 15% PMME5000 and 600 mM Li2SO4. Protein crystals grew to approximately 0.8 × 0.1 × 0.1 mm in 2 weeks time. The cryopreservative was made using 80% reservoir solution and 20% glycerol. Crystals were flash-frozen in liquid nitrogen for data collection after quick passage through the cryopreservative. Diffraction data were collected at the Industrial Macromolecular Crystallography Association beamline ID-17 at Argonne National Laboratory and processed to 0.32-nm resolution using HKL2000.14 The crystals are space group P212121 and unit cell parameter a = 117.95, b = 156.17, and c = 164.20, with 3 Fab's bound to 3 EPORs in the asymmetric unit based on Matthews parameter calculations.
Structure determination and refinement
The structure was solved using a combination of Phaser15 version 1.3 and Molrep16 version 8.2 within the Collaborative Computational Project Number 4 program suite (Daresbury Laboratory, Cheshire, United Kingdom) for molecular replacement. The search model used in Phaser for the Fab fragment was 1JPT,17 and an ensemble of EPOR structures 1CN4,7 1EBA,18 1EBP,19 and 1EER7 was used to search for EPOR portions. This procedure identified 2 Fab/EPOR complexes in the asymmetric unit. One of these Fab/EPOR complexes was then used as a search model in Molrep to identify the third Fab/EPOR complex in the asymmetric unit, with the first 2 complexes from Phaser held fixed. The resulting structure shows well-determined electron density for 3 copies of EPOR and 2 well-defined copies of the ABT007 Fab, whereas the third copy has well-defined density of the light and heavy chains in the complementarity-determining regions. The conserved domains of the light and heavy chains of the third copy are solvent exposed and not well ordered. Refinement was initiated with multiple rounds of visual inspection and manual fitting in Quanta version 20 000 (Accelrys Software, San Diego CA) and refinement using CNX20,21 version 2000 (Accelrys Software, San Diego, CA) followed by a final refinement using Refmac22 version 5.2 within the Computational Project Number 4 program suite (Daresbury Laboratory) to refine the structure to 0.32-nm resolution with an Rwork = 23% and Rfree = 32%.
Results
ABT007 stimulates erythropoiesis
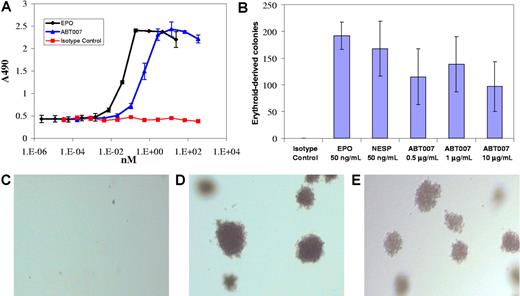
BIAcore analysis confirmed that ABT007 binds to EPOR with a Kd value of 30 nM and a fast off rate of 4.8 × 10−3 s−1 (S.E.L. and E.B.R., unpublished data, December 2003). ABT007 stimulated the proliferation of the F36E EPO-dependent cell line8 with maximal proliferative activity similar to that observed with EPO (Figure 1A). The maximal response occurred at concentrations approximately 10-fold higher than that of EPO on a molar basis. Increasing concentrations of both EPO and ABT007 resulted in a bell-shaped activation curve, most likely explained by involvement of EPOR-ligand/antibody interactions in nonproductive 1:1, not 2:1, complexes.5,6 Similar results were observed with UT-7/EPO, an EPO-dependent human megakaryoblastic leukemia cell line.23 Since growth and differentiation of erythroid progenitor cells depend on EPO, ABT007 was tested for its ability to support the formation of erythroid colonies from human bone marrow containing CD34+ progenitor cells. The addition of ABT007 to hematopoietic progenitor cells induced the formation of erythroid colonies, which were readily identified by the hemoglobinization of cells in the colony (Figure 1B,D-E). The maximum colony number observed with ABT007 was somewhat reduced compared with the maximum colony number observed with EPO and required a 5- to 40-fold greater concentration on a molar equivalent basis.
ABT007 stimulates in vitro erythropoiesis. (A) ABT007 stimulates the proliferation of EPO-responsive F36E human erythroleukemic cells.8 Error bars represent standard deviation (SD) calculated from the average of duplicate counts. (B) ABT007 supports the formation of erythroid colonies from hematopoietic precursor cells. Error bars represent standard deviation (SD) calculated from the average of duplicate counts. Typical colonies, identified microscopically, are shown below for (C) the isotype control–, (D) EPO-, and (E) ABT007-treated cells. The colonies were red in color.
ABT007 stimulates in vitro erythropoiesis. (A) ABT007 stimulates the proliferation of EPO-responsive F36E human erythroleukemic cells.8 Error bars represent standard deviation (SD) calculated from the average of duplicate counts. (B) ABT007 supports the formation of erythroid colonies from hematopoietic precursor cells. Error bars represent standard deviation (SD) calculated from the average of duplicate counts. Typical colonies, identified microscopically, are shown below for (C) the isotype control–, (D) EPO-, and (E) ABT007-treated cells. The colonies were red in color.
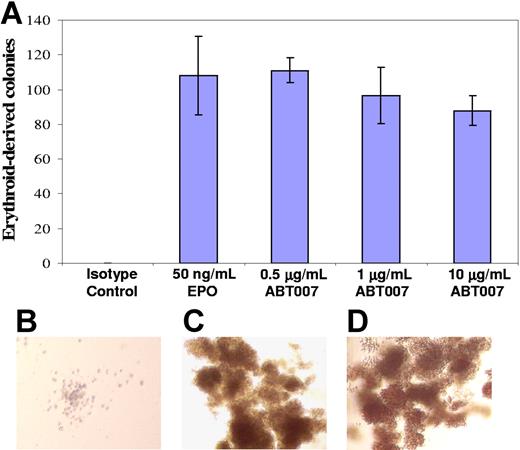
ABT007 does not recognize rodent EPOR (E.B.R, unpublished data, December 2003), so transgenic mice generated by rescuing genetic knockout mice, which lack the murine EPOR gene but have the human EPOR transgene,24,25 were used to establish in vivo efficacy of ABT007. These mice exhibit the transgene in hematopoietic tissues at levels comparable to endogenous murine EPOR, and the reticulocyte and hematocrit responses to dosing with EPO observed in these animals are similar to those seen with inbred strains of mice.25 ABT007 supported the formation of erythroid colonies from transgenic mouse–derived hematopoietic precursors comparable to the formation seen with human progenitor cells, indicating that ABT007 does recognize the human transgene receptor (Figure 2).
ABT007 stimulates formation of transgenic mouse erythroid colonies. (A) ABT007 supports the growth of erythroid colonies from bone marrow cells of transgenic mice. Error bars represent SD calculated from the average of duplicate counts. Typical colonies, identified microscopically, are shown on the right for (B) the isotype control–, (C) EPO-, and (D) ABT007-treated cells. The colonies were red in color. All images are at the same magnification.
ABT007 stimulates formation of transgenic mouse erythroid colonies. (A) ABT007 supports the growth of erythroid colonies from bone marrow cells of transgenic mice. Error bars represent SD calculated from the average of duplicate counts. Typical colonies, identified microscopically, are shown on the right for (B) the isotype control–, (C) EPO-, and (D) ABT007-treated cells. The colonies were red in color. All images are at the same magnification.
ABT007 dosed every 4 weeks sustains hematocrit increases in animals
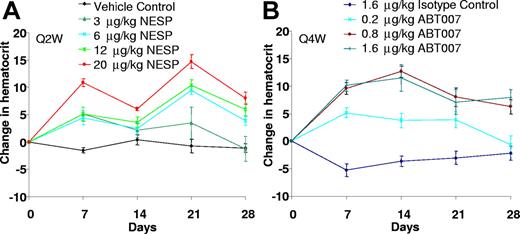
The ability of ABT007 to elevate hematocrit in a dose- and time-responsive manner was compared with that of NESP, a long-acting, hyperglycosylated analog of rHu-EPO currently in clinical use for anemia treatment as darbepoetin alfa.2 Successful antibody therapy generally requires higher dose requirements than other protein therapeutics. A single administration of ABT007, at concentrations greater than 0.2 mg/kg, results in a dose-dependent rise in hematocrit that is sustained for at least 4 weeks (Figure 3). The hematocrit achieved with a single dose of ABT007 is at least equivalent to that observed with a clinically relevant dose (3 μg/kg) of NESP26 administered every 2 weeks. ABT007 dosing results in more stable erythropoiesis with less fluctuation than that observed following NESP dosing (Figure 3). Additionally, increases in hematocrit were similar following either subcutaneous or intravenous administration of ABT007 (E.B.R., unpublished data, December 2003).
ABT007 dosed every 4 weeks is comparable to NESP dosed every 2 weeks. Male transgenic mice were dosed subcutaneously either with NESP (A) on days 0 and 14 or antibody (ABT007 or isotype control; B) on day 0 at the concentrations indicated. Blood samples were collected weekly, and hematocrit was measured using a HESKA Vet ABC-Diff hematology analyzer. Data represent change in hematocrit mean (± standard error) of 4 to 6 mice per treatment group. A typical NESP dose in practice is 3 μg/kg every 2 weeks.26
ABT007 dosed every 4 weeks is comparable to NESP dosed every 2 weeks. Male transgenic mice were dosed subcutaneously either with NESP (A) on days 0 and 14 or antibody (ABT007 or isotype control; B) on day 0 at the concentrations indicated. Blood samples were collected weekly, and hematocrit was measured using a HESKA Vet ABC-Diff hematology analyzer. Data represent change in hematocrit mean (± standard error) of 4 to 6 mice per treatment group. A typical NESP dose in practice is 3 μg/kg every 2 weeks.26
ABT007 Fab interacts with novel EPOR binding site
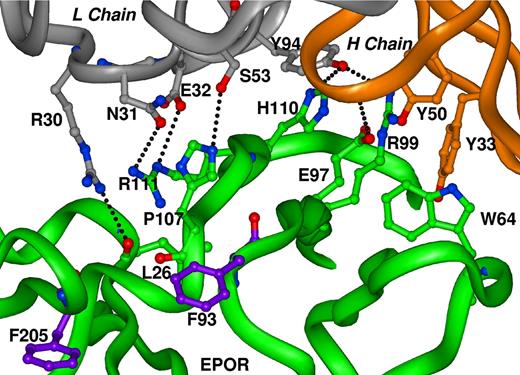
Protein fragment complementation assays and crystallographic studies indicate that EPOR exists as a preformed dimer.19,27 EPO binding to the receptor, through nonequivalent high- and low-affinity binding sites,28 triggers the switch between a self-associated, inactive conformation and an active, ligand-bound conformation.19 A full-length IgG-type antibody raised to EPOR ECD, by virtue of its bivalency, may also induce the interaction of 2 receptors and stabilize the active conformation. The in vivo potency of ABT007 may be at least partially attributed to the enhanced serum half-life of the fully human antibody. In fact, it has been demonstrated that NESP, despite its lower affinity for EPOR, has greater in vivo activity than that of EPO, due to a longer serum half-life.29 We postulated, however, that the binding conformation imposed by ABT007 might also contribute to the activation of the receptor and subsequent enhanced erythropoiesis. In fact, ABT007 binds to EPOR under nondenaturing conditions, but not under denaturing conditions, suggesting that the epitope recognized by ABT007 is a nonlinear, conformational epitope. In order to map the EPOR binding site and elucidate the molecular basis of this interaction, a soluble form of rHu-EPOR ECD9 was generated, and the crystal structure of human monomeric EPOR ECD complexed with a single Fab fragment of ABT007 (Fab-EPOR) was determined at 0.32-nm resolution by molecular replacement. The crystal structure of Fab-EPOR (Figure 4) confirmed that ABT007 binds EPOR through a nonlinear, conformationally defined epitope that includes residues from EPOR region E25-V112 (Table 1). The primary hydrophobic interactions are extended cation/π stacking interactions involving residues Y33 from antibody heavy chain, R99 from EPOR, H110 from EPOR, and H91 from antibody light chain. In addition to the extended stacking interactions, there is a series of hydrogen bonds between light chain N31 to EPOR-R111, light chain E32 to EPOR-R111, light chain R30 to both EPOR-E25 and the main chain carbonyl of EPOR-L26, and heavy chain L100 main chain to EPOR-E97 that further stabilize the complex. There are additional van der Waals interactions between other residues of both the light and heavy chains and residues V112, P107, and H110 of EPOR that complete the interactions between the Fab and EPOR. Finally, W64 of EPOR is an additional contact residue that interacts with heavy chain Y33 and may also interact with EPOR R99 side chain, thereby stabilizing the EPOR conformation. Comparison of the Fab-EPOR complex with the previously determined crystal structure of EPO complexed to EPOR7 shows that there is no overlap of contact residues (Figure 4). F93 and F205 of EPOR, which are the basis of essential hydrophobic interactions with EPO,7,27 do not participate in the interaction with Fab. Coordinates of the x-ray structure of the ABT007 Fab/EPOR complex have been deposited in the RCSB Protein Data Bank under accession number 2JIX.
Interaction of ABT007 Fab-EPOR. Crystal structure of the binding region of a single Fab-EPOR monomeric complex. Gray represents the ABT007 Fab light chain, brown represents the ABT007 Fab heavy chain, and green represents EPOR. Highlighted residues are directly involved in Fab/EPOR binding. Residues F93 and F205 of EPOR, highlighted in purple, are key residues involved in binding EPO and are not involved in Fab binding.
Interaction of ABT007 Fab-EPOR. Crystal structure of the binding region of a single Fab-EPOR monomeric complex. Gray represents the ABT007 Fab light chain, brown represents the ABT007 Fab heavy chain, and green represents EPOR. Highlighted residues are directly involved in Fab/EPOR binding. Residues F93 and F205 of EPOR, highlighted in purple, are key residues involved in binding EPO and are not involved in Fab binding.
EPOR and ABT007 residues involved in interaction
| EPOR . | Chain . | Type of interaction . |
|---|---|---|
| Heavy chain | ||
| R99 | Y33 | Cation/π stacking |
| R99 | Y50 | Cation/π stacking |
| W64 | Y33 | π stacking |
| E97 | L100 (main chain) | Hydrogen bond |
| V112 | L100 | Van der Waals |
| P107 | Y94 | Van der Waals |
| Light chain | ||
| H110 | H91 | π stacking |
| P107 | Y94 | Van der Waals |
| R111 | N31 | Hydrogen bond |
| R111 | E32 | Hydrogen bond |
| H114 | S53 | Hydrogen bond |
| E25 | R30 | Hydrogen bond |
| L26 (main chain) | R30 | Hydrogen bond |
| V112 | A50 | Van der Waals |
| EPOR . | Chain . | Type of interaction . |
|---|---|---|
| Heavy chain | ||
| R99 | Y33 | Cation/π stacking |
| R99 | Y50 | Cation/π stacking |
| W64 | Y33 | π stacking |
| E97 | L100 (main chain) | Hydrogen bond |
| V112 | L100 | Van der Waals |
| P107 | Y94 | Van der Waals |
| Light chain | ||
| H110 | H91 | π stacking |
| P107 | Y94 | Van der Waals |
| R111 | N31 | Hydrogen bond |
| R111 | E32 | Hydrogen bond |
| H114 | S53 | Hydrogen bond |
| E25 | R30 | Hydrogen bond |
| L26 (main chain) | R30 | Hydrogen bond |
| V112 | A50 | Van der Waals |
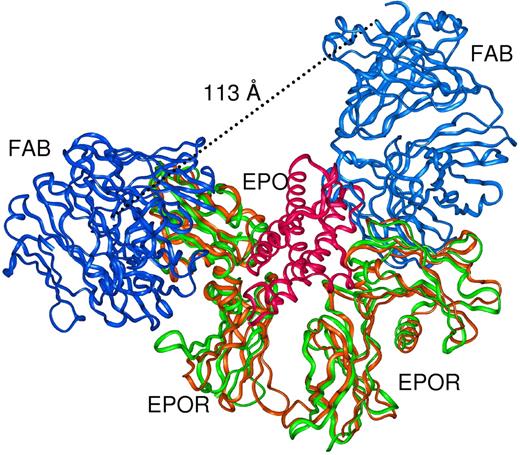
It has been demonstrated that the activating efficiency of EPOR is dependent on the orientation imposed by bound ligands and that asymmetric molecules may be required for optimal EPOR activation.7,19,27 The fact that ABT007 is a symmetric molecule that binds to a distinct Fab-EPOR binding site suggests that ABT007 may induce a unique conformation on the receptor and provide a basis for a novel mechanism of activation. To explore this possibility further, as shown in Figure 5, we superimposed 2 Fab-EPOR complexes (to mimic the bivalency of ABT007) onto the conformation of 2 adjacent receptors induced by EPO binding.7 Although it is conceivable that 1:1 monomeric antibody-receptor complexes could support this model, this is unlikely since the bell-shaped activation curve observed for ABT007 (Figure 1) is consistent with nonproductive binding at high antibody concentrations where 1:1 complexes are likely to predominate. Additionally, in this model (Figure 5) the distance between the carboxyl termini of the 2 antigen-specific Fabs is at least 11.3 nm and cannot be accommodated by a single antibody molecule. A more attractive model of activation is based on a conformation induced onto EPOR by ABT007 in a 2:1 ratio that is different from that caused by EPO. Additional experimental findings support a distinct EPO binding site and activation mechanism, since ABT007 treatment of EPO-dependent F36E cells results in an altered profile of STAT proteins compared with that observed upon EPO binding (R.L.S. and E.R.B, unpublished data, January 2005).
Comparison of the Fab-EPOR complex with the EPO-activated EPOR crystal structure. Two copies of ABT007 Fab (blue) complexed to EPO-activated EPOR dimer (green) are superimposed onto the EPO-activated (red) EPOR dimer (brown, 1EER)7 complex. Two independent Fab fragments can be accommodated on the EPO-activated form of EPOR, but the carboxyl termini of the Fab fragments are too distant (11.3 nm) to be derived from a single IgG.
Comparison of the Fab-EPOR complex with the EPO-activated EPOR crystal structure. Two copies of ABT007 Fab (blue) complexed to EPO-activated EPOR dimer (green) are superimposed onto the EPO-activated (red) EPOR dimer (brown, 1EER)7 complex. Two independent Fab fragments can be accommodated on the EPO-activated form of EPOR, but the carboxyl termini of the Fab fragments are too distant (11.3 nm) to be derived from a single IgG.
Discussion
Potent, safe EPO mimics that offer advantages with respect to a more sustained, less pulsatile elevation of hematocrit may offer both medical benefits and improved patient convenience. Other EPO mimics, including both peptides30 and activating antibodies,5,6 that activate EPOR by binding to regions outside the EPO binding site have been described. The potency of previously described antibody mimics is suboptimal compared with EPO and unsuitable for therapeutic use. In contrast, our results demonstrate that ABT007 is a potent, in vivo, stimulator of erythropoiesis that requires less frequent dosing than NESP and has a preclinical profile consistent with therapeutic intervention of anemia associated with kidney failure and cancer.31 To our knowledge, this is the first demonstration that an EPO mimic antibody stimulates and sustains erythropoiesis in vivo. Additionally, ABT007 dosing in transgenic mice results in more efficient and stable erythropoiesis than that observed following NESP dosing. Fluctuation of hemoglobin levels is highly correlated with clinical complications in patients with end-stage renal disease.32 It is also unlikely that a human antibody against EPOR would induce antibody-mediated pure red cell aplasia associated with some rHu-EPO therapies.4
Previous results demonstrate that optimal EPOR signaling requires asymmetric EPO as its ligand.7,18,27 In contrast, results presented herein suggest that a symmetric molecule, ABT007, also effectively activates the EPOR. As a symmetric molecule, ABT007 may bind and activate the receptor in a manner distinct from EPO. In support of this hypothesis, crystal structure resolution of the Fab-EPOR complex identified a unique binding site for ABT007 (Figure 4). Additionally, based on modeling deduced from the Fab-EPOR crystal structure, the size of ABT007 precludes it from assuming a conformation similar to that induced by EPO binding (Figure 5). Our observation that other activating human anti-EPOR IgG2 antibodies are less effective in stimulating erythropoiesis in vivo provides additional support that the unique receptor conformation imposed by ABT007 plays a critical role in supporting erythropoiesis.
Other attributes of ABT007, including extended serum half-life, may also contribute to its in vivo potency. For example, ABT007 has similar pharmacokinetic profiles in mouse EPOR−/−/human EPOR+ transgenic mice and cynomolgus monkeys (data not shown). Its serum half-life of approximately 6 days, significantly longer than that of either rHuEPO or NESP,33 is consistent with in vivo results, indicating that ABT007 supports monthly dosing (Figure 3). Interspecies scaling has been used successfully for the prediction of clearance for protein drugs in humans.34 Additionally, the in vivo potency of ABT007 is also influenced by its on-off rate kinetics and binding affinity. Repeated binding and dissociation from the receptor may permit continuous stimulation of erythropoiesis.
In summary, our findings indicate that ABT007 has several potential dosing and safety features that make it an attractive alternative for the treatment of anemia. Transgenic mice, expressing the human EPOR transgene in hematopoietic tissues at levels comparable to endogenous murine EPOR, provide a relevant model for predicting human dosing and mitigating the risk associated with overshooting safe hematocrit. The correlation observed in animals between dose and subsequent increases in hematocrit offers another attractive clinical feature of ABT007. This characteristic may prove extremely valuable in predicting human dosing. The value of this therapeutic will ultimately be realized upon human testing.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr C. Noguchi for providing breeding pairs of mouse EPOR−/−, human EPOR+ transgenic mice and Dr S. Fesik for comments on the manuscript. We also acknowledge the support of Amgen Freemont (Freemont, CA; formerly Abgenix) in the generation of the ABT007 precursor antibody. For crystal structure analysis, data were collected at beamline 17-BM in the facilities of the Industrial Macromolecular Crystallography Association Collaborative Access Team at the Advanced Photon Source. These facilities are supported by the companies of the Industrial Macromolecular Crystallography Association.
Authorship
Contribution: Z.L., R.L.S., R.R.L., and E.B.R. designed experiments and analyzed data. Z.L., V.S.S., P.J.D., C.G.J., N.X., S.E.L., D.A.E., and J.E.H. performed experiments and analyzed data. E.B.R. wrote the paper.
Conflict-of-interest disclosure: All of the authors are employees of Abbott Laboratories.
Correspondence: Edward B. Reilly, Abbott Laboratories, R4CD, AP31-4, 200 Abbott Park Rd, Abbott Park, IL 60064-6199; e-mail:ed.reilly@abbott.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal