To the editor:
We report here the clinical outcome for 2 chronic myeloid leukemia (CML) patients who acquired the T315I mutation while on imatinib, both of whom were treated successfully, one with recombinant interferon-alpha (rIFN) and the other with semisynthetic homoharringtonine (HHT).
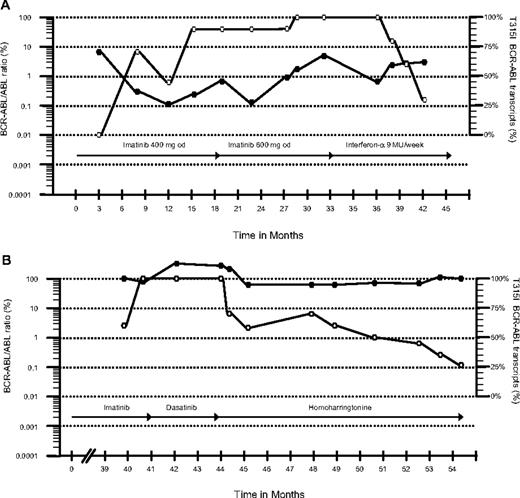
Patient 1 was a 75-year-old man with CML diagnosed in October 2003 who started imatinib 400 mg daily. After 4 months he achieved a complete cytogenetic remission (CCyR) with low BCR-ABL transcript numbers (0.3%) quantified by real-time quantitative polymerase chain reaction (RQ-PCR).1 He maintained a good response for 12 months but then BCR-ABL transcripts started to increase progressively and a T315I mutation was identified. The earlier samples were then analyzed retrospectively. Thirty months after starting imatinib, cytogenetics revealed loss of CCyR (2% Philadelphia chromosome [Ph]–positive cells) and the patient started rIFN 9 MU/week. Ten months later the patient was still in complete hematologic remission (CHR) with low BCR-ABL transcripts (3%), a level that is just consistent with CCyR. Interestingly, on rIFN, the percentage of mutant transcripts measured by quantitative single-nucleotide polymorphism pyrosequencing1 decreased from 100% to 30% (Figure 1A).
Total and T315I BCR-ABL in vivo kinetics. The figure shows BCR-ABL transcript levels measured by RQ-PCR and the relative size of the mutant clone. ● and ○ represent the total BCR-ABL transcripts and the percentage of the mutant clone, respectively. The type and duration of therapy are represented by the arrows.
Total and T315I BCR-ABL in vivo kinetics. The figure shows BCR-ABL transcript levels measured by RQ-PCR and the relative size of the mutant clone. ● and ○ represent the total BCR-ABL transcripts and the percentage of the mutant clone, respectively. The type and duration of therapy are represented by the arrows.
The second patient was a 60-year-old woman diagnosed with chronic-phase CML in 1997. She received rIFN and thereafter imatinib 400 mg daily from October 2002. She achieved a major cytogenetic response (15% Ph-positive cells) at 12 months but lost her response 2 years later. Bone marrow examination then revealed myelofibrotic transformation with 100% Ph positivity. Imatinib was increased to 600 mg/day, but she went into accelerated phase at 40 months, and thus received dasatinib 70 mg twice a day. Four months later a bone marrow aspirate still showed accelerated-phase leukemia, and a T315I mutation was identified. The earlier samples were then analyzed and the mutated clone was detected before starting dasatinib. The patient started on HHT at 1.25 mg/m2 subcutaneously twice daily for 5 consecutive days every month. Five months later she achieved a CHR. The marrow showed a minor cytogenetic response, and at 10 months the percentage of the mutant clone had decreased from 100% to 27% (Figure 1B).
The finding of a T315I mutation in the BCR-ABL gene characteristic of CML is associated with clinical resistance to imatinib, nilotinib, and dasatinib.2,–4 The only established salvage option for patients harboring this mutation is allogeneic stem cell transplantation (allo-SCT)5 ; the role of the aurora kinase inhibitor MK-0457 is not yet clear.6 Because the mechanisms of action of rIFN and HHT differ from that of tyrosine kinase inhibitors (TKIs), we evaluated their effect in these 2 patients with a predominant T315I clone.2,7,8 Although longer follow-up is needed, both patients showed a significant decrease in the percentage of the mutated clone along with a continuing disease response for a follow-up of 10 months. The question whether T315I-expressing CML cells are more “aggressive” than cells expressing native protein is still debated.9,10 However, it is possible that the decrease in size of the mutant subclone was a consequence of removing the selection pressure by stopping TKI. Thus rIFN or HHT should be considered for CML patients harboring the T315I mutation.2
Authorship
H.d.L. was supported by a grant from the “Fondation de France” (Paris, France).
Contribution: H.d.L. collected and analyzed clinical data and wrote the manuscript; J. S. Khorashad performed the molecular studies, assembled the molecular data, and commented on the manuscript; H.P.D. recruited the first patient, provided clinical care, and commented on the manuscript; D. Milojkovic provided clinical care and commented on the manuscript; J. S. Kaeda supervised the day-to-day running of the Minimal Residual Disease laboratory and commented on the manuscript; J.M.G. commented on and revised the manuscript; J.F.A. was responsible for coordinating the CML program and commented on the manuscript; and D. Marin supervised patient care and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John Goldman, Department of Haematology, Imperial College London, Du Cane Road, London W12 0NN, United Kingdom; e-mail: jgoldman@imperial.ac.uk.