Abstract
Although thalidomide has been shown to improve anemia in some patients with myelodysplastic syndromes and stimulates erythropoietin in patients with multiple myeloma, thalidomide's specific effects on γ-globin gene expression during erythroid differentiation have not been studied. Here, we investigated the effects of thalidomide on γ-globin gene expression and the involved signaling pathway using an ex vivo culture system of primary human CD34+ cells. We found that thalidomide induced γ-globin mRNA expression in a dose-dependent manner, but had no effect on β-globin expression. We also demonstrated that intracellular reactive oxygen species (ROS) levels were increased by treatment with thalidomide for 48 hours (from day 3 to day 5). Western blot analysis demonstrated that thalidomide activated the p38 mitogen-activated protein kinase (MAPK) signaling pathway in a time- and dose-dependent manner and increased histone H4 acetylation. Pretreatment of cells with the antioxidant enzyme catalase and the intracellular hydroxyl scavenger dimethylthiourea (DMTU) abrogated the thalidomide-induced p38 MAPK activation and histone H4 acetylation. Moreover, pretreatment with catalase and DMTU diminished thalidomide-induced γ-globin gene expression. These data indicate that thalidomide induces increased expression of the γ-globin gene via ROS-dependent activation of the p38 MAPK signaling pathway and histone H4 acetylation.
Introduction
Thalidomide is a synthetic glutamic acid derivative that was originally prescribed as a sedative and antinausea medicine, but later withdrawn from the market due to its teratogenic effects.1 However, thalidomide has made a remarkable comeback since the discovery of its immunomodulatory and anti-inflammatory effects, which have led to its use as a treatment for various proinflammatory and autoimmune conditions.2,3 It is currently being used to treat a number of diseases, including dermatologic, infectious, autoimmune, and hematologic disorders, especially multiple myeloma.4 Thalidomide has shown promising results in the treatment of multiple myeloma due to its antiangiogenic activity.5 The precise mechanisms whereby thalidomide exerts its therapeutic effect are still unknown
Thalidomide's effects may be related to its ability to repress cytokine-induced nuclear factor-κB (NF-κB), tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), and prostaglandin E2 (PGE2) synthesis, as well as its ability to increase production of reactive oxygen species (ROS) that play a role in vitro.3,6-9 ROS can induce or suppress the expression of several genes and can affect cell-signaling pathways by altering the activities of certain protein kinases and transcription factors. The pathway by which thalidomide exerts its teratogenicity is also under debate. However, it has been observed that the teratogenic properties of thalidomide may be due to a species-specific conversion to free-radical intermediates that result in DNA damage.10,11 In addition to these biologically based effects, recent clinical trials have confirmed that thalidomide may improve anemia and, less frequently, other cytopenias in a proportion of younger patients with low-risk myelodysplastic syndromes, and stimulates erythropoiesis in patients with multiple myeloma.12,13 Moreover, thalidomide and its derivatives have been reported to reduce or even eliminate the need for red blood cell transfusions in some anemic patients with myelodysplasia.14 Hence, it is of special interest to evaluate the effect of thalidomide on fetal hemoglobin synthesis in adult erythropoiesis using an in vitro culture system.
Induction of fetal hemoglobin (HbF) is a most promising therapeutic for treatment of β-thalassemia and sickle cell disease because HbF can substantially ameliorate the clinical symptoms of these genetic disorders. Several pharmacologic agents, such as hydroxyurea,15 5-deoxyazacytidine,16 and the histone deacetylase (HDAC) inhibitors butyrate and trichostatin A have been shown to induce γ-globin activity.17,18 However, the therapeutic use of these substances may be complicated due to several limitations. For example, induction of the γ-globin gene is relatively weak and is not effective in patients with severe disease. In addition, some of these fetal globin-inducing agents may aggravate apoptosis by inhibiting cell proliferation and arresting cell growth in the G1 phase, resulting in irreversible apoptosis in certain cell types. Therefore, research efforts continue to identify additional clinically useful compounds that might be able to induce γ-globin gene expression, as well as stimulate erythroid proliferation and prolong erythroblast survival. To establish additional and more effective therapeutic approaches, studies have focused on elucidating the cell-signaling mechanism for drug-mediated γ-globin gene induction. Increasing evidence has emerged that demonstrates the important role for p38 mitogen-activated protein kinase (MAPK) signaling in γ-globin induction by HDAC inhibitors.19-21 More recent data from Hsiao et al22 have shown that HbF is induced by HDAC inhibitors through generation of ROS.
Given that thalidomide may increase hemoglobin in patients with multiple myeloma as well as induce the generation of ROS (which subsequently act as mediators to inhibit angiogenesis), we hypothesize that thalidomide may induce the γ-globin gene through the ROS-p38 MAPK signaling pathway during adult human erythropoiesis. As demonstrated here, thalidomide induces γglobin expression through a mechanism that involves ROS-dependent activation of p38 MAPK cell signaling and histone H4 acetylation.
Materials and methods
Cell culture and reagents
Human peripheral blood CD34+ cells from healthy donors were isolated and cultured in medium containing 4U/mL erythropoietin (EPO; Amgen, Thousand Oaks, CA) at an initial density of 105 cells/mL for 14 days as described previously.23 Thalidomide (Calbiochem, San Diego, CA) was dissolved in dimethyl sulphoxide (DMSO; Sigma, St Louis, MO) to prepare a stock concentration of 500 mM. The stock solution was diluted with culture medium and added to the cells at a final concentration of 0.01 to 100 μM thalidomide for the indicated time periods. For all experimental timepoints, the final DMSO concentration was less than 0.1% DMSO; 0.1% DMSO was used as a control. For Western blot experiments, the antioxidants catalase (4000 U/mL) 1,3-dimethyl-2-thiourea (DMTU; 10 mM), or the p38 MAPK inhibitor SB203580 (5, 10, 20, 50 μM) was added to day-7 cells for 30 minutes, then coincubated with 100 μM thalidomide in the presence of indicated inhibitors for 30 minutes. For analysis of the expression of γ- or β-globin mRNA or HbF experiments, catalase, DMTU, and SB203580 were added to cell cultures with thalidomide (100 μM) from days 6 to 14. Catalase and DMTU were purchased from Sigma. SB203580 was obtained from Calbiochem. For cell growth experiments, cells were counted after 2 weeks of being cultured with different concentrations of thalidomide. Cells obtained from at least 3 separate donors were assayed for each experiment. All the antibodies for Western blots were purchased from Cell Signaling Technology (Beverly, MA). Approval was obtained from the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health (NIDDK/NIH) Institutional Review Board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Quantitative PCR analysis
Quantitative real-time polymerase chain reaction (PCR) assays of γ- and β-globin were carried out using gene-specific TaqMan double fluorescent–labeled probes in an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). On day 14 of culture, total RNA was prepared from 1 × 106 cells using the RNeasy Mini kit (Qiagen, Valencia, CA). For reverse transcription (RT), 1 μg total RNA per sample was used as a template for cDNA synthesis using Superscript II (Invitrogen, Carlsbad, CA) following the manufacturer's guidelines. The quantitative PCR was performed as described previously,23 and all samples were run in triplicate. A plasmid DNA encoding γ- and β-globin templates was used to generate the standard curve (20 to 2 000 000 copies) for determination of copy number. Statistical significance for all experiments was determined by Student paired t test analyses.
Flow cytometry analysis
After 14 days of culture, erythroid progenitors were collected, washed in ice-cold Dulbecco phosphate-buffered saline (PBS; CellGro; MediaTech, Herndon, VA) containing 0.1% bovine serum albumin (BSA; Sigma), and pelleted. The cells were fixed for 10 minutes at room temperature in 0.05% glutaraldehyde (Sigma), washed 3 times, and then permeabilized by 0.1% Triton/PBS/BSA solution for 5 minutes at room temperature. Cells were stained with anti-HbF–TRI-COLOR–conjugated monoclonal antibody (Caltag Laboratories, Burlingame, CA) for 15 minutes in the dark. TRI-COLOR–conjugated murine IgG1 monoclonal antibody of unrelated specificity was used as an isotype control. After staining, cells were analyzed by EPICS ELITE ESP flow cytometry (Beckman Coulter, Hialeah, FL).
Colony formation assay
CD34+ cells were seeded onto 35-mm plastic culture dishes (Corning, Acton, MA; 1 × 103 cells per dish) in 1 mL EPO-containing methylcellulose media (Stem Cell Technologies, Vancouver, BC) and cultured in the presence of thalidomide (1, 10, or 100 μM) or 0.1% DMSO (control). Cells were incubated in 5% CO2 with high humidity at 37°C for 14 days. At day 7, colony forming unit–erythroids (CFU-Es) were evaluated, and at day 14, burst forming unit–erythroids (BFU-Es) were evaluated.
Western blotting analysis
CD34+ cells were treated with thalidomide (0.01-100 μM) on day 7 of cell culture or following pretreatment for 30 minutes with indicated inhibitors and harvested at different time points. Extracted total protein was prepared using Protein Extraction Reagent (M-PER; Pierce Biotechnology, Rockford, IL) as recommended by the manufacturer. Proteins (30 μg/lane) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and then analyzed with rabbit polyclonal phosphorylated p38 MAPK (no. 9211; Thr180/182) and total p38 MAPK (no. 9212) antibodies or rabbit polyclonal acetylated histone H4 (no. 2591; Lys12) or total histone H4 (no. 2592). Horseradish peroxidase–conjugated secondary antibodies were used for chemiluminescent detection of protein (enhanced chemiluminescence [ECL] kit; Amersham Biosciences, Piscataway NJ).
Measurement of intracellular ROS
Intracellular ROS was detected using the oxidation-sensitive fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA; Invitrogen, San Diego, CA). Briefly, CD34+ cells were initially cultured in EPO medium. On day 3, thalidomide (0.01-100 μM) was added to the medium. After 48 hours, cells were incubated with 2 μM DCFH-DA at 37°C for 15 minutes in the dark and washed once with PBS. Samples were analyzed by flow cytometry using a Beckman Coulter Epics-XL model equipped with an excitation wavelength of 480 nm and an emission wavelength of 525 nm. Results were expressed as the percentage increase in mean channel fluorescence of drug-treated cells over untreated cells for each time point. Results were reported as an average of 3 independent experiments.
Results
Thalidomide increases the proliferation of erythroid cells in vitro
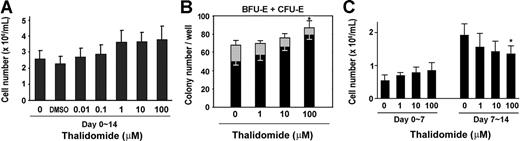
We first tested the effect of thalidomide on erythroid progenitor cell growth by measuring cell numbers and using a colony-forming assay. As shown in Figure 1A, thalidomide increased the cell number by 1.4-fold over control growth at a concentration of 1 to 100 μM. In the colony-forming assay, thalidomide induced a dose-dependent inhibition of CFU-E colonies formation by 58.8% in cells treated with 100 μM thalidomide; however, a dose-dependent increase in the number of BFU-E colonies (1.6-fold compared with control; Figure 1B). To further investigate the effect of thalidomide on early or late erythroid progenitor growth, we conducted time-course experiments. CD34+ cells were grown in EPO-only medium or treated with different concentrations of thalidomide (1, 10, or 100 μM) either for the first or second week of the 2-week culture period. As shown in Figure 1C, the number of day-7 cells was enhanced by 1.6-fold relative to the control number of cells when thalidomide (100 μM) was added during the first week. Conversely, when the drug was added during the second week, a dose-dependent decrease of the cell number was observed. Thus, thalidomide exhibited a selective activation of different effects at distinct stages of erythroid cell development.
Effects of thalidomide on cell growth and erythroid colony formation. (A) Human CD34+ cells were cultured in erythropoietin (EPO) medium with the indicated concentration of thalidomide for 2 weeks and counted on day 14. (B) Erythroid colonies (BFU-E + CFU-E) were calculated as numbers of colonies per well. CD34+ cells (1 × 103 cells/well) were cultured in methylcellulose with the indicated concentration of thalidomide. CFU-Es (▩) were evaluated at day 7 and BFU-Es (■) were evaluated at day 14. (C) Thalidomide (1-100 μM) was added to CD34+ cell cultures either for only the first week (days 0-7) or the second week (days 7-14), and the cells were counted on day 7 or day 14. Results are shown as means (± SD) from 3 different donors. *P < .05 versus untreated cells.
Effects of thalidomide on cell growth and erythroid colony formation. (A) Human CD34+ cells were cultured in erythropoietin (EPO) medium with the indicated concentration of thalidomide for 2 weeks and counted on day 14. (B) Erythroid colonies (BFU-E + CFU-E) were calculated as numbers of colonies per well. CD34+ cells (1 × 103 cells/well) were cultured in methylcellulose with the indicated concentration of thalidomide. CFU-Es (▩) were evaluated at day 7 and BFU-Es (■) were evaluated at day 14. (C) Thalidomide (1-100 μM) was added to CD34+ cell cultures either for only the first week (days 0-7) or the second week (days 7-14), and the cells were counted on day 7 or day 14. Results are shown as means (± SD) from 3 different donors. *P < .05 versus untreated cells.
Thalidomide induces γ-globin gene expression in primary human erythroid progenitors in a dose-dependent manner
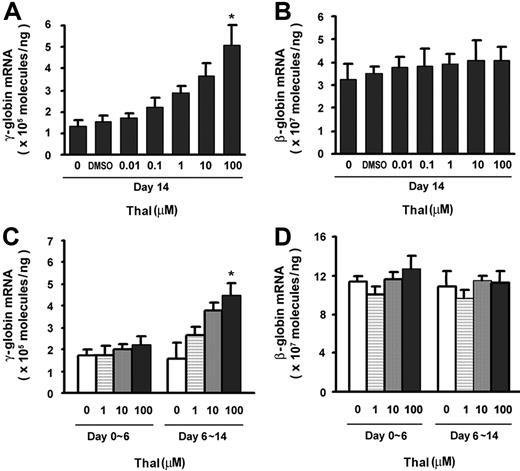
To evaluate the effects of thalidomide on γ- and β-globin expression, CD34+ cells were cultured with different concentrations (0.01-100 μM) of thalidomide for some or all of a 14-day period. The γ- and β-globin mRNA expression levels were determined by quantitative real-time RT-PCR. As shown in Figure 2A, thalidomide induced a dose-dependent increase in γ-globin expression of 3.8-fold (from 1.34 ± 0.87 × 105 molecules/ng in the control up to 5.12 ± 1.1 × 105 molecules/ng) at 100 μM after 2 weeks of treatment, while there was no effect on β-globin mRNA expression by thalidomide (Figure 2B). The γ/γ + β mRNA ratio increased 3.8-fold in the presence of 100 μM thalidomide (data not shown). Thus, thalidomide appears to exhibit relative specificity for γglobin mRNA expression during adult erythropoiesis.
Effects of thalidomide on expression of γ- and β-globin mRNA in erythroid progenitor cells. CD34+ cells were cultured for 14 days in EPO and treated with different concentrations of thalidomide (0.01-100 μM) over the indicated time periods (days 0-14, days 0-6, or days 6-14). (A,C) γ-globin and (B,D) β-globin mRNA levels were quantitated by real-time PCR. Results are shown as means (± SD) from 3 different donors. *P < .05 versus untreated cells.
Effects of thalidomide on expression of γ- and β-globin mRNA in erythroid progenitor cells. CD34+ cells were cultured for 14 days in EPO and treated with different concentrations of thalidomide (0.01-100 μM) over the indicated time periods (days 0-14, days 0-6, or days 6-14). (A,C) γ-globin and (B,D) β-globin mRNA levels were quantitated by real-time PCR. Results are shown as means (± SD) from 3 different donors. *P < .05 versus untreated cells.
To further investigate whether the effect of thalidomide on γ-globin gene expression is an early or late event in erythroid differentiation, we performed a time-course experiment to compare the effects of thalidomide on the early stage (proliferating erythroid) versus later stage (differentiated erythroid) of erythroid progenitor cell development. Surprisingly, there is no effect on γ-globin expression compared with the EPO control when thalidomide was present during days 0 to 6 of culture; however, when thalidomide was added during days 6 to 14, the γ-globin mRNA expression increased by 2.9-fold (from 1.58 ± 5.30 × 105 molecules/ng in the control up to 4.58 ± 3.74 × 105 molecules/ng; Figure 2C). There was no effect on β-globin expression during erythroid differentiation by thalidomide (Figure 2D). These results revealed that thalidomide's effect on γ-globin expression appeared to be greater at later stages of erythroblast maturation.
Thalidomide activates p38 MAPK and acetylation of histone H4
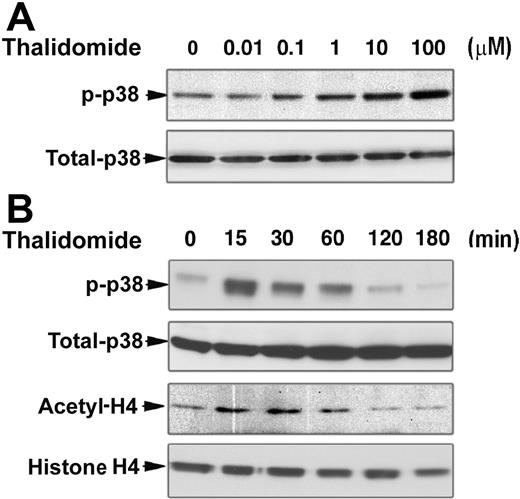
To explore the molecular mechanisms underlying γ-globin induction by thalidomide, we examined the effect of thalidomide on the kinase activity of p38 MAPK and acetylation of histone H4 in erythroid progenitor cells. Based on our results demonstrating that thalidomide induction of γ-globin gene is effective from days 6 to 14 in cultured CD34+ cells, we performed Western blot analysis of the p38 MAPK phosphorylation and histone H4 acetylation status of day-7 cells. As shown in Figure 3A and B, thalidomide induced the dose- and time-dependent phosphorylation of p38 MAPK in day-7 erythroid progenitor cells. We found that thalidomide increased p38 MAPK phosphorylation at 15 minutes; this activation was not seen at timepoints beyond 1 hour of exposure to thalidomide. Similar results were obtained for histone H4 acetylation. An increase in histone H4 acetylation was also evident following a 15-minute incubation with thalidomide. Acetylated histone H4 reached its maximum at 30 minutes and returned to basal levels after 1 hour. These results indicate that the activation of p38 MAPK pathway and acetylation of histone H4 might be necessary for thalidomide induction of γ-globin gene expression.
Thalidomide induces activation of p38 MAPK and acetylation of histone H4. CD34+ cells were cultured in EPO alone medium for 7 days prior to the addition of thalidomide. (A) Cells were treated with different concentrations of thalidomide for 30 minutes. (B) Cells were treated with 100 μM thalidomide for the indicated times. Cellular protein (30 μg) was analyzed by Western blot using phosphorylated p38 MAPK and acetyl-histone H4 antibodies. The membranes were stripped and reprobed with total p38 MAPK and histone H4 antibodies as indicated to confirm that similar amounts of protein extracts were analyzed in each lane.
Thalidomide induces activation of p38 MAPK and acetylation of histone H4. CD34+ cells were cultured in EPO alone medium for 7 days prior to the addition of thalidomide. (A) Cells were treated with different concentrations of thalidomide for 30 minutes. (B) Cells were treated with 100 μM thalidomide for the indicated times. Cellular protein (30 μg) was analyzed by Western blot using phosphorylated p38 MAPK and acetyl-histone H4 antibodies. The membranes were stripped and reprobed with total p38 MAPK and histone H4 antibodies as indicated to confirm that similar amounts of protein extracts were analyzed in each lane.
Thalidomide induces intracellular ROS
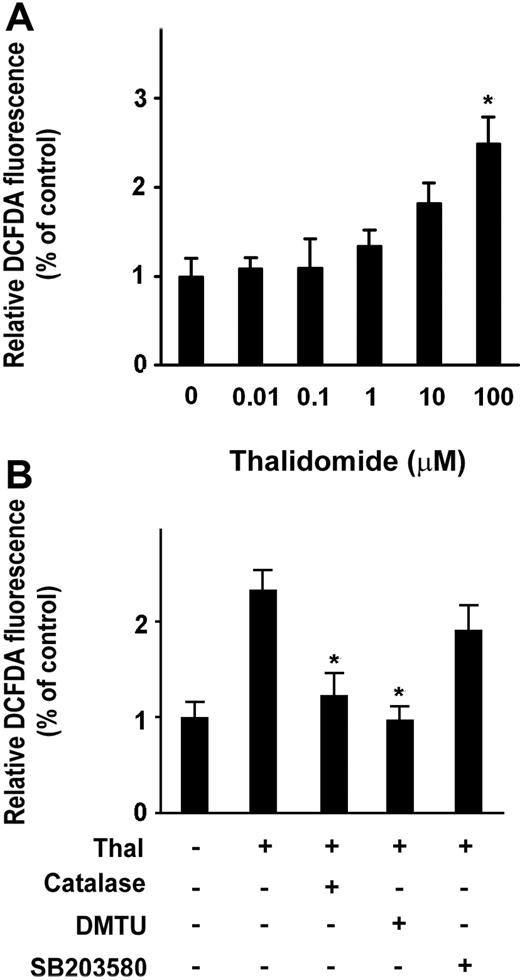
Thalidomide induced an increase in γ-globin mRNA, activation of p38 MAPK, and acetylation of histone H4 (Figures 2,3), suggesting a possibility that thalidomide might induce the γ-globin gene through the generation of ROS, which could then phosphorylate p38 MAPK and acetylate histone H4. We therefore examined the ability of thalidomide to increase intracellular ROS in erythroid progenitor cells using the cell permeant oxidant-sensitive fluorescent probe DCFDA. As shown in Figure 4A, treatment with 100 μM thalidomide increased the intracellular ROS level by 2.5-fold after 2 days of treatment (from day 3 to day 5 of culture) compared with thalidomide-untreated cells. We next tested how thalidomide-induced ROS production was affected by catalase, an enzymatic antioxidant that primarily scavenges hydrogen peroxide in vivo, and DMTU, a nonenzymatic antioxidant that strongly scavenges several ROS (specifically, hydroxyl radical [−OH], superoxide anion [O2−], and hypochlorous acid [HOCI]). As shown in Figure 4B, increased production of ROS induced by 100 μM thalidomide was significantly reduced by antioxidants catalase (4000 U/mL) and DMTU (10 mM). The inhibitory effect of DMTU on ROS (97.6% ± 13.5%) appeared to be stronger than that of catalase (123.2% ± 23%). In contrast, the p38 MAPK inhibitor SB023580 did not significantly inhibit thalidomide-induced production of ROS. These data suggest that thalidomide specifically generated hydroxyl radicals, which act upstream of the p38 MAPK signaling pathway.
Thalidomide induces intracellular ROS in erythroid progenitor cells. CD34+ cells were treated with the indicated concentration of thalidomide (A) or with 100 μM thalidomide in the presence or absence of catalase (4000 U/mL), DMTU (10 mM), or SB203580 (5 μM) (B) from day 3 to day 5 for 48 hours, followed by treatment with the DCFDA probe for 10 minutes. The intracellular reactive oxygen species (ROS) production was measured by flow cytometry. ROS levels induced by thalidomide were normalized to those of untreated cells as determined by monitoring the increased fluorescence in the cells. Results are shown as means (± SD) from 3 independent experiments. *P < .05 versus untreated cells.
Thalidomide induces intracellular ROS in erythroid progenitor cells. CD34+ cells were treated with the indicated concentration of thalidomide (A) or with 100 μM thalidomide in the presence or absence of catalase (4000 U/mL), DMTU (10 mM), or SB203580 (5 μM) (B) from day 3 to day 5 for 48 hours, followed by treatment with the DCFDA probe for 10 minutes. The intracellular reactive oxygen species (ROS) production was measured by flow cytometry. ROS levels induced by thalidomide were normalized to those of untreated cells as determined by monitoring the increased fluorescence in the cells. Results are shown as means (± SD) from 3 independent experiments. *P < .05 versus untreated cells.
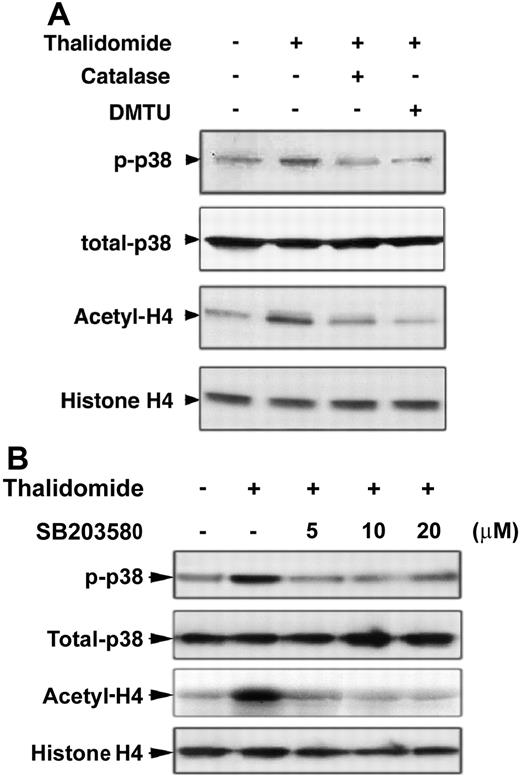
The antioxidants catalase and DMTU inhibit p38 MAPK phosphorylation and histone H4 acetylation induced by thalidomide
Because thalidomide induced ROS production and was inhibited by the antioxidants catalase and DMTU, we next evaluated whether thalidomide-induced phosphorylation of p38 MAPK and acetylation of histone H4 occurs through ROS generation. As shown in Figure 5A, pretreatment of day-7 cells with the antioxidants catalase (4000 U/mL) and DMTU (10 mM) for 30 minutes prevented thalidomide-induced p38 phosphorylation and histone H4 acetylation, demonstrating an involvement of ROS in these processes. Figure 5B demonstrates that p38 phosphorylation induced by thalidomide was also inhibited by the p38 MAPK inhibitor SB203580 at 5 or 10 μM. However, higher concentrations of SB203580 (30-50 μM) failed to inhibit the increased phosphorylation of p38 MAPK (data not shown).
Increased p38 MAPK phosporylation and acetylation of histone H4 by thalidomide inhibited by the antioxidants catalase and DMTU, and p38 inhibitor SB203580. CD34+ cells cultured in EPO medium for 7 days were pretreated with the antioxidants catalase (4000 U/mL) or DMTU (10 mM) (A) or with 5, 10, or 20 μM of the p38 MAPK inhibitor SB203580 (B) for 30 minutes, then coincubated with 100 μM thalidomide in the presence of indicated inhibitors for 30 minutes. Cell lysates (30 μg) were analyzed by Western blot using phosphorylated p38 MAPK and acetyl-histone H4 antibodies. Total p38 MAPK and histone H4 were analyzed as loading controls as described in Figure 3.
Increased p38 MAPK phosporylation and acetylation of histone H4 by thalidomide inhibited by the antioxidants catalase and DMTU, and p38 inhibitor SB203580. CD34+ cells cultured in EPO medium for 7 days were pretreated with the antioxidants catalase (4000 U/mL) or DMTU (10 mM) (A) or with 5, 10, or 20 μM of the p38 MAPK inhibitor SB203580 (B) for 30 minutes, then coincubated with 100 μM thalidomide in the presence of indicated inhibitors for 30 minutes. Cell lysates (30 μg) were analyzed by Western blot using phosphorylated p38 MAPK and acetyl-histone H4 antibodies. Total p38 MAPK and histone H4 were analyzed as loading controls as described in Figure 3.
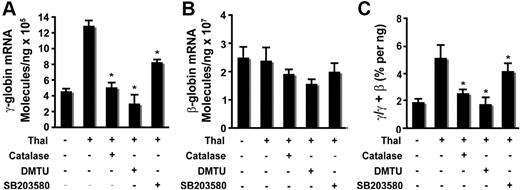
Thalidomide-induced generation of ROS is required for γ-globin gene activation and HbF induction
Our studies demonstrated that generation of ROS is required for thalidomide-induced activation of p38 MAPK and acetylation of histone H4. Therefore, to elucidate the mechanism by which thalidomide induced γ-globin through the ROS-dependent p38 MAPK signaling pathway, we used real-time PCR to determine the effects of antioxidants and p38 MAPK inhibitor SB203580 on downstream γ-globin gene expression in cultured CD34+ cells. On day 6, thalidomide was added to the culture medium of cells and incubated with or without antioxidant (4000 U/mL catalase or 10 μM DMTU) or p38 MAPK inhibitor SB203580 (5 μM) for 8 days (from days 6-14), and mRNA was collected on day 14 for analysis by quantitative RT-PCR. As shown in Figure 6A, the antioxidants catalase or DMTU or the p38 MAPK inhibitor SB203580 separately significantly reduced the thalidomide induction of γ-globin mRNA levels on day 14 (12.8 ± 0.66 × 105 molecules/ng in thalidomide-treated cells compared with 4.97 ± 0.47 × 105 molecules/ng in thalidomide plus catalase–treated cells (P < .01); 3.01 ± 0.98 × 105 molecules/ng in thalidomide plus DMTU–treated cells (P < .01); 8.27 ± 0.25 × 105 molecules/ng in thalidomide plus SB203580–treated cells (P < .01). These 2 antioxidants or the p38 MAPK inhibitor also slightly decreased β-globin mRNA compared with thalidomide-treated cells (Figure 6B), but those differences did not achieve statistical significance. The γ/γ + β ratio averaged 5.10% ± 0.95% in thalidomide-treated cells compared with 2.53% ± 0.28% in thalidomide plus catalase–treated cells (P < .05); 1.89% ± 0.46% in thalidomide plus DMTU (P < .05); and 4.01% ± 0.51% in thalidomide plus SB203580 (P < .05) (Figure 6C). These data suggest that thalidomide-induced γ-globin gene expression was through a mechanism that involves ROS-dependent activation of p38 MAPK cell signaling.
Thalidomide-induced γ-globin expression requires enhanced ROS and mediated p38 MAPK activation. CD34+ cells grown in EPO medium for 6 days were treated with 100 μM thalidomide in the presence or absence of the antioxidants catalase (4000 U/mL) or DMTU (10 mM) or the p38 MAPK inhibitor SB203580 (5 μM) from days 6 to 14. RNA extracted from cells harvested on day 14 was amplified by quantitative PCR to determine γ- and β-globin expression levels. (A) Average copy number of γ-globin molecules per 1 ng total RNA. (B) Average copy number of β-globin molecules per 1 ng total RNA. (C) Average ratio of γ/γ + β-globin percentages. Results are shown as means (± SD) from 3 independent donors that were analyzed in separate experiments. *P < .05 versus untreated cells.
Thalidomide-induced γ-globin expression requires enhanced ROS and mediated p38 MAPK activation. CD34+ cells grown in EPO medium for 6 days were treated with 100 μM thalidomide in the presence or absence of the antioxidants catalase (4000 U/mL) or DMTU (10 mM) or the p38 MAPK inhibitor SB203580 (5 μM) from days 6 to 14. RNA extracted from cells harvested on day 14 was amplified by quantitative PCR to determine γ- and β-globin expression levels. (A) Average copy number of γ-globin molecules per 1 ng total RNA. (B) Average copy number of β-globin molecules per 1 ng total RNA. (C) Average ratio of γ/γ + β-globin percentages. Results are shown as means (± SD) from 3 independent donors that were analyzed in separate experiments. *P < .05 versus untreated cells.
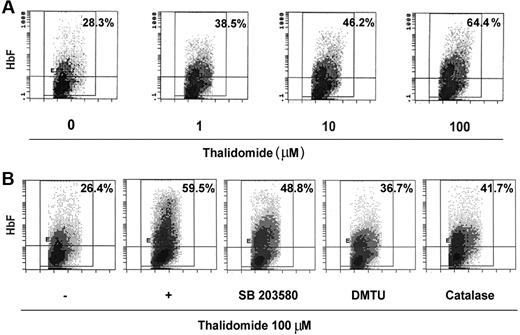
The effect of thalidomide on γ-globin mRNA prompted us to study the distribution of HbF-expression of cells during erythroid maturation. Because of thalidomide's significant effects on γglobin mRNA that occur during the second week of culture, we treated the CD34+ cells with 1 to 100 μM thalidomide from days 6 to 14. On day 14, cells were stained with a specific anti-HbF antibody and analyzed by flow cytometry. As shown in Figure 7A, thalidomide induced a dose-related HbF increase from 28.3% ± 3.2% HbF-containing cells with EPO alone up to 64.4% ± 8.2% with EPO and 100 μM of thalidomide. Treatment of day-6 cells with thalidomide (100 μM) in the presence or absence of DMTU or catalase from days 6 to 14 demonstrated that these inhibitors significantly inhibited the thalidomide-induced increase in HbF (59.5% ± 4.9% in EPO + 100 μM of thalidomide compared with 36.7% ± 6.8% in EPO + thalidomide plus 10 mM DMTU; 41.7% ± 5.2% in EPO + thalidomide plus 4000 U/mL catalase; Figure 7B). SB203580 partially inhibited the induction of HbF by thalidomide. These results are consistent with the quantitative RT-PCR studies (Figure 6) in demonstrating that the ROS-dependent p38 MAPK signaling involves the γ-globin gene and HbF induction.
Thalidomide induced fetal hemoglobin expression. Cells were cultured in EPO on days 0 to 6 and then were treated with the indicated concentration of thalidomide (A) or 100 μM thalidomide in the presence or absence of catalase (4000 U/mL), DMTU (10 mM), or SB203580 (5 μM) (B) from days 6 to 14. On day 14, cells were stained with fluorescently labeled anti-HbF antibodies and analyzed by flow cytometry. Panels represent fluorescence signal (y-axis) versus cell size (x-axis); the percentage of positive cells is shown in the top right corner of each panel. The panels shown are representative of the results for cultures from 3 separate donors.
Thalidomide induced fetal hemoglobin expression. Cells were cultured in EPO on days 0 to 6 and then were treated with the indicated concentration of thalidomide (A) or 100 μM thalidomide in the presence or absence of catalase (4000 U/mL), DMTU (10 mM), or SB203580 (5 μM) (B) from days 6 to 14. On day 14, cells were stained with fluorescently labeled anti-HbF antibodies and analyzed by flow cytometry. Panels represent fluorescence signal (y-axis) versus cell size (x-axis); the percentage of positive cells is shown in the top right corner of each panel. The panels shown are representative of the results for cultures from 3 separate donors.
Discussion
This study was undertaken to investigate the effect of thalidomide on γ-globin gene expression and the involved signaling pathway(s) that might provide further insights into the mechanism of up-regulation of the γ-globin gene. Herein, we demonstrated that thalidomide induced γ-globin gene expression and increased HbF protein production are associated with activation of p38 MAPK and acetylation of histone H4. Moreover, thalidomide induced the generation of ROS in erythroid progenitor cells, which we speculate activates p38 MAPK and acetylates histone H4, leading to subsequent γ-globin gene expression. This is the first report describing the role of ROS in thalidomide-induced γ-globin gene expression.
In this study, primary human erythroid progenitor cells were exposed to thalidomide at concentrations of 0.01 to 100 μM, which is the thalidomide dose most often used in ex vivo studies.24,25 We demonstrated that thalidomide reduced CFU-E colony growth, which is in accordance with previous findings that H2O2 inhibits CFU-E colony formation in CFU-E–enriched cells derived from peripheral blood.26 The most pronounced effects of thalidomide are achieved at 100 μM concentration; at this concentration, no cell toxicity was observed. Our quantitative RT-PCR results showed that thalidomide induced dose-dependent up-regulation of γ-globin expression, with this effect occurring upon the addition of thalidomide to late progenitors just prior to the terminal stages of differentiation. This unexpected result is similar to that of Urula et al, who earlier reported that stem cell factor (SCF) induced γ-globin expression effectively in the late stages of erythropoiesis.27
The role of cell signaling mechanisms in drug-mediated γglobin induction are becoming better defined. Stat5 signaling is involving in sodium butyrate (NaB)–mediated erythroid differentiation28 and γ-globin induction by short-chain fatty acids.29 Several HDAC inhibitors, including trichostatin A 30,31 and scriptaid32 induce γ-globin expression via p38 MAPK signaling. The p38 MAPK pathway is known to be activated by a variety of stimuli, including growth factors and cytokines, but also by physical or chemical stressing such as radiation, osmotic shock, anticancer drugs, heat shock, and oxidative stress. In this work, we provide evidence to demonstrate that thalidomide activated the p38 MAPK pathway in a time- and dose-dependent manner in erythroid progenitors, and this transient activation was reduced by the p38 MAPK inhibitor SB203580 at 5 to 10 μM. However, higher concentrations of SB203580 (30-50 μM) did not inhibit p38 MAPK activation and γ-globin up-regulation, but rather caused cell death (data not shown). These results suggest that transient activation of p38 MAPK appears to involve γ-globin gene induction, and also might evoke the survival signaling in this situation. Indeed, activation of p38 MAPK is also known to play important roles in cell survival and/or differentiation.33,34 Nagata et al35 have reported that transient activation of p38 MAPK is required for environmental stress-induced erythroid differentiation; however, prolonged activation of p38 MAPK by these stresses results in apoptosis in SKT6 cells. These observations, coupled with the ability of HDAC inhibitors to increase γ-globin expression, suggest that different stresses via distinct sensing pathways that converge on an upstream activator stimulate p38 MAPK activity, which might directly contribute to induction of γ-globin expression.
It has been proposed that chromatin remodeling is also involved in the induction of γ-globin gene expression by HDAC inhibitors.17,32 Considerable experimental evidence suggests that chromatin remodeling is involved in the developmental control of globin gene expression.36,37 Consistent with these and other previous studies,17,21,32 we also observed that increased phosphorylation of p38 MAPK coincides with increased acetylation of histone H4 after treatment with thalidomide, and inhibition of p38 MAPK activation by SB203580 reduced the increased acetylation of histone H4. These results suggest that activated p38 MAPK at least in part stimulated the histone acetylation, and this action might be involved in thalidomide-induced γ-globin expression.
ROS are generated by many physiologic processes and can affect signal transduction cascades by altering the activities of certain protein kinases and transcription factors, which can subsequently affect differentiation, proliferation, apoptosis, and gene expression. Several previous studies have shown that thalidomide leads to increased ROS production in vitro and in vivo.9,24 Other studies have demonstrated that oxidative stress activates p38 MAPK signaling and also causes increased histone acetylation in vitro.38,39 Therefore, we speculate that the action of thalidomide on erythroid progenitor cells may be due to generation of free radicals, which are then responsible for γ-globin gene induction through activation of p38 MAPK and acetylation of histone H4. In this study, our results clearly show that thalidomide enhances the ROS level in day 5 cells after 48 hours of treatment, and that this effect was significantly inhibited by the antioxidants catalase and DMTU. Moreover, our data revealed that catalase and DMTU dramatically abolished the thalidomide-induced activation of p38 MAPK and hyperacetylation of histone H4, and nearly completely diminish thalidomide-induced elevation of the ratio of γ-globin versus total globin in this culture system. These results strongly suggest that hydroxyl radicals may be the most important molecules among the ROS generated by thalidomide action, which are then responsible for γ-globin gene induction through activation of p38 MAPK and acetylation of histone H4.
Surprisingly, when cells were cocultured with the most widely used oxidant scavenger N-acetylcysteine (NAC), which is postulated to exert its effects by increasing intracellular glutathione (GSH) levels,40 NAC failed to inhibit the thalidomide-induced elevation of the ratio of γ-globin versus total globin in this culture system, even at a higher concentration (10 mM). However, at higher concentrations (5-10 mM), NAC completely abolished the control levels of both γ- and β-globin gene expression and, moreover, dramatically suppressed cell proliferation and terminal differentiation (data not shown). Our findings are in agreement with previous reports that have implicated the antioxidant NAC in suppressing EPO- and interleukin-3–induced intracellular signaling events, as well as cell proliferation and differentiation.41 Taken together, these data strongly support the idea that a physiologic concentration of ROS is important in the activation and propagation of intracellular signaling to maintain normal cellular function, such as cell proliferation and differentiation.
Recently, it has been reported that, consistent with our data, HDAC inhibitor–induced γ-globin expression involved ROS generation,22 suggesting that increased ROS might activate common p38 MAPK-signaling effectors that target the specific nuclear factors essential for γ-globin induction. A number of proteins have been identified which bind the promoter region for regulating γ-globin gene expression in adult erythropoiesis. Hence, therapeutic γ-globin reactivation could be accomplished by inhibition of repressor proteins to prevent silencing or enforced expression of trans-activators. Indeed, recent evidence has shown that γ-globin induction by HDAC inhibitors involves trans-activation of ATF2 and CREB1 via p38 MAPK signaling,42 and short-chain fatty acids induce γ-globin gene expression through displacement of a HDAC3-NCoR repressor complex.43 Further investigation will be required to determine whether thalidomide induced γ-globin expression through these factors or other mechanisms.
In summary, our results strongly suggest that thalidomide induced γ-globin expression in adult erythroid progenitor cells, which is mediated by ROS-dependent activation of the p38 MAPK pathway and acetylation of histone H4. These findings may lead not only to the quest for a better and complete understanding of the precise signals that regulate drug-mediated γ-globin expression, but also shed light on the identification of new molecular therapeutic targets for hemoglobinopathies.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Nicole M. Gantt in the Molecular Medicine Branch/NIDDK, NIH, for her skillful help with flow cytometry analyses.
Authorship
Contribution: W.A. designed and performed the research, analyzed data, and wrote the paper; J.Z. performed critical RT-PCR studies and analysis; Z.G. contributed to the analysis of the flow cytometry data; K.C. performed critical RT-PCR studies and analysis; and G.P.R. contributed to the design, interpretation, and writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Griffin P. Rodgers, Molecular and Clinical Hematology Branch, Bldg 10, Rm 9N-119, National Institutes of Health, Bethesda, MD 20892-2560; e-mail: gr5n@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal