Abstract
Individuals with systemic lupus erythematosus (SLE) have a striking increase in premature atherosclerosis of unclear etiology. Accelerated endothelial cell apoptosis occurs in SLE and correlates with endothelial dysfunction. Endothelial progenitor cells (EPCs) and myelomonocytic circulating angiogenic cells (CACs) are crucial in blood vessel repair after vascular damage, and decreased levels or abnormal function of EPCs/CACs are established atherosclerosis risk factors. We investigated if vascular repair is impaired in SLE. We report that SLE patients display abnormal phenotype and function of EPCs/CACs. These abnormalities are characterized by significant decreases in the number of circulating EPCs (310 ± 50 EPCs/mL of blood in SLE versus 639 ± 102 in controls) and significant impairments in the capacity of EPCs/CACs to differentiate into mature ECs and synthesize adequate levels of the proangiogenic molecules vascular endothelial growth factor (VEGF) and hepatic growth factor (HGF). These abnormalities are triggered by interferon-α (IFN-α), which induces EPC and CAC apoptosis and skews myeloid cells toward nonangiogenic phenotypes. Lupus EPCs/CACs have increased IFN-α expression and their supernatants promote higher induction of IFN-inducible genes. Importantly, neutralization of IFN pathways restores a normal EPC/CAC phenotype in lupus. SLE is characterized by an imbalance between endothelial cell damage and repair triggered by type I IFNs, which might promote accelerated atherosclerosis.
Introduction
Individuals with systemic lupus erythematosus (SLE) have up to a 50-fold increase in the incidence of cardiovascular disease (CVD) when compared with age- and sex-matched controls.1 While increases in CVD are secondary to premature atherosclerosis, Framingham risk factors do not account for this increased propensity.2 It is generally accepted that immune dysregulation plays a significant role in premature atherosclerosis development in SLE; however, the exact mechanisms leading to this complication have not been well characterized. We previously reported that SLE is characterized by accelerated endothelial cell (EC) apoptosis,3 which strongly correlates with endothelial dysfunction and with increased tissue factor generation. These findings indicate that accelerated vascular damage occurs in SLE and might participate in promotion of premature atherosclerosis, as apoptotic ECs are prothrombotic and the denudation of the endothelium promotes plaque formation.4 A crucial aspect to preserve endothelial integrity and prevent plaque formation following vascular damage/apoptosis is maintaining normal blood vessel repair.5 Vascular repair appears to be primarily mediated by 2 cell subsets: bone marrow (BM)–derived endothelial progenitor cells (EPCs)5 and myelomonocytic circulating angiogenic cells (CACs).6 Individuals with decreased circulating EPC numbers have higher rates of CV events and of hospitalization from CV complications compared with those with normal EPC levels.7 Patients with CV risk factors or diseases characterized by premature atherosclerosis display aberrant vascular repair, decreased EPC levels, and abnormal EPC/CAC function.8,9 Whether a similar phenomenon occurs in SLE and contributes to vascular damage is unclear. Here we report significant abnormalities in the phenotype and function of lupus EPCs/CACs. Importantly, we report for the first time that IFN-α, previously proposed to play a crucial role in SLE pathogenesis,10 promotes abnormal vascular repair in lupus and could potentially be involved in triggering premature atherosclerosis in this disease. These results point at an imbalance between EC damage and repair in lupus triggered by IFN-α
Patients, materials, and methods
Patient population
The University of Michigan institutional review board (IRB) approved this study. Subjects gave informed consent in accordance with the Declaration of Helsinki. Patients fulfilled the revised American College of Rheumatology criteria for SLE,11 had no history of atherosclerotic disease, and were enrolled at the University of Michigan and from the Michigan Lupus Cohort. Healthy controls were recruited by advertisement and from University of Michigan Women's Health Registry. Lupus disease activity was assessed by SLE Disease Activity Index (SLEDAI).12 Of all the SLE patients studied, 4% had diabetes mellitus, 20% had systemic hypertension, 15% had current and/or past smoking history, 8% had low high-density lipoprotein (HDL) levels, 14% had high low-density lipoprotein (LDL) levels, and all patients had normal homocysteine levels at the time of blood collection. For all specific experiments, controls and SLE patients matched for demographic and metabolic parameters and there were no significant differences between these groups.
Characterization of BM-derived EPCs
EPCs were characterized following described protocols, with some modifications.8 Peripheral blood mononuclear cells (PBMCs) were obtained from blood by Ficoll-Hypaque gradient and stained with anti-CD34–FITC (Ancell, Bayport, MN) and anti-CD133–PE (Miltenyi, Auburn, CA), in combination with a cocktail of PE/cy5-conjugated antibodies recognizing CD3, CD79b, and CD56 (BD, San Jose, CA). Immunofluorescence was measured using a Coulter XL flow cytometer (Hialeah, FL). EPCs were identified in the lymphocyte population as CD34+/CD133+ cells in the CD3−/CD79b−/CD56− gate. The number of positive events detected by fluorescence-activated cell sorter (FACS) was divided by the sum of lymphocytes plus monocyte events obtained during the acquisition. The percentage of CD34+/CD133+ cells in the PBMC pool (CD34/CD133 divided by lymphocytes plus monocytes) was used to calculate the total number of CD34+/CD133+ cells recovered from the PBMC isolation and then divided by the initial blood volume to determine CD34+/CD133+ cells/mL blood.
In vitro differentiation into mature ECs
PBMCs (4 × 106/mL) were cultured in EC-specific enrichment medium (EBM2; Cambrex, East Rutherford, NJ) supplemented with 20% FBS, bovine brain extract, and epithelial growth factor (EGF) as described.8 Media was changed 120 hours after plating, then every 3 days. On days 7 to 21, cells were incubated with markers of mature ECs, including 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (diI)–acetylated LDL (Biomedical Technologies, Stoughton, MA), FITC–anti–von Willebrand factor (VWF; Serotec, Raleigh, NC), and Texas Red–Ulex europaeus agglutinin-1 (UEA-1; ICN, Irvine, CA). To assess nuclear morphology, cells were stained with Hoechst 33342 (Invitrogen, Carlsbad, CA). Cells were analyzed by fluorescent microscopy using a Leica DMIRB fluorescent inverted microscope (Bannockburn, IL). The VWF-FITC images were acquired on fixed permeabilized cell monolayers, washed, and imaged in PBS. All other images were acquired at room temperature using live cells in PBS without mounting media. Images were acquired with an objective magnification of × 5, × 10, or × 20 (for × 50, × 100, or × 200 total magnification, respectively). The numeric apertures for the objective lenses of the fluorescent microscope were as follows: × 5 = 0.15; × 10 = 0.3; × 20 = 0.4. Images were acquired with an Olympus DP30BW camera (Olympus Corporation, Tokyo, Japan) using the acquisition software Olympus-BSW (Olympus). Final processing was done with Adobe Photoshop CS2 (San Jose, CA).
For expression of myelomonocytic markers, cells were stained with anti-CD14–PE and anti–HLA-DR, anti–HLA-P, and anti–HLA-Q–FITC (Ancell) and analyzed by FACS.
When indicated, cells were cultured in the presence or absence of graded concentrations of either blocking antihuman–IFN-α (R & D, Minneapolis, MN), blocking anti–type I IFN receptor (IFN-R; PBL, Piscataway, NJ), a mixed monoclonal pool of neutralizing IFN-α antibodies (Abs; Chemicon, Temecula, CA), polyclonal anti–IFN-α (PBL), neutralizing antihuman TRAIL (Biolegend, San Diego, CA), or respective isotype controls before assessing phenotype. In additional experiments, cells were similarly cultured in the presence or absence of 20% heat-inactivated lupus or control allogeneic serum without FBS, graded concentrations of recombinant human vascular endothelial growth factor (VEGF, National Cancer Institute [NCI], Bethesda, MD), or recombinant IFN-α2b (Schering, Kenilworth, NJ).
Toll-like receptor 7 (TLR7) and/or TLR9 inhibitors were used at 0.3 μM and prepared as described.13 Prototypes for the immunoregulatory sequences (IRS) classes used for TLR inhibition were as follows: TLR9 inhibitor (IRS 869), 5′-TCC TGG AGG GGT TGT-3′; TLR-7 inhibitor (IRS 661), 5′-TGC TTG CAA GCT TGC AAG CA-3′; and TLR-7 and 9 inhibitor (IRS 954), 5′-TGC TCC TGG AGG GGT TGT-3′. Control oligonucleotide (ODN) was 5′-TCC TGC AGG TTA AGT-3′. All were phosphorothioate ODNs and had less than 5 endotoxin U/mg ODN, determined by Limulus amebocyte lysate assay (Cambrex).
mRNA isolation, reverse transcriptase–polymerase chain reaction, real-time PCR, and quantification of proangiogenic molecules and IFN-inducible genes
Total RNA prepared with Tri-pure (Roche, Indianapolis, IN) was reverse transcribed to cDNA. Oligonucleotide primers (Integrated DNA Technologies, Coralville, IA) used in the reactions were as follows: VWF: 5′-CATGACACTGAAGCGTGATGA-3′ (forward), 5′-GCCTGGCAGTGATGTCGTT-3′ (reverse); VEGF-A: 5′-ATCTTCAAGCCATCCTGTGTGC-3′ (forward), 5′-GCTCACCGCCTCGGCTTGT-3′ (reverse); VEGF-B: 5′-CCTGACGATGGCCTGGAGTGT-3′ (forward), 5′-GCCATGTGTCACCTTCGCAG-3′ (reverse); VEGF-C: 5′-ATGTTTTCCTCGGATGCTGGA-3′ (forward), 5′-CATTGGCTGGGGAAGAGTTTG-3′ (reverse); VEGF-D: 5′-GTATGGACTCTCGCTCAGCAT-3′ (forward), 5′-AGGCTCTCTTCATTGCAACAG-3′ (reverse); hepatic growth factor (HGF): 5′-TTCTTTCACCCAGGCATCTC-3′ (forward), 5′-ATTAGCACATTGGTCTGCAG-3′ (reverse); GAPDH: 5′-CCA CCC ATG GCA AAT TCC ATG GCA-3′ (forward), 5′-TCT AGA CGG CAG GTC AGG TCC ACC-3′ (reverse).
Cycling conditions were as follows: denaturing at 95°C for 1 minute, annealing at 60°C for 1 minute, and extension at 72°C for 1 minute for 30 cycles for all except for VEGF-B and VEGF-C, where denaturing at 95°C for 1 minute, annealing at 56°C for 1 minute, and extension at 72°C for 1 minute for 35 cycles was performed.
Induction of IFN-inducible genes by EPC/CAC supernatants and autologous sera was determined using a described bioassay14 that measures the functional effects of serum or cell supernatant components on the gene expression of cultured target cells (an epithelial cell line). To this end, the epithelial cell line HeLa was cultured in DIFCO/10% FBS/nonessential amino acids/10 mM Hepes at 37°C in 5% CO2; plated at 2 × 105 cells/well in a 24-well plate; and exposed to 100% lupus or control EC monolayer–conditioned media, 50% lupus or control sera, or recombinant IFN-α (1 kU/well, used as positive control) or recombinant IFN-γ (200 ng/well, used as negative control for type I IFN-inducible genes; Peprotech, Rockyhill, NJ) for 6 hours. TriPure was added and cells were stored at −70°C until RNA extraction. cDNA was made with Superscript II reverse transcriptase (Invitrogen). Real-time PCR reactions were run on an ABI PRISM 7900HT in duplicate using 2 × SYBR GREEN PCR master mix (Applied Biosystems, Foster City, CA) and primers previously described,14 at 2.5 μM concentration. The type I IFN-inducible genes quantified by this assay were IFN-induced protein-44 (IFI44), myxovirus resistance-1 (MX1), IFN-induced protein with tetratricopeptide repeats 1 (IFIT1), and double-stranded RNA-activated protein kinase (PRKR). Primers for these genes have been previously described.14 Samples were normalized to media alone after normalization to housekeeping gene HPRT-1, and results were reported as fold induction/media.15
Serum proangiogenic factors
Serum VEGF, HGF, EGF, and angiostatin were quantified by enzyme-linked immunosorbent assay (ELISA; R&D), following manufacturer's instructions.
Matrigel assay
A matrigel tube formation assay was performed to assess the ability of EPCs to incorporate into EC vascular structures, which is considered important in new vessel formation.16 Matrigel was spread onto 4- or 16-well chamber slides. Human umbilical vein endothelial cells (HUVECs) were labeled with Hoeschst 33342 (2 ng/mL). Day 7 EPCs were stained with Hoeschst 33342 (2 ng/mL) and CFSE (carboxy fluoroscein succinimidyl ester; 4.5 nM). HUVECs (22 × 103 cells/cm2) were mixed with EPCs (11 × 103cells/cm2) in complete EGM media, plated on matrigel, and incubated at 37°C for 18 to 24 hours. Tubules were quantified as previously described.16 EPC incorporation was quantified by counting the total number of nuclei and the number of nuclei colocalizing with CSFE-labeled cells in 10 random fields.
Quantification of intracellular IFN-α
Control and SLE PBMCs were plated in complete EC media for 24 to 72 hours; washed; treated with brefeldin A (BD) for 4 hours; fixed; permeabilized; incubated with goat antihuman IFN-α monoclonal antibody (mAb), rabbit polyclonal anti–IFN-α, or control Abs; washed; incubated with PE goat antimouse or FITC goat antirabbit IgG; and analyzed by FACS.
Apoptosis assessment
Control PBMCs were obtained by leukapheresis and Ficoll gradient. Monocytes and T, B, and natural killer (NK) cells were depleted by positive selection with magnetic beads; remaining cells were stained with anti-PE–CD133 (Miltenyi), positively sorted by FACS, and cultured on fibronectin-coated wells in EC-specific medium, in the presence or absence of recombinant IFN-α2b for up to 5 days. Viability was analyzed by incubating cells with annexin V–FITC (BD), and the percentage of annexin V+ cells was measured by FACS. Similar experiments were performed using BM CD133+ cells (Stem-Cell Technologies, Vancouver, BC, Canada) or with total PBMCs. IFN-treated PBMCs were also analyzed for expression of CD11c, DC-SIGN, CD86, and CD14 using fluorophore-labeled mAbs (BD).
Statistical analysis
Difference between means was analyzed using Student t test or analysis of variance (ANOVA), with Stata v.9 (StataCorp, College Station, TX) or SPSS v.14 (SPSS, Chicago, IL). To determine whether treatment with immunosuppressants was associated with phenotypic/functional abnormalities, univariate linear regression was performed. Vascular repair markers were modeled separately as dependent variables, with medications modeled as dichotomous independent predictors. For the IFN-inducible gene experiments, 2-group comparisons of continuous data that had a normal distribution were assessed using t tests. The Kruskal-Wallis nonparametric test was used to compare the study groups for the values of the IFN-inducible genes because the data were not normally distributed.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Lupus patients have decreased levels of circulating EPCs
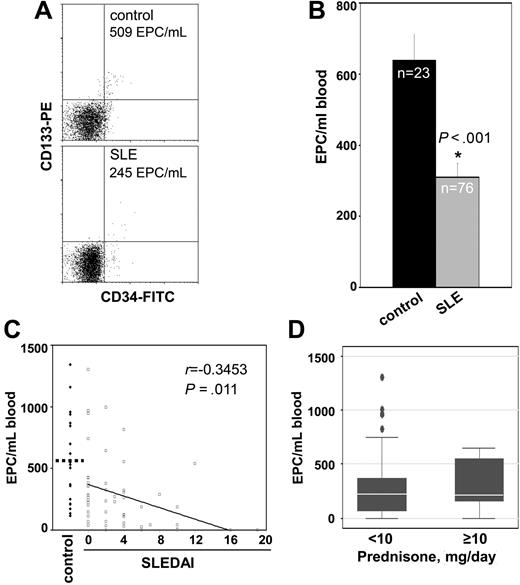
Compared with healthy controls, SLE patients displayed significantly decreased circulating EPC levels (Figure 1A,B), which suggests that BM responses to vascular injury are impaired. Since the majority of the SLE patients are women, we also compared EPC levels in control females versus SLE females and confirmed a significant decrease in the SLE group (SLE = 306 ± 38 EPCs/mL of blood vs control = 770 ± 104 EPCs/mL of blood; P < .001). Decreased EPC numbers correlated with SLEDAI. However, even those individuals with no clinical or serologic disease activity (SLEDAI = 0) had pronounced EPC decreases compared with controls (Figure 1C). Similar significant correlations were found when females only were analyzed (r = −0.42, P = .01). Demographic and clinical characteristics of patients and controls are presented in Table 1. No correlation was found between the use of specific medications or daily corticosteroid doses and EPC numbers (Figure 1D). While previous studies have reported that kidney dysfunction is associated with decreased circulating EPCs,17 we found no correlation between low EPC numbers and serum creatinine level (r = −0.02, P = .87); protein-creatinine ratio (r = −0.83, P = .65); or a kidney biopsy consistent with lupus nephritis (r = 0.08, P = .49). Given that this was a cohort of patients with overall low Framingham scores, we found no significant statistical correlation between low levels of EPCs and specific Framingham risk factors (low HDL level, P = .9; high LDL level, P = .75; smoking, P = .34; diabetes, P = .64; or homocysteine levels, P = .4), indicating that lupus-specific mechanisms drive the abnormal vascular repair responses. Also, there was no correlation between presence and/or levels of antiphospholipid (APL) Abs, anti–β-2 glycoprotein, and/or lupus anticoagulant with decreased EPCs (not shown).
SLE EPCs are decreased in peripheral blood. (A) Dot plots of 1 representative control and 1 representative SLE patient displaying no. of EPCs/mL of blood. Total events for acquisition were as follows: for controls, 127 000 ± 18 000; for SLE, 87 000 ± 6400 (mean ± SEM). (B) Results represent the mean (± SEM). (C) Decreased EPC numbers in SLE correlate with disease activity. EPCs are plotted as a function of SLEDAI score in individual patients. EPC levels in controls also exceed those in lupus patients with SLEDAI scores of zero (639 EPC/mL blood in control vs 370 EPC/mL blood in SLEDAI = 0, P < .05). (D) Lack of association between circulating EPCs and daily prednisone dose in SLE. Error bars are 95% CI whiskers.
SLE EPCs are decreased in peripheral blood. (A) Dot plots of 1 representative control and 1 representative SLE patient displaying no. of EPCs/mL of blood. Total events for acquisition were as follows: for controls, 127 000 ± 18 000; for SLE, 87 000 ± 6400 (mean ± SEM). (B) Results represent the mean (± SEM). (C) Decreased EPC numbers in SLE correlate with disease activity. EPCs are plotted as a function of SLEDAI score in individual patients. EPC levels in controls also exceed those in lupus patients with SLEDAI scores of zero (639 EPC/mL blood in control vs 370 EPC/mL blood in SLEDAI = 0, P < .05). (D) Lack of association between circulating EPCs and daily prednisone dose in SLE. Error bars are 95% CI whiskers.
Demographic characteristics of patients
| Variable . | Control, n = 60 . | SLE, n = 135 . |
|---|---|---|
| Females, % | 55 | 95 |
| Age, mean (SEM) | 38 (3) | 41 (1.3) |
| Disease activity SLEDAI, mean | — | 4.8 (5.2) |
| (SEM) | ||
| SLEDAI less than 2, % | — | 39 |
| SLEDAI 2 or greater, % | — | 61 |
| Medications, % | — | |
| Antimalarials | — | 66 |
| Azathioprine | — | 4 |
| Mycophenolate | — | 11 |
| Cyclophosphamide | — | 3 |
| Methotrexate | — | 7 |
| Prednisone less than 0.5 mg/kg/d | — | 58 |
| Prednisone 0.5–1 mg/kg/d | — | 6 |
| Prednisone greater than 1 mg/kg/d | — | 1 |
| No immunosuppression | — | 12 |
| Past or current lupus nephritis, % | — | 25 |
| Positive antiphospholipid Ab, β2-glycoprotein antibody, and/or lupus anticoagulant, % | — | 15 |
| Variable . | Control, n = 60 . | SLE, n = 135 . |
|---|---|---|
| Females, % | 55 | 95 |
| Age, mean (SEM) | 38 (3) | 41 (1.3) |
| Disease activity SLEDAI, mean | — | 4.8 (5.2) |
| (SEM) | ||
| SLEDAI less than 2, % | — | 39 |
| SLEDAI 2 or greater, % | — | 61 |
| Medications, % | — | |
| Antimalarials | — | 66 |
| Azathioprine | — | 4 |
| Mycophenolate | — | 11 |
| Cyclophosphamide | — | 3 |
| Methotrexate | — | 7 |
| Prednisone less than 0.5 mg/kg/d | — | 58 |
| Prednisone 0.5–1 mg/kg/d | — | 6 |
| Prednisone greater than 1 mg/kg/d | — | 1 |
| No immunosuppression | — | 12 |
| Past or current lupus nephritis, % | — | 25 |
| Positive antiphospholipid Ab, β2-glycoprotein antibody, and/or lupus anticoagulant, % | — | 15 |
— indicates not applicable.
Abnormal in vitro EPC/CAC function in SLE
To assess the in vitro phenotype and function of lupus EPCs and myelomonocytic CACs, the capacity of PBMCs (which contain both CACs and EPCs18 ) to become mature ECs under conditions that promote this phenotype was examined using described protocols.9 Control PBMCs clearly differentiated into mature ECs with significant diI-acetylated LDL uptake and expression of VWF and UEA-1 (Figures 2A, 3A), indicating that culture conditions were appropriate and consistent with previous publications. Furthermore, VWF expression at the mRNA level did not differ between SLE and control cells (not shown). Strikingly, while a mature EC monolayer was formed by 96% of healthy controls, only 22% of lupus patients did so (Figure 2C). While comparable numbers of EPCs (control, 143 000 ± 25 000/well; SLE, 103 000 ± 30 000/well; P = not significant) and of total PBMCs were initially plated, most lupus patients typically displayed scattered or clustered ECs but no monolayer formation by day 15 (Figures 2B, 3A). Lupus patients whose cells formed EC monolayers had lower SLEDAIs than patients whose cells could not form mature ECs (1.75 ± 0.70 vs 4.04 ± 0.76, respectively; mean ± SEM; P = .04). Furthermore, significantly fewer SLE EPCs were incorporated into tubules in a matrigel assay compared with the normal control EPCs (36% reduction in EPC incorporation, P = .04). There was no correlation between the capacity to differentiate into mature ECs and lupus nephritis, Framingham risk factors, homocysteine levels, or APL Abs. Similarly, no correlation was found between medication use and the inability to form EC monolayers. These results suggest that lupus EPCs and/or CACs, present in the PBMC fraction, are abnormal in their capacity to become mature ECs under proangiogenic stimulation.
Lupus PBMCs fail to form mature EC monolayers. (A) Control PBMCs were plated into fibronectin-coated wells in complete EC media. On day 15, cells were stained with anti–VWF-FITC, UEA-1–Texas red, and Hoechst 33342 and analyzed by fluorescent microscopy. Images are from 1 representative healthy control and show single fluorophores and a merged image (bottom; × 20 objective magnification). (B) On day 15, wells were examined for EC monolayer development. Representative images of PBMC-derived cells from a healthy control and a lupus patient (× 5 objective magnification). See “In vitro differentiation into mature ECs” for more image acquisition information. (C) The y-axis represents the percentage of controls (13/14) or lupus patients (7/33) that formed an EC monolayer.
Lupus PBMCs fail to form mature EC monolayers. (A) Control PBMCs were plated into fibronectin-coated wells in complete EC media. On day 15, cells were stained with anti–VWF-FITC, UEA-1–Texas red, and Hoechst 33342 and analyzed by fluorescent microscopy. Images are from 1 representative healthy control and show single fluorophores and a merged image (bottom; × 20 objective magnification). (B) On day 15, wells were examined for EC monolayer development. Representative images of PBMC-derived cells from a healthy control and a lupus patient (× 5 objective magnification). See “In vitro differentiation into mature ECs” for more image acquisition information. (C) The y-axis represents the percentage of controls (13/14) or lupus patients (7/33) that formed an EC monolayer.
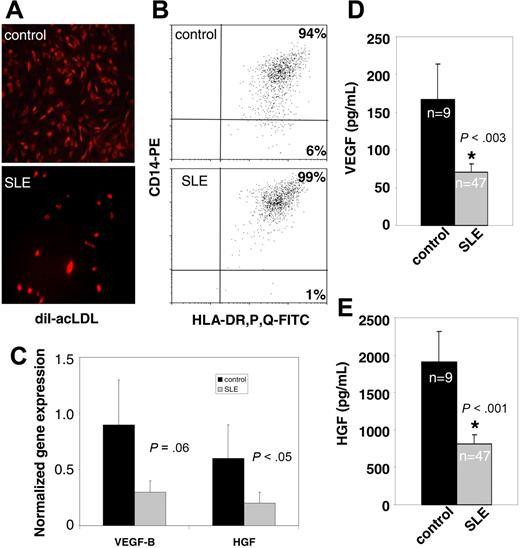
EC monolayer phenotype in SLE and controls. (A) No differences in acetylated-LDL uptake were detected between SLE and controls, although there were significantly fewer cells in SLE cultures at day 15 (× 10 objective magnification). See “In vitro differentiation into mature ECs” for more image acquisition information. (B) Day 15 EC monolayers were analyzed for expression of HLA-DP, -R, and -Q and CD14. Dot plots display a representative healthy control and lupus patient. MHC class II and CD14 expression is consistent with CD14-derived CACs and did not differ between SLE and controls. (C) mRNA expression of proangiogenic factors on day 15 ECs from controls or SLE, normalized to GAPDH. HGF and VEGF-B expression was lower in lupus patients (1-tailed t test, P < .05 for HGF and P = .06 for VEGF-B). Results are mean (± SEM) of 8 patients and 4 controls. (D-E) Proangiogenic molecules are decreased in lupus sera. Results represent mean (± SEM) of 9 controls and 47 SLE patients.
EC monolayer phenotype in SLE and controls. (A) No differences in acetylated-LDL uptake were detected between SLE and controls, although there were significantly fewer cells in SLE cultures at day 15 (× 10 objective magnification). See “In vitro differentiation into mature ECs” for more image acquisition information. (B) Day 15 EC monolayers were analyzed for expression of HLA-DP, -R, and -Q and CD14. Dot plots display a representative healthy control and lupus patient. MHC class II and CD14 expression is consistent with CD14-derived CACs and did not differ between SLE and controls. (C) mRNA expression of proangiogenic factors on day 15 ECs from controls or SLE, normalized to GAPDH. HGF and VEGF-B expression was lower in lupus patients (1-tailed t test, P < .05 for HGF and P = .06 for VEGF-B). Results are mean (± SEM) of 8 patients and 4 controls. (D-E) Proangiogenic molecules are decreased in lupus sera. Results represent mean (± SEM) of 9 controls and 47 SLE patients.
EPCs/CACs from SLE patients display aberrant production of proangiogenic molecules
Phenotypic analysis of the cells in culture indicates that greater than 90% of the cells that constitute the EC monolayers in healthy controls and SLE at day 15 are derived from myelomonocytic CACs. Indeed, these cells display a phenotype consistent with an EC (expressing VWF and UEA-1, taking up acetylated LDL; Figures 2A, 3A and data not shown) but also express the myeloid markers CD14 and class II major histocompatibility complex (MHC; Figure 3B). Similar findings have been described in other patient populations by other groups.19 While there were significantly fewer ECs in the SLE than in control cultures, lupus cells express similar amounts of VWF, UEA-1, and acLDL uptake and equivalent levels of expression of myeloid markers (Figures 2A, 3A,B). In addition to their capacity to differentiate into ECs, both CACs and EPCs are endowed with the capability of secreting proangiogenic factors, including VEGF and HGF, that promote EPC BM release, homing, and incorporation into blood vessels.19 Synthesis of specific proangiogenic molecules by EPCs/CACs was analyzed at the mRNA level. After plating equal numbers of PBMCs containing comparable amounts of EPCs, SLE patients displayed decreased VEGF-B and HGF mRNA compared with controls (Figure 3C). These in vitro results correlated with serum levels, as significantly decreased levels of VEGF and HGF were detected in SLE (Figure 3D,E). Other proangiogenic molecules tested at the mRNA and protein level were not significantly different between SLE and controls (not shown). These results indicate that lupus EPCs/CACs have aberrant production of proangiogenic factors crucial in angiogenesis.
IFN-α expression is increased in lupus EPCs/CACs
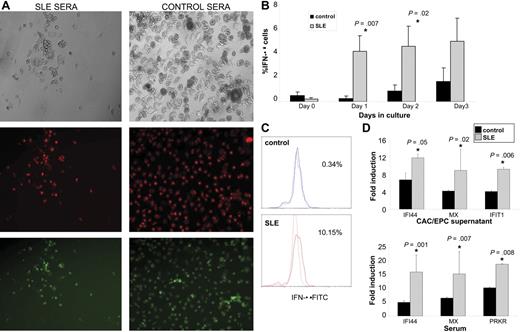
Different soluble or cellular factors may potentially be involved in the induction of abnormal vasculogenesis in SLE. To assess the potential role of a soluble factor present in SLE serum promoting this abnormal phenotype, we proceeded to treat control PBMCs with 20% allogeneic control or SLE serum when cells were undergoing proangiogenic stimulation to become mature ECs. As shown in Figure 4A, control cells treated with SLE (but not with control) sera were not capable of forming an EC monolayer at day 15 and acquired a similar phenotype to that seen in SLE EPCs/CACs. Given that a soluble factor present in SLE serum appears to be involved in the induction of abnormal vasculogenesis, we investigated whether a number of cytokines potentially involved in SLE pathogenesis could induce EPC/CAC cytotoxicity and impair EC monolayer formation. After analyzing 10 different cytokines (IL-2, IL-3, IL-4, IL-6, IL-10, IL-13, TNF-α, IFN-α, IFN-γ, and G-MCSF), using a range that included the maximum concentration described to induce specific effects on PBMCs, ECs, and/or EPCs,20-26 only IFN-α induced significant cytotoxicity in healthy control EPC/CACs. IFN-α is a multifunctional cytokine capable of interfering with viral infection, inhibiting cell proliferation, regulating cell differentiation, and modulating immune responses.27 Importantly, type I IFNs are inhibitors of angiogenesis/vasculogenesis in normal and pathologic conditions.28-30 Recently, different groups have proposed that IFN-α plays a crucial role in SLE pathogenesis by triggering many of the immune abnormalities seen in this disease.10 Given the link of IFN-α with impaired angiogenesis on one hand and with lupus pathogenesis on the other, we tested whether the antiangiogenic responses seen in SLE were secondary to the effects of this molecule. We first tested if cells in the EPC/CAC monolayer expressed intracellular IFN-α and, if so, whether higher levels of IFN-α were expressed by lupus EPCs/CACs compared with healthy controls. IFN-α expression was detectable by FACS in the monocyte/CAC gate within 24 hours and was significantly more pronounced in SLE patients both as percentage of cells expressing IFN-α (Figure 4B,C) and as mean fluorescent intensity (control, 10 ± 2 vs SLE, 31 ± 9; P = .04 on day 1; control, 24 ± 12 vs SLE, 45 ± 10; P = .05 on day 2). Since currently available ELISAs to quantify soluble IFN-α in SLE have been reported to be unreliable31 and to further compare the effects of IFN-α production, we quantified the effect of EPC/CAC supernatants or autologous sera on the induction of type I IFN-inducible genes on epithelial HeLa cells following a published protocol.14 Compared with healthy controls, supernatants from lupus EC monolayers induced significantly higher induction of the IFN-α–inducible genes IFI44, MX1, and IFIT1. As expected, autologous lupus sera also induced higher expression of the IFN-inducible genes IFI44, MX1, and PRKR (Figure 4D). These results suggest an increased production of IFN-α within a subset of cells in lupus EPCs/CACs.
Increased IFN-α expression in lupus EPCs/CACs. (A) Lupus serum prevents monolayer formation by healthy control EPCs/CACs. Control PBMCs were plated on fibronectin-coated wells with complete EC media in the presence or absence of 20% allogeneic control or SLE sera. At day 15, EC monolayer formation was assessed. Images are representative of 7 of 8 SLE serum samples and 4 of 4 control serum samples used in 5 allogeneic control PBMCs. Images represent bright field (top), diI–ac-LDL (middle), and UEA-1–FITC (bottom) of allogeneic control cells exposed to 1 representative SLE serum sample (left) and 1 representative control serum sample (right; × 10 objective magnification). See “In vitro differentiation into mature ECs” for more image acquisition information. (B) Graphs represent percentage IFN-α expression (± SEM) of 13 SLE and 6 controls in PBMCs cultured under proangiogenic conditions. (C) IFN-α expression on day 1–cultured cells of a representative control and SLE patient. (D) EPC/CAC supernatants and autologous serum from SLE patients induce higher expression of IFN-α–inducible genes in epithelial cell lines than controls. Results represent fold induction of IFN-inducible genes (mRNA) and are presented as mean (± SEM) of supernatants or sera from 8 controls and 23 SLE patients. Data are normalized to HPRT-1.
Increased IFN-α expression in lupus EPCs/CACs. (A) Lupus serum prevents monolayer formation by healthy control EPCs/CACs. Control PBMCs were plated on fibronectin-coated wells with complete EC media in the presence or absence of 20% allogeneic control or SLE sera. At day 15, EC monolayer formation was assessed. Images are representative of 7 of 8 SLE serum samples and 4 of 4 control serum samples used in 5 allogeneic control PBMCs. Images represent bright field (top), diI–ac-LDL (middle), and UEA-1–FITC (bottom) of allogeneic control cells exposed to 1 representative SLE serum sample (left) and 1 representative control serum sample (right; × 10 objective magnification). See “In vitro differentiation into mature ECs” for more image acquisition information. (B) Graphs represent percentage IFN-α expression (± SEM) of 13 SLE and 6 controls in PBMCs cultured under proangiogenic conditions. (C) IFN-α expression on day 1–cultured cells of a representative control and SLE patient. (D) EPC/CAC supernatants and autologous serum from SLE patients induce higher expression of IFN-α–inducible genes in epithelial cell lines than controls. Results represent fold induction of IFN-inducible genes (mRNA) and are presented as mean (± SEM) of supernatants or sera from 8 controls and 23 SLE patients. Data are normalized to HPRT-1.
IFN-α is toxic to EPCs/CACs
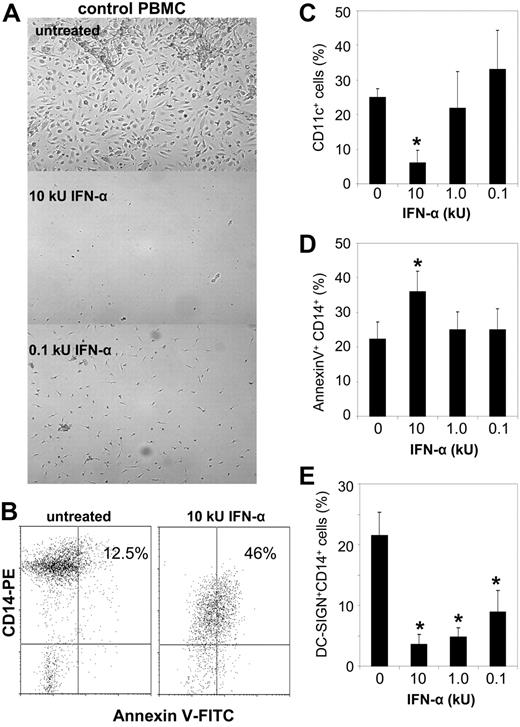
To further assess if IFN-α is toxic to EPCs/CACs, lupus and control PBMCs were grown under proangiogenic conditions in the presence or absence of graded concentrations of recombinant IFN-α. IFN-α–treated cells from controls acquired the phenotype of lupus cells and were unable to form an EC monolayer. Higher concentrations (10 kU/mL) of IFN-α were overtly toxic on day 5 of culture by inducing significant increases in myeloid cell apoptosis (assessed by CD14 and CD11c expression), explaining the lack of monolayer formation by day 15 (Figure 5A-D). While lower concentrations of IFN-α were not overtly toxic by day 5, they altered the expression of surface developmental markers on myelomonocytic cells, consistent with skewing the differentiation of these cells from angiogenic CACs to IFN-α–derived dendritic cells (DCs)10,32 (Figure 5E). Indeed, IFN-α induced a significant reduction in DC-SIGN+CD14+ cells, CD86 up-regulation, and overall down-regulation of CD14. To assess if type I IFNs would be similarly toxic to BM-derived EPCs, we treated CD133+ cells with recombinant IFN-α. There was a striking apoptosis induction on CD34+ CD133+ cells using graded concentrations of IFN-α (0.1–10 kU/mL), which was observed 24 hours after treatment (Figure 6A-C). These results suggest that type I IFNs are toxic to both cell types involved in vascular repair.
IFN-α treatment prevents EC monolayer formation in healthy controls. (A) Control PBMCs were plated on fibronectin-coated wells with complete EC media in the presence or absence of graded concentrations of recombinant IFN-α. At day 15, EC monolayer formation was assessed. Images are from experiments from 4 representative controls. Similar results were seen in 4 SLE patients using similar concentrations of IFN-α. Magnifications are × 10. See “In vitro differentiation into mature ECs” for more image acquisition information. (B-E) IFN-α induces CAC apoptosis and skewing toward other myeloid cell subsets. Representative dot plots display percentage apoptotic CD14+ CACs in untreated and IFN-α–treated cells. Graphs represent mean (± SEM) percentage expression of myeloid subsets on the EC monolayer of 5 controls after IFN-α treatment. * indicates P < .05.
IFN-α treatment prevents EC monolayer formation in healthy controls. (A) Control PBMCs were plated on fibronectin-coated wells with complete EC media in the presence or absence of graded concentrations of recombinant IFN-α. At day 15, EC monolayer formation was assessed. Images are from experiments from 4 representative controls. Similar results were seen in 4 SLE patients using similar concentrations of IFN-α. Magnifications are × 10. See “In vitro differentiation into mature ECs” for more image acquisition information. (B-E) IFN-α induces CAC apoptosis and skewing toward other myeloid cell subsets. Representative dot plots display percentage apoptotic CD14+ CACs in untreated and IFN-α–treated cells. Graphs represent mean (± SEM) percentage expression of myeloid subsets on the EC monolayer of 5 controls after IFN-α treatment. * indicates P < .05.
IFN-α induces EPC apoptosis. (A,B) Representative dot plots show increased BM and circulating CD133+ cytotoxicity by IFN-α. (C) Graph is representative of 2 independent experiments from healthy controls.
IFN-α induces EPC apoptosis. (A,B) Representative dot plots show increased BM and circulating CD133+ cytotoxicity by IFN-α. (C) Graph is representative of 2 independent experiments from healthy controls.
Type I IFNs have prominent pleiotropic effects on proangiogenic responses. Potential mechanisms by which this cytokine inhibits in vitro vasculogenesis were sought. Because IFN-α can inhibit the VEGF promoter,33 we tested whether adding recombinant VEGF to lupus PBMC cultures could restore a normal monolayer phenotype. However, exogenous addition of VEGF did not induce monolayer formation, suggesting that the effects of IFN-α on EPCs/CACs are not explained solely by VEGF promoter inhibition. IFN-α up-regulates TRAIL mRNA in myeloid cells and promotes increased release of soluble TRAIL. TRAIL expression is increased in SLE and induces accelerated monocyte apoptosis in this disease.34 However, blocking TRAIL in vitro did not promote EC monolayer formation in SLE, suggesting that TRAIL is not significantly involved in the increased apoptosis induced by type I IFNs in vitro (not shown).
IFN-α blockade restores a normal angiogenic phenotype in SLE
Further supporting the hypothesis that IFN-α promotes abnormal vascular repair in SLE, when graded concentrations of blocking anti–IFN-α mAbs (but not isotype control) were added to lupus PBMCs stimulated to become ECs in vitro, these cells acquired the phenotype of healthy controls, formed a normal EC monolayer, and expressed mature EC markers (Figure 7A). The normalization of the phenotype was observed only in samples from SLE patients whose cells failed to form an EC monolayer in the absence of anti–IFN-α. Blocking other cytokines (IFN-γ, IL-4, or IL-10, at 0.5–2 μg/mL; not shown) did not result in restoration of the normal phenotype. Confirming these results, blocking the type I IFN-R promoted similar improvements in EC phenotype in SLE patients who were not EC monolayer formers (Figure 7B).
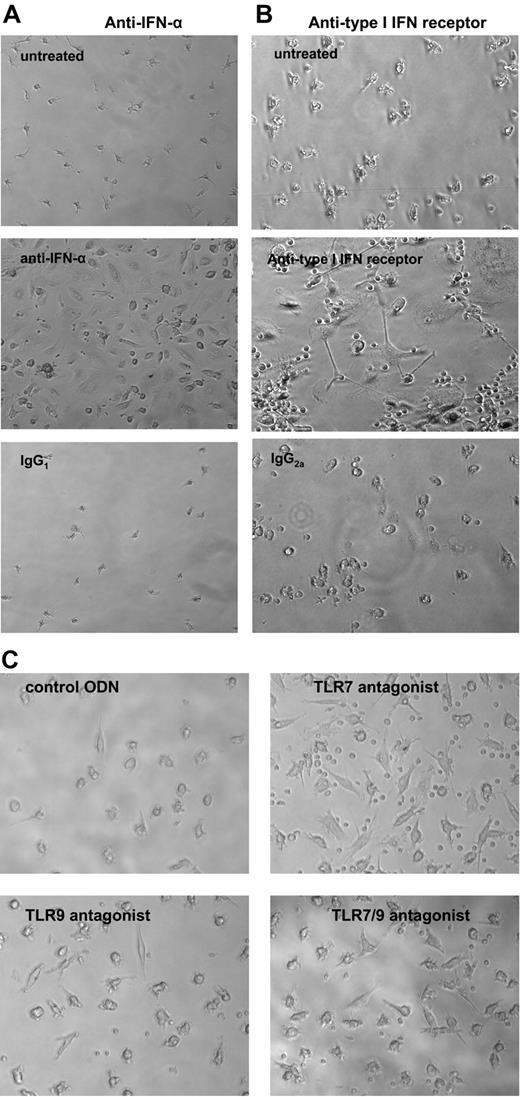
IFN-α blockade promotes EC monolayer formation in SLE. Lupus PBMCs were cultured under proangiogenic conditions in the presence or absence of (A) blocking antihuman IFN-α mAb or isotype control or (B) antihuman type I IFN-R or isotype control (all 2 μg/mL). At day 15, anti–IFN-α– or anti–type I IFN-R–treated cells, but not controls, formed EC monolayers. Results are representative of independent experiments in SLE patients who failed to form EC monolayers, which corrected with anti–IFN-α (n = 9) or with anti–type I IFN-R (n = 6). (C) TLR7 and/or TLR9 blockade promotes EC monolayer formation in SLE. Lupus PBMCs were cultured in EGM20 supplemented with a control ODN, a TLR7 antagonist, a TLR9 antagonist, or a TLR7/9 antagonist (1 μM). Images were acquired after 15 days in culture. Results show cells from a representative SLE patient. All magnifications are × 20. See “In vitro differentiation into mature ECs” for more image acquisition information.
IFN-α blockade promotes EC monolayer formation in SLE. Lupus PBMCs were cultured under proangiogenic conditions in the presence or absence of (A) blocking antihuman IFN-α mAb or isotype control or (B) antihuman type I IFN-R or isotype control (all 2 μg/mL). At day 15, anti–IFN-α– or anti–type I IFN-R–treated cells, but not controls, formed EC monolayers. Results are representative of independent experiments in SLE patients who failed to form EC monolayers, which corrected with anti–IFN-α (n = 9) or with anti–type I IFN-R (n = 6). (C) TLR7 and/or TLR9 blockade promotes EC monolayer formation in SLE. Lupus PBMCs were cultured in EGM20 supplemented with a control ODN, a TLR7 antagonist, a TLR9 antagonist, or a TLR7/9 antagonist (1 μM). Images were acquired after 15 days in culture. Results show cells from a representative SLE patient. All magnifications are × 20. See “In vitro differentiation into mature ECs” for more image acquisition information.
TLR7 and TLR9 are key molecules involved in the induction of IFN-α synthesis in specific cell subsets.35 To further support the role of IFN-α in abnormal vasculogenesis in SLE, we used ODN-based inhibitors of TLR7 and TLR935 and tested whether TLR7 and/or TLR9 are involved in an abnormal EC monolayer phenotype in SLE. Blocking TLR7 and/or TLR9, separately or in combination (but not a control ODN), restored the ability of lupus cells to form a normal EC monolayer (4 of 7 samples were completely rescued by treatment with any of the TLR antagonists and an additional 2 of those 7 samples were partially rescued by TLR7 antagonist; Figure 7C).
Discussion
The number of studies supporting an important pathogenic role for type I IFNs in lupus has significantly increased over the past few years.10 IFN-α levels increase during SLE flares and multiple organ involvement.10 Type I IFN–inducible genes are the most significantly overexpressed on lupus microarrays36 and many of the IFN-α effects on immune function mimic the altered immune function characteristic of SLE.10
While it is known that IFN-α can also have profound effects on the endothelium (with data primarily derived from the cancer literature), a link between type I IFNs and premature atherosclerosis in SLE had not previously been made. IFN-α is a potent antiangiogenic factor, as shown by inhibition of angiogenic responses in matrigel,37 decreased EC migration, and down-regulation of proangiogenic molecules.29,38 IFN-α has been used as adjuvant antiangiogenic therapy in neoplasias,29 diabetic retinopathy,39 hemangiomas, and Kaposi sarcoma.28,40 While the exact pathways by which IFN-α inhibits angiogenesis are not well characterized and have focused primarily on tumor cell lines, potential mechanisms include VEGF gene transcription inhibition33 and induction of TRAIL secretion and Fas expression on myelomonocytic cells, with potential angiogenesis impairment via apoptosis.41,42 In our experiments, neither of these mechanisms accounted for the antiangiogenic effects of IFN-α and, given the pleiotropic effects of IFN-α on angiogenesis genes,43 it is likely that the mechanisms are multifactorial. IFN-α has antiproliferative effects on hepatic progenitor cells,43 but little is known about its effects on EPCs/CACs. IFN-α can decrease colony formation of hematopoietic progenitor cells,44 further suggesting its implication in EPC decreases in SLE. Indeed, increased apoptosis of BM CD34+ cells has been reported in SLE, but the precise etiology was uncharacterized.45
Our study reports the following findings. (1) BM EPCs are decreased in the circulation of lupus patients despite the previous evidence of accelerated EC damage3 (a factor that should normally enhance BM EPC release).46 Since EPCs can lead to a mature endothelium after EC damage, EPC decreases may significantly affect vascular repair. Our results confirm a recent study in which a small number of SLE patients in clinical remission were found to have decreased circulating EPCs.47 (2) Lupus EPCs and CACs have impaired function characterized by an inability to form adequate numbers of mature ECs under proangiogenic stimulation and incorporate into vascular structures. (3) Lupus EPCs/CACs have a decreased capacity to synthesize proangiogenic factors in vitro and, likely, in vivo. It is possible that these decreases may impair BM EPC release and their proper homing into damaged vasculature to repair blood vessels. (4) Importantly, we have found for the first time that IFN-α promotes the abnormal vasculogenesis observed in SLE by inducing apoptosis of cells involved in blood vessel function and, possibly, by skewing monocytes and other myeloid angiogenic precursors toward nonangiogenic myelomonocytic cells (like DCs). Supporting our results, other studies have reported a toxic role of IFN-α in APCs, including macrophages.48 Since different cells produce IFN-α in response to various stimuli, including pDCs, macrophages, B cells, and BM progenitors, it is possible that both EPCs and CACs are involved in type I IFN production in SLE monolayers.
Our results implicate that IFN-α not only contributes to lupus pathogenesis but might also play a significant role in the premature atherosclerosis observed in this disease. We have reported that SLE patients have accelerated EC apoptosis, which strongly correlates with endothelial dysfunction and tissue factor generation.3 Since mechanisms of blood vessel repair after EC damage occurs are crucial in atherosclerosis prevention, we propose that an imbalance between EC damage and repair in SLE may strongly promote accelerated atherosclerosis. This hypothesis is supported by multiple observations. Individuals with low EPC numbers have higher rates of CV events, and patients with conditions known to be associated with premature atherosclerosis display decreased EPC levels and abnormal EPC/CAC function.8,9 BM-derived EPCs can proliferate, migrate, and differentiate into mature ECs and are largely responsible for generating neovascularization in damaged tissues.49 Myelomonocytic CACs release potent proangiogenic growth factors, improve vascular growth, and can differentiate into endothelium-like cells, and a functional link between peripheral blood monocyte concentration and enhancement of arteriogenesis has been reported.18,50
Overall, our results strongly suggest that IFN-α may play an important role in abnormal vascular repair in SLE and, potentially, in promoting the marked increase in premature CVD seen in this patient population. Our conclusions are supported by preliminary data from Kirou et al51 that suggest that activation of IFN pathways is associated with rapid atherosclerosis progression in SLE. In addition, IFN-α–producing pDCs are present in a significant proportion of carotid atheromas in idiopathic atherosclerosis and may enhance cytotoxic T lymphocyte (CTL) capabilities toward vascular smooth muscle cells (VSMCs), potentially providing an additional mechanism by which this cytokine might be deleterious to the vasculature.52
Our results extend the clinical relevance of type I IFN pathways in lupus and suggest that future interventions aimed at abrogating the effects of IFN-α in autoimmunity could also prevent the development of accelerated vascular events in these patients. The relevance of our findings may extend beyond lupus into other conditions associated with higher production of type I IFNs. Chronic infections may play a significant role in atherosclerosis development and our results suggest that IFN-α could be an important culprit.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jennifer Johnson and Joel Joshua for patient recruitment.
This study was supported by the Alliance for Lupus Research, the Lupus Research Institute, the Anthony S. Gramer Fund in Inflammation Research, and National Institutes of Health (NIH) grant AR048235 (all to M.J.K.) and by the Carol and Herb Amster Lupus Research Fund and the Klein Lupus Research Fund (W.J.M.). S.T. was supported by NIH training grant T32-A107413. This work was also supported also in part by NIH through the University of Michigan's Cancer Center Support Grant (P30 CA46592) and the Rheumatic Disease Core Center Grant (P30 AR48310).
National Institutes of Health
Authorship
Contribution: M.F.D. designed and performed research, analyzed data, and wrote the paper. S.T. performed research, analyzed data, and wrote the paper. H.M. performed research. E.C.S. analyzed data. T.D. analyzed data. F.J.B. contributed vital new reagents and wrote the paper. W.J.M. designed research and wrote the paper. M.J.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mariana J. Kaplan, Division of Rheumatology, University of Michigan Medical School, 1550 W. Medical Center Dr, 5520 MSRBI, Ann Arbor, MI 48109; e-mail: makaplan@umich.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal