In this issue of Blood, Geletu and colleagues demonstrate that the dominant-negative effect of C/EBPαp30 on full-length C/EBPαp42 is mediated through up-regulation of Ubc9, which sumoylates p42 and thereby inhibits its ability to activate transcription.
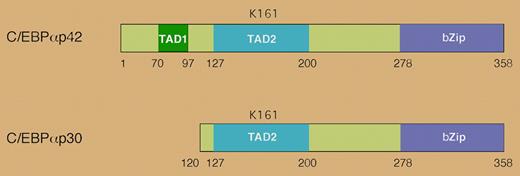
Mutations in the CEBPA gene occur in approximately 10% of acute myelogenous leukemia (AML) cases.1 CEBPA codes for the transcription factor C/EBPα, which is necessary for myeloid differentiation.2 Two protein isoforms of C/EBPα are produced through alternative translational initiation: a 42-kDa form (p42) and a shorter, 30-kDa form (p30) (see the figure). The p30 isoform retains the DNA binding domain but lacks the N-terminal transcriptional activation domain of p42. It acts as a dominant negative, blocking p42's ability to bind DNA, activate transcription, and induce differentiation of hematopoietic cells.3 Many CEBPA mutations associated with AML increase the expression of the p30 isoform.
In this issue of Blood, Geletu and colleagues addressed the mechanism of the p30 dominant-negative effect using a proteomic approach. Proteins up-regulated by p30 were identified using 2D-gel electrophoresis of proteins isolated from K562 cells expressing an inducible version of p30 and primary AML cells expressing endogenous p30. Sixteen proteins were identified that changed expression in both samples.
One of the proteins identified was Ubc9, an E2-conjugating enzyme essential for sumoylation. Ubc9 is up-regulated in several tumors and binds p42 in vitro.4,5 Additionally, sumoylation of p42 inhibits both its ability to activate transcription and its ability to inhibit proliferation of hepatocytes.6 Geletu and colleagues hypothesized that Ubc9 induction by p30 is responsible for the p30 dominant-negative effect on p42 in myeloid differentiation. Expression of p30 or Ubc9 led to increased sumoylation of p42 and repression of p42's ability to promote transcription. Additionally, siRNA knockdown of Ubc9 blocked p30-induced sumoylation and transcriptional repression of p42.
To determine whether Ubc9 is necessary for p30 to inhibit granulocytic differentiation, the authors examined the differentiation of U937 cells and CD34+ human hematopoietic progenitors. In both U937 cells treated with retinoic acid and CD34+ cells treated with granulocyte–colony-stimulating factor (G-CSF), Ubc9 siRNA antagonized the p30 differentiation block. Additionally, expression of Ubc9 alone in U937 cells reduced granulocyte differentiation approximately 2-fold. Ubc9 was not as effective at blocking granulocyte differentiation as p30.
It is still unclear how p30 increases the ex-pression of Ubc9. UBC9 transcription was increased by p30, but not p42, expression. However, it is not clear if this effect is due to direct or indirect regulation by p30. This is the first report of C/EBPα sumoylation playing a role in hematopoietic differentiation. Importantly, it provides a mechanism for the p30 dominant-negative effect on p42. Further investigation into how p30 upregulates Ubc9 could reveal a novel pathway to target for therapeutic treatment of AML.
The human CEBPA gene encodes 2 distinct isoforms that are generated by alternative use of an initiator AUG codon. The p30 isoform lacks the N-terminal transactivation domain and antagonizes the function of p42. Lysine 161 in human C/EBPα is a site for sumoylation.
The human CEBPA gene encodes 2 distinct isoforms that are generated by alternative use of an initiator AUG codon. The p30 isoform lacks the N-terminal transactivation domain and antagonizes the function of p42. Lysine 161 in human C/EBPα is a site for sumoylation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal