Abstract
Lymphangiogenesis is involved in tumor cell metastasis and plays a major role in chronic inflammatory disorders. To investigate the role of lymphangiogenesis in inflammation, we induced and maintained delayed-type hypersensitivity (DTH) reactions in the ears of mice and then analyzed the resulting lymphangiogenesis in the inflamed tissue and draining lymph nodes (LNs) by quantitative fluorescence-activated cell sorting (FACS) and by immunofluorescence. Long-lasting inflammation induced a significant increase in the number of lymphatic endothelial cells, not only in the inflamed ears but also in the ear-draining auricular LNs. Inflammation-induced lymphangiogenesis was potently blocked by systemic administration of a vascular endothelial growth factor (VEGF)-A neutralizing antibody. Surprisingly, tissue inflammation specifically induced LN lymphangiogenesis but not LN angiogenesis. These findings were explained by analysis of both VEGF-A protein and mRNA levels, which revealed that VEGF-A was expressed at high mRNA and protein levels in inflamed ears but that expression was increased only at the protein level in activated LNs. Inflammation-induced lymphangiogenesis in LNs was independent of the presence of nodal B lymphocytes, as shown in B cell-deficient mice. Our data reveal that chronic inflammation actively induces lymphangiogenesis in LNs, which is controlled remotely, by lymphangiogenic factors produced at the site of inflammation.
Introduction
The lymphatic system is important in the physiologic processes of immune surveillance, tissue fluid homeostasis, and fat absorption.1 Lymphatic vessels are also involved in many pathologic processes including tumor cell metastasis, wound healing, and chronic inflammation.2-4 Recent evidence indicates that, similar to angiogenesis, lymphangiogenesis might also occur during certain inflammatory and autoimmune conditions. For example, the chronic inflammatory skin disease psoriasis is characterized by extensive lymphangiogenesis,5 and lymphatic hyperplasia is frequently found in rejected renal transplants.6,7 Furthermore, in mice, bacterial infection of airway epithelia was shown to induce a strong, vascular endothelial growth factor (VEGF)-C- and VEGF-D-mediated lymphangiogenic response.8 Although the functional implications of these findings are unclear, it appears that lymphatic vessels participate in the regulation of the immune response through their role in antigen transport and leukocyte migration to draining lymph nodes (LNs).
Recent results indicate that during pathologic conditions, the lymphatic system is not only remodeled in the diseased tissue but also in the LNs that drain it. Importantly, malignant tumors can induce lymphangiogenesis of sentinel LNs even before they metastasize to these sites, probably facilitating their further metastatic spread.9,10 Furthermore, expansion of lymphatic vessels within inflamed LNs enhances dendritic cell mobilization after immunization with complete Freund adjuvant (CFA).11 However, it is unclear whether LN lymphangiogenesis occurs during chronic tissue inflammation and whether expansion of lymphatic networks in the draining LN is remotely controlled by factors released in peripheral tissues or by local release of lymphangiogenic factors within the LN.
In this study, we have investigated the (lymph)angiogenic response in peripheral tissues and draining LNs during inflammation. To this end, we induced and maintained delayed-type hypersensitivity (DTH) reactions in the ears of VEGF-A transgenic (Tg)12 and wild-type (WT) mice for 9 days. Immunofluorescence and quantitative fluorescence-activated cell sorting (FACS) analysis of single-cell suspension samples from these tissues allowed us to quantitatively analyze the effect of inflammation on the number of lymphatic endothelial cells (LECs) and blood vascular endothelial cells (BECs) in ears and draining LNs. We found that prolonged inflammation induced proliferation of LECs and BECs in the ears of VEGF-A Tg mice and WT mice with DTH reactions. Importantly, the numbers of LECs, but not of BECs, also significantly increased in the auricular LNs that drained the inflamed ears, and administration of a neutralizing monoclonal antibody against VEGF-A (anti–VEGF-A) potently blocked inflammation-induced ear and LN lymphangiogenesis. Surprisingly, although elevated levels of VEGF-A protein were detected in tissue lysates from inflamed LNs, in situ hybridization and reverse transcription–polymerase chain reaction (RT-PCR) analysis detected no increase in VEGF-A mRNA in inflamed LNs. In contrast, VEGF-A was induced both at the mRNA and at the protein level in the inflamed ears of DTH-challenged mice. Thus, during DTH-induced inflammation, the inflamed peripheral tissue is the prime source of VEGF-A; lymphangiogenesis in the LN is remotely controlled by VEGF-A drainage from the inflamed peripheral site to the LN. Collectively, these findings reveal that lymphangiogenesis in the LN is induced by factors produced remotely, at the site of inflammation.
Materials and methods
Mice
WT female FVB mice were purchased from Charles River Laboratories (Sulzbach, Germany), and severe combined immunodeficiency (SCID)/beige mice were obtained from Taconic (Bomholt, Denmark). Hemizygous VEGF-A Tg mice (FVB background)12 were bred and housed in the animal facility of ETH. B cell-deficient C57BL/6 mice (JHT) were kindly provided by Daniel Pinschewer (University Hospital, Zurich, Switzerland). Experiments were performed under our animal protocol (Nr. 123/2005) and approved by the Kantonales Veterinäramt Zürich.
Induction of DTH response
Mice were randomly assigned to groups of 6 to 8 mice each. The DTH response was induced in the ear skin of 6- to 8-week-old female mice as described.5 Briefly, on study day −5, mice were anesthetized by intraperitoneal administration of medetomidine (0.2 mg/kg) and ketamine (80 mg/kg) and sensitized by topical application of 2% oxazolone (4-ethoxymethylene-2 phenyl-2-oxazoline-5-one; Sigma, St. Louis, MO) in acetone/olive oil (4:1 vol/vol) on the shaved abdomen (50 μL) and on each paw (5 μL). Five days after sensitization (study day 0), 10 μL of a 1% oxazolone solution was applied topically to each side of the ears. VEGF-A Tg mice were only challenged once (study day 0). In the case of WT and JHT mice, animals were challenged with 1% oxazolone on the ears every other day (study days 0, 2, 4, 6, and 8). In all experiments, mice of the control group were neither sensitized nor challenged with oxazolone.
FACS analysis of cell suspensions from LNs and ears
Ears and draining auricular LNs were analyzed by fluorescence-activated cell sorting (FACS) analysis on study days 2 and 9. All mice were anesthetized, and their ear thickness was measured using calipers. The mice were then killed and their ears and auricular LNs were collected, weighed, and digested in phosphate-buffered saline (PBS) containing 0.4% collagenase IV (Invitrogen, Basel, Switzerland) at 37°C for 45 minutes. Tissue suspensions were passed through a cell strainer (BD Biosciences, San Jose, CA). LN cell suspensions were counted using a hematocytometer, washed with PBS, and resuspended in PBS containing 1% fetal bovine serum (FBS) (FACS buffer). Ear cell suspensions were passed twice through a cell strainer, washed, and resuspended in FACS buffer. All experiments were performed twice unless otherwise noted.
The antibodies used for FACS analysis included the following: rat antimouse CD31 and fluorescein isothiocyanate (FITC)–conjugated antimouse CD45.1 or CD45.2 (BD Biosciences); hamster antimouse podoplanin (clone 8.1.1; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); antirat-allophycocyanin (APC) and antihamster-phycoerythrin (PE) and isotype control antibodies (CALTAG/Invitrogen, Basel, Switzerland). FACS was performed using a BD FACSCanto (Becton Dickinson, Basel, Switzerland) using FACSDiva software. Data were analyzed with Flowjo software (Treestar, Ashland, TN).
For quantification of total LEC and BEC numbers in LN samples, the fraction of LECs and BECs among all live cells (gated) was determined and multiplied by the total number of LN cells counted. For quantification of LECs and BECs in ear samples, a defined aliquot of each sample was stained and acquired by FACS.
Administration of anti–VEGF-A or of VEGFR3-Fc
On study day 0 (day of challenge), WT FVB mice were given intraperitoneal injections of anti–VEGF-A (50 μg, clone G6–31,13 kind gift of Germaine Fuh, Genentech, South San Francisco, CA), of a mouse VE6F receptor (VEGFR)3-Fc fusion protein14 (65 μg; R&D Systems, Minneapolis, MN) or of a human control immunoglobulin G (IgG) (50 μg or 65 μg, respectively; Southern Biotech, Birmingham, AL). Oxazolone was applied to the ears of these mice 5 hours later and afterwards every other day (study days 2, 4, 6, and 8). Anti–VEGF-A, VEGFR3-Fc, or IgG was readministered on study days 4 and 7. Control WT FVB mice were neither exposed to oxazolone nor antibody. Ears and LNs of all mice were analyzed on day 9 of the study. Each experiment was performed once with 5 to 7 mice in the inflamed groups (exposed to either anti–VEGF-A, VEGFR3-Fc, or control IgG) and 4 to 6 mice in the control group.
Immunofluorescence analysis
On study days 2 and 9, mice were killed and ears and auricular LNs were harvested, embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA), and frozen on dry ice. Immunofluorescence was performed on 6 μm cryostat sections as described15 using the following reagents: anti-mouse LYVE-1 (Angiobio, Del Mar, CA, or Upstate/Millipore, Billerica MA), anti-mouse MECA-32 (BD Biosciences), anti-mouse podoplanin (clone 8.1.1), Alexa488- or Alexa594-coupled secondary antibodies, and the nuclear dye Hoechst 33342 (Molecular Probes/Invitrogen, Basel, Switzerland). For Ki67 staining of LNs, tissue was fixed in 4% paraformaldehyde for 1 hour before freezing in OCT. Antigen retrieval was performed by boiling the sections for 10 minutes in 0.01 M citrate buffer (pH 6) followed by immunofluorescent staining as described.15 Anti-mouse Ki67 was purchased from DakoCytomation (Zug, Switzerland).
Stained sections were washed in PBS and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA). Images were captured on an Axioskop 2 mot plus microscope (Carl Zeiss, Feldbach, Switzerland), equipped with an AxioCam MRc camera (Zeiss) and the following objectives: Plan-APOCHROMAT 10×/0.45 MA. Plan-NEOPLAN 20×/0.5 NA (both from Zeiss). Image acquisition in the individual fluorescent channels was accomplished using Axio Vision4.4 software (Zeiss). Adobe Photoshop CS2 (Adobe Systems, San Jose, CA) was used to adjust image brightness and for image overlay.
In situ hybridization and quantitative RT-PCR
In situ hybridization on LN and ear sections (n = 2 mice per group) was performed as described.15 Antisense and sense single-stranded sulfur 35 (35S)–labeled RNA probes for VEGF-A and VEGF-C were used as described.15 For RT-PCR analysis, total RNA was extracted from LN and salivary glands (n = 2–5 mice per group) using TRIZOL reagent (Invitrogen) and incubated with RQ RNase-free DNase (Catalys, Wallisellen, Switzerland) and RNase-Inhibitor (Applied Biosystems, Rotkreuz, Switzerland). Salivary glands, which constitutively express VEGF-A,16 were assayed as positive control. The expression levels of VEGF-A were determined using 5′-ggagatccttcgaggagcactt-3′ (sense) and 5′-ggcgatttagcagcagatataagaa-3′ (antisense) primers17 and Power SYBR Green PCR Master Mix (Applied Biosystems). β-actin levels were determined using primers (part number 4352341E) and TaqMan One-Step RT-PCR Master Mix from Applied Biosystems. Reactions were performed on a 7900HT Fast Real-Time PCR System (Applied Biosystems), and VEGF-A expression was normalized to the expression of β-actin.
VEGF-A ELISA
WT FVB and VEGF-A Tg mice (n = 3 mice per group, 1 experiment performed) were killed on study days 2 and 9. Ears and auricular LNs were dissected, weighed, and homogenized in lysis buffer (150 mM NaCl, 50 mM Tris, pH 7.5, with a protease inhibitor cocktail [Roche Diagnostics, Mannheim, Germany]). Homogenates were centrifuged for 10 minutes at 4°C (14 000g), and supernatants were assayed using a VEGF-A enzyme-linked immunosorbent assay (ELISA) (Quantikine; R&D Systems).
Statistical analysis
Data were analyzed using a Student t test and are presented as means (± standard error [SEM]). Differences were considered statistically significant when P is less than .05.
Results
The numbers of BECs and LECs are increased in the inflamed skin of VEGF-A Tg mice
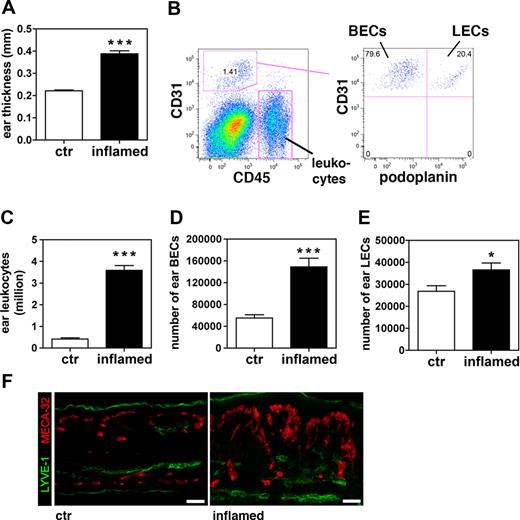
We have recently described a new mouse model of psoriasis, namely VEGF-A Tg mice, in which murine VEGF-A164 is constitutively expressed in the epidermis under the control of the keratinocyte-specific keratin 14 (K14) promoter.18 Unlike WT mice, homozygous and hemizygous VEGF-A Tg mice are unable to down-regulate DTH-induced skin inflammation and develop chronic, psoriasis-like inflammatory skin lesions, characterized by epidermal hyperproliferation, leukocyte infiltration, and vascular remodeling.5,12 In a first experiment, we used FACS analysis to quantify the vascular response to inflammation in these mice. To this end, DTH reactions were induced in the ears of VEGF-A Tg mice using oxazolone as a sensitizing agent. On day 9 after challenge, ears from DTH-inflamed VEGF-A Tg mice were significantly more swollen (1.8-fold) than ears from mice in which DTH reactions had not been induced (Figure 1A). FACS analysis was performed on single cell suspensions obtained from ears, using the leukocyte marker CD45, the endothelial cell marker CD31,19 and the LEC marker podoplanin20 to differentiate between leukocytes (CD45+CD31−podoplanin−), BECs (CD45−CD31+podoplanin−), and LECs (CD45−CD31+podoplanin+) (Figure 1B). We observed that leukocytes were more abundant in inflamed ears (8.7-fold), compared with controls (Figure 1C). Moreover, the numbers of BECs and LECs were significantly increased (2.7-fold and 1.4-fold, respectively), compared with controls (Figure 1D,E). In agreement with these findings, immunofluorescence revealed an increased number of MECA-32+ blood vessels in inflamed ears and an expansion of the LYVE-1+ lymphatic network (Figure 1F).
The number of LECs increases in inflamed ears of VEGF-A Tg mice. A DTH response to oxazolone was induced in the ears of VEGF-A Tg mice. Ear tissue was analyzed 9 days after challenge. (A) At this time point, the inflamed ears were markedly increased in thickness compared with ears from control (ctr) animals. (B) FACS analysis was performed on cells derived from enzymatically digested ears (example shown: inflamed ears). Cells were stained for CD45, CD31, and podoplanin to differentiate between leukocytes (CD45+CD31−podoplanin−), BECs (CD45+CD31+podoplanin−), and LECs (CD45+CD31+podoplanin+). (C-E) Quantitative FACS analysis revealed that total numbers of leukocytes (C), BECs (D), and LECs (E) were increased in cell suspensions of inflamed ears compared with those of control ears. *P < .05; ***P < .001 (compared with control). (F) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that vascularization was increased in inflamed compared with control ears. Scale bars represent 50 μm. Error bars are SE.
The number of LECs increases in inflamed ears of VEGF-A Tg mice. A DTH response to oxazolone was induced in the ears of VEGF-A Tg mice. Ear tissue was analyzed 9 days after challenge. (A) At this time point, the inflamed ears were markedly increased in thickness compared with ears from control (ctr) animals. (B) FACS analysis was performed on cells derived from enzymatically digested ears (example shown: inflamed ears). Cells were stained for CD45, CD31, and podoplanin to differentiate between leukocytes (CD45+CD31−podoplanin−), BECs (CD45+CD31+podoplanin−), and LECs (CD45+CD31+podoplanin+). (C-E) Quantitative FACS analysis revealed that total numbers of leukocytes (C), BECs (D), and LECs (E) were increased in cell suspensions of inflamed ears compared with those of control ears. *P < .05; ***P < .001 (compared with control). (F) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that vascularization was increased in inflamed compared with control ears. Scale bars represent 50 μm. Error bars are SE.
The lymphatic network is expanded in the LNs that drain inflamed tissues of VEGF-A Tg mice
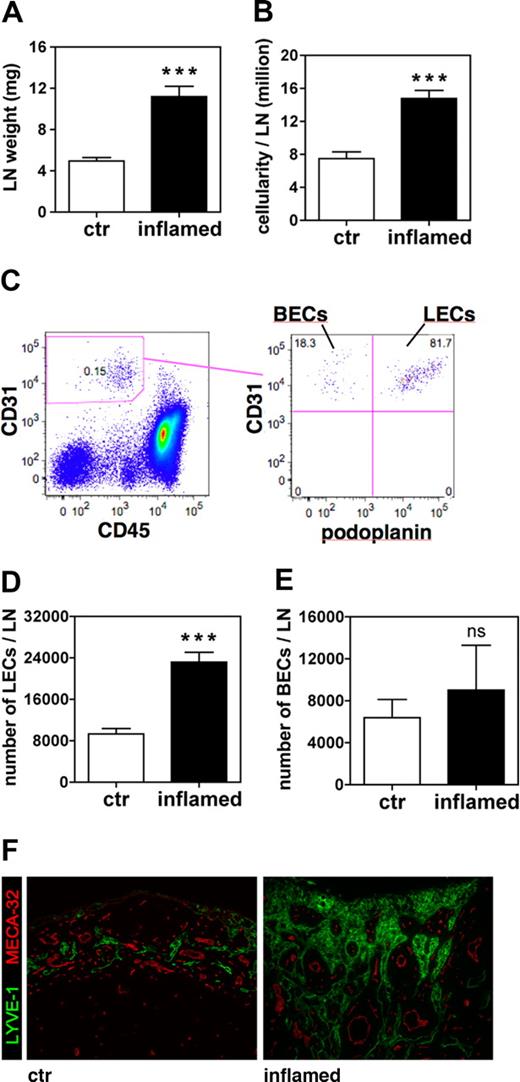
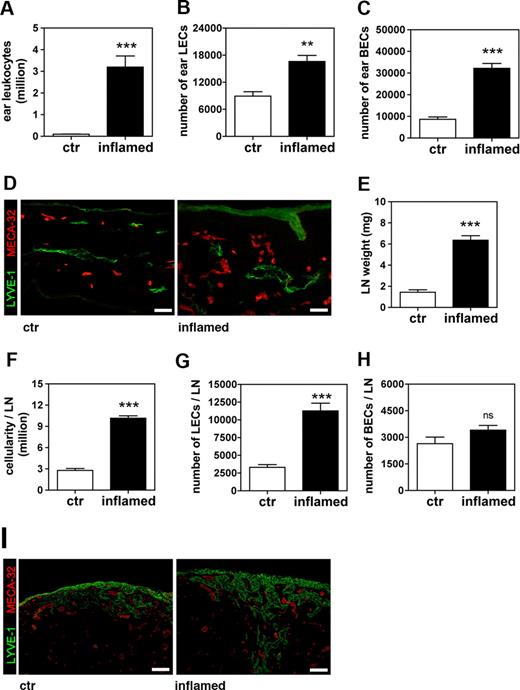
We next quantified inflammation-induced lymphangiogenesis in the ear-draining auricular LNs of DTH-inflamed and control VEGF-A Tg mice 9 days after challenge. LNs of mice with inflamed ears had more than doubled in weight and in cellularity (Figure 2A,B). Quantitative FACS analysis of LN cell suspensions (Figure 2C) revealed significantly increased numbers of LECs in the LNs that drained inflamed ears (2.5-fold more than control; Figure 2D), whereas no significant increase in the number of BECs could be detected (Figure 2E). This differential effect of tissue inflammation on the lymphatic, but not on the blood vascular network, was also apparent when we analyzed LN sections by immunofluorescence (Figure 2F): Whereas LYVE-1+ lymphatic structures were markedly increased in number and size in inflamed LNs, there were no major differences in the appearance of MECA-32+ blood vessels when inflamed and control LNs were compared. Identical observations were made comparing LN sections stained with podoplanin and CD31 (data not shown).
The number of LECs increases in auricular LNs that drain the inflamed ears of VEGF-A Tg mice. Auricular LNs were analyzed 9 days after induction of a DTH response to oxazolone in the ears of VEGF-A Tg mice. (A,B) At this time point, LN weight (A) and cellularity (B) were markedly increased in LNs draining inflamed ears compared with those draining control (ctr) ears. (C) FACS analysis of cell suspensions of auricular LN was used to differentiate between leukocytes, BECs, and LECs. (D,E) Quantitative FACS analysis revealed that the total number of LECs (D) was increased in inflamed compared with control LNs, whereas the total number BECs (E) remained unchanged. ***P < .001 (compared with control). (F) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that lymphatic structures were markedly expanded in LNs draining inflamed compared with control ears. Scale bars represent 100 μm. Error bars are SE.
The number of LECs increases in auricular LNs that drain the inflamed ears of VEGF-A Tg mice. Auricular LNs were analyzed 9 days after induction of a DTH response to oxazolone in the ears of VEGF-A Tg mice. (A,B) At this time point, LN weight (A) and cellularity (B) were markedly increased in LNs draining inflamed ears compared with those draining control (ctr) ears. (C) FACS analysis of cell suspensions of auricular LN was used to differentiate between leukocytes, BECs, and LECs. (D,E) Quantitative FACS analysis revealed that the total number of LECs (D) was increased in inflamed compared with control LNs, whereas the total number BECs (E) remained unchanged. ***P < .001 (compared with control). (F) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that lymphatic structures were markedly expanded in LNs draining inflamed compared with control ears. Scale bars represent 100 μm. Error bars are SE.
No increase in LEC numbers was observed when analyzing auricular LNs 2 days after induction of a DTH response, although at this time point LN weight and cellularity had already reached levels similar to day 9 (Figure S1A-E, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, expansion of the lymphatic network in the LN mediated by LEC proliferation requires more than 2 days to reach levels detectable by quantitative FACS analysis.
(Lymph)angiogenesis occurs in the inflamed ears of WT mice after repeated induction of the DTH response
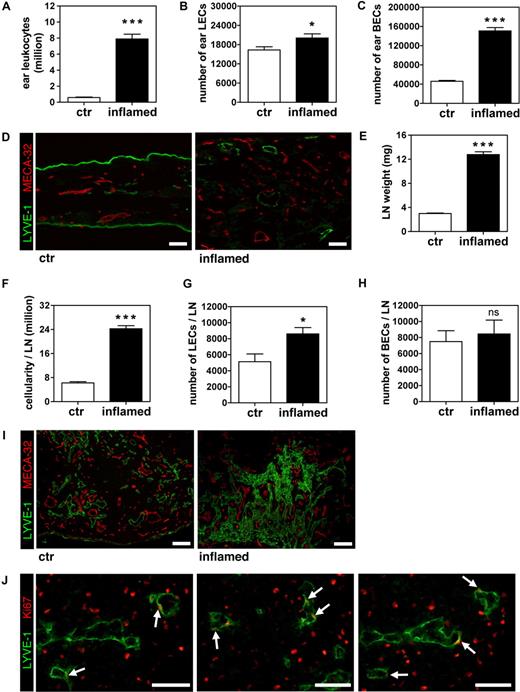
To rule out the possibility that the dramatic effect of tissue inflammation on LN lymphangiogenesis in VEGF-A Tg mice might simply be caused by the constitutive expression of high levels of VEGF-A in the skin, we performed similar experiments in WT mice. Given that WT mice generally clear DTH-induced ear inflammation within 1 week, we repeatedly exposed the mice to oxazolone every other day, to mimic chronic/subacute inflammation. On study day 9, the ears of these mice where markedly swollen and covered by dried exudates (data not shown). FACS analysis of ear single-cell suspensions revealed a massive accumulation of CD45+ leukocytes in the inflamed ears (13.5-fold increase more than controls, in which the DTH response had not been induced; Figure 3A). Furthermore, the number of LECs was significantly increased, although to a lesser extent than that of BECs (1.2-fold and 3.3-fold more than control, respectively; Figure 3B,C). This stimulatory effect of inflammation on lymphatic and blood vascular structures was also detectable by immunofluorescence of ear sections (Figure 3D).
The number of LECs increases in auricular LNs that drain inflamed ears of WT mice subjected to repeated DTH challenges. A DTH response to oxazolone was induced in the ears of WT mice and maintained by repeatedly applying oxazolone on the ears for 9 days. (A-C) After 9 days, the total numbers of leukocytes (A) and of LECs (B) and BECs (C) were significantly increased in inflamed ears over control (ctr) ears, as determined by quantitative FACS analysis. (D) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that vascularization was increased in inflamed compared with control ears. Scale bars represent 50 μm. (E,F) Analysis of ear draining auricular LNs revealed that LN weight (E) and cellularity (F) was markedly increased in inflamed compared with control animals. (G,H) Quantitative FACS analysis detected elevated numbers of LECs (G) in LNs draining inflamed compared with control ears, but no change in BEC (H) numbers. (I) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that lymphatic structures were markedly expanded in LNs draining inflamed compared with control ears. Scale bars represent 100 μm. (J) Differential immunofluorescence staining for LYVE-1 (green) and Ki67 (red) revealed the presence of proliferating LECs (white arrows) in inflamed LNs. Scale bars represent 25 μm. *P < .05; ***P < .001 (compared with control). Error bars are SE.
The number of LECs increases in auricular LNs that drain inflamed ears of WT mice subjected to repeated DTH challenges. A DTH response to oxazolone was induced in the ears of WT mice and maintained by repeatedly applying oxazolone on the ears for 9 days. (A-C) After 9 days, the total numbers of leukocytes (A) and of LECs (B) and BECs (C) were significantly increased in inflamed ears over control (ctr) ears, as determined by quantitative FACS analysis. (D) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that vascularization was increased in inflamed compared with control ears. Scale bars represent 50 μm. (E,F) Analysis of ear draining auricular LNs revealed that LN weight (E) and cellularity (F) was markedly increased in inflamed compared with control animals. (G,H) Quantitative FACS analysis detected elevated numbers of LECs (G) in LNs draining inflamed compared with control ears, but no change in BEC (H) numbers. (I) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that lymphatic structures were markedly expanded in LNs draining inflamed compared with control ears. Scale bars represent 100 μm. (J) Differential immunofluorescence staining for LYVE-1 (green) and Ki67 (red) revealed the presence of proliferating LECs (white arrows) in inflamed LNs. Scale bars represent 25 μm. *P < .05; ***P < .001 (compared with control). Error bars are SE.
The lymphatic network is expanded in LNs that drain inflamed tissues of WT mice
In a manner similar to that of VEGF-A Tg mice, there was a significant increase in the weight and cellularity (4.3-fold and 3.9-fold, respectively) of the auricular LNs of DTH-inflamed WT mice, compared with controls (Figure 3E,F). Importantly, quantitative FACS analysis revealed a significant increase in LEC numbers in inflamed LNs (1.7-fold more than control LNs, P = .015; Figure 3G). However, as in the experiments with VEGF-A Tg mice, the number of BECs in the LNs was not affected by ear inflammation (Figure 3H). Immunofluorescence analysis of LYVE-1 and MECA-32 expression confirmed the differential effects of peripheral tissue inflammation on LEC and BEC numbers in draining LNs (Figure 3I). In fact, when costaining LN sections for LYVE-1 and the proliferation marker Ki67, numerous lymphatic vessels harboring Ki67-positive LECs could be detected (Figure 3J), confirming that LECs actively proliferate in the LNs draining inflamed tissues.
Interestingly, no increase in the number of LECs in auricular LNs of WT mice could be detected by FACS on day 2 after induction of inflammation, but Ki67 staining already revealed the presence of proliferating LECs at this time point (Figure S2A-F). Collectively, these findings indicate that inflammatory events in peripheral tissues induce a proliferative expansion of the lymphatic, but not of the blood vascular network, in draining LNs.
LN lymphangiogenesis can be blocked by anti–VEGF-A
The fact that LN lymphangiogenesis was more pronounced in LNs draining inflamed ears of VEGF-A Tg mice, compared with LNs draining ears of WT mice in which a DTH had repeatedly been induced, indicated a potential involvement of VEGF-A in this process. In further support of this hypothesis, a cross-comparison of our FACS experiments revealed that both LEC and BEC numbers were significantly increased in the ears of VEFG-A Tg mice, compared with ears of WT FVB mice. In addition, similarly to our observations made in DTH-inflamed LNs, we found that LEC numbers where increased in the draining LNs of VEGF-A Tg, compared with those of WT FVB mice (data not shown). To test whether VEGF-A was the main trigger of inflammation-induced LN lymphangiogenesis, we therefore treated mice with inflamed ears with a VEGF-A neutralizing antibody.13 To this end, a DTH response was induced in the ears of WT mice, and the mice were then exposed to anti-VEGF-A or to IgG as a negative control. On study day 9, the weight and cellularity of the auricular LNs draining inflamed ears of mice that had been exposed to anti–VEGF-A or to IgG were significantly higher than those of mice in which the DTH response had not been induced (control mice) (Figure 4A,B). However, both LN weight and cellularity was significantly reduced in mice that had been treated with anti–VEGF-A, compared with mice treated with control IgG (Figure 4A,B). Furthermore, numbers of LECs were significantly higher (1.7-fold) in the LNs of IgG-treated mice with inflamed ears, compared with those of mice with uninflamed ears (Figure 4C). In mice treated with anti–VEGF-A, lymphangiogenesis in the LNs draining inflamed ears was almost completely blocked (89%; Figure 4C), although there was no effect on BEC numbers in ear-draining LNs (data not shown).
Blockade of VEGF-A, but not of VEGF-C, inhibits inflammation-induced LN lymphangiogenesis. A DTH response to oxazolone was induced in the ears of WT mice and maintained by repeatedly applying oxazolone for 9 days. Mice with inflamed ears were exposed to anti–VEGF-A Ab (A-F), VEGFR3-Fc (G-L), or control IgG (A-L), and the effects were compared with those observed in mice in which inflammation had not been induced (ctr). (A-F) Effect of anti–VEGF-A: Weight (A) and cellularity (B) of the auricular LN was significantly increased in mice with inflamed ears, exposed to anti–VEGF-A or IgG. This effect was less pronounced in inflamed LNs of mice that had been administered anti–VEGF-A compared with IgG. (C) Exposure to anti–VEGF-A potently blocked the increase in LEC numbers in LNs draining inflamed ears, whereas exposure to IgG had no effect. (D) Inflammation led to a significant increase in leukocyte numbers in the ears of both anti–VEGF-A or IgG treated mice, compared with uninflamed the ears of control animals. (E, F) Administration of anti–VEGF-A potently blocked the increase in LEC (E) and BEC (F) numbers in inflamed ears of mice. (G-L) Effect of VEGFR3-Fc: Weight (G) and cellularity (H) of the auricular LN was similarly increased in mice with inflamed ears exposed to VEGFR3-Fc or IgG. (I) Administration of VEGFR3-Fc did not block the increase in LEC numbers in LNs draining inflamed ears. (J) Leukocyte numbers were strongly increased in inflamed ears of mice exposed to IgG. Administration of VEGFR3-Fc led to a significant reduction in the numbers of leukocytes in inflamed ears. Administration of VEGFR3-Fc had no effect on the numbers of LECs (K) or BECs (L) in inflamed ears of mice. *P < .05, **P < .01, ***P < .001 (compared with control [ctr]); #P < .05, ##P < .01, ###P < .001 (anti–VEGF-A or VEGFR3-Fc, compared with IgG). Error bars are SE.
Blockade of VEGF-A, but not of VEGF-C, inhibits inflammation-induced LN lymphangiogenesis. A DTH response to oxazolone was induced in the ears of WT mice and maintained by repeatedly applying oxazolone for 9 days. Mice with inflamed ears were exposed to anti–VEGF-A Ab (A-F), VEGFR3-Fc (G-L), or control IgG (A-L), and the effects were compared with those observed in mice in which inflammation had not been induced (ctr). (A-F) Effect of anti–VEGF-A: Weight (A) and cellularity (B) of the auricular LN was significantly increased in mice with inflamed ears, exposed to anti–VEGF-A or IgG. This effect was less pronounced in inflamed LNs of mice that had been administered anti–VEGF-A compared with IgG. (C) Exposure to anti–VEGF-A potently blocked the increase in LEC numbers in LNs draining inflamed ears, whereas exposure to IgG had no effect. (D) Inflammation led to a significant increase in leukocyte numbers in the ears of both anti–VEGF-A or IgG treated mice, compared with uninflamed the ears of control animals. (E, F) Administration of anti–VEGF-A potently blocked the increase in LEC (E) and BEC (F) numbers in inflamed ears of mice. (G-L) Effect of VEGFR3-Fc: Weight (G) and cellularity (H) of the auricular LN was similarly increased in mice with inflamed ears exposed to VEGFR3-Fc or IgG. (I) Administration of VEGFR3-Fc did not block the increase in LEC numbers in LNs draining inflamed ears. (J) Leukocyte numbers were strongly increased in inflamed ears of mice exposed to IgG. Administration of VEGFR3-Fc led to a significant reduction in the numbers of leukocytes in inflamed ears. Administration of VEGFR3-Fc had no effect on the numbers of LECs (K) or BECs (L) in inflamed ears of mice. *P < .05, **P < .01, ***P < .001 (compared with control [ctr]); #P < .05, ##P < .01, ###P < .001 (anti–VEGF-A or VEGFR3-Fc, compared with IgG). Error bars are SE.
Inflammation induced massive accumulation of leukocytes in the ears of mice exposed to anti–VEGF-A or IgG (Figure 4D). Inflammation also induced an increase in the number of LECs in mice exposed to IgG; by contrast, this could be blocked by administration of anti–VEGF-A (Figure 4E). Furthermore, the number of BECs was increased in inflamed ears of mice exposed to IgG (4.3-fold more than controls; Figure 4F), but this increase was significantly reduced by anti–VEGF-A (61%; Figure 4F).
Because VEGF-A has been shown to induce expression of VEGF-C,21,22 we next wanted to address whether some of the effects of VEGF-A on inflammation-induced LN lymphangiogenesis were indirectly mediated by VEGF-C. In analogy to the experiment performed in DTH-challenged mice with anti–VEGF-A Ab (Figure 4A-F), we therefore repeatedly administered a mouse VEGFR3-Fc fusion protein, which sequesters VEGF-C, or control IgG to the mice. Administration of VEGFR3-Fc did not diminish inflammation-induced increases in LN weight, cellularity, or in LN LEC numbers, compared with IgG treatment (Figure 4G-I). However, leukocyte numbers were significantly reduced in inflamed ears of VEGFR3-Fc-treated mice, possibly due to reduced recruitment of VEGFR3-expressing inflammatory cells (Figure 4J).23 LEC and BEC numbers in the inflamed ears were unaffected by treatment with VEGFR3-Fc (Figure 4K,L). Overall, these data suggest that VEGF-A, but not VEGFC-C, mediates the expansion of the lymphatic network in inflamed tissues and in the LNs that drain them.
The site of inflammation, rather than the draining LN, is the major source of VEGF-A
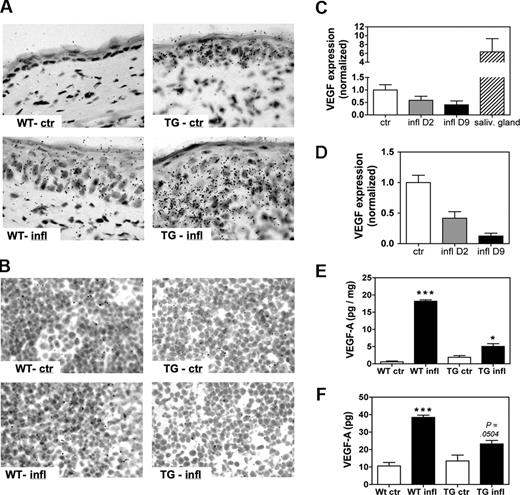
Having determined that VEGF-A is a crucial mediator of lymphangiogenesis in the LNs that drain DTH-inflamed tissues, we set out to localize the source(s) of this growth factor. For this, we performed in situ hybridization on ear and LN sections obtained from inflamed and control WT and VEGF-A Tg mice on study day 9. VEGF-A was weakly expressed in the ears of control WT mice and was markedly induced in ears of inflamed WT mice (Figure 5A). Basal levels of VEGF-A were slightly higher in ear sections from control VEGF-A Tg mice, and high levels of VEGF-A mRNA expression were observed in inflamed ears of VEGF-A Tg mice (Figure 5A). In both strains, the mRNA expression was primarily confined to the epidermis, indicating that keratinocytes are the main producers of VEGF-A in the inflamed skin, as previously described.24 Surprisingly, in situ hybridization detected virtually no VEGF-A expression in LN sections prepared from inflamed or from control mice (Figure 5B). Likewise, quantitative RT-PCR of total RNA extracted from auricular LN homogenates did not reveal an increase of VEGF-A mRNA in inflamed, compared with control, LNs on study days 2 or 9 (Figure 5C,D).
VEGF-A is expressed in the inflamed ears but not in the draining LNs. A DTH response was induced and maintained in the ears of WT or of VEGF-A Tg mice, and VEGF-A mRNA and protein levels in ears and draining auricular LN were determined on study days 2 and 9. (A) In situ hybridization on study day 9 revealed that VEGF-A mRNA was up-regulated in inflamed ears of both WT mice (WT-infl) and of VEGF-A Tg (TG-infl) mice compared with WT and VEGF-A Tg control mice (WT-ctr and TG-ctr, respectively). (B) Virtually no VEGF-A mRNA expression was detected in LN sections from inflamed or control VEGF-A Tg (TG-infl and TG-ctr) and WT mice (WT-infl and WT-ctr). (C,D) Quantitative RT-PCR was performed on RNA extracted from auricular LNs of WT (C) or of VEGF-A Tg (D) mice. No increase in VEGF-A mRNA expression in inflamed over control LNs could be detected, neither when analyzing RNA extracted on study day 2 (D2) nor on study day 9 (D9). VEGF-A mRNA levels in salivary glands were assayed as positive control. (E,F) A VEGF-A ELISA was performed on tissue homogenates of ears (E) and draining LNs (F) on study day 9. (E) VEGF-A protein concentration was significantly elevated in homogenates of inflamed ears, compared with controls. (F) The amount of VEGF-A protein was significantly higher in homogenates from inflamed LNs compared with control LNs. *P < .05; ***P < .001 (compared with control). Error bars are SE.
VEGF-A is expressed in the inflamed ears but not in the draining LNs. A DTH response was induced and maintained in the ears of WT or of VEGF-A Tg mice, and VEGF-A mRNA and protein levels in ears and draining auricular LN were determined on study days 2 and 9. (A) In situ hybridization on study day 9 revealed that VEGF-A mRNA was up-regulated in inflamed ears of both WT mice (WT-infl) and of VEGF-A Tg (TG-infl) mice compared with WT and VEGF-A Tg control mice (WT-ctr and TG-ctr, respectively). (B) Virtually no VEGF-A mRNA expression was detected in LN sections from inflamed or control VEGF-A Tg (TG-infl and TG-ctr) and WT mice (WT-infl and WT-ctr). (C,D) Quantitative RT-PCR was performed on RNA extracted from auricular LNs of WT (C) or of VEGF-A Tg (D) mice. No increase in VEGF-A mRNA expression in inflamed over control LNs could be detected, neither when analyzing RNA extracted on study day 2 (D2) nor on study day 9 (D9). VEGF-A mRNA levels in salivary glands were assayed as positive control. (E,F) A VEGF-A ELISA was performed on tissue homogenates of ears (E) and draining LNs (F) on study day 9. (E) VEGF-A protein concentration was significantly elevated in homogenates of inflamed ears, compared with controls. (F) The amount of VEGF-A protein was significantly higher in homogenates from inflamed LNs compared with control LNs. *P < .05; ***P < .001 (compared with control). Error bars are SE.
Interestingly, in situ hybridization for VEGF-C yielded similar results: VEGF-C mRNA levels were slightly up-regulated in ear sections of inflamed WT and VEGF-A Tg mice, compared with control mice, but virtually no VEGF-C mRNA expression was detected in LNs from mice with DTH inflammation or from control mice (Figure S3A,B).
In agreement with this analysis, ELISA experiments revealed that VEGF-A protein concentrations were highly elevated in tissue homogenates prepared from inflamed ears of VEGF-A Tg mice and from repeatedly challenged ears of WT mice, compared with ears of control mice (Figure 5E and Figure S4A). VEGF-A protein was significantly elevated in tissue homogenates of inflamed LNs, compared with control LNs (Figure 5F and Figure S4B). Thus, although a rise in VEGF-A protein was coupled with an increase in VEGF-A mRNA levels at the site of inflammation, the increase in VEGF-A protein did not coincide with an increase in VEGF-A mRNA in the draining auricular LN. These results indicate that the inflamed ears were the predominant source of VEGF-A and that VEGF-A produced at the sites of inflammation was subsequently transported to the draining LNs through afferent lymphatics.
LN lymphangiogenesis does not depend on the presence of B cells
To rule out the possibility that VEGF-A might be expressed by LN-resident B cells11 at subdetectable levels, we examined whether the absence of B cells had any effect on LN lymphangiogenesis in our model of DTH-induced skin inflammation. For this, JHT mice, which are deficient in B cells, were subjected to repeated rounds of DTH challenge with oxazolone, and ears and draining LNs were analyzed after 9 days. Induction of inflammation in the ear occurred with similar kinetics and intensity as in WT mice, in agreement with the fact that cutaneous DTH responses are predominantly T cell-mediated.25 In addition to an increased number of leukocytes, the numbers of LECs and BECs were also increased in inflamed ears of JHT mice (Figure 6A-D). The LNs that drained the inflamed ears had increased in weight and cellularity (4.4- and 3.7-fold, respectively; Figure 6E,F), and also the number of LECs had increased significantly (3.4-fold; Figure 6G), whereas the number of BECs was unchanged, compared with control mice (Figure 6H). Immunofluorescence analysis confirmed an expansion of the lymphatic network in LN sections from mice with inflamed ears, compared with LN sections from control mice (Figure 6I). Thus, DTH-induced lymphangiogenesis in draining LNs occurs independently from the presence of B cells.
Lymphangiogenesis in LNs that drain inflamed ears occurs in the absence of nodal B cells. A DTH response to oxazolone was induced in the ears of B cell-deficient JHT mice and maintained by repeatedly challenging the ears of the mice with oxazolone over 9 days. Ears and draining auricular LNs were analyzed on study day 9. (A-C) Quantitative FACS analysis detected a significant increase in the numbers of leukocytes (A) and of LECs (B) and BECs (C) in cell suspensions of inflamed compared with control (ctr) ears. (D) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that vascularization was increased in inflamed compared with control ears. Scale bars represent 50 μm. (E,F) Analysis of ear draining auricular LNs revealed that LN weight (E) and cellularity (F) was significantly increased in LNs of inflamed animals compared with LNs of control animals. (G,H) Quantitative FACS analysis detected elevated numbers of LECs (G) in LNs draining inflamed compared with LNs draining control ears, but no change in BEC numbers (H). (I) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that lymphatic structures were markedly expanded in LNs draining inflamed compared with control ears. Scale bars represent 100 μm. **P < .01; ***P < .001 (compared with control). Error bars are SE.
Lymphangiogenesis in LNs that drain inflamed ears occurs in the absence of nodal B cells. A DTH response to oxazolone was induced in the ears of B cell-deficient JHT mice and maintained by repeatedly challenging the ears of the mice with oxazolone over 9 days. Ears and draining auricular LNs were analyzed on study day 9. (A-C) Quantitative FACS analysis detected a significant increase in the numbers of leukocytes (A) and of LECs (B) and BECs (C) in cell suspensions of inflamed compared with control (ctr) ears. (D) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that vascularization was increased in inflamed compared with control ears. Scale bars represent 50 μm. (E,F) Analysis of ear draining auricular LNs revealed that LN weight (E) and cellularity (F) was significantly increased in LNs of inflamed animals compared with LNs of control animals. (G,H) Quantitative FACS analysis detected elevated numbers of LECs (G) in LNs draining inflamed compared with LNs draining control ears, but no change in BEC numbers (H). (I) Immunofluorescence analysis of LYVE-1 (green) and MECA-32 (red) expression confirmed that lymphatic structures were markedly expanded in LNs draining inflamed compared with control ears. Scale bars represent 100 μm. **P < .01; ***P < .001 (compared with control). Error bars are SE.
Because repeated application of an irritant substance like oxazolone could potentially also elicit an antigen-nonspecific inflammatory response in the skin, we decided to test whether inflammation and LN lymphangiogenesis observed in our model were dependent on specific antigen recognition. To this end, we applied our protocol of repeated oxazolone challenges to SCID/beige mice. These mice are deficient in lymphocytes and contain dysfunctional natural killer (NK) cells and, consequently, do not mount a DTH response to a single challenge with a contact sensitizer.26
Interestingly, we found that 9 days after the first challenge, the ears of SCID/beige mice were reddened and significantly swollen (Figure S5A), but inflammation was less pronounced than in ears of repeatedly challenged WT mice (no dried exudates were present; data not shown). Immunofluorescence of ear skin revealed an increase in blood vessels and an expansion of lymphatic vessels (Figure S5B). Furthermore, the weight of the draining auricular LN was markedly increased, and also lymphatic structures in the draining LNs were expanded, as assessed by immunofluorescence (Figure S5C,D). These results indicate that antigen-nonspecific inflammatory events contribute to the LN lymphangiogenesis observed in our model.
Discussion
Our study reveals that prolonged tissue inflammation potently promotes lymphangiogenesis in the inflamed tissue and also in the tissue-draining LNs. As a model of inflammation, we induced DTH responses to the contact sensitizer oxazolone in the ears of VEGF-A Tg18 and WT mice. VEGF-A Tg mice are unable to down-regulate DTH-induced inflammation and, as a consequence, develop psoriasis-like symptoms in the skin.5,12 Because WT mice typically clear DTH-induced inflammation within 1 to 2 weeks, we repeatedly restimulated the ears of these mice with oxazolone to maintain a strong inflammatory response for a 9-day observation period. In both the VEGF-A Tg and WT models of DTH-induced inflammation, we observed a robust proliferative response of the lymphatic network in the ear-draining auricular LNs, as measured by quantitative FACS analysis and by double immunofluorescence with Ki67.
The concept that the lymphatic network is not only remodeled at sites of tumor growth or inflammation, but also in LNs that drain these tissues, has only recently been established, mainly based on immunofluorescence analyses of LN sections.9,11,27 Using such a qualitative approach, it is, however, impossible to determine whether the observed expansion of the lymphatic network is caused by passive dilation of preexisting vessels or by active proliferation of LECs. Quantification of LECs and BECs in tissue single-cell suspensions by FACS analysis therefore represents an important tool to analyze the nature of the observed vascular response.
Because VEGF-A Tg mice constitutively express VEGF-A18 in the skin and induce a strong, possibly supraphysiologic up-regulation of this growth factor during inflammation, an important finding of this study was that inflammation-induced LN lymphangiogenesis not only occurred in this model but also in LNs of WT mice after induction of a chronic DTH response. It is therefore likely that inflammation-induced LN lymphangiogenesis also occurs in patients with persisting inflammatory disorders such as psoriasis, rheumatoid arthritis, Crohn's disease, or ulcerative colitis, during which VEGF-A levels are reportedly elevated in the inflamed tissue.28-30
We identified VEGF-A as the prime mediator of inflammation-induced LN lymphangiogenesis; anti-VEGF-A almost completely blocked LEC proliferation in LNs that drained the inflamed ears of WT mice, as well as (lymph)angiogenesis in the inflamed ear tissues. Perhaps the most surprising finding was that VEGF-A expression was increased in activated LNs at the protein level but not at the mRNA level, as determined by in situ hybridization and quantitative RT-PCR. These results indicate that VEGF-A is transported to the inflamed LN from another, distant site. Indeed, we found that both VEGF-A mRNA and protein were strongly induced in the inflamed ears of VEGF-A Tg and WT mice. Collectively, these findings suggest that during chronic inflammation, VEGF-A is produced in the inflamed tissue and subsequently drains to the LN, where it induces lymphangiogenesis. In fact, a similar mechanism was recently shown to regulate the action of the inflammatory chemokine MCP-1.31 In this study, the authors showed that inflammation in a peripheral tissue leads to induction of MCP-1, which subsequently drains to the downstream LN where it regulates nodal recruitment of monocytes.
A recent study using a CFA-based model of subcutaneous immunization reported that LN lymphangiogenesis depended on VEGF-A produced within the LN by recruited B cells.11 However, our studies in B cell-deficient mice did not reveal any contribution of B cells to DTH-induced LN lymphangiogenesis. This observation is further supported by our in situ and RT-PCR experiments, which showed that VEGF-A mRNA levels did not increase in activated LNs. Even so, we cannot rule out that B cells contribute to DTH-induced lymphangiogenesis at earlier or later time points than the one analyzed by us. In fact, a recent study by Liao and Ruddle27 reported that LN lymphangiogenesis was delayed but not abolished in B cell-deficient mice after immunization with oxazolone.
Interestingly, we found that LN lymphangiogenesis even occurred when repeatedly applying oxazolone to the ears of SCID/beige mice. These mice have no lymphocytes and harbor dysfunctional NK cells and, consequently, are incapable of mounting an antigen-specific DTH response.26 This observation therefore implies that the inflammatory condition elicited in our DTH model is in part independent of the induction of an adaptive immune response. Overall, this finding indicates that also chronic inflammation elicited by an antigen-nonspecific stimulus induces lymphangiogenesis in the draining LN.
It is well known that during the inflammatory response, tissue macrophages32 and activated keratinocytes33 can produce high levels of VEGF-A. In the model used in our study, the activated keratinocytes of the epidermis are probably the prime source of VEGF-A, as demonstrated by in situ hybridization. The fact that inflammation specifically promotes lymphangiogenesis, but not angiogenesis, in the draining LN might be somewhat surprising. However, this absence of BEC proliferation in the activated LNs fits with our data showing that VEGF-A is not produced in the LN but probably only drains to this site via the afferent lymphatics from its place of production: the inflamed ears. Therefore, given the spatial confinement of VEGF-A to the lymphatic vessels when entering the LN, it seems likely that VEGF-A acts mainly on LECs because of its proximity to this cell type. The distribution of lymph-borne proteins to different compartments of the LN has been shown to largely depend on their molecular weight (MW).34 Although the MW of the predominant VEGF-A isoform, VEGF-164 (46 kDa), should still allow its penetration from the lymphatic vessels into the nodal tissue, it is generally thought that such free diffusion of VEGF-164 is compromised by its heparin-binding domain, which preferentially anchors the molecule to cell surfaces and to the extracellular matrix.35,36
It has recently been shown that VEGF-A can induce VEGF-C expression, either by increasing the recruitment of VEGF-C-producing inflammatory cells21 or by inducing VEGF-C expression in endothelial cells.22 In fact, in situ hybridization revealed that VEGF-C mRNA was slightly up-regulated in inflamed ears but absent from tissue-draining LNs. However, administration of a VEGFR3-Fc fusion protein, which sequesters VEGF-C, did not block inflammation-induced LN lymphangiogenesis in our model. This suggests that VEGF-C does not play an important role in this process. The fact that anti-VEGF-A almost completely inhibited LEC proliferation in the activated LNs suggests that VEGF-A is the key trigger of LN lymphangiogenesis.
Little is known about the biologic role of lymphangiogenesis in inflamed tissues or in draining LNs. It is perceivable that in these tissues, an increase in lymphatic vessel density and surface area could improve tissue fluid homeostasis and alleviate edema formation. However, it has not been determined whether the newly formed lymphatics, especially those in the LN, are functional. Some studies have indicated that the lymphangiogenic response leads to increased antigen drainage and migration of antigen presenting cells to the draining LN, thereby boosting the immune response.11,37,38 In this regard, it will be important to determine whether anti-lymphangiogenic agents can alleviate certain inflammatory disorders, or whether stimulation of the lymphatic system could be used to increase the potency of the immune response during vaccination.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jana Zielinski and Sarah Hofmann for technical assistance. C.H. gratefully acknowledges support from the Swiss National Fund (grant 310000-116128) and the Prof. Dr. Max Cloetta Foundation.
This work was supported by the National Institutes of Health (grants CA69184 and CA92644), the Swiss National Fund (grant 3100A0-108207), the Austrian Science Fund (grant S9408-B11), and the Commission of the European Communities (grant LSHC-CT-2005-518178) (M.D.).
National Institutes of Health
Authorship
Contribution: C.H. designed and performed research, analyzed data, and wrote the paper; N.E.T., B.V., and L.F.B. performed research and analyzed data; and M.D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Detmar, Swiss Federal Institute of Technology, ETH, Wolfgang-Pauli-Str 10, HCI H303, 8093 Zurich, Switzerland; e-mail: michael.detmar@pharma.ethz.ch.

![Figure 4. Blockade of VEGF-A, but not of VEGF-C, inhibits inflammation-induced LN lymphangiogenesis. A DTH response to oxazolone was induced in the ears of WT mice and maintained by repeatedly applying oxazolone for 9 days. Mice with inflamed ears were exposed to anti–VEGF-A Ab (A-F), VEGFR3-Fc (G-L), or control IgG (A-L), and the effects were compared with those observed in mice in which inflammation had not been induced (ctr). (A-F) Effect of anti–VEGF-A: Weight (A) and cellularity (B) of the auricular LN was significantly increased in mice with inflamed ears, exposed to anti–VEGF-A or IgG. This effect was less pronounced in inflamed LNs of mice that had been administered anti–VEGF-A compared with IgG. (C) Exposure to anti–VEGF-A potently blocked the increase in LEC numbers in LNs draining inflamed ears, whereas exposure to IgG had no effect. (D) Inflammation led to a significant increase in leukocyte numbers in the ears of both anti–VEGF-A or IgG treated mice, compared with uninflamed the ears of control animals. (E, F) Administration of anti–VEGF-A potently blocked the increase in LEC (E) and BEC (F) numbers in inflamed ears of mice. (G-L) Effect of VEGFR3-Fc: Weight (G) and cellularity (H) of the auricular LN was similarly increased in mice with inflamed ears exposed to VEGFR3-Fc or IgG. (I) Administration of VEGFR3-Fc did not block the increase in LEC numbers in LNs draining inflamed ears. (J) Leukocyte numbers were strongly increased in inflamed ears of mice exposed to IgG. Administration of VEGFR3-Fc led to a significant reduction in the numbers of leukocytes in inflamed ears. Administration of VEGFR3-Fc had no effect on the numbers of LECs (K) or BECs (L) in inflamed ears of mice. *P < .05, **P < .01, ***P < .001 (compared with control [ctr]); #P < .05, ##P < .01, ###P < .001 (anti–VEGF-A or VEGFR3-Fc, compared with IgG). Error bars are SE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/9/10.1182_blood-2007-01-066811/7/m_zh80210708450004.jpeg?Expires=1767700243&Signature=sz5yralb9LgCj4GED8My5deV8JZERdoa0eoxf4z4Q0qzDXVAdNqBbfDJSR3ahL-VZ0XZMeNz0OeBpOBdpCUl-vTk-mTIk9yS2WAPW~-2ajjzZO7HDSHKMwTxthYMlAdVaVp~-8Mc4ZxZ~5AQgGgYZnCW5XIp0nXRA4O5mE3qq2byO7oPbkRSX6fjbp0A73Pm43jXq7jEw3v2cklhcEie0N1UT~MO~zei2mK5gfsmOYIHcbu7cM2gR4I3Dr~uDbDgztGBrUyK984jgushPIrq4Z8tZZc8gfutsXZ8HAbOhiIkL14qQuBjEbg7EK7WF1ziwAYl4XtnxVKeqWNF4E13gw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal