Abstract
CD4+CD25+Foxp3+ regulatory T (Treg) cells have been implicated in the lack of effective antitumor immunity. Denileukin diftitox (DAB389IL-2), a fusion protein of interleukin 2 (IL-2) and diphtheria toxin, provides a means of targeting Treg cells. In this study, we examined (1) the effect of denileukin diftitox on the deletion of Treg cells in various lymphoid compartments and (2) the dose scheduling of denileukin diftitox in combination with a recombinant poxviral vaccine to enhance antigen-specific immune responses. Treg cells in spleen, peripheral blood, and bone marrow of normal C57BL/6 mice were variously reduced after a single intraperitoneal injection of denileukin diftitox; the reduction was evident within 24 hours and lasted approximately 10 days. Injection of denileukin diftitox 1 day before vaccination enhanced antigen-specific T-cell responses above levels induced by vaccination alone. These studies show for the first time in a murine model (1) the differential effects of denileukin diftitox on Treg cells in different cellular compartments, (2) the advantage of combining denileukin diftitox with a vaccine to enhance antigen-specific T-cell immune responses, (3) the lack of inhibition by denileukin diftitox of host immune responses directed against a live viral vector, and (4) the importance of dose scheduling of denileukin diftitox when used in combination with a vaccine.
Introduction
Naturally occurring regulatory T (Treg) cells, characterized by expression of the interleukin 2 (IL-2) receptor α chain (CD25), the transcription factor Foxp3, cytotoxic T-lymphocyte antigen-4 (CTLA-4), and glucocorticoid-induced tumor necrosis factor (TNF) receptor (GITR), constitute 5% to 10% of peripheral CD4+ T cells.1,2 Treg cells represent an important mechanism of peripheral T-cell tolerance through their inhibition of self-reactive effector T cells.1 Treg cells also have been implicated in the lack of effective antitumor immunity, having been shown to be increased in both peripheral blood and tumors in a wide variety of human cancers.3-6 Moreover, the increase in Treg cells in patients with cancer has been associated with poorer prognosis and reduced survival.7,8
Denileukin diftitox (DAB389IL-2, ONTAK) is a fusion protein of human IL-2 and the enzymatically active and membrane-translocating domains of diphtheria toxin.9 It preferentially binds to cells expressing the high-affinity IL-2R, consisting of CD25 (IL-2Rα), CD122 (IL-2Rβ), and CD132 (γc).10 After binding to the IL-2R, denileukin diftitox is internalized by endocytosis and inhibits protein synthesis, ultimately leading to cell death.9 The United States Food and Drug Administration has approved the use of denileukin diftitox for the treatment of cutaneous T-cell lymphoma, in which up to 60% of tumors express IL-2R, and the clinical response to denileukin diftitox has been shown to correlate with the level of CD25 expression.9,11 The efficacy of denileukin diftitox on other tumor types without CD25 expression suggested that denileukin diftitox also could affect tumors indirectly, perhaps through deletion of CD4+CD25+ Treg cells.12,13 Several studies have since investigated the use of denileukin diftitox to delete Treg cells, with generally consistent results. Denileukin diftitox was shown to delete blood Treg cells in patients with late-stage ovarian cancer, melanoma, and renal cell carcinoma.14-16 However, another study of patients with metastatic melanoma showed only minimal reduction of Foxp3 expression in purified CD4+ cells after denileukin diftitox treatment.17 In a mouse model for breast cancer, multiple doses of 5 μg denileukin diftitox were shown to reduce Treg cells in spleen at a single time point.18
The first aim of the study reported here was to examine comprehensively in a murine model the effect of different dosing regimens of denileukin diftitox on Treg cells in different cellular compartments. We show that a single intraperitoneal injection of denileukin diftitox partially deleted Treg cells in spleen, peripheral blood, and bone marrow of normal C57BL/6 mice, a deletion evident within 24 hours of injection. Thereafter, the Treg-cell population recovered, reaching normal levels in approximately 10 days after denileukin diftitox administration. The second aim of this study was to determine whether the transient decrease in Treg cells caused by denileukin diftitox administration could enhance antigen-specific T-cell responses to a viral-based vaccine consisting of recombinant poxviral vectors (vaccinia or fowlpox) expressing a specific antigen and a triad of costimulatory molecules (B7–1, intercellular adhesion molecule 1 [ICAM-1], and leukocyte function associated antigen 3 [LFA-3], designated TRICOM).19-21 We show that denileukin diftitox injection 1 day before a poxviral-based vaccine increased antigen-specific T-cell responses above levels induced by vaccination alone, in both a self-antigen system, with CEA as antigen, and a foreign-antigen system, with Influenza A/PR/8/34 nucleoprotein as antigen. These studies show for the first time in a murine model (1) the differential deletion by denileukin diftitox of Treg cells in different cellular compartments, (2) the combination of denileukin diftitox with a vaccine to enhance antigen-specific T-cell immune responses, (3) the lack of inhibition by denileukin diftitox of host immune responses directed against a live viral vector, and (4) the importance of dose scheduling of denileukin diftitox when used in combination with a vaccine.
Materials and methods
Mice were used in this study after institutional review board approval. Animal care was in compliance with recommendations of the Guide for Care and Use of Laboratory Animals, National Research Council.
Denileukin diftitox
Denileukin diftitox was generously provided by Ligand Pharmaceuticals (San Diego, CA). It was supplied as a sterile, frozen solution at a concentration of 150 μg/mL in citrate buffer. For in vivo treatment, mice were injected intraperitoneally with denileukin diftitox diluted in 100 μL HBSS at doses indicated.
Mice
Female C57BL/6 mice were obtained from the National Cancer Institute, Frederick Cancer Research Facility (Frederick, MD). Female C57BL/6 mice transgenic for human CEA were derived from a breeding pair provided by Dr John E. Shively (Division of Immunology, Beckman Research Institute of the City of Hope, Duarte, CA).22 Mice were used at 8 to 12 weeks of age.
Human PBMCs
Peripheral blood was collected from healthy donors after institutional review board approval by the National Institutes of Health Clinical Center. Informed consent was obtained in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were isolated from leukapheresis samples by centrifugation on a Ficoll density gradient (LSM Lymphocyte Separation Medium; ICN Biochemicals, Aurora, OH).
Fluorescence-activated cell sorting (FACS) analysis
Antibodies to CD4, CD25, CD8, CD19, CD49b, and all corresponding isotype controls were purchased from BD Biosciences Pharmingen (San Diego, CA). Foxp3 staining was performed with antibody and fixation or permeabilization buffers from eBioscience (San Diego, CA). Annexin V-FITC Apoptosis Detection Kit was purchased from BD Biosciences Pharmingen.
In vivo proliferation
Mice were placed on 0.8 mg/mL bromodeoxyuridine (BrdU) water, changed daily, for 5 days before being killed. At time of being killed, cells were stained for CD4, CD25, and BrdU incorporation (FITC BrdU Flow Kit; BD Biosciences Pharmingen).
In vitro suppressor assay
CD4+CD25− and CD4+CD25+ cells were purified by magnetic beads (for mouse; Miltenyi Biotec, Auburn, CA) or by FACS (for humans; FACSVantage; Becton Dickinson, San Jose, CA). On 96-well round-bottom plates precoated with 1 μg/mL anti-CD3 (BD Biosciences Pharmingen; eBioscience), control CD4+CD25− cells were incubated at a 1:1 ratio with CD4+CD25+ cells (control or denileukin diftitox treated), with irradiated syngeneic antigen-presenting cells (APCs). Cells were cultured for 4 days. 3H-thymidine (1 μCi [0.037 MBq]/well) was added for the final 18 hours before harvesting.
Recombinant poxvirus vaccines
Vaccines used were recombinant vaccinia (rV-) or fowlpox (rF-) viruses containing the genes encoding human CEA protein or Influenza A/PR/8/34 nucleoprotein and 3 murine costimulatory molecules: B7–1, ICAM-1, and LFA-3 (designated TRICOM). These vaccines were termed rV-CEA/TRICOM, rV-Inf(A)NP34/TRICOM, and rF-Inf(A)NP34/TRICOM. A description of the construction of the recombinant vaccinia and fowlpox viruses has been reported.19,23 The fowlpox virus containing the gene for murine granulocyte macrophage colony-stimulating factor (GM-CSF) has been described previously.24 All recombinant poxviruses were kindly provided by Therion Biologics Corporation (Cambridge, MA).
CD4+ T-cell proliferation and cytokine production
All CD4+ proliferation assays were performed as described previously.25 Human CEA protein was obtained from Aspen Bio (Littleton, CO); β-gal protein (Prozyme, San Leandro, CA) was used as a corresponding negative control. Human Influenza A–purified virus was obtained from Advanced Biotechnologies (Columbia, MD) and inactivated using a Stratalinker (Stratagene, La Jolla, CA). Wild-type vaccinia virus was inactivated by photochemical DNA crosslinking using psoralen (Sigma-Aldrich, St Louis, MO) and UV light, as previously described.26 To assess cytokine production, supernatants were removed at 72 hours. Levels of IFN-γ, TNF-α, IL-2, and IL-5 were analyzed using the Mouse Th1/Th2 Cytometric Bead Array kit (BD Biosciences).
CD8+ T-cell IFN-γ production
Splenocytes (5 × 105) were cultured on 96-well round-bottom plates with the indicated concentrations of H-2Db-restricted nonamer peptide NP34 (ASNENMETM; Biosynthesis, Lewisville, TX) from the nucleoprotein of the influenza strain A/PR/8/34. Supernatants were removed at 48 hours. Levels of IFN-γ were determined using the Mouse IFN-γ Quantikine enzyme-linked immunoabsorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN). An H-2Db-restricted VSV peptide (RGYVYQGL; Biosynthesis) was used as a negative control.
ELISA for anti-Flu and antivaccinia antibodies
Microtiter plates were coated overnight with either 60 ng/well inactivated Flu virus or 5 × 105 plaque-forming unit (pfu)/well wild-type vaccinia virus. Otherwise, ELISA was performed as described previously.19
Results
Deletion of murine CD4+CD25+Foxp3+ regulatory T cells by denileukin diftitox in vitro
To our knowledge, no reports exist on the effect of denileukin diftitox on murine Treg cells in vitro. To determine the level of denileukin diftitox–mediated deletion of CD4+CD25+Foxp3+ Treg cells in vitro, whole murine splenocytes were treated with varying doses of denileukin diftitox for 2 hours, then washed, and incubated at 37°C for various time points. The 2-hour treatment was chosen to approximate the situation in vivo, because the half-life of denileukin diftitox in humans after intravenous injection has been reported to be 72 minutes.27 Although staining with CD4, CD25, and Foxp3 antibodies showed no evidence of Treg-cell deletion at 24 hours after treatment (data not shown), deletion was observed at 48 hours and, to a greater extent, at 72 hours (Figure 1A). The lowest dose of denileukin diftitox used, 25 ng/mL (0.4 nM), reduced CD4+CD25+Foxp3+ regulatory T cells by 60% at 72 hours after treatment (Figure 1A). Increasing treatment doses of 75, 225, and 675 ng/mL (1.3, 3.9, and 11.6 nM) resulted in greater percentages of Treg-cell deletion at 72 hours, of 70%, 78%, and 89%, respectively (Figure 1A). In summary, denileukin diftitox deleted murine CD4+CD25+Foxp3+ regulatory T cells in vitro in a dose-dependent manner.
Effect of denileukin diftitox on murine and human Treg cells in vitro. (A) Whole murine splenocytes from C57BL/6 mice were treated with the indicated doses of denileukin diftitox for 2 hours, then washed, and incubated at 37°C for 72 hours. Splenocytes were stained for CD4+CD25+Foxp3+ Treg cells. The plots shown were gated on CD4+ T cells. Percentage of CD25+Foxp3+ cells is indicated in the upper right quadrant of each plot. (B-D) PBMCs from healthy human donors were treated with 290 ng/mL (5 nM) denileukin diftitox for 72 hours. Cells were stained for CD4 and CD25. CD4+CD25+ cells were gated into CD25high and CD25low subpopulations based on CD25 mean fluorescent intensity. The low threshold for the CD25high gate was set above the population of CD4−CD25+ cells; the low threshold for the CD25low gate was set above the isotype control. (B) Percentage CD25high, CD25low, and CD25+ of total CD4+ T cells is shown in the left, center, and right graphs, respectively, for untreated and denileukin diftitox-treated samples from 6 donors. (C) Representative plots for untreated and denileukin diftitox-treated samples from one donor are shown. Percentage CD25high of total CD4+ T cells is indicated on each plot. (D) CD4+CD25+ cells were isolated from untreated and denileukin diftitox-treated PBMC samples by FACS. Purified CD4+CD25− effector T cells (2.5 × 104) from untreated PBMCs were then cultured in the presence or absence of the isolated CD4+CD25+ cells at a 1:1 ratio (with irradiated untreated PBMCs as antigen-presenting cells [APCs; 1.25 × 105]) on an anti–CD3-coated 96-well plate. Mean proliferation (± SD) was measured by 3H-thymidine incorporation of triplicate wells.
Effect of denileukin diftitox on murine and human Treg cells in vitro. (A) Whole murine splenocytes from C57BL/6 mice were treated with the indicated doses of denileukin diftitox for 2 hours, then washed, and incubated at 37°C for 72 hours. Splenocytes were stained for CD4+CD25+Foxp3+ Treg cells. The plots shown were gated on CD4+ T cells. Percentage of CD25+Foxp3+ cells is indicated in the upper right quadrant of each plot. (B-D) PBMCs from healthy human donors were treated with 290 ng/mL (5 nM) denileukin diftitox for 72 hours. Cells were stained for CD4 and CD25. CD4+CD25+ cells were gated into CD25high and CD25low subpopulations based on CD25 mean fluorescent intensity. The low threshold for the CD25high gate was set above the population of CD4−CD25+ cells; the low threshold for the CD25low gate was set above the isotype control. (B) Percentage CD25high, CD25low, and CD25+ of total CD4+ T cells is shown in the left, center, and right graphs, respectively, for untreated and denileukin diftitox-treated samples from 6 donors. (C) Representative plots for untreated and denileukin diftitox-treated samples from one donor are shown. Percentage CD25high of total CD4+ T cells is indicated on each plot. (D) CD4+CD25+ cells were isolated from untreated and denileukin diftitox-treated PBMC samples by FACS. Purified CD4+CD25− effector T cells (2.5 × 104) from untreated PBMCs were then cultured in the presence or absence of the isolated CD4+CD25+ cells at a 1:1 ratio (with irradiated untreated PBMCs as antigen-presenting cells [APCs; 1.25 × 105]) on an anti–CD3-coated 96-well plate. Mean proliferation (± SD) was measured by 3H-thymidine incorporation of triplicate wells.
Deletion of human CD4+CD25high T cells by denileukin diftitox in vitro
There are conflicting reports about the efficacy of denileukin diftitox on human regulatory T cells both in vitro and in vivo.14-17 Complicating analyses of the effect of denileukin diftitox on regulatory T cells in humans is that, unlike the situation with mouse Treg cells, no unique marker exists for human Treg cells. Suppressive activity appears to correlate only with those CD4+ T cells expressing the highest levels of CD25,28 and, unlike in the mouse system, the Foxp3 transcription factor is expressed by activated T cells as well as Treg cells.29,30 Denileukin diftitox was previously shown to reduce CD4+CD25high T cells, with minimal effects on the total CD4+CD25+ population.15-17 We examined the action of denileukin diftitox on CD4+CD25high, CD4+CD25low, and total CD4+CD25+ cells from human PBMCs from 6 healthy donors in vitro. When the PBMCs were treated with doses of denileukin diftitox ranging from 25 to 675 ng/mL (0.4-11.6 nM) for 2 hours, then washed, and followed for an additional 48 to 72 hours, analogous to the treatment of murine splenocytes, no effect was observed on the CD4 subpopulations (data not shown). In contrast, after continuous treatment with 290 ng/mL (5 nM) denileukin diftitox, reduction of the CD4+CD25high population was observed at 48 hours (data not shown) and, more so, at 72 hours (Figure 1B-C). Although the overall percentage of CD4+CD25+ or CD4+CD25low T cells was not significantly changed after denileukin diftitox treatment, the fraction of CD4+CD25high T cells was markedly deleted by denileukin diftitox at 72 hours after treatment (from an average of 0.89% to 0.17% of total CD4+ T cells for all 6 donors tested) (Figure 1B,C).
To examine functionality of the CD4+CD25+ population, CD4+CD25+ cells were purified from untreated and denileukin diftitox-treated PBMCs (290 ng/mL, 72-hour treatment). Purified CD4+CD25− effector T cells from untreated PBMCs were then cultured in the presence or absence of the isolated CD4+CD25+ cells at a 1:1 ratio. As shown in Figure 1D, whereas CD4+CD25+ cells from untreated PBMCs suppressed the proliferation of the effector cells by 27%, CD4+CD25+ cells from denileukin diftitox-treated PBMCs exhibited no such suppression. In fact, because the denileukin diftitox-treated CD4+CD25+ population now lacked the putative CD4+CD25high Treg-cell subset (refer to Figure 1C), these cells alone proliferated to equal or better levels than the CD4+CD25− effector cells, suggesting that the remaining CD4+CD25low cells were effector cells. These results show that denileukin diftitox deletes the CD4+CD25high Treg-cell subset from human PBMCs in vitro.
Deletion of murine CD4+CD25+Foxp3+ regulatory T cells by denileukin diftitox in vivo
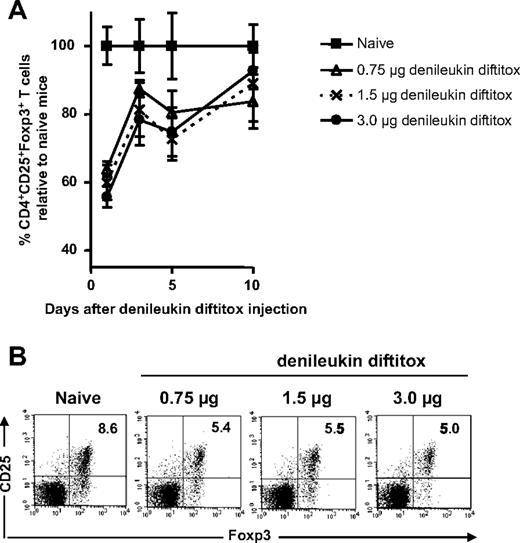
Denileukin diftitox was previously shown to delete Treg cells in BALB/c mice when administered for a total of 6 doses.18 Studies were conducted here to determine whether denileukin diftitox deleted Treg cells in C57BL/6 mice after a single intraperitoneal injection. Denileukin diftitox was administered once to normal C57BL/6 mice, and spleen cells were stained for CD4+CD25+Foxp3+ T cells on various days after the injection. After denileukin diftitox administration (0.75, 1.5, or 3.0 μg dose), the greatest deletion of Treg cells relative to naive mice was seen on day 1 after injection (Figure 2A); levels almost normalized by day 10 after injection (Figure 2A). A single injection of 0.75 μg denileukin diftitox reduced Treg cells by 36.2% (± 2.2%; mean ± SD) relative to naive on day 1 after injection (Figure 2A). As shown in Figure 2B for a representative sample, Treg cells were reduced from 8.6% of total CD4+ cells in naive mice to approximately 5.0% in denileukin diftitox-treated mice. Increasing the dose of denileukin diftitox to 1.5 or 3.0 μg did not result in a significant enhancement of Treg-cell deletion at any time point analyzed (Figure 2A,B); therefore, the 0.75 μg dose was used in further experiments.
Dosing of denileukin diftitox for deletion of splenic Treg cells in vivo. A single intraperitoneal injection of denileukin diftitox (0.75, 1.5, or 3.0 μg) was administered to C57BL/6 mice. (A) Spleen cells from individual mice were stained for CD4+CD25+Foxp3+ Treg cells on days 1, 3, 5, and 10 after the injection. Mean values for percentage of Treg cells (± SD) are shown on the graph as percentage relative to naive mice; actual values for percentage Treg cells of total CD4+ T cells averaged 9.7% (range, 7.3%-12.0%) for the naive group. (B) One representative plot for each group is shown for day 1 after denileukin diftitox injection. The plots shown were gated on CD4+ T cells. Percentage of CD25+Foxp3+ cells is indicated in the upper right quadrant of each plot.
Dosing of denileukin diftitox for deletion of splenic Treg cells in vivo. A single intraperitoneal injection of denileukin diftitox (0.75, 1.5, or 3.0 μg) was administered to C57BL/6 mice. (A) Spleen cells from individual mice were stained for CD4+CD25+Foxp3+ Treg cells on days 1, 3, 5, and 10 after the injection. Mean values for percentage of Treg cells (± SD) are shown on the graph as percentage relative to naive mice; actual values for percentage Treg cells of total CD4+ T cells averaged 9.7% (range, 7.3%-12.0%) for the naive group. (B) One representative plot for each group is shown for day 1 after denileukin diftitox injection. The plots shown were gated on CD4+ T cells. Percentage of CD25+Foxp3+ cells is indicated in the upper right quadrant of each plot.
To examine whether multiple doses of denileukin diftitox were more effective at deleting Treg cells in C57BL/6 mice compared with a single administration, an intraperitoneal injection of 0.75 μg was given on a single day, on 2 consecutive days, or on 3 consecutive days, and spleen cells were stained for Treg cells on various days after the final injection. There was no significant difference in deletion of Treg cells between 1, 2, or 3 injections of denileukin diftitox (data not shown). Thus, a single administration of the 0.75 μg dose was used in future experiments.
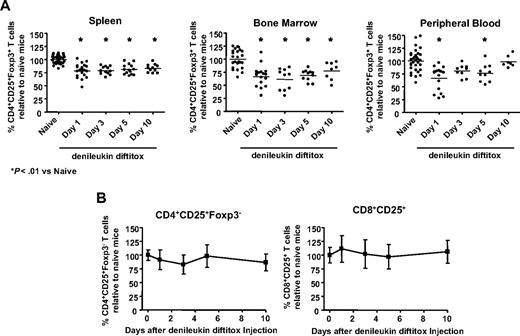
The single report in the literature on the effect of denileukin diftitox on Treg cells in a murine model examined deletion in spleen at a single time point.18 Thus, we next sought to investigate the effect of a single intraperitoneal administration of denileukin diftitox on Treg cells in other lymphoid compartments at various time points; 0.75 μg denileukin diftitox reduced CD4+CD25+Foxp3+ regulatory T cells in spleen to 78.6% (± 12.8%) of the levels seen in naive mice on day 1 after injection (Figure 3A, left). The percentage of Treg cells relative to naive remained depressed on days 3, 5, and 10 after injection, averaging 79.0% (± 7.2%), 81.1% (± 9.7%), and 83.1% (± 7.6%), respectively (Figure 3A, left). The percentage of lymph node Treg cells was decreased by denileukin diftitox treatment similarly to that in spleen (data not shown). In bone marrow, a single injection of denileukin diftitox reduced Treg cells to 66.4% (± 19.5%) of the naive levels on day 1 after injection (Figure 3A, middle). As in spleen, the percentage of bone marrow Treg cells relative to naive mice remained decreased on days 3, 5, and 10 after injection, averaging 61.5% (± 20.6%), 68.7% (± 10.5%), and 77.5% (± 16.9%), respectively (Figure 3A, middle). In peripheral blood, denileukin diftitox reduced Treg cells to 66.8% (± 23.0%) of the naive levels on day 1 after injection, whereas levels normalized by day 10 after injection (Figure 3A, right). These studies thus show the differential regulation of Treg cells by denileukin diftitox in different cellular compartments.
Specificity of denileukin diftitox for deletion of Treg cells versus activated T cells. A single intraperitoneal injection of 0.75 μg denileukin diftitox was administered to C57BL/6 mice (n ≥ 10/group). Flow cytometry analysis was performed on tissues on days 1, 3, 5, and 10 after injection. (A) Spleen, bone marrow, and peripheral blood cells from individual mice were stained for CD4+CD25+Foxp3+ Treg cells. Individual values and means for percentage of Treg cells are represented as percentage relative to naive mice; actual values for percentage Treg cells of total CD4+ T cells in the naive group averaged 8.9% for spleen (range, 5.6%-12%), 8.4% for bone marrow (range, 3.7%-11.1%), and 5.0% for peripheral blood (range, 2.2%-7.8%). Statistical evaluation was performed by one-way ANOVA using GraphPad Prism (GraphPad Software, San Diego, CA). (B) Spleen cells from individual mice were stained for CD4+CD25+Foxp3− and CD8+CD25+ activated T cells. Mean values (± SD) are shown on the graph as percentage relative to naive mice. Actual values for percentage activated CD4+ of total CD4+ T cells and activated CD8+ of total CD8+ T cells averaged 1.4% (range, 0.6%-2.9%) and 0.9% (range, 0.2%-1.6%), respectively, for the naive group.
Specificity of denileukin diftitox for deletion of Treg cells versus activated T cells. A single intraperitoneal injection of 0.75 μg denileukin diftitox was administered to C57BL/6 mice (n ≥ 10/group). Flow cytometry analysis was performed on tissues on days 1, 3, 5, and 10 after injection. (A) Spleen, bone marrow, and peripheral blood cells from individual mice were stained for CD4+CD25+Foxp3+ Treg cells. Individual values and means for percentage of Treg cells are represented as percentage relative to naive mice; actual values for percentage Treg cells of total CD4+ T cells in the naive group averaged 8.9% for spleen (range, 5.6%-12%), 8.4% for bone marrow (range, 3.7%-11.1%), and 5.0% for peripheral blood (range, 2.2%-7.8%). Statistical evaluation was performed by one-way ANOVA using GraphPad Prism (GraphPad Software, San Diego, CA). (B) Spleen cells from individual mice were stained for CD4+CD25+Foxp3− and CD8+CD25+ activated T cells. Mean values (± SD) are shown on the graph as percentage relative to naive mice. Actual values for percentage activated CD4+ of total CD4+ T cells and activated CD8+ of total CD8+ T cells averaged 1.4% (range, 0.6%-2.9%) and 0.9% (range, 0.2%-1.6%), respectively, for the naive group.
We then compared in a controlled experiment the ability of a single dose of 0.75 μg denileukin diftitox to delete Treg cells in C57BL/6 versus BALB/c mice. In C57BL/6 mice, Treg cells were deleted in spleen, bone marrow, and blood, averaging 76.9% (± 4.6), 76.1% (± 13.4), and 78.4% (± 4.4) of the naive levels, respectively, on day 1 after injection (Table 1). This deletion was maintained in spleen and bone marrow on days 3, 5, and 10 after injection (Table 1). In contrast, in BALB/c mice, Treg cells were transiently reduced only in blood, averaging 85.7% (± 5.6) of the naive levels on day 1 after injection (Table 1). No deletion of Treg cells was observed in spleen or bone marrow in BALB/c mice (Table 1).
Effect of a single intraperitoneal injection of denileukin diftitox on Treg cells in various lymphoid compartments in C57BL/6 versus BALB/c mice
| . | Naive . | Day 1 . | Day 3 . | Day 5 . | Day 10 . |
|---|---|---|---|---|---|
| C57BL/6 mice | |||||
| Spleen | 9.9 ± 0.5 | 7.6 ± 0.5* | 7.3 ± 0.4* | 8.3 ± 0.6* | 8.2 ± 1.0* |
| Blood | 6.1 ± 0.4 | 4.8 ± 0.3* | 5.6 ± 0.5 | 5.6 ± 0.8 | 5.6 ± 0.2 |
| Bone marrow | 10.2 ± 0.6 | 7.7 ± 1.4* | 7.8 ± 0.7 | 7.6 ± 0.2* | 6.8 ± 1.9* |
| BALB/c mice | |||||
| Spleen | 8.3 ± 1.1 | 9.0 ± 0.3 | 8.5 ± 0.9 | 8.3 ± 0.9 | 8.2 ± 0.4 |
| Blood | 5.9 ± 0.3 | 5.1 ± 0.3* | 5.4 ± 0.2 | 5.6 ± 0.4 | 5.6 ± 0.5 |
| Bone marrow | 10.9 ± 1.8 | 10.3 ± 2.0 | 6.4 ± 1.9 | 8.2 ± 3.3 | 11.1 ± 4.5 |
| . | Naive . | Day 1 . | Day 3 . | Day 5 . | Day 10 . |
|---|---|---|---|---|---|
| C57BL/6 mice | |||||
| Spleen | 9.9 ± 0.5 | 7.6 ± 0.5* | 7.3 ± 0.4* | 8.3 ± 0.6* | 8.2 ± 1.0* |
| Blood | 6.1 ± 0.4 | 4.8 ± 0.3* | 5.6 ± 0.5 | 5.6 ± 0.8 | 5.6 ± 0.2 |
| Bone marrow | 10.2 ± 0.6 | 7.7 ± 1.4* | 7.8 ± 0.7 | 7.6 ± 0.2* | 6.8 ± 1.9* |
| BALB/c mice | |||||
| Spleen | 8.3 ± 1.1 | 9.0 ± 0.3 | 8.5 ± 0.9 | 8.3 ± 0.9 | 8.2 ± 0.4 |
| Blood | 5.9 ± 0.3 | 5.1 ± 0.3* | 5.4 ± 0.2 | 5.6 ± 0.4 | 5.6 ± 0.5 |
| Bone marrow | 10.9 ± 1.8 | 10.3 ± 2.0 | 6.4 ± 1.9 | 8.2 ± 3.3 | 11.1 ± 4.5 |
C57BL/6 or BALB/c mice were either untreated (naive) or treated with 0.75 μg denileukin diftitox intraperitoneally. Mice were killed on the indicated days after denileukin diftitox injection. Cells from individual mice were stained for CD4+CD25+Foxp3+ Treg cells; value shown is mean percentage CD25+Foxp3+ of total CD4+ cells (n = 4).
Statistical evaluation was performed by one-way ANOVA using GraphPad Prism.
P < .05 versus naive.
Because T cells (both CD4+ and CD8+) up-regulate the expression of CD25 on their cell surface after activation, denileukin diftitox may also mediate deletion of these cell populations.10,15 There was no significant change in CD4+CD25+Foxp3− and CD8+CD25+ activated T cells in spleen, lymph node, blood, or bone marrow of C57BL/6 mice on any day after injection in the denileukin diftitox-treated animals (shown for spleen in Figure 3B). Further, there were no significant changes in the relative numbers of CD4+ or CD8+ T cells, B cells, or natural killer cells in any of these compartments after treatment (data not shown). In summary, a single intraperitoneal injection of 0.75 μg denileukin diftitox into normal mice mediated partial deletion of Treg cells in spleen, bone marrow, and peripheral blood. The deletion was somewhat more dramatic in bone marrow and blood, although those compartments also exhibited higher variability between individual animals. There were no significant decreases in activated CD4+ or CD8+ T cells after denileukin diftitox treatment.
Analyses of proliferation, survival, and functionality of Treg cells not deleted by denileukin diftitox
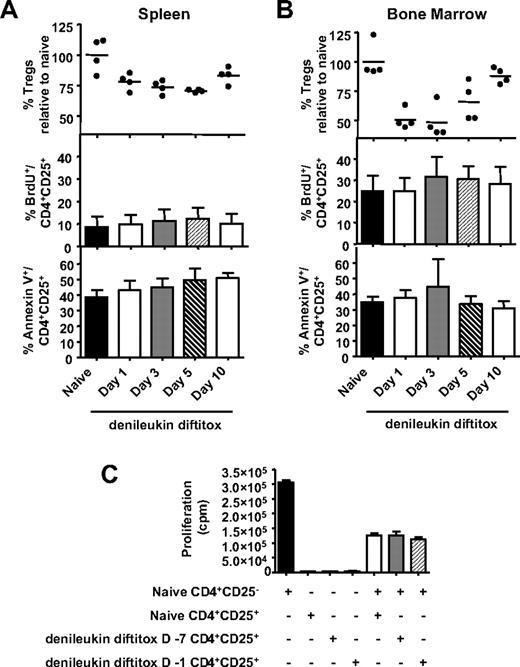
Previous studies using low-dose cyclophosphamide have shown that, in addition to reducing numbers of Treg cells, cyclophosphamide also reduced the functionality of nondeleted Treg cells.31,32 To examine whether the Treg cells that remain after denileukin diftitox treatment are adversely affected, we examined the effect of denileukin diftitox on the in vivo proliferation, apoptosis, and functionality of Treg cells. Mice were fed BrdU water for 5 days before being killed; animals in the denileukin diftitox-treated groups received 1 intraperitoneal injection of denileukin diftitox (0.75 μg) 1, 3, 5, or 10 days before being killed. Staining confirmed that Treg cells were deleted in both spleen and bone marrow after denileukin diftitox treatment (Figure 4A,B, top rows). Staining for CD4, CD25, and BrdU showed that 9% and 25% of Treg cells were proliferating in the spleen and bone marrow, respectively, of untreated animals (Figure 4A,B, middle). This BrdU incorporation by CD4+CD25+ cells was not altered by denileukin diftitox treatment in either compartment on any day after injection (Figure 4A,B, middle). The Foxp3 stain confirmed that the CD4+CD25+ T-cell population was largely composed of Treg cells, as more than 90% expressed Foxp3 (data not shown).
Effect of denileukin diftitox on proliferation, apoptosis, and functionality of Treg cells. (A,B) A single intraperitoneal injection of 0.75 μg denileukin diftitox was administered to C57BL/6 mice. Flow cytometry analysis was performed on tissues on days 1, 3, 5, and 10 after injection. Animals were fed BrdU water for 5 days before being killed. At time of being killed, spleen (A) and bone marrow (B) cells from individual mice were stained for CD4+CD25+Foxp3+ Treg cells (individual values and means represented as percentage relative to naive mice, top), CD4+CD25+BrdU+ cells (mean values ± SD, middle), and CD4+CD25+AnnexinV+ cells (mean values ± SD, bottom). (C) Splenic CD4+CD25+ cells were isolated from naive mice, as well as from mice that were treated with 0.75 μg denileukin diftitox on day −7 or day −1 before being killed on day 0. Purified CD4+CD25− effector T cells (5 × 104) from naive mice were then cultured in the presence or absence of the various isolated Treg cells (CD4+CD25+) at a 1:1 ratio (with 105 irradiated naive T-cell–depleted splenocytes as APCs) on an anti–CD3-coated 96-well plate. Mean proliferation (± SD) was measured by 3H-thymidine incorporation of triplicate wells.
Effect of denileukin diftitox on proliferation, apoptosis, and functionality of Treg cells. (A,B) A single intraperitoneal injection of 0.75 μg denileukin diftitox was administered to C57BL/6 mice. Flow cytometry analysis was performed on tissues on days 1, 3, 5, and 10 after injection. Animals were fed BrdU water for 5 days before being killed. At time of being killed, spleen (A) and bone marrow (B) cells from individual mice were stained for CD4+CD25+Foxp3+ Treg cells (individual values and means represented as percentage relative to naive mice, top), CD4+CD25+BrdU+ cells (mean values ± SD, middle), and CD4+CD25+AnnexinV+ cells (mean values ± SD, bottom). (C) Splenic CD4+CD25+ cells were isolated from naive mice, as well as from mice that were treated with 0.75 μg denileukin diftitox on day −7 or day −1 before being killed on day 0. Purified CD4+CD25− effector T cells (5 × 104) from naive mice were then cultured in the presence or absence of the various isolated Treg cells (CD4+CD25+) at a 1:1 ratio (with 105 irradiated naive T-cell–depleted splenocytes as APCs) on an anti–CD3-coated 96-well plate. Mean proliferation (± SD) was measured by 3H-thymidine incorporation of triplicate wells.
Staining of CD4+CD25+ T cells with Annexin V showed no differences in viability between Treg cells from untreated animals and Treg cells remaining at day 1, 3, 5, or 10 after denileukin diftitox treatment (Figure 4A,B, bottom). To examine functionality, splenic CD4+CD25+ cells were isolated from naive mice, as well as from mice that were treated with denileukin diftitox 7 days or 1 day before being killed. Purified CD4+CD25− effector T cells from naive mice were then cultured in the presence or absence of the various isolated Treg cells (CD4+CD25+) at a 1:1 ratio. As shown in Figure 4C, Treg cells from denileukin diftitox-treated mice (day −7 or day −1) were as efficient as Treg cells from naive mice in suppressing the proliferation of CD4+CD25− effector cells. These results show that Treg cells which remained after denileukin diftitox treatment exhibited proliferation, viability, and functionality similar to that of Treg cells from untreated mice.
Effect of denileukin diftitox on vaccine-mediated immune responses
To determine whether denileukin diftitox could enhance antigen-specific T-cell responses to a live viral-based vaccine and, if so, the optimal timing for denileukin diftitox administration in relation to vaccine, denileukin diftitox was administered to mice before (day −4), concurrent with (day 0), or after (day 3) a subcutaneous vaccination on day 0 with rV-CEA/TRICOM (admixed with rF-GM-CSF). CEA transgenic mice, which express CEA in the gastrointestinal tract, were used to examine the vaccine response in a self-antigen system. Immune assays were performed on day 28 after the vaccine.
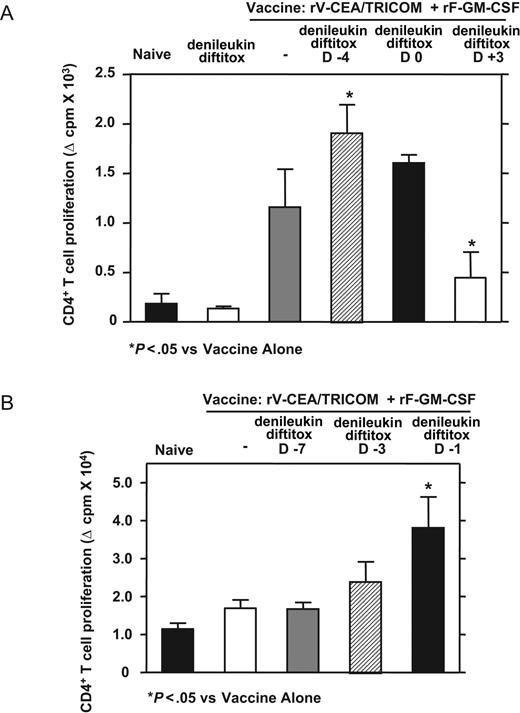
Pretreatment of mice with denileukin diftitox on day −4 relative to vaccine enhanced CD4+ T-cell proliferation to CEA protein compared with vaccine alone (Figure 5A). Treatment with denileukin diftitox on day 0 relative to vaccine had a minimal effect on proliferation, whereas the addition of denileukin diftitox on day 3 decreased the CD4 response to vaccine, suggestive of denileukin diftitox-mediated deletion of freshly activated T cells (Figure 5A). T-cell proliferation to CEA protein was not seen after treatment with denileukin diftitox alone (Figure 5A).
Scheduling of denileukin diftitox treatment for optimization of CD4+ T-cell proliferation to rV-CEA/TRICOM (+rF-GM-CSF) vaccination in a self-antigen system. CEA transgenic mice were subcutaneously vaccinated on day 0 with 1 × 108 pfu rV-CEA/TRICOM (admixed with 1 × 107 pfu rF-GM-CSF). On day 28, CD4+ T cells were added to proliferation assays with 50 μg/mL CEA protein. Mean proliferation (± SD) was measured by 3H-thymidine incorporation of triplicate wells; nonspecific proliferation to β-Gal protein was subtracted from CEA-specific proliferation. A single intraperitoneal injection of 0.75 μg denileukin diftitox was administered to indicated groups (A) before (day −4), concurrent with (day 0), or after (day 3) vaccination or (B) on day −7, −3, or −1 before vaccination. Statistical evaluation was performed by repeated-measures one-way ANOVA using GraphPad Prism.
Scheduling of denileukin diftitox treatment for optimization of CD4+ T-cell proliferation to rV-CEA/TRICOM (+rF-GM-CSF) vaccination in a self-antigen system. CEA transgenic mice were subcutaneously vaccinated on day 0 with 1 × 108 pfu rV-CEA/TRICOM (admixed with 1 × 107 pfu rF-GM-CSF). On day 28, CD4+ T cells were added to proliferation assays with 50 μg/mL CEA protein. Mean proliferation (± SD) was measured by 3H-thymidine incorporation of triplicate wells; nonspecific proliferation to β-Gal protein was subtracted from CEA-specific proliferation. A single intraperitoneal injection of 0.75 μg denileukin diftitox was administered to indicated groups (A) before (day −4), concurrent with (day 0), or after (day 3) vaccination or (B) on day −7, −3, or −1 before vaccination. Statistical evaluation was performed by repeated-measures one-way ANOVA using GraphPad Prism.
Because denileukin diftitox treatment before vaccine resulted in the enhancement of immunity, we sought to more accurately determine the optimal day for treatment. Denileukin diftitox was administered to CEA transgenic mice on day −7, −3, or −1 relative to a subcutaneous vaccination on day 0 with rV-CEA/TRICOM (admixed with rF-GM-CSF). Although the addition of denileukin diftitox on day −3 slightly enhanced CD4 response to vaccine, pretreatment with denileukin diftitox on day −1 relative to vaccine significantly enhanced CEA-specific CD4 proliferation compared with vaccine alone (Figure 5B). Treatment with denileukin diftitox on day −7 had no effect on proliferation (Figure 5B). IFN-γ production by CD4+ T cells in response to CEA protein also showed optimal responses when denileukin diftitox was administered on day −1 relative to vaccine (data not shown). These results show the potential importance of scheduling of denileukin diftitox in relation to vaccine.
We next sought to determine whether denileukin diftitox would similarly enhance antigen-specific T-cell responses in a foreign antigen system, using recombinant poxviral vectors expressing the Influenza A/PR/8/34 nucleoprotein and TRICOM in normal mice. The optimal timing of denileukin diftitox administration in relation to vaccine as determined in the CEA self-antigen system was used. Thus, denileukin diftitox was administered to mice on day −1 relative to a subcutaneous vaccination on day 0 with rV-Inf(A)NP34/TRICOM (admixed with rF-GM-CSF). Fourteen days later, mice were boosted subcutaneously with rF-Inf(A)NP34/TRICOM (and rF-GM-CSF). Twenty-one days after the boost, immune assays were performed.
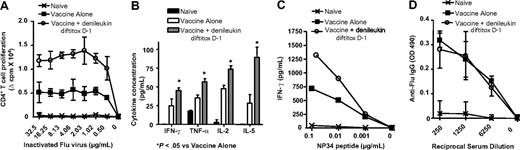
Pretreatment with denileukin diftitox on day −1 relative to rV-Inf(A)NP34/TRICOM significantly increased CD4+ T-cell proliferation to inactivated Influenza A virus compared with vaccine alone (Figure 6A). Further, CD4+ T cells showed enhanced antigen-specific production of several cytokines, including IFN-γ, TNF-α, IL-2, and IL-5, when denileukin diftitox was administered 1 day before the vaccine prime (Figure 6B). IFN-γ production by CD8+ T cells in response to NP34 peptide was similarly enhanced with denileukin diftitox treatment before vaccination (Figure 6C). In contrast to the T-cell responses, the use of denileukin diftitox 1 day before the vaccine prime did not alter serum anti-Flu IgG levels compared with vaccine alone (Figure 6D).
Immune responses to Flu antigen after pretreatment with denileukin diftitox and vaccination with rV-Inf(A)NP34/TRICOM (+ rF-GM-CSF) in a foreign antigen system. C57BL/6 mice were subcutaneously vaccinated on day 0 with 5 × 107 pfu rV-Inf(A)NP34/TRICOM (admixed with 1 × 107 pfu rF-GM-CSF) and boosted on day 14 with 5 × 107 pfu rF-Inf(A)NP34/TRICOM (admixed with 1 × 107 pfu rF-GM-CSF) (vaccine alone). One group additionally received an intraperitoneal injection of 0.75 μg denileukin diftitox 1 day before the vaccine prime (vaccine + denileukin diftitox, day −1). Immune assays were performed on day 35, 3 weeks after the boost. Background-subtracted values are shown. (A) CD4+ T-cell proliferation to the indicated concentrations of inactivated Influenza A virus was measured by 3H-thymidine incorporation. Values are represented as mean proliferation (± SD) of triplicate wells. Statistical evaluation was performed by repeated-measures one-way ANOVA using GraphPad Prism. All values for the group pretreated with denileukin diftitox on day −1 relative to vaccine prime were statistically significant (P < .01) compared with vaccine alone. (B) CD4+ T cells were stimulated with 32.5 μg/mL inactivated Influenza A virus for 72 hours. Mean cytokine concentration in supernatants (± SD) was measured by Cytometric Bead Array analysis of triplicate samples. Statistical evaluation was performed by repeated-measures one-way ANOVA using GraphPad Prism. (C) CD8+ T cells were stimulated with the indicated concentrations of NP34 peptide for 48 hours. IFN-γ concentration in supernatants was measured by ELISA. (D) Mean serum levels of anti-Flu IgG (± SD) were measured by ELISA.
Immune responses to Flu antigen after pretreatment with denileukin diftitox and vaccination with rV-Inf(A)NP34/TRICOM (+ rF-GM-CSF) in a foreign antigen system. C57BL/6 mice were subcutaneously vaccinated on day 0 with 5 × 107 pfu rV-Inf(A)NP34/TRICOM (admixed with 1 × 107 pfu rF-GM-CSF) and boosted on day 14 with 5 × 107 pfu rF-Inf(A)NP34/TRICOM (admixed with 1 × 107 pfu rF-GM-CSF) (vaccine alone). One group additionally received an intraperitoneal injection of 0.75 μg denileukin diftitox 1 day before the vaccine prime (vaccine + denileukin diftitox, day −1). Immune assays were performed on day 35, 3 weeks after the boost. Background-subtracted values are shown. (A) CD4+ T-cell proliferation to the indicated concentrations of inactivated Influenza A virus was measured by 3H-thymidine incorporation. Values are represented as mean proliferation (± SD) of triplicate wells. Statistical evaluation was performed by repeated-measures one-way ANOVA using GraphPad Prism. All values for the group pretreated with denileukin diftitox on day −1 relative to vaccine prime were statistically significant (P < .01) compared with vaccine alone. (B) CD4+ T cells were stimulated with 32.5 μg/mL inactivated Influenza A virus for 72 hours. Mean cytokine concentration in supernatants (± SD) was measured by Cytometric Bead Array analysis of triplicate samples. Statistical evaluation was performed by repeated-measures one-way ANOVA using GraphPad Prism. (C) CD8+ T cells were stimulated with the indicated concentrations of NP34 peptide for 48 hours. IFN-γ concentration in supernatants was measured by ELISA. (D) Mean serum levels of anti-Flu IgG (± SD) were measured by ELISA.
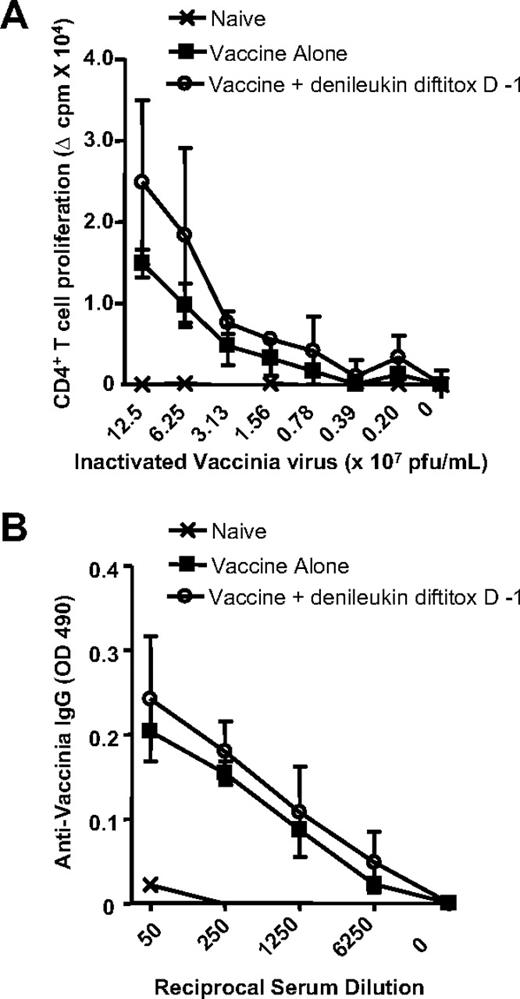
Because denileukin diftitox may kill activated lymphocytes10,15 and thus impair immune function, its use before immunization with a live vector such as vaccinia virus potentially could result in enhanced vector replication and resulting adverse effects. To evaluate this, the CD4 proliferation and antibody response to vaccinia virus was measured after pretreatment with denileukin diftitox and immunization with rV-Inf(A)NP34/TRICOM. Denileukin diftitox treatment 1 day before the vaccine prime did not impair CD4+ T-cell proliferation to inactivated vaccinia virus compared with vaccine alone (Figure 7A). Similarly, the antivaccinia IgG response was unchanged by denileukin diftitox pretreatment (Figure 7B). Examination of multiple tissues, including spleen, lymph node, heart, liver, kidney, stomach, and intestine, by histology at 30 days after vaccination revealed no tissue abnormalities resulting from the combination of denileukin diftitox and a vaccinia prime (data not shown), thus ruling out uncontrolled vaccinia virus replication as a consequence of denileukin diftitox pretreatment. These results collectively show that denileukin diftitox administration 1 day before vaccine priming with rV-Inf(A)NP34/TRICOM (and rF-GM-CSF) enhanced CD4 and CD8 responses to vaccine in a foreign antigen system. Importantly, there was no evidence of compromised immunity to vaccinia virus.
Immune responses to vaccinia virus after pretreatment with denileukin diftitox and vaccination with rV-Inf(A)NP34/TRICOM (+ rF-GM-CSF) in a foreign antigen system. C57BL/6 mice were subcutaneously vaccinated on day 0 with 5 × 107 pfu rV-Inf(A)NP34/TRICOM (admixed with 107 pfu rF-GM-CSF) and boosted on day 14 with 5 × 107 pfu rF-Inf(A)NP34/TRICOM (admixed with 107 pfu rF-GM-CSF) (vaccine alone). One group additionally received an intraperitoneal injection of 0.75 μg denileukin diftitox 1 day before the vaccine prime (vaccine + denileukin diftitox, day −1). Immune assays were performed on day 35, 3 weeks after the boost. Background-subtracted values are shown. (A) Mean CD4+ T-cell proliferation to the indicated concentrations of inactivated vaccinia virus (± SD) was assessed by 3H-thymidine uptake of triplicate wells. (B) Mean serum levels of antivaccinia IgG (± SD) were measured by ELISA.
Immune responses to vaccinia virus after pretreatment with denileukin diftitox and vaccination with rV-Inf(A)NP34/TRICOM (+ rF-GM-CSF) in a foreign antigen system. C57BL/6 mice were subcutaneously vaccinated on day 0 with 5 × 107 pfu rV-Inf(A)NP34/TRICOM (admixed with 107 pfu rF-GM-CSF) and boosted on day 14 with 5 × 107 pfu rF-Inf(A)NP34/TRICOM (admixed with 107 pfu rF-GM-CSF) (vaccine alone). One group additionally received an intraperitoneal injection of 0.75 μg denileukin diftitox 1 day before the vaccine prime (vaccine + denileukin diftitox, day −1). Immune assays were performed on day 35, 3 weeks after the boost. Background-subtracted values are shown. (A) Mean CD4+ T-cell proliferation to the indicated concentrations of inactivated vaccinia virus (± SD) was assessed by 3H-thymidine uptake of triplicate wells. (B) Mean serum levels of antivaccinia IgG (± SD) were measured by ELISA.
Discussion
Whereas the physiologic role of Treg cells is to protect the host against the development of autoimmunity, in the context of cancer Treg cells might prevent the development of an effective antitumor immune response directed against “self” tumor antigens. Previous studies in several models of mouse tumors have indeed shown that deletion of Treg cells by anti-CD25 mAb enhanced the development of antitumor immunity and could lead to tumor rejection.33-35 Further, it has been previously shown that antigen-specific CD4+ and CD8+ T-cell immune responses induced by poxviral vaccines were augmented by simultaneous administration of anti-CD25 mAb in mice.36 In humans, however, 2 available anti-CD25 mAbs, daclizumab and basiliximab, have not yet been characterized in terms of their ability to delete Treg cells. Among alternative modalities that can be used in humans to delete Treg cells is denileukin diftitox. Denileukin diftitox was previously shown to reduce Treg cells and lead to an objective clinical response in patients with ovarian cancer.14 Moreover, antitumor immunotherapy regimens combining denileukin diftitox-mediated deletion of Treg cells followed by vaccination with RNA-transfected dendritic cells or tumor antigen peptides have been shown to improve tumor-specific T-cell responses in patients with renal cell cancer and patients with melanoma, respectively.15,16 However, another previous report has shown no effect of denileukin diftitox in reducing Treg cells in patients with melanoma.17 These conflicting results may be due to dose and scheduling effects in the case of denileukin diftitox alone or in combination with vaccine.
In the studies reported here, we sought to investigate for the first time the potential of combining denileukin diftitox with poxviral vaccines in the development of antigen-specific immune responses in mouse models. First, we investigated the kinetics of Treg-cell deletion in C57BL/6 mice. We have shown that a single intraperitoneal injection of denileukin diftitox was sufficient to decrease the frequency of Treg cells in spleen, peripheral blood, and bone marrow of treated mice. The reduction was evident within 24 hours of denileukin diftitox injection and lasted approximately 10 days, with gradual recovery of the Treg-cell population. A previous report showed that multiple doses of denileukin diftitox greater than 1 μg were required to reduce splenic Treg cells in BALB/c mice.18 Our results show strain-specific differences between BALB/c and C57BL/6 mice when administering denileukin diftitox; we observed only limited deletion of blood Treg cells and no deletion of splenic or bone marrow Treg cells in BALB/c mice with a single dose of 0.75 μg in a controlled experiment.
Bone marrow has recently attracted renewed interest as a central organ in T-lymphocyte trafficking and immunity. A part of the lymphocyte recirculation network, it has been shown to act as a reservoir for functional Treg cells that can traffic to the periphery to maintain homeostasis.37 Bone marrow also has been shown to be a preferential homing site for antigen-specific memory T cells, as well as an important site for T-cell priming against bloodborne antigens, including tumor antigens.38,39 Thus, we hypothesize that the reduction of Treg cells in the bone marrow by denileukin diftitox treatment could be beneficial for the induction of antigen-specific T-cell immune responses.
Unlike previous observations with low-dose cyclophosphamide or anti-CD25 mAb which have been shown to reduce both number and functionality of Treg cells in mice,31,32,40 the Treg cells that remained after denileukin diftitox treatment in vivo exhibited normal levels of proliferation, apoptosis, and functionality. Because denileukin diftitox effected maximal deletion of Treg cells in spleen, blood, and bone marrow by 24 hours after injection, measurement of Annexin V staining on day 1 after injection or later may have “missed” loss of viability attributable to denileukin diftitox. Moreover, the gradual normalization of Treg-cell levels after denileukin diftitox-mediated deletion was not accompanied by an increase in homeostatic proliferation of the Treg cells in spleen or bone marrow. Therefore, we hypothesize that other mechanisms might account for the recovery of the Treg-cell population. Possible mechanisms are trafficking of Treg cells from other sites, as from bone marrow,37 or peripheral generation of Treg cells from CD4+CD25− T cells.41
The studies presented here show the importance of dose scheduling of denileukin diftitox when used in combination with vaccine, because denileukin diftitox injection 1 day before a poxviral-based vaccine optimally increased antigen-specific T-cell responses above levels induced by vaccination alone. Previous studies in patients with cancer likewise showed enhanced T-cell responses to vaccine after denileukin diftitox pretreatment, when denileukin diftitox was administered 4 days or 1 day before RNA-transfected dendritic cells or tumor antigen peptides, respectively.15,16 Optimal timing may vary depending on the type of vaccine. For example, a peptide vaccine may present antigen to APCs for a few hours to up to 2 days, whereas some viral vectors such as vaccinia can present antigen to APCs for up to 1 week.
The use of denileukin diftitox before immunization with a replication-competent vaccinia virus could raise concerns about potential adverse effects resulting from denileukin diftitox-mediated killing of activated lymphocytes and consequent immune impairment.10,15 Protective immunity against vaccinia virus in mice was previously shown to be dependent on virus-specific antibody, with a contribution of CD4+ helper T cells to antibody production.42,43 We have shown here that the use of denileukin diftitox 1 day before a recombinant vaccinia vector did not impair the CD4+ T-cell proliferation or antibody response to vaccinia virus. In fact, the proliferative response to inactivated vaccinia virus was slightly enhanced in mice that received denileukin diftitox before vaccine. Moreover, examination of multiple tissues from mice that received denileukin diftitox 1 day before a recombinant vaccinia vector showed no evidence of abnormal tissue histology, showing that viral replication was controlled. Collectively, these results indicate that immunity to vaccinia virus was not compromised by denileukin diftitox pretreatment and should help to allay concerns about combination therapy with denileukin diftitox and recombinant vaccinia vectors.
These results extend findings by our own laboratory showing that deletion of Treg cells via anti-CD25 mAb resulted in the enhancement of antigen-specific immune responses.36 In those studies, anti-CD25 mAb, local tumor radiation, or vaccine alone did not eliminate established tumors, whereas therapy with all 3 modalities did.36 Denileukin diftitox may similarly be combined with vaccine or other types of therapy to increase antitumor efficacy in mouse models. In these studies, dose scheduling will also be an important consideration.
There have been conflicting reports about the efficacy of denileukin diftitox on human regulatory T cells in vitro, complicated by differences in dosing of drug, method of evaluation of cell death, method for detection of Treg cells, and examined time points.15-17 Although 2 previous reports showed that denileukin diftitox induced death of human CD4+CD25high T cells in vitro,15,16 another report found no reduction in CD25+-expressing cells after in vitro treatment of human PBMCs with denileukin diftitox.17 Our results have shown that denileukin diftitox treatment of human PBMCs from healthy donors in vitro selectively deleted the population of CD4+CD25high T cells at 72 hours. We speculate that some of the inconsistencies from previous reports may result from differential gating on CD4+CD25high versus total CD4+CD25+ populations, because we have shown a consistent decrease in CD4+CD25high T cells but observed no reproducible change in the percentage of total CD4+CD25+ T cells after denileukin diftitox treatment. Unlike in the mouse, where Treg cells account for the majority of CD4+CD25+ T cells, T cells with regulatory properties in humans mainly reside in the CD4+ T-cell fraction that constitutively expresses high levels of CD25.28 Thus, denileukin diftitox mediates selective deletion of the regulatory CD4+CD25high T-cell fraction from human PBMCs in vitro.
In conclusion, our results confirm and extend findings on the use of denileukin diftitox for the deletion of Treg cells in a mouse model and in human in vitro studies. Further, these results show for the first time in a murine model (1) the differential effects of denileukin diftitox on Treg cells in different cellular compartments, (2) the advantage of combining denileukin diftitox with a vaccine to enhance antigen-specific T-cell immune responses, (3) the lack of inhibition by denileukin diftitox of host immune responses directed against a live viral vector, and (4) the importance of dose scheduling of denileukin diftitox when used in combination with a vaccine. These studies thus form the rationale for clinical studies in the use of denileukin diftitox with live viral-based vaccines.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Margie Duberstein, Bertina Gibbs, and Eileen Thompson for technical assistance. We thank Elizabeth Wansley for critical reading of the manuscript. We thank Debra Weingarten for editorial assistance.
This work was supported in part by the National Institutes of Health Intramural Research Program, Center for Cancer Research, National Cancer Institute. Dr. Curiel was supported by NIH grants CA100425 and CA105207, FDA grant RO1 FD003118, and The Rippel Foundation.
National Institutes of Health
Authorship
Contribution: M.T.L. designed and performed research, analyzed and interpreted data, and wrote the manuscript; R.F. performed research and analyzed data; T.J.C. contributed vital new reagents and analytical tools and wrote the manuscript; D.W.G. performed research and analyzed data; J.S. designed research, analyzed and interpreted data, and wrote manuscript; C.P. designed and performed research, analyzed and interpreted data, and wrote manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey Schlom, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 10 Center Dr, Rm 8B09, MSC 1750, Bethesda, MD 20892; e-mail: js141c@nih.gov.

![Figure 1. Effect of denileukin diftitox on murine and human Treg cells in vitro. (A) Whole murine splenocytes from C57BL/6 mice were treated with the indicated doses of denileukin diftitox for 2 hours, then washed, and incubated at 37°C for 72 hours. Splenocytes were stained for CD4+CD25+Foxp3+ Treg cells. The plots shown were gated on CD4+ T cells. Percentage of CD25+Foxp3+ cells is indicated in the upper right quadrant of each plot. (B-D) PBMCs from healthy human donors were treated with 290 ng/mL (5 nM) denileukin diftitox for 72 hours. Cells were stained for CD4 and CD25. CD4+CD25+ cells were gated into CD25high and CD25low subpopulations based on CD25 mean fluorescent intensity. The low threshold for the CD25high gate was set above the population of CD4−CD25+ cells; the low threshold for the CD25low gate was set above the isotype control. (B) Percentage CD25high, CD25low, and CD25+ of total CD4+ T cells is shown in the left, center, and right graphs, respectively, for untreated and denileukin diftitox-treated samples from 6 donors. (C) Representative plots for untreated and denileukin diftitox-treated samples from one donor are shown. Percentage CD25high of total CD4+ T cells is indicated on each plot. (D) CD4+CD25+ cells were isolated from untreated and denileukin diftitox-treated PBMC samples by FACS. Purified CD4+CD25− effector T cells (2.5 × 104) from untreated PBMCs were then cultured in the presence or absence of the isolated CD4+CD25+ cells at a 1:1 ratio (with irradiated untreated PBMCs as antigen-presenting cells [APCs; 1.25 × 105]) on an anti–CD3-coated 96-well plate. Mean proliferation (± SD) was measured by 3H-thymidine incorporation of triplicate wells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/9/10.1182_blood-2007-06-094615/7/m_zh80210708260001.jpeg?Expires=1765900314&Signature=znDAqFdL5-fPr3bo8kYUUvzApRZr~nJePm3uhIcDVBa8AVNMR3hOSrT3~33VfOY6t0zAWLs~cLico0vSKKuztnsCPbFeBmbAKkekg6cmxsBNTnrRIqX7BedCzULvQaX3flqYWgkslJwGwMxoT8GBaZAcq4fWj6XVtFU1R~WyP-OqXovL1B6gUAwwjjXCBxxaBMDpfbgZLR1YGVeTlzeZx4vyxWxYpdhNySbJu-ynyGvvdIb1P~pFt~vRrtNdvGiQXMY7jrIa3Y8Gbxzx-KkXsh4WbQdethOKOhTavgHNJLMtur3Q9P1rkSb108DUMDbyPnG7tziHZ-rtLSL4UVZGOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal