Abstract
CCAAT/enhancer-binding protein α (C/EBPα) is a critical regulator for early myeloid differentiation. Mutations in C/EBPα occur in 10% of patients with acute myeloid leukemia (AML), leading to the expression of a 30-kDa dominant-negative isoform (C/EBPαp30). In the present study, using a global proteomics approach to identify the target proteins of C/EBPαp30, we show that Ubc9, an E2-conjugating enzyme essential for sumoylation, is increased in its expression when C/EBPαp30 is induced. We confirmed the increased expression of Ubc9 in patients with AML with C/EBPαp30 mutations compared with other subtypes. We further confirmed that the increase of Ubc9 expression was mediated through increased transcription. Furthermore, we show that Ubc9-mediated enhanced sumoylation of C/EBPαp42 decreases the transactivation capacity on a minimal C/EBPα promoter. Importantly, overexpression of C/EBPαp30 in granulocyte colony-stimulating factor (G-CSF)–stimulated human CD34+ cells leads to a differentiation block, which was overcome by the siRNA-mediated silencing of Ubc9. In summary, our data indicate that Ubc9 is an important C/EBPαp30 target through which C/EBPαp30 enhances the sumoylation of C/EBPαp42 to inhibit granulocytic differentiation.
Introduction
The transcription factor CCAAT/enhancer-binding protein α (C/EBPα) is crucial for granulocytic differentiation.1-3 Alterations of the function of C/EBPα are a common feature of leukemic cells.4,5 It was discovered in 10% of patients with acute myeloid leukemia (AML) that the CEBPA gene is mutated.6,7 These mutations are found in AMLs with a myeloblast phenotype (French-American-British [FAB]–M1 and –M2 subtypes). The mutated gene results in the predominant expression of a 30-kDa protein initiated at an internal AUG start codon. This mutated isoform lacks the N-terminal transactivation domain 1 (TAD1). However, it possesses the intact bZIP protein-protein interaction domain and can interact with activators and repressors that affect its biological roles. The mutated 30-kDa isoform has been shown to act in a dominant-negative manner over the wild-type isoform.5 The ratio of p30/p42 is critical for a physiologic granulopoiesis.5,8 In contrast to C/EBPαp42, C/EBPαp30 fails to induce myeloid cell differentiation. It inhibits the expression of the endogenous granulocyte colony-stimulating factor (G-CSF) receptor and leads to an enhanced proliferation.9,10 Recently, it was reported that C/EBPαp30 directly interacts with the BCL2 promotor to fulfill this role.11 Relatively little is understood about how C/EBPαp30 exerts its dominant-negative effect over C/EBPαp42 and how it inhibits C/EBPαp42 during normal myeloid lineage development. We applied high-throughput proteomics to identify the target proteins of C/EBPαp30. In our screen, we identified the ubiquitin-conjugating enzyme (Ubc9) as a novel target of C/EBPαp30
Ubc9 is an essential E2 enzyme required for small ubiquitin-related modifier (SUMO) conjugation, or sumoylation.12,13 Ubc9 is known to play a central role in sumoylation-regulated cellular pathways, regulating many events, including transformation, cell-cycle regulation,14,15 mitosis, and recovery from DNA damage or S-phase arrest.16,17 The role of Ubc9 in a number of human malignancies, such as ovarian carcinoma, melanoma, and lung adenocarcinoma,18,19 has been reported. Moreover, Ubc9 is believed to play a role in DNA metabolism and repair, and in apoptosis pathways by physically interacting with topo I20 Rad51,21,22 and p53,23,24 respectively. It was recently reported that C/EBPα is sumoylated at the lysine residue of a synergy control motif that causes an inhibitory effect on its transcriptional activity.25 Sumoylated C/EBPα failed to induce proliferation arrest.26 These data indicate that sumoylation of C/EBPα may play a key role in the regulation of gene expression during myeloid differentiation. However, whether sumoylation plays a role in the C/EBPαp30-mediated differentiation block has not been investigated so far. Our proteomic screen revealed Ubc9 to be up-regulated by C/EBPαp30, suggesting that sumoylation may also play a crucial role in modulating C/EBPαp30 functions.
In the present study, we show that enhanced sumoylation of C/EBPαp42 through C/EBPαp30 via Ubc9 decreases the transactivation capacity on a minimal C/EBPα promoter. Furthermore, overexpression of C/EBPαp30 in G-CSF–stimulated human CD34+ cells leads to a differentiation block, which was overcome by siRNA-mediated silencing of Ubc9.
Materials and methods
Construction of His C/EBPα mutant—K161R
A polymerase chain reaction (PCR) mutagenesis kit (Stratagene, La Jolla, CA) was used for the generation of His C/EBPα K161R mutants from the His PCDNA6Ap42 plasmid. The primers used for the generation of the C/EBPα K161R mutant were the following: forward, 5′-CCG CTG GTG ATC AGG CAG GAG CCC-3′; and reverse, 5′-GGG GCT CCT GCC TGA TCA CCA GCG-3′. The plasmid constructs were verified by sequencing.
Cell line, cell culture, transfection, and immunoblotting
K562 C/EBPαp30–estrogen receptor (ER) cells5,10,27 were maintained in RPMI (without phenol red) supplemented with 10% charcoal-treated FBS (Hyclone, Logan, UT) and 2 μg/mL puromycin, 2 mM glutamine, and 1% penicillin/streptomycin at 37°C with 5% CO2 enrichment. Human kidney 293T cells were maintained in Dulbecco modified Eagle medium (DMEM; Gibco, Aidenbach, Germany) supplemented with 10% fatal bovine serum (FBS), 2 mM glutamine, and 1% penicillin/streptomycin (all from Gibco). Human monoblastic U937 cells were cultured in RPMI 1640 medium (PAN Biotech, Aidenbach, Germany) supplemented with 10% FBS, 2 mM glutamine, and 1% penicillin/streptomycin. Human erythroleukemia K562 cells were cultured in RPMI 1640 medium (PAN Biotech) supplemented with 10% FBS, 2 mM glutanine, and 1% penicillin/streptomycin. 293T and K562 cells were transfected using Lipofectamine plus (Life Technologies, Bethesda, MD) and cell line nucleofactor transfection reagent (AMAXA, Cologne, Germany), respectively, as described by the manufacturer. Firefly luciferase activities from the constructs p(C/EBP)2TK and renilla luciferase activity from the internal control plasmid PRL-null were determined 24 hours after the transfection using the Dual Luciferase Reporter Assay System (Promega, Madison, WI). Firefly luciferase activities were normalized to renilla luciferase values of PRL-null.28 Results are given as means plus or minus standard error of the mean (SEM) of at least 3 independent experiments. For this study, we used bone marrow samples from patients with AML with C/EBPαp30 mutations after obtaining their written consent and receiving Institutional Review Board approval from the Laboratory for Leukemia Diagnostics, Department of Medicine III, Klinikum Grosshadern, LMU, Germany. K562 C/EBPαp30-ER and K562 C/EBPαp42-ER cells were lysed in RIPA lysis buffer and nuclear extract was also performed by subcellular proteome extraction kit (catalog no. 539790; Calbiochem, San Diego, CA) at different time points of β-estradiol induction. A total of 60 μg lysate was subjected to electrophoresis on 10% SDS-PAGE gels. The Western blotting procedure was performed, and the blots were detected with the enhanced chemiluminescence (ECL) system as described previously.28 Immunoblot for C/EBPα (sc-61; Santa Cruz Biotechnology, Santa Cruz, CA), Ubc9 (sc-10759; Santa Cruz Biotechnology), and Calreticulin (C-4606; Sigma, St Louis, MO) was performed. Anti–β-tubulin antibody (sc-9104; Santa Cruz Biotechnology) was used as internal loading control on the same blot after stripping. For sumoylation experiments, 2 × 106 293T cells were transfected with 2.5 μg of pCDNA3 p42 His (wild-type [WT]) alone, 2.5 μg His pCDNA3 p42 (WT) with 2.5 μg pCDNA3p30 or 2.5 μg pCDNA6A p42K161R His (Mut) alone, or 2.5 μg His pCDNA6Ap42 K161R (Mut) with 2.5 μg pCDNA3p30 along with 2.5 μg of pSG(Flag)3c-myc–SUMO-1 using Polyfect (Qiagen, Valencia, CA) as described by the manufacturer. After 48 hours, cells were lysed with lysis buffer (8 M urea, 0.5 M NaCl, 45 mM Na2HPO4, 5 mM NaH2PO4, and 10 mM imidazole) as described previously25 with protease and phosphate inhibitor cocktail (Sigma). The cells were incubated for 1 hour in the rotator at room temperature and ultracentrifuged for 2 hours at 15°C. Cell lysates were prepared, and total protein concentration was determined by Bio-Rad assay (Hercules, CA) as described previously.29 Lysates (400 μg protein) were incubated with 100 μL of nickel nitrilotriacetic acid (Ni2+-NTA resin; Invitrogen, Carlsbad, CA) for 1 hour at room temperature while rotating. The resin was washed 3 times with 1 mL of buffer I (8 M urea, 0.4 M NaCl, 17.6 mM Na2HPO4, 32.4 mM NaH2PO4, 10 mM imidazol [pH 6.75]) and 3 times with 1 mL of buffer II (buffer I without urea and with 150 mM NaCl). Protein were eluted by incubating at 90°C in elution buffer for 10 minutes (100 mM Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 500 mM imidazole, 0.015% bromphenel blue, and 10 mM dithiothreitol) and resolved by 8% SDS-PAGE. Immunoblot for FLAG-M2 (F-3165; Sigma), C/EBPα (sc-61; Santa Cruz Biotechnology), and Anti-His6 (1 922 416; Roche, Indianapolis, IN) was performed.
Flow cytometric analysis
Human CD34+ hematopoietic cells were isolated from human cord blood (CB) cells. Briefly, CB was collected on delivery with informed consent obtained in accordance with the Declaration of Helsinki. The blood was diluted with magnetic-activated cell sorter (MACS) buffer at a ration of 1:5, and mononuclear cells were isolated by lymphocyte separation medium (LSM 1077, J 15–004; PAA Laboratories, Pasching, Austria). The lymphocyte ring was collected, transferred to a new tube, and washed with MACS buffer. Pellets were dissolved in 300 μL MACS buffer and 100 μL Fc receptor (FcR) blocking reagent and incubated for 10 minutes. at 4°C. A total of 100 μL of CD34 microbeads were added and incubated for 30 minutes at 4°C. The cells were washed, and CD34+ cells were sorted by the MACS system (Miltenyi Biotec, Auburn, CA). More than 70% of the cells were required to express the CD34 antigen (Figure S2A, available on the Blood website; see the Supplemental Materials link at the top of the online article). An aliquot containing 5 × 105 CD34+ cells was cultured in Iscove modified Dulbecco medium with 10% heat-inactivated FBS, 50 ng/mL Flt3-ligand (Flt3-L), 50 ng/mL stem cell factor (SCF), 50 ng/mL thrombopoietin (TPO), 10 ng/mL IL-3, 10 U/mL penicillin/streptomycin, and 2 mM l-glutamine. The cells were transfected with C/EBPαp30 (1 μg), Ubc9 siRNA (500 ng, sc-36773; Santa Cruz Biotechnology), and control siRNA (500 ng, sc-37007; Santa Cruz Biotechnology) using AMAXA nucleofection technology as described by the manufacturer. Transfection efficiency was analyzed using a plasmid with an enhanced green fluorescent protein (eGFP) marker (2 μg) as was calculated by the GFP expression. The percentage of transfection reached by this method was 50% for CD34+ cells and more than 80% for U937 cells (Figure S2B,C). After 12 hours, the cells were spun down and incubated with media containing 50 ng/mL G-CSF, 50 ng/mL SCF, 10 ng/mL IL-3, 50 ng/mL TPO, and 50 ng/mL Flt3-L. A total of 106 U937 cells were transfected with C/EBPαp30 (1 μg), Ubc9 siRNA (500 ng), control siRNA (500 ng), and HA-Ubc9 (3 μg) by AMAXA. At 12 hours after transfection, the cells were stimulated with retinoic acid (RA; Sigma) at a concentration of 10−6 M to induce granulocytic differentiation. Fluorescence-activated cell sorter (FACS) analysis was performed after 6 and 3 days for CD34+ and U937 cells, respectively, on a flow cytometer (Becton Dickinson, San Jose, CA) using FITC-labeled CD15 antibodies (55401; BD Pharmingen, San Diego, CA) and IgG-FITC (555742; BD Pharmingen) as an isotype control. The Annexin-V assay was carried out in conjunction with propodium iodide (PI) staining according to the manufacture protocol (BD Pharmingen). Briefly, to detect apoptotic cells, 106 cells were transfected with control siRNA and Ubc9 siRNA by AMAXA nucleofection and treated with RA. After 48 hours, the cells were washed 2 times in cold PBS. Cells were resuspended with 100 μL of Annexin-V–binding buffer, 5 μL Annexin-V–FITC (catalog no. 51–65874X; BD Pharmingen), and 5 μL PI (catalog no. 51–66211E; BD Pharmingen) and incubated in the dark for 15 minutes at room temperature. A total of 400 μL of Annexin-V–binding buffer were added, and FACS analysis was performed.
Quantitative real-time PCR analysis
RNA isolation was performed using TRIZOL (Invitrogen, Germany). RNA was isolated from K562 C/EBPαp30-ER and K562 C/EBPαp42-ER at different time points after β-estradiol induction and from CD34+ and U937 cells transfected with C/EBPαp30 and siRNA against Ubc9 as described previously. RNA was transcribed to cDNA using standard conditions. Equal amounts of cDNA were used for all conditions, and gene expression was quantified by real-time PCR in a Rotor-Gene RG-3000 (Corbett Research, Leipzig, Germany) using a SYBR Green kit (Qiagen, Düsseldorf, Germany). Methods were used according to the manufacturer's protocol. The following primer sequences were used: Ubc9, 5′-ATT ATC CAT CTT CGC CAC CA-3′ (forward) and 5′-CTC TGC TTG AGC TGG GTC TT-3′ (reverse); G-CSFR, 5′-AAG AGC CCC CTT ACC CAC TAC ACC ATC TT-3′ (forward) and 5′-TGC TGT AGC CTG GGT CTG GGA CAC TT-3′ (reverse); and myeloperoxidase (MPO), 5′-TCG GTA CCC ATG TCA GGA AG-3′ (forward) and 5′-CCA GGT TCA ATG CAG GAA GT-3′ (reverse). To determine the relative expression level of each sample, GAPDH expression levels were measured as internal controls. The delta ct value (Δct) was calculated from the given ct value by the formula Δct = (ct sample − ct control). The fold change was calculated as (= 2−Δct).
2D gel electrophoresis
A total of 6 gels with or without β-estradiol induction were run in a pH range of 4 to 7, 3 to 10, and 6 to 11, respectively. K562C/EBPαp30-ER and WT K562 cells were induced with β-estradiol at a concentration of 2 μM for 6 hours. Whole-cell lysates were prepared (8 M urea), and total protein concentration was determined by Bio-Rad assay as described previously.29 Isoelectric focusing was performed with 1 mg of protein samples using immobilized pH gradient (IPG) strips (Amersham, Arlington Heights, IL). Proteins were separated in the second dimension by 12% SDS-PAGE, and protein spots were visualized by colloidal coomassie blue staining. Furthermore, 2 gels in a pH range of 3 to 10 were run using bone marrow samples from patients with AML with C/EBPαp30 mutations. The gels were scanned as 16-bit images and analyzed with the ProteomWeaver software version 2.1 (Definiens, Munich, Germany). Analysis with ProteomWeaver was performed with threshold intensity (average spot volume) of 2.0 to determine whether a protein is induced or suppressed. To overcome gel-to-gel and staining technique variations, each protein spot was normalized to a number of other protein spots in the same gel whose expression did not vary under a particular experimental condition (values in Table S1A, representing normalized average spot values). The pair match-based normalization function of the program enabled us to remove nonexpression-related variations in the spot intensity. With the support of statistical analysis, we could detect and analyze differentially expressed proteins in induced conditions compared with those of native conditions.
Protein identification by mass spectrometry
The spots were carefully excised from the gels with a pipette tip manually, placed in Eppendorf tubes, and destained by washing in 50 mM ammonium bicarbonate–acetronitrile solution (1:1) for 15 minutes, followed by 2 alternative washing steps with 50% acetronitrile and 50 mM ammonium bicarbonate. The gel pieces were dehydrated at room temperature with 100% acetronitrile and covered with 10 μL of trypsin (working solution: 5 ng/μL prepared from 100 ng/μL stock solution in 50 mM ammonium bicarbonate) overnight at 37°C. The spots were crushed, and peptides were extracted in 10 μL of 70% acetronitrile. The eluate was dried using a vacuum centrifuge and stored at −80°C. The peptides were resuspended in 5 μL of 20% acetronitrile and 0.1% TFA and sonicated for 3 minutes before processing for mass spectrometry.30 Measurements were performed using a ReflexIII matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) instrument (Bruker Daltonic, Bremen, Germany) and confirmed by using tandem mass spectrometry (MS-MS) with the AB4700 GPS explorer software (Applied Biosystems, Foster City, CA).
Statistical analysis
The P value was calculated using the Student t test by comparing the means of 2 different conditions in each experiment. A P value of .05 or less was considered significant.
Results
C/EBPαp30 regulates the expression of 60 different proteins
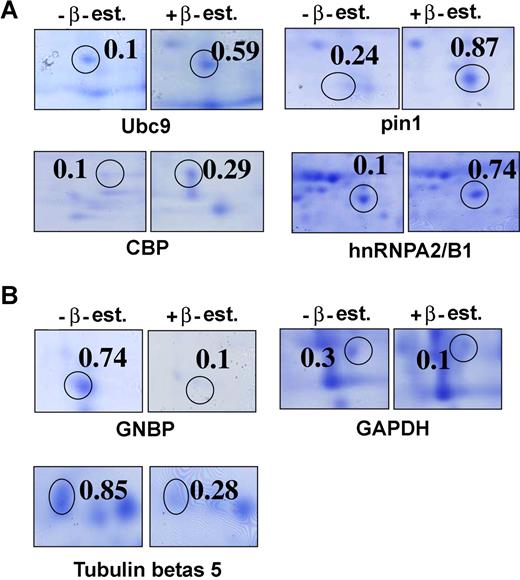
Identification of the target proteins of C/EBPαp30 was conducted by using 2D gel electrophoresis in K562C/EBPαp30-ER cells and in patients with AML carrying C/EBPαp30 mutations. In K562C/EBPαp30-ER cells, C/EBPαp30-ER is constitutively expressed in the cytoplasm. Samples were lysed 6 hours after β-estradiol induction and subjected to isoelectric focusing (IEF) and 2D gel electrophoresis (Figure 1A,B; Figure S1A-C). Samples from patients with AML carrying C/EBPαp30 mutations were also used (Figure S2D,E). In total, 600 spots were manually excised from the gels and processed for mass spectrometry–based identification. This analysis identified 60 proteins as differentially regulated by C/EBPαp30. By using spot analyzer ProteomWeaver software (Definiens), we identified 40 proteins as up-regulated and 20 proteins as down-regulated (Table S1A-B). In addition, we compared the proteins that were more than 2-fold regulated in the K562 C/EBPαp30 cell line after β-estradiol induction and in patients with AML with the C/EBPαp30 mutation. Interestingly, we found 16 regulated proteins common to both K562 C/EBPαp30 and patients with AML with the C/EBPαp30 mutation (Table S1C), establishing the relevancy of our model system.
The identification of target proteins of C/EBPαp30 from 2D gels with or without β-estradiol induction. Enlarged view of some of the spots taken from the gel image. Each spot represents the zoom view of 2D gel spots induced by C/EBPαp30 in K562C/EBPαp30-ER cells after β-estradiol induction. (A) Protein spots identified as up-regulated targets. (B) Protein spots identified as down-regulated targets of C/EBPαp30 after β-estradiol induction. The value(s) are calculated as a mean normalized spot volume obtained from the ProteomWeaver software.
The identification of target proteins of C/EBPαp30 from 2D gels with or without β-estradiol induction. Enlarged view of some of the spots taken from the gel image. Each spot represents the zoom view of 2D gel spots induced by C/EBPαp30 in K562C/EBPαp30-ER cells after β-estradiol induction. (A) Protein spots identified as up-regulated targets. (B) Protein spots identified as down-regulated targets of C/EBPαp30 after β-estradiol induction. The value(s) are calculated as a mean normalized spot volume obtained from the ProteomWeaver software.
Ubc9 is up-regulated in patients with AML with the C/EBPαp30 mutation and in K562C/EBPαp30-ER cells
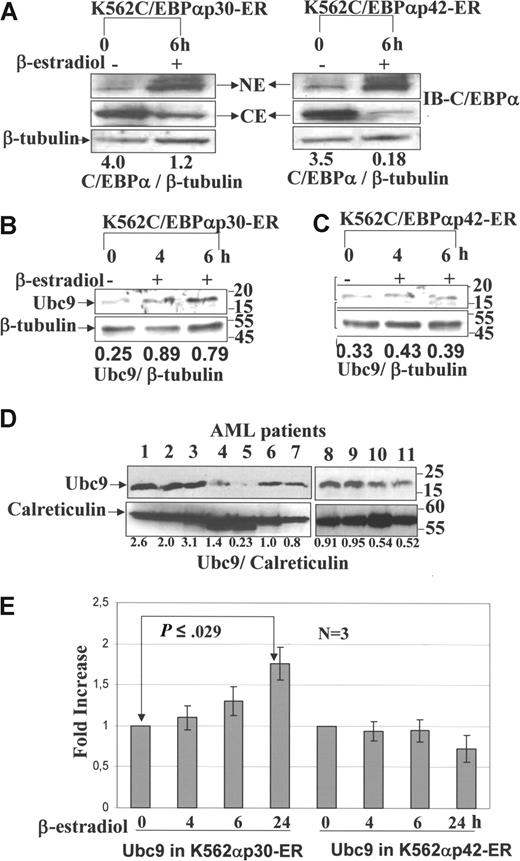
Our proteome data identify Ubc9 as a target of C/EBPαp30, which is 6-fold up-regulated. Nuclear translocation is induced by treatment with β-estradiol (Figure 2.5,10,27 In order to confirm the expression and regulation of Ubc9, Western blot analysis was performed from the whole-cell lysates of the K562C/EBPαp30-ER and K562C/EBPαp42-ER cell lines after β-estradiol induction at different time points as indicated. The results showed that Ubc9 expression increased significantly 6 hours after β-estradiol induction (Figure 2B), whereas no change in expression was detected under the same conditions in the K562 C/EBPαp42-ER–inducible cell line (Figure 2C). The induction of Ubc9 expression was further confirmed in AML patient samples with C/EBPαp30 mutations that show the expression of Ubc9 to be higher compared with normal bone marrow and other subtypes of leukemia (Figure 2D). We further confirmed that the increase of Ubc9 was mediated through increased transcription by performing quantitative real-time PCR at different time points. We observed an increased expression of Ubc9 transcription between 4-hour and 24-hour time points in K562C/EBPαp30 after β-estradiol induction (Figure 2E). However, in the K562C/EBPαp42-ER cell line, we do not observe any effect on Ubc9 transcription. Taken together, this suggests a possible role of C/EBPαp30-induced expression of Ubc9 in leukemogenesis.
Validation of Ubc9 expression by Western blot and quantitative RT-PCR. Western blot analysis was performed from nuclear extract and cytoplasm extract of K562C/EBPαp30-ER and p42 cell line after β-estradiol induction at different time points using anti-C/EBPα antibody. Western blot analysis was also performed from whole-cell lysates of AML patient cells, K562 C/EBPαp30-ER cells, and C/EBPαp42-ER cells before and after β-estradiol induction at different time points using anti-Ubc9 antibody. (A) Nuclear translocation is induced by treatment with β-estradiol. (B,C) Ubc9 expression in K562C/EBPαp30-ER and K562C/EBPαp42-ER cells after β-estradiol induction. (D) Patients with AML with different subtypes (lanes 1-3, 8, and 9: patients with AML with C/EBPαp30 mutation; lanes 4 and 10: t(8;21); lane 5: Inv(6); lanes 6 and 11: Inv3; and lane 7: normal bone marrow). The numbers underneath the blot are the densitometric values calculated as protein–β-tubulin ratios using ImageJ 1.36 software (National Institutes of Health, Bethesda, MD). (E) Quantitative real-time PCR for the expression of Ubc9 mRNA after β-estradiol induction at different time points. The fold increase for expression was calculated using Δct = (Ct sample − Ct control), and ΔCt values for each sample were standardized by GAPDH Ct value. The fold change was calculated as (= 2−Δct). Values are expressed as means (± SEM) for 3 independent experiments, with P values shown on histograms.
Validation of Ubc9 expression by Western blot and quantitative RT-PCR. Western blot analysis was performed from nuclear extract and cytoplasm extract of K562C/EBPαp30-ER and p42 cell line after β-estradiol induction at different time points using anti-C/EBPα antibody. Western blot analysis was also performed from whole-cell lysates of AML patient cells, K562 C/EBPαp30-ER cells, and C/EBPαp42-ER cells before and after β-estradiol induction at different time points using anti-Ubc9 antibody. (A) Nuclear translocation is induced by treatment with β-estradiol. (B,C) Ubc9 expression in K562C/EBPαp30-ER and K562C/EBPαp42-ER cells after β-estradiol induction. (D) Patients with AML with different subtypes (lanes 1-3, 8, and 9: patients with AML with C/EBPαp30 mutation; lanes 4 and 10: t(8;21); lane 5: Inv(6); lanes 6 and 11: Inv3; and lane 7: normal bone marrow). The numbers underneath the blot are the densitometric values calculated as protein–β-tubulin ratios using ImageJ 1.36 software (National Institutes of Health, Bethesda, MD). (E) Quantitative real-time PCR for the expression of Ubc9 mRNA after β-estradiol induction at different time points. The fold increase for expression was calculated using Δct = (Ct sample − Ct control), and ΔCt values for each sample were standardized by GAPDH Ct value. The fold change was calculated as (= 2−Δct). Values are expressed as means (± SEM) for 3 independent experiments, with P values shown on histograms.
C/EBPαp30 enhances sumolyation of C/EBPαp42 at lysine 161— K161—and thereby inhibits its transcriptional activity
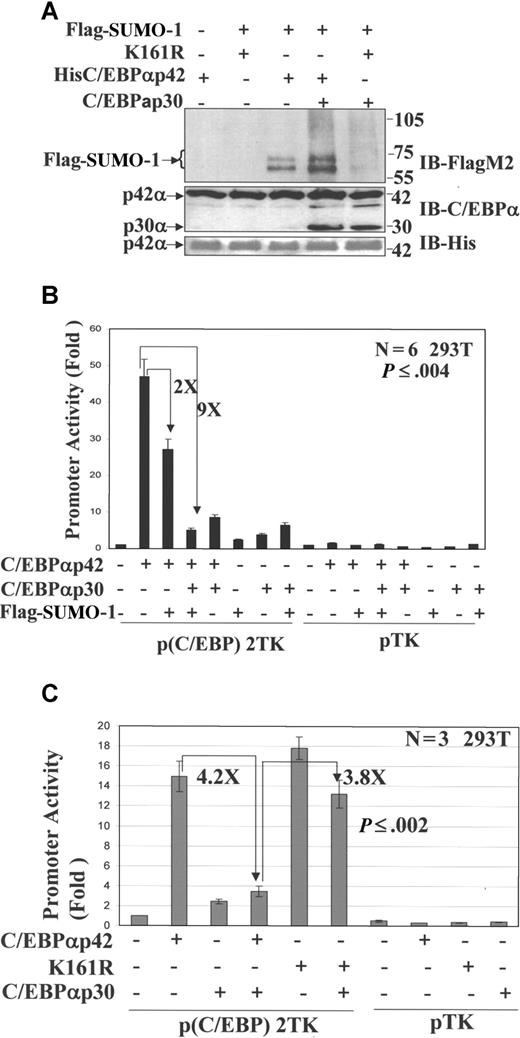
Ubc9-mediated sumoylation has previously been reported to lead to the transcriptional inhibition of number of target proteins and is overexpressed in a number of cancers.19 Since WT C/EBPα is inhibited by dominant-negative C/EBPαp30 in AML, we hypothesize that C/EBPαp30 induced up-regulation of Ubc9, and subsequent sumoylation of C/EBPαp42, may be a mechanism behind its transcriptional suppression. To test this hypothesis, we used the 293T cell line, which lacks endogenous C/EBPα.31 We cotransfected 293T cells with the expression vectors for His-tagged WT C/EBPα, His-tagged C/EBPα K161R mutant (SUMO-deficient mutant), C/EBPαp30,5,10 and Flag-tagged SUMO-1.32 At 48 hours after transfection, His-tagged C/EBPα forms were purified via Ni-NTA+ pull-down assays and immunoblotted with FLAG-M2 antibody. The results demonstrate that sumoylation of C/EBPαp42 (Figure 3A; lane 4) at K161 was enhanced when C/EBPαp30 was coexpressed. However, no sumoylation was detected when C/EBPαp30 was cotransfected with the SUMO-deficient mutant (K161R; Figure 3A; lane 5). The expression of C/EBPαp42 and C/EBPαp30 is shown after His purification (Figure 3A, middle panel). The expression of C/EBPαp42 was further confirmed by immunoblotting against His (Figure 3A, bottom panel). We next asked whether sumoylation of C/EBPαp42 could lead to its transcriptional suppression. We transiently transfected 293T cells with a minimal thymidine kinase (TK) promoter containing 2 C/EBP binding sites cloned upstream of the luciferase reporter gene along with various expression plasmids. Expression of the luciferase reporter gene was determined 24 hours after transfection. Transfection of C/EBPαp30 and Flag-SUMO expression construct significantly reduced the ability of C/EBPα to transactivate a minimal C/EBP promoter by almost 9-fold, whereas Flag-SUMO alone could reduce up to 2-fold (Figure 3B). No significant change was observed in pTK-luc empty vector. In line with our hypothesis, the transcriptional activity of C/EBPαp42 K161R mutant was not inhibited by C/EBPαp30 cotransfection (Figure 3C). These results indicate that sumoylation of C/EBPαp42 at lysine 161 is critical for C/EBPαp30-mediated transcriptional repression.
C/EBPαp30 enhances the sumoylation of C/EBPαp42 at K161, leading to its reduced transcriptional activity. His-tagged proteins purified using nickel Ni2+-NTA resin. (A) Immunoblot for the eluted proteins using anti-Flag M2 (top panel), anti-C/EBPα (middle panel), and Anti-His6 (bottom panel) antibodies. The arrows indicate the position of SUMO-1–modified proteins. (B) Transient transfection assay performed in 293T cells with a reporter construct of a minimal TK promoter with CEBP binding sites and expression plasmids for His C/EBPαp42 (WT), C/EBPαp30, and Flag–SUMO-1. (C) His C/EBPαp42 K161R (Mut), His C/EBPαp42 (WT), and C/EBPαp30. pTK (without CEBP sites) was used as control. Luciferase activities were measured 24 hours after transfection, and the values were normalized by using Renilla luciferase PRL-null. Values are expressed as means (± SEM) for 3 to 5 independent experiments, with P value shown as histograms.
C/EBPαp30 enhances the sumoylation of C/EBPαp42 at K161, leading to its reduced transcriptional activity. His-tagged proteins purified using nickel Ni2+-NTA resin. (A) Immunoblot for the eluted proteins using anti-Flag M2 (top panel), anti-C/EBPα (middle panel), and Anti-His6 (bottom panel) antibodies. The arrows indicate the position of SUMO-1–modified proteins. (B) Transient transfection assay performed in 293T cells with a reporter construct of a minimal TK promoter with CEBP binding sites and expression plasmids for His C/EBPαp42 (WT), C/EBPαp30, and Flag–SUMO-1. (C) His C/EBPαp42 K161R (Mut), His C/EBPαp42 (WT), and C/EBPαp30. pTK (without CEBP sites) was used as control. Luciferase activities were measured 24 hours after transfection, and the values were normalized by using Renilla luciferase PRL-null. Values are expressed as means (± SEM) for 3 to 5 independent experiments, with P value shown as histograms.
Ubc9 knockdown prevents sumoylation of C/EBPαp42 and enhances its transcriptional activity in the presence of the dominant-negative C/EBPαp30 isoform
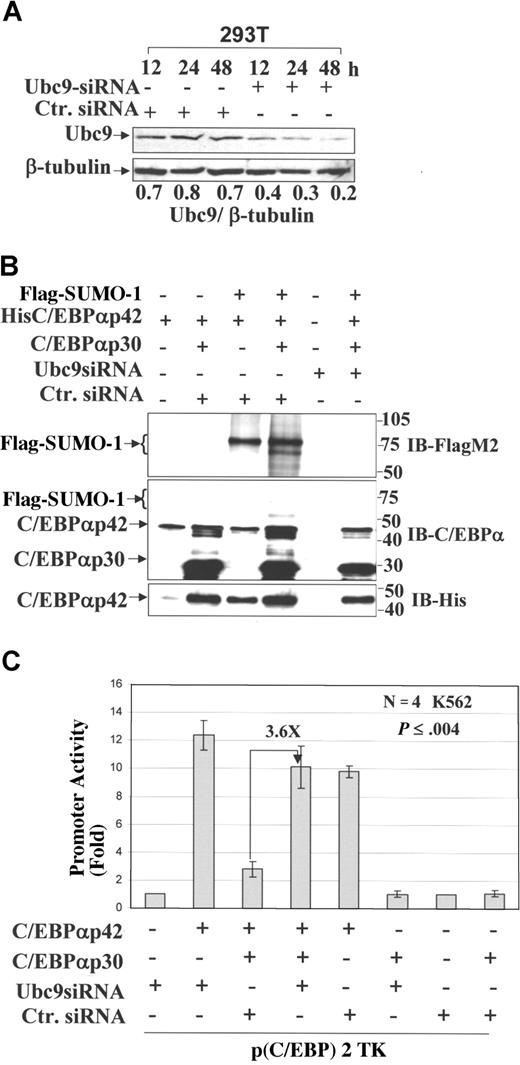
We investigated whether the prevention of C/EBPαp42 sumoylation by knockdown of endogenous Ubc9 is able to overcome the block in its transactivation. To address this, we performed transient transfection of Ubc9 siRNA in 293T cells and analyzed the expression of Ubc9 at different time points. Western blot analysis showed that Ubc9 expression was considerably reduced after 48 hours; β-tubulin was used as a loading control (Figure 4A). We again performed sumoylation assays in 293T cells after transient transfection of Ubc9 siRNA, siRNA control together with His-tagged C/EBPαp42, C/EBPαp30, and Flag–SUMO-1. At 48 hours after transfection, lysates were prepared, subjected to His purification, and immunoblotted with FLAG-M2 antibody (Figure 4B, top panel). The results showed that siRNA directed against Ubc9 significantly reduced sumoylation of C/EBPαp42 (Figure 4B, lane 6), whereas siRNA control had no effect (Figure 4B, lanes 3,4). The expression of His-purified C/EBPαp42 and C/EBPαp30 are shown for all the conditions (Figure 4B, middle and bottom panels). Next, we posed the question whether Ubc9-mediated inhibition of sumoylation of C/EBPαp42 is able to overcome the block in its transactivation. A transient transfection assay was performed in K562 cells using a minimal TK promoter plasmid along with C/EBPαp42, C/EBPαp30, Ubc9 siRNA, and control siRNA. Reporter gene expression assays 24 hours after transfection show that inhibition of Ubc9 by siRNA against Ubc9 increases the transcriptional activity of C/EBPαp42 3.6-fold in the presence of dominant-negative C/EBPαp30 (Figure 4C, lane 4). The dominant-negative transcriptional repression of C/EBPαp42 by C/EBPαp30 is shown (Figure 4C, lane 3).5 These data show conclusively that the dominant-negative effect of C/EBPαp30 over C/EBPαp42 is facilitated by Ubc9-mediated sumoylation, which can be overcome by the knockdown of Ubc9. Furthermore, we calculated the P value from the Student t test, which showed that Ubc9 siRNA–mediated increased transactivation potential of C/EBPαp42 was significant compared with that of control siRNA (Figure 4C, lanes 3,4).
Ubc9 knockdown prevents sumoylation of C/EBPαp42 and enhances its transcriptional activity in the presence of the dominant-negative C/EBPαp30 isoform. (A) Western blot analysis performed for the expression of Ubc9 in 293T cells at different time points after the overexpression of Ubc9 siRNA and a nonsilencing siRNA control. The numbers underneath the blot are the densitometric values calculated as protein–β-tubulin ratios. (B) A sumoylation assay performed after the overexpression of His C/EBPαp42 (WT), C/EBPαp30, Flag–SUMO-1, siRNA against Ubc9, and control siRNA, and immunoblot for the eluted proteins using anti–Flag M2 (top panel), anti-C/EBPα (middle panel), and anti-His6 (bottom panel) antibodies. (C) Transient transfection assay performed in K562 cells using the Nucleofector Kit (AMAXA) with the reporter construct of a minimal TK promoter with CEBP-binding sites and expression plasmids for C/EBPαp42, C/EBPαp30, Ubc9 siRNA, and control siRNA. Values are expressed as means (± SEM) for 3 independent experiments, with P values shown on histograms.
Ubc9 knockdown prevents sumoylation of C/EBPαp42 and enhances its transcriptional activity in the presence of the dominant-negative C/EBPαp30 isoform. (A) Western blot analysis performed for the expression of Ubc9 in 293T cells at different time points after the overexpression of Ubc9 siRNA and a nonsilencing siRNA control. The numbers underneath the blot are the densitometric values calculated as protein–β-tubulin ratios. (B) A sumoylation assay performed after the overexpression of His C/EBPαp42 (WT), C/EBPαp30, Flag–SUMO-1, siRNA against Ubc9, and control siRNA, and immunoblot for the eluted proteins using anti–Flag M2 (top panel), anti-C/EBPα (middle panel), and anti-His6 (bottom panel) antibodies. (C) Transient transfection assay performed in K562 cells using the Nucleofector Kit (AMAXA) with the reporter construct of a minimal TK promoter with CEBP-binding sites and expression plasmids for C/EBPαp42, C/EBPαp30, Ubc9 siRNA, and control siRNA. Values are expressed as means (± SEM) for 3 independent experiments, with P values shown on histograms.
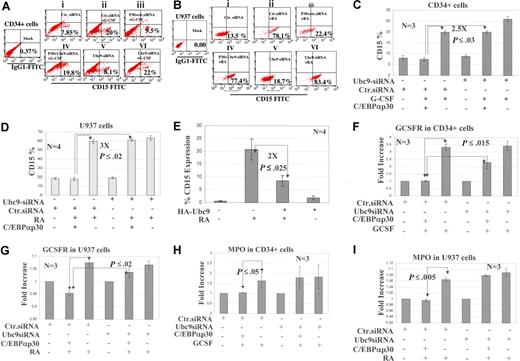
Knocking down of Ubc9 expression in CD34+ and U937 leads to enhanced granulocytic differentiation in the presence of C/EBPαp30
Next, we asked whether the inhibition of C/EBPαp42 sumoylation and the subsequent overcoming of dominant-negative block in transactivation by C/EBPαp30 can restore its WT functions, mainly granulocytic differentiation. We performed overexpression studies using 2 different experimental settings: human hematopoietic CD34+ cells and U937 cells. Ubc9 siRNA and C/EBPαp30 were overexpressed in these cells, and at 12 hours after transfection, CD34+ cells were induced with G-CSF and U937 cells were induced with RA to enforce granulocytic differentiation.1,33 The data show that G-CSF and RA, as expected, lead to a significant increase in CD15 expression up to 3-fold compared with native cells (Figure 5Ai vs Aii and 5Bi vs Bii). However, when cotransfected with C/EBPαp30, CD15 expression was impaired (Figure 5Aii vs Aiii and 5Bii vs Biii). Interestingly, siRNA against Ubc9 in these cells restores the CD15 expression 2-fold for CD34+ and 4-fold for U937 cells compared with the controls, C/EBPαp30-transfected cells with control siRNA (Figure 5Aiii vs 5Aiv and 5Biii vs Biv). Compared with cells alone (without p30), there is a 2.5-fold increase in CD15 expression for CD34+ and a 3-fold increase for U937 cells (Figure 5Av vs 5Avi and Figure 5Bv vs 5Bvi). In order to validate these findings, 3 different experiments were performed; the significant increase of CD15 expression is plotted as a histogram representing the mean standard deviation (SD) with standard error of the mean (Figure 5C,D). We confirmed whether differentiation block was due to up-regulation of Ubc9 by p30. We overexpressed HA-Ubc9 in U937 cells followed by RA induction, and CD15 expression was analyzed. The data indicated that overexpression of Ubc9 inU937 cells leads to down-regulation of CD15 expression up to 2-fold compared with untransfected cells (Figure 5E). The enhanced granulocytic differentiation was further validated in a similar experimental setup by checking the expression levels of the GCSFR and MPO genes by quantitative real-time PCR. The expression of both genes (GCSFR and MPO) was relatively high in G-CSF– and RA-induced cells compared with cells alone and cells with C/EBPαp30 cotransfection (Figure 5F-I).
Knockdown of Ubc9 expression in CD34 + and U937 leads to enhanced granulocytic differentiation. (A,B) Flow cytometric analysis performed for CD15 expression at day 6 (CD34+ cells) and day 3 (U937 cells) after the cotransfection of Ubc9 siRNA, a control siRNA and C/EBPαp30 in CD34+ and U937 cells and induced with G-CSF and RA, respectively. The fold changes are expressed as percentages and shown as a dotplot representative of 1 experiment. siRNA control was also used in all the experiments and is shown. (C,D) The percentage of the population of CD15+ cells from 3 independent experiments are represented as means (± SEM), with P values shown as histograms. (E) The percentage of the population of CD15+ cells after HA-Ubc9 overexpression in U937 cells and RA treatments. (F-I) Quantitative real-time PCR for the expression of G-CSF receptor and MPO. The fold increase for expression was calculated using Δct = (Ct sample − Ct control), and ΔCt values for each sample were standardized by GAPDH Ct value. The fold change was calculated as (= 2−Δct). Values are expressed as means (± SEM) for 3 independent experiments, with P values shown on histograms. The transfection efficiency in U937 cells and CD34+ cells after GPF nucleofection technology was calculated to be 80% and 50%, respectively (Figure S2B,C).
Knockdown of Ubc9 expression in CD34 + and U937 leads to enhanced granulocytic differentiation. (A,B) Flow cytometric analysis performed for CD15 expression at day 6 (CD34+ cells) and day 3 (U937 cells) after the cotransfection of Ubc9 siRNA, a control siRNA and C/EBPαp30 in CD34+ and U937 cells and induced with G-CSF and RA, respectively. The fold changes are expressed as percentages and shown as a dotplot representative of 1 experiment. siRNA control was also used in all the experiments and is shown. (C,D) The percentage of the population of CD15+ cells from 3 independent experiments are represented as means (± SEM), with P values shown as histograms. (E) The percentage of the population of CD15+ cells after HA-Ubc9 overexpression in U937 cells and RA treatments. (F-I) Quantitative real-time PCR for the expression of G-CSF receptor and MPO. The fold increase for expression was calculated using Δct = (Ct sample − Ct control), and ΔCt values for each sample were standardized by GAPDH Ct value. The fold change was calculated as (= 2−Δct). Values are expressed as means (± SEM) for 3 independent experiments, with P values shown on histograms. The transfection efficiency in U937 cells and CD34+ cells after GPF nucleofection technology was calculated to be 80% and 50%, respectively (Figure S2B,C).
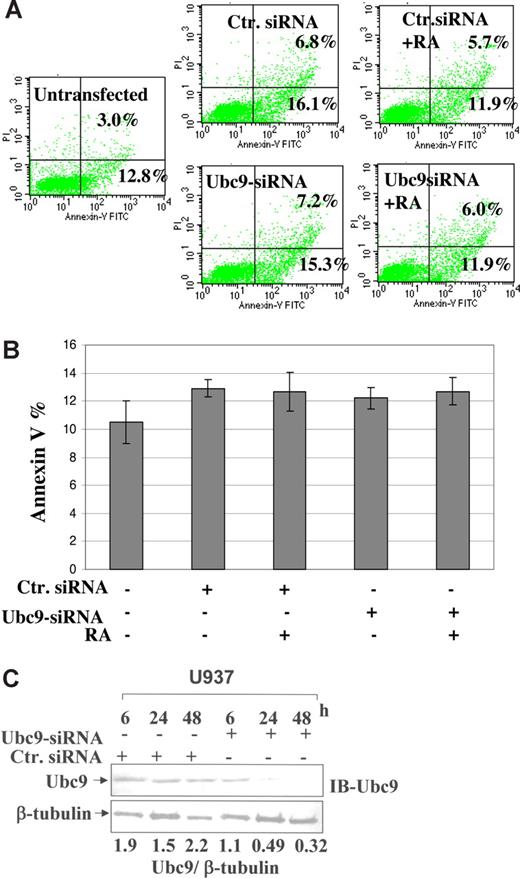
Ubc9 knockdown in U937 cells does not lead to significant apoptosis
In order to confirm that Ubc9 siRNA does not lead to cell death in U937 cells, we performed double-staining for Annexin-V and PI exclusion. For this assay, we transfected Ubc9 siRNA, and a control siRNA into U937 cells, and after 24 hours, cells were further induced with RA for another 24 hours. FACS analysis at the 48-hour time point showed that there were no significant apoptotic cells compared with untransfected cells (Figure 6A). The same results are plotted as a standard deviation calculated for 3 separate experiments (Figure 6B), and under similar experimental settings, cells were also stained with CD15 marker, which showed significant increases (data not shown). In order to further confirm that effects shown are specific for Ubc9 degradation, we immunoblotted the same lysates for Ubc9; our results confirmed that there was a significant decrease of Ubc9 expression (7-fold) compared with control siRNA (Figure 6C) after densitometric analysis. Our results are summed up as a model shown in Figure 7.
Detection of apoptosis in U937 cells. (A) Dual staining of FITC-labeled Annexin-V and PI. To detect apoptotic cells, U937 cells were transfected with 500 ng of control siRNA and Ubc9 siRNA by AMAXA nucleofection and treated with RA. After the 48-hour time point, cells were stained with Annexin-V and PI. Numbers in the lower right quadrant of each plot represent the percentage of cells in early apoptosis (Annexin-V+ and PI−). Numbers in the top right quadrant of each plot represent the percentage of dead cells (Annexin-V+ and PI+). (B) The histogram represented the values calculated for the Annexin-V staining for the above experimental settings. Values shown here from 3 independent experiments represented as means (± SEM). (C) Western blot analysis performed for the expression of Ubc9 in U937 cells at different time points after overexpression of Ubc9 siRNA and a nonsilencing siRNA control.
Detection of apoptosis in U937 cells. (A) Dual staining of FITC-labeled Annexin-V and PI. To detect apoptotic cells, U937 cells were transfected with 500 ng of control siRNA and Ubc9 siRNA by AMAXA nucleofection and treated with RA. After the 48-hour time point, cells were stained with Annexin-V and PI. Numbers in the lower right quadrant of each plot represent the percentage of cells in early apoptosis (Annexin-V+ and PI−). Numbers in the top right quadrant of each plot represent the percentage of dead cells (Annexin-V+ and PI+). (B) The histogram represented the values calculated for the Annexin-V staining for the above experimental settings. Values shown here from 3 independent experiments represented as means (± SEM). (C) Western blot analysis performed for the expression of Ubc9 in U937 cells at different time points after overexpression of Ubc9 siRNA and a nonsilencing siRNA control.
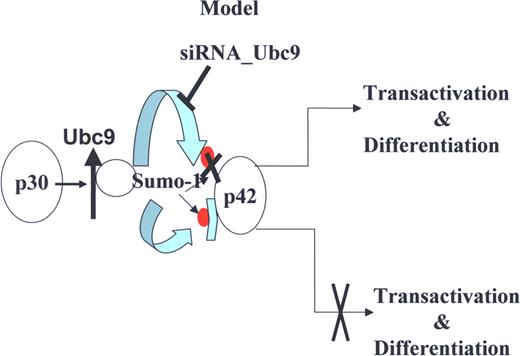
A proposed model for the role of Ubc9 in AML with C/EBPα mutation. Model depicting how C/EBPαp30 enhances C/EBPαp42 sumoylation via up-regulation of Ubc9 and blocks the transcription and differentiation potential of C/EBPαp42, and how Ubc9 siRNA overcomes the C/EBPαp30-mediated block of transcription and differentiation potential of C/EBPαp42.
A proposed model for the role of Ubc9 in AML with C/EBPα mutation. Model depicting how C/EBPαp30 enhances C/EBPαp42 sumoylation via up-regulation of Ubc9 and blocks the transcription and differentiation potential of C/EBPαp42, and how Ubc9 siRNA overcomes the C/EBPαp30-mediated block of transcription and differentiation potential of C/EBPαp42.
Discussion
Mutations of transcription factor C/EBPα occur in 10% of patients with AML.5-7 The mutant 30-kDa isoform protein blocks WT C/EBPα DNA binding and transactivation of granulocyte genes in a dominant-negative manner and fails to induce granulocytic differentiation.5 The underlying mechanism for C/EBPαp30's dominant-negative role over WT was addressed in this study. We analyzed C/EBPαp30 target proteins by inducing C/EBPαp30 expression in the erythroleukemic K562C/EBPαp30-ER cell line as a model system and directing proteome analysis of patients with the dominant-negative 30-kDa C/EBPα mutations. This systematic proteome analysis leads to the identification of many proteins that are differentially regulated by C/EBPαp30. Among these, we focused on Ubc9, which is up-regulated almost 6-fold by C/EBPαp30 and is required for sumoylation. Recently, it has been reported that Ubc9 is up-regulated in an increasing number of human malignancies, such as ovarian carcinoma, melanoma, and lung adenocarcinoma.18,19 Moreover, Ubc9-mediated SUMO-1 modification of the TEL-AML1 fusion protein and MKL1 has been reported to lead to the abnormal subcellular localization and decrease transcriptional capacity.34,35 In our analysis, we found Ubc9 expression to be markedly higher in patients with dominant-negative 30-kDa C/EBPα expression compared with other AML subtypes, for example, AML carrying t(8;21) and Inv(3) as well as normal bone marrow mononuclear cells. We observed an increased transcription of Ubc9 by C/EBPαp30 in an inducible cell line model. To further confirm whether the increased transcription was driven by the physical binding of C/EBPαp30 to the Ubc9 promotor, we performed electrophoretic mobility shift assay (EMSA) to determine whether either WT (C/EBPαp42) or C/EBPαp30 CEBPα interacts with the 5′ flank of the gene. We did not observe, however, any binding to the Ubc9 probe derived from the Ubc9 promotor, which has a CCAAT site −500 bp upstream of the initiating ATG (data not shown). This indicates that the effect may involve some indirect mechanism.
Posttranslational modification (sumoylation) has been reported to affect biological roles of many transcription factors.36 Most cases imply that conjugation of SUMO represses the activity of transcriptional activators.37-40 In several transcription factors, a synergetic control region has been identified, and putative synergetic control factors have been proposed.25,36,41 The activity of C/EBPα has been shown to be modulated by posttranslational modifications that are also critical in specifying lineage-commitment decisions.27,42 Recently, it has been reported that C/EBPαp42 can be sumoylated and thereby alter the transcriptional activity.25,26 Here, we show that C/EBPαp30 enhances sumoylation of C/EBPαp42 via up-regulation of Ubc9, which results in its decreased transcriptional activity. This is in agreement with the reports that sumoylated C/EBPα recruits histone deacetylase (HDACs) and prevents recruitment of SW1/SNF complex to the promoter,43,44 resulting in its transcriptional repression. Sumoylation is further reported to be an important ubiquitin-like modification of proteins affecting protein stability, enzymatic activity, nucleocytoplasmic trafficking, and protein-protein interactions. Like ubiquitination, sumoylation also affects many important cellular pathways.45 However, in contrast to ubiquitination, sumoylation46 does not necessarily commit proteins for degradation. In fact, SUMO may even function as an antagonist of ubiquitin in the degradation of selected proteins. Site-directed mutagenesis has confirmed lysine 161 as the major sumoylation site for human C/EBPαp42. Interestingly, we show that in a WT human C/EBPαp42, the Lys161 → Arg point mutant could not get sumoylated, which in turn restores the transcriptional activity of C/EBPαp42. As such, C/EBPαp30 does not have any dominant-negative effect.
The disruption of endogenous Ubc9 expression using RNAi has been successfully used to inhibit its expression, which disrupts sumoylation of its substrates.47 We used a 21-nucleotide siRNA that specifically silenced the human Ubc9 coding region in 293T cells at a 48-hour time point after transfection to study the biological effect on C/EBPαp42. This silencing resulted in the reduction of the steady-state level of SUMO-conjugated C/EBPαp42 and increased the transcriptional activity of C/EBPαp42 alone or C/EBPαp42 cotransfected with C/EBPαp30. We further validated these findings by inducing CD34+ cells with G-CSF and U937 cells with RA to induce granulocytic differentiation.48,49 However, cotransfection of these cells with C/EBPαp30 and their induction either with G-CSF or RA could not induce granulocytic differentiation, as has also been previously reported.33 Here, we show that the knockdown of Ubc9 by the overexpression of Ubc9-specific siRNA in these cells enhanced the granulocyte differentiation as indicated by the enhanced expression of GCSFR and MPO genes in the presence of C/EBPαp30 (Figure 5F-I)9,10 These data point to the fact that Ubc9-mediated sumoylation of C/EBPαp42 inhibits its WT functions, primarily blocking the granulopoiesis in human hematopoietic systems. Our data also confirmed that lack of Ubc9 does not result in cell death at the 48-hour time point. Very recent reports from Sato et al has shown that sumoylated C/EBPα failed to induce proliferation arrest in hepatocytes.26 Recently, it has been reported that inhibition of Ubc9 expression impairs melanoma cell proliferation, and lack of Ubc9 has been reported to induce cell death in vivo in animal models.50-52
In summary, we dissected a novel mechanism of C/EBPαp42 transcriptional repression in leukemia by Ubc9-mediated sumoylation. Interestingly, either by mutating the SUMO site or by disrupting Ubc9 we could overcome the C/EBPαp30-mediated block of transcription and the differentiation potential of C/EBPαp42. Therefore, understanding such mechanisms leads to the development of new therapeutics that stimulates C/EBPα activity to overcome the differentiation block observed in AML.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof S. K. Bohlander for providing AML patient samples. We are grateful to Profs Claessens and Callewaert for providing the pSG5 (Flag) 3c.myc SUMO-1 construct, Dr Jürgen Dittmer for allowing us to use his facility, and Prof Jorge A. Iniguez-Lluhi and Dr Subramanian for the HA-Ubc9 construct.
This work was supported by a Krebshilfe grant to G.B.
Authorship
Contribution: M.G. performed the research, collected and analyzed the data, and wrote the manuscript; M.Y.B. performed experiments and wrote the manuscript; A.A.P.Z. performed statistical analysis and helped write the manuscript; A.K.T. and J.A.P. performed experiments; M.C. collected patient samples and performed the experiments; D.J.T. analyzed the data; and G.B. designed the research, supervised, and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerhard Behre, Universitätsklinikum Halle (Saale), Klinik für Innere Medizin IV–Onkologie/Hämatologie -Ernst-Grube-Straße 40, 06120 Halle, Germany; e-mail: gerhard.behre@medizin.uni-halle.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal