Abstract
CXCL13 is a homeostatic chemokine for lymphocyte homing and positioning within follicles of secondary lymphoid tissues, acting through its cognate receptor, CXCR5. Moreover, the CXCR5-CXCL13 axis plays a unique role in trafficking and homing of B1 cells. Here, we report that chronic lymphocytic leukemia (CLL) B cells express high levels of functional CXCR5. CXCR5 expression levels were similar on CLL B cells and normal CD5+ B cells, and higher compared with normal CD5− B cells, follicular B-helper T cells (TFH cells), or neoplastic B cells from other B-cell neoplasias. Stimulation of CLL cells with CXCL13 induces actin polymerization, CXCR5 endocytosis, chemotaxis, and prolonged activation of p44/42 mitogen-activated protein kinases. Anti-CXCR5 antibodies, pertussis toxin, and wortmannin inhibited chemotaxis to CXCL13, demonstrating the importance of Gi proteins and PI3 kinases for CXCR5 signaling. Moreover, CLL patients had significantly higher CXCL13 serum levels than volunteers, and CXCL13 levels correlated with β2 microglobulin. We detected CXCL13 mRNA expression by nurselike cells, and high levels of CXCL13 protein in supernatants of CLL nurselike cell cultures. By immunohistochemistry, we detected CXCL13+ expression by CD68+ macrophages in situ within CLL lymph nodes. These data suggest that CXCR5 plays a role in CLL cell positioning and cognate interactions between CLL and CXCL13-secreting CD68+ accessory cells in lymphoid tissues.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is characterized by the accumulation of a monoclonal population of CD5+ neoplastic B cells in blood, secondary lymphoid tissues, and the marrow.1 Chemokines are essential for lymphocyte trafficking and homing during immune surveillance.2 However, the mechanisms that regulate the dissemination of CLL cells to different tissue compartments are largely unknown. Earlier, we reported that the chemokine receptor CXCR4 induces spontaneous migration of CLL cells beneath marrow stromal cells.3 More recently, the importance of CXCR4 for homing of neoplastic B cells to the marrow was confirmed in vivo.4 We also reported that a small proportion of monocyte-derived cells from the blood of CLL patients differentiates into large, adherent nurselike cells (NLCs) that attract CLL cells and protect them from undergoing spontaneous or drug-induced cell death.5-7 NLCs express high levels of CD68 and can be detected in secondary lymphoid tissues from patients with CLL.6 NLCs therefore appear to be an integral part of the CLL microenvironment within secondary lymphoid tissues, and may be comparable with lymphoma-associated macrophages (LAMs) in follicular lymphoma.8 The molecules involved in the cross talk between CLL cells and NLCs are only partially understood. NLCs secrete CXCL12 and thereby attract CLL cells and support their survival.5,9 Moreover, NLCs express B-cell–activating factor of the tumor necrosis factor (TNF) family (BAFF), a proliferation-inducing ligand (APRIL),9 CD31, and plexin-B110 that also protect CLL cells from apoptosis, indicating that NLCs use multiple distinct pathways to support CLL cell survival
In contrast to CXCR4, which is broadly expressed by normal and malignant hematopoietic and nonhematopoietic cells,11 the chemokine receptor CXCR5 is expressed only by mature recirculating B cells, a small subset of CD4+ and CD8+ T cells, and skin-derived migratory dendritic cells (reviewed in Muller et al12 ). CXCR5 initially was isolated from Burkitt lymphoma and designated Burkitt lymphoma receptor 1 (BLR1).13 CXCR5 gene–deleted mice display defective formation of primary follicles and germinal centers in the spleen and Peyer patches, and lack inguinal lymph nodes.14 Subsequently, the ligand for CXCR5 was identified and termed B-cell–attracting chemokine 1 (BCA-1).15,16 According to our current chemokine classification, BCA-1 now is designated CXCL13.17 CXCL13 is a homeostatic chemokine that is constitutively secreted by stromal cells in B-cell areas of secondary lymphoid tissues (follicles), where B cells encounter antigen and differentiate. Generally, CXCR5 induces recruitment of circulating naive B cells to follicles.15,18-20 Regarding the microanatomic positioning within the germinal center (GC), dark and light zones of the GC can be distinguished, and centroblasts localize to the dark zones via CXCR4. There, centroblasts rapidly divide and undergo somatic hypermutation of the antibody variable region genes. Subsequently, they become smaller, nondividing centrocytes and migrate to light zones of the GC in a CXCR5-dependent fashion, where selection for B cells with high-affinity binding surface antibody occurs (affinity maturation).21-23 A unique 3-dimensional network of CXCL13-expressing stromal cells provides the underlying roads that B cells actively follow for localization within the follicles.24
In addition to regulating the complex lymphocyte migration and territoriality in secondary lymphoid tissues, the CXCR5-CXCL13 axis appears to have a particularly important role in trafficking and homing of B1 B cells. B1 B cells are characterized and distinguished from the majority of recirculating B cells (B2 cells) by their distinct immunophenotype, tissue distribution, capacity for self-renewal, and role in autoantibody production (reviewed in Hardy25 ). Initially, B1 cells were described as a possible normal counterparts of CLL B cells,26 characterized by the expression of high levels of surface IgM, low CD23 and surface IgD, and CD5 (B1a cells). B1 cells are predominant in body cavities, but almost absent in secondary lymphoid organs, and play a key role in production of nonspecific, natural IgM antibodies for early protection form infections. B1 cells display higher CXCR5 surface expression than B2 cells, and are preferentially chemoattracted to CXCL13.27,28 CXCL13 is also produced by peritoneal macrophages, and B1 cells home to the peritoneal cavity in a CXCL13-dependent manner.28,29 As such, it has been suggested that the primordial function of CXCL13 may be the recruitment of primitive B cells to body cavities for T-independent responses, prior to its involvement in the complex lymphocyte positioning during T-dependent antibody responses.28
In addition to its function in specific and nonspecific immunity, CXCL13 is also an interesting molecule due to its capability for inducing ectopic lymphoidlike tissues (lymphoid neogenesis) frequently observed in chronic inflammatory processes and autoimmune diseases.30 Ectopic CXCL13 expression in the pancreas is sufficient to induce lymphoid neogenesis,31 and CXCR5/CXCL13 has been implicated in ectopic lymphoid follicle formation in rheumatoid arthritis,32,33 myasthenia gravis,34 and other autoimmune35,36 and chronic inflammatory diseases.37,38 Aberrant B1-cell trafficking to CXCL13 plays a role in development of lupus in aged (NZB × NZW)F1 (BWF1) mice. In these mice, myeloid dendritic cells overexpress CXCL13 in the thymus, kidneys, and spleen, inducing B1-cell recruitment to these target organs.27,39
In CLL patients, lymphadenopathy is common, and pseudofollicles, a histologic hallmark,40 are considered the proliferative compartment of this disease. In these proliferation centers, CLL cells are in close contact with stromal cells and activated T cells, and the interaction between these cell types is hypothesized to have a major impact on the progression of the disease (reviewed in Caligaris-Cappio41 ). Because of the importance of the CXCR5-CXCL13 axis for B-cell follicle formation, and its particular role in B1-cell trafficking and homing, we investigated the expression and function of this receptor-ligand pair in CLL.
Materials and methods
Approval was obtained from the Freiburg University Hospital and the University of Texas M. D. Anderson Cancer Center institutional review boards for these studies.
Chemokine, antibodies, flow cytometry, and immunohistochemistry
Synthetic, human CXCL13 was purchased from R&D Systems (Wiesbaden, Germany), human CXCL12 SDF-1alpha (1–67) was purchased from Upstate (Dundee, United Kingdom). The following monoclonal antibodies (mAbs) specific for human surface antigens were used: anti–CXCR5-phycoerythrin (PE) from R&D Systems, and anti–CXCR4-PE (12G5), anti–CD3-fluorescein isothiocyanate (FITC), anti–CD5-FITC, anti–CD19-allophycocyanine (APC), and the appropriate isotype controls from BD Biosciences (Heidelberg, Germany). For flow cytometry, the cells were adjusted to a concentration of 5 × 106 cells/mL in RPMI 1640 with 0.5% bovine serum albumin (BSA). A total of 5 × 105 cells were stained with saturating antibody concentrations for 30 minutes at 4°C, washed 2 times, and then analyzed on a FACSCalibur (Becton Dickinson, Mountain View, CA). To determine CXCR5 expression on normal CD5+ B cells, buffy coat cells from 3 different donors were obtained from the Blood Bank at M. D. Anderson Cancer Center. Lymphocytes were isolated by gradient centrifugation over Ficoll Paque, and stained with saturating concentrations of anti-CD5, anti-CXCR5, and anti-CD19 mAbs, or respective isotype control antibodies. This set of antibodies also allows us to determine frequency and CXCR5 expression levels on the CXCR5-positive T-cell subset designated follicular B-helper T cells (TFH cells).42 Due to the low frequency of CD5+ B cells within the population of circulating CD19+ B cells, we acquired 500 000 or more CD19+ events for each of the tested buffy coat samples. To determine whether mutational status of the immunoglobulin heavy chain variable region (IgVH) and/or CD38 expression affects CXCR5 expression levels, CXCR5 expression was determined in a series of selected CLL patients who either (a) displayed mutated IgH variable regions (> 2% deviation from the germ-line sequence) and lacked expression of CD38 (< 20% CD38+ CLL cells), or (b) displayed nonmutated IgH variable regions (< 2% deviation from the germ-line sequence) and were positive for expression of CD38 (> 20% CD38+ CLL cells). CXCR5 expression was determined by flow cytometry after gating on the CD19+ cells. Flow cytometry data were analyzed using the FlowJo software (Tree Star, San Carlos, CA).
Cell purification, cell lines, serum samples, and CXCL13 enzyme-linked immunosorbent assay (ELISA)
After informed consent in accordance with the Declaration of Helsinki, peripheral blood samples were obtained from patients fulfilling diagnostic and immunophenotypic criteria for common B-cell CLL at Freiburg University Hospital, Germany, and the Leukemia Department, University of Texas, M. D. Anderson Cancer Center, Houston, TX. Peripheral blood mononuclear cells (PBMCs) were isolated via density gradient centrifugation over Ficoll Paque (Pharmacia, Freiburg, Germany). Cells were used fresh or viably frozen in fetal calf serum (FCS) plus 10% dimethyl sulfoxide (DMSO) for storage in liquid nitrogen, stained with anti-CXCR5 and anti-CD19 mAbs, washed twice, and analyzed by flow cytometry. CXCR5 expression on malignant B cells from other B-cell lymphoma and multiple myeloma was examined as part of the diagnostic immunophenotyping by flow cytometry of blood or marrow samples from patients who fulfilled the respective diagnostic criteria at Freiburg University Hospital. The neoplastic cells were generally defined by gating on the CD19+ cells in cases of proven leukemic or marrow involvement, or by gating on the CD38+/CD138+ cells in cases of multiple myeloma. For CXCR5 expression on CD19+ B cells from healthy individuals, volunteers provided anticoagulated blood samples that were stained after Ficoll separation similarly to CLL cell samples, and CXCR5 expression was determined after gating on the CD19+ cells.
CLL nurselike cells were generated as described previously.5 Briefly, PBMCs from CLL patients were suspended in RPMI 1640 supplemented with 10% FCS and penicillin-streptomycin-glutamine (Gibco, Carlsbad, CA) to a final concentration of 1 × 107 cells/mL in tissue culture–treated 12-well plates (2 mL/well). The CLL cells then were cultured at 37°C in 5% CO2 in air for 14 days, and cultures were examined by phase-contrast microscopy for the outgrowth of adherent NLCs. The follicular dendritic HK cell line was obtained from Dr Yong Sung Choi (Ochsner Medical Foundation, New Orleans, LA) and cultured as described.43 Conditioned medium from HK cells and NLCs was used to assay for secretion of CXCL13. For this purpose, the culture medium was replaced with fresh medium when cells had reached 70% confluence (HK cells) or after 14 days (NLCs). After 3 days, the supernatants were removed and assayed for CXCL13 by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions (R&D Systems). Serum samples were obtained from patients with CLL at Freiburg University (n = 36) and M. D. Anderson Cancer Center (n = 64), and serum samples from healthy blood donors were provided by the Department of Transfusion Medicine (Freiburg University Hospital).
CXCR5 receptor endocytosis assay.
Time course and dose-dependency of CXCR5 receptor endocytosis in response to CXCL13 were examined as described.3 Briefly, CLL cells were adjusted to 5 × 106/mL in RPMI 1640 with 0.5% BSA. For the time course experiment, cells were incubated with 1 μg/mL CXCL13 at 37°C in 5% CO2 in air, and aliquots were removed at different time points (5, 10, 20, 60 minutes), placed on ice, stained at 4°C with saturating concentrations of anti-CD19 and anti-CXCR5 mAbs, washed twice with ice-cold RPMI/BSA, and analyzed by flow cytometry. To determine dose dependency of CXCR5 endocytosis, CLL cells were incubated with CXCL13 at various concentrations for 1 hour at 37°C in 5% CO2 in air. Cells were washed with a 20-fold volume of ice-cold buffer without FCS, stained at 4°C with saturating concentrations of anti-CXCR5 mAbs, and placed on ice until analysis by flow cytometry. Moreover, to determine the effect of CXCL13 in CLL cocultures with NLCs, we examined for CXCR5 expression (and other surface markers) on CLL B cells before and after 14 and 35 days of coculture with NLCs. CLL cell samples were removed from NLC cultures at the indicated time points, and CXCR5 staining was performed as described in the first paragraph of the materials and methods section.
Actin polymerization assay.
Actin polymerization was tested as described.3 Briefly, cells (1.25 × 106/mL) were suspended in RPMI 1640 medium with 0.5% BSA at 37°C and incubated with 200 ng/mL CXCL13 for various amounts of time. To determine actin polymerization in CLL B cells, CLL cells were prelabeled with anti-CD19 mAbs in selected cases. At the indicated time points, 400 μL of the cell suspension was added to 100 μL of a solution containing 4 × 10−7 M FITC-labeled phalloidin, 0.5 mg/mL 1-alpha-lysophosphatidylcholine (both from Sigma, St Louis, MO), and 7% formaldehyde in phosphate-buffered saline (PBS). The fixed cells were analyzed by flow cytometry on a FACSCalibur, and all time points are plotted relative to the mean relative fluorescence of the sample before addition of the chemokine.
Chemotaxis assay.
The chemotaxis assay across bare polycarbonate was preformed as described.3 Briefly, CLL PBMCs were isolated by density gradient centrifugation over Ficoll Paque and suspended in RPMI 1640 with 0.5% BSA. A total of 100 μL, containing 5 × 105 cells, was added to the top chamber of a 6.5-mm diameter Transwell culture inserts (Corning Costar, Bodenheim, Germany) with a pore size of 5 μm. Filters then were transferred to wells containing medium with or without CXCL13 or CXCL12. The chambers were incubated for 2 hours at 37°C in 5% CO2. After this incubation, the cells in the lower chamber were suspended and divided into aliquots for counting with a FACSCalibur for 20 seconds at 60 μL/min in triplicates or for immunophenotyping. A 1:20 dilution of input cells was counted under the same conditions. For pertussis toxin treatment, cells were preincubated with 200 ng/mL pertussis toxin (Sigma) at 37°C for 2 hours, washed twice, and subsequently applied to the top chamber of the chemotaxis assay. For anti-CXCR5 treatment, CLL cells were preincubated with 20 μg/mL anti-CXCR5 mAbs (clone no. 51505; R&D Systems). For inhibition of phosphatidylinositol 3-kinase (PI-3 kinase), p38, and p44/42 MAP kinases, B-CLL cells were incubated with either 500 or 2500 nM wortmannin, 20 μM SB 203580, or 20 μM PD 98059, respectively (all from Calbiochem, San Diego, CA) at 37°C for 30 minutes, and then assayed for chemotaxis in response to 1000 ng/mL CXCL13, as described above.
p44/42 mitogen-activated protein kinase Western blot analysis
The p44/42 mitogen-activated protein kinase (MAPK) immunoblot assays were performed as described.5 Briefly, CLL cells were serum-starved for 2 hours, and then lysates from 1 × 107 CLL cells per sample were prepared after stimulation with 200 ng/mL CXCL12 or 1 μg/mL CXCL13 at the indicated time points. Protein content was determined using the Pierce (Rockford, IL) Coomassie Protein Assay Reagent. Equal amounts of protein were separated by polyacrylamide gel electrophoresis (PAGE) and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, München, Germany). Western blot analysis was performed using the phospho-p44/42 MAPK rabbit polyclonal antibody Thr202/Tyr204 that specifically recognizes the phosphorylated (active) form of p44/42 MAPK protein, and a p44/42 MAP kinase control antibody (both from Cell Signaling Technology, Danvers, MA). Immunoreactive bands were visualized using horseradish peroxidase–conjugated goat-antirabbit secondary antibody (New England BioLabs, Beverly, MA) and the enhanced chemiluminescence system (ECL; Amersham, Arlington Heights, IL).
RT-PCR for CXCL12 and CXCL13 expression of nurselike cells
NLC cultures were established as described above. Nonadherent lymphoid cells then were removed and the NLC layer was washed 2 times with PBS. The complete removal of lymphocytes was verified by phase-contrast microscopy. Using Trizol, NLCs were lysed in the culture flasks according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Extraction of total RNA was performed with the PureLink Micro-to-Midi Total RNA Purification System as described by the manufacturer (Invitrogen). For each sample 4 μg total RNA was used for the reverse-transcription (RT) reaction with the SuperScript III First-Strand Synthesis System for RT–polymerase chain reaction (RT-PCR) (Invitrogen). CXCL12 (SDF-1b)–specific and CXCL13 (BCA-1)–specific primers for the PCR reactions are (all in 5′ to 3′ direction) as follows: SDF-1b 5′ primer, ATG AAC GCC AAG GTC GTG GTC; SDF-1b 3′ primer, GCT TCG GGT CAA TGC ACA C; BCA-1 5′ primer, CTC TGC TTC TCA TGC TGC TG; BCA-1 3′ primer, CAG CTT GAG GGT CCA CAC AC. The annealing temperature was 64°C, and the reaction proceeded for 35 cycles. To normalize for the amount of RNA, RT reactions for the human glyceraldehydes-3-phosphatase dehydrogenase (GA3PD) were performed, as described,44 using the following modified primers: 5′ primer, AAA TCC CAT CAC CAT CTT CC; 3′ primer, CTG TGG TCA TGA GTC CTT CC. To exclude that RNA from nonremoved CLL B cells caused the amplification signals, CLL B cells from 3 representative patients were purified at day 1 and day 14 with CD19 MicroBeads according to the manufacturer's instructions (Miltenyi, Bergisch Gladbach, Germany).
Immunohistochemistry
For CXCL13 immunohistochemistry, we used the EnVision G2 Doublestain System, according to the manufacturer's instructions (Dako, Hamburg, Germany). Briefly, paraffin-embedded 3-μm sections from lymph nodes from patients with CLL were mounted on microscope slides, dewaxed in xylene, and rehydrated through graded alcohol to water. Antigen retrieval was carried out using a steamer and EDTA buffer at a pH of 6. Then, dual endogenous enzyme block is applied to block endogenous alkaline phosphatase, peroxidase, and pseudoperoxidase activity. Tissue samples then were incubated with a 1:200 dilution of mouse antihuman CD68 primary mAbs (clone PG-M1; Dako), followed by incubation with an HRP-conjugated dextran polymer that also carries antibodies to mouse and rabbit immunoglobulins (polymer/HRP reagent), and subsequent visualization with the DAB+ chromogen. Then, samples were blocked with the block reagent before incubation with a 1:80 dilution of mouse antihuman CXCL13 primary mAbs (clone no. 53610; R&D Systems). After incubation, a dextran polymer carrying antibodies to mouse and rabbit immunoglobulins (rabbit/mouse LINK reagent) is added, followed by incubation with the polymer/AP reagent, which is visualized with the permanent red chromogen. This staining procedure results in brown staining for the first primary antibody (CD68) and red staining for the second primary antibody (CXCL13). Immunohistochemistry staining procedures were performed with the Eridan automated slide stainer, according to the manufacturer's instructions (Dako).
Data analysis and statistics
Results are shown as mean plus or minus standard deviation (SD) or standard error about the mean (SEM) of at least 3 experiments each. For statistical comparison between groups, the Student paired t test or Bonferroni t test were used. Analyses were performed using the Biostatistics software developed by Stanton A. Glantz (UC San Francisco), and Statistica 6.1 software (StatSoft, Tulsa, OK). Flow cytometry data were analyzed using the FlowJo software.
Results
Expression of surface CXCR5 on CLL B cells, normal CD5+ and CD5− B cells, follicular B-helper T cells, and other B-cell neoplasias
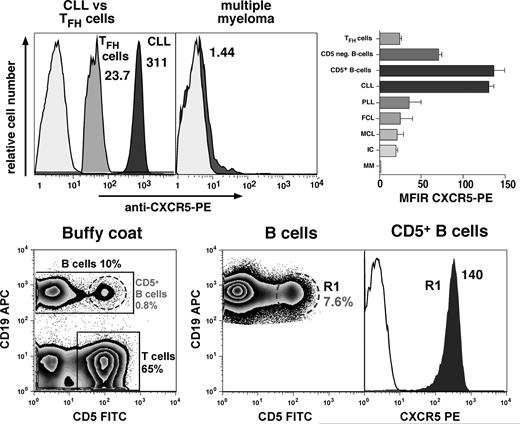
Using flow cytometry, we found that CLL B cells displayed high surface expression of CXCR5 with a mean fluorescence intensity ratio (MFIR) of 130 (± 8, mean ± SEM, n = 49). This is similar to the CXCR5 expression on circulating CD5+ B cells, which displayed a CXCR5 MFIR of 135.9 (± 19.2, mean ± SEM, n = 3), and higher than CXCR5 expression on normal CD5− B cells with a CXCR5 MFIR of 69.9 (± 5.4, mean ± SEM, n = 11, P < .01), or follicular B-helper T cells (TFH cells), which displayed a CXCR5 MFIR of 23.4 (± 1.5, mean ± SEM, n = 3). Neoplastic B cells from other B-cell lymphomas or multiple myeloma had relatively lower or absent CXCR5 expression, respectively. The mean CXCR5 MFIRs (± SEM) were 24.3 (± 15.7, n = 2) for follicular lymphoma, 19.3 (n = 1) for immunocytoma, 34.9 (± 15.2, n = 3) for prolymphocytic leukemia, 20.1 (± 9.1, n = 2) for mantle cell lymphoma, and 1.2 (± 0.3, n = 3) for multiple myeloma. Figure 1 displays representative histograms that depict the relative CXCR5 fluorescence intensities of a representative CLL and multiple myeloma cell sample, CXCR5 expression on TFH cells and CD5+ B cells, as well as the mean MFIRs (± SEM) for each of these cell populations. Mutational status of the IgH variable region and/or CD38 expression did not affect CXCR5 expression levels on the CLL cells. The CXCR5 expression levels, mutational status, and CD38 expression are displayed in Table 1; the mean (± SD) CXCR5 fluorescence for mutated, CD38− CLL cases was 257 (± 65.6, n = 6), compared with 264.8 (± 101.5) for nonmutated, CD38+ cases (n = 5).
High-level CXCR5 surface expression on CLL cells and normal CD5+ B cells. The top panel displays overlay histograms that depict the relative red fluorescence intensity after staining of CLL B cells, CXCR5-positive T cells (TFH cells), or neoplastic B cells from a patient with multiple myeloma with PE-labeled anti-CXCR5 mAbs (dark-gray histograms) or PE-conjugated isotype controls (light-gray histograms). CLL cells were identified by gating on the CD19-expressing cells, TFH cells by gating on the CD5+ and CXCR5-positive and CD19− lymphocytes, whereas multiple myeloma cells were gated by costaining with anti-CD38 and anti-CD138 mAbs. The CXCR5 mean fluorescence intensity ratio (MFIR) values are displayed next to each CXCR5 histogram. Compared with other B-cell neoplasias, TFH cells, or CD5− B cells, CLL B cells and normal CD5+ B cells consistently expressed higher levels of surface CXCR5, and multiple myeloma cells did not express surface CXCR5. The right-hand side box in the top panel displays a bar diagram that depicts the mean (± SEM) CXCR5 MFIRs for TFH cells (n = 3), normal CD5− B cells (n = 11), normal CD5+ B cells (n = 3), CLL B cells (n = 49), prolymphocytic leukemia cells (PLL, n = 3), follicular lymphoma cells (FCL, n = 2), mantle cell lymphoma cells (MCL, n = 2), immunocytoma (IC, n = 1), and multiple myeloma (MM, n = 3). The bottom panel displays contour plots that depict the staining and gating of buffy coat cells to determine CXCR5 expression on normal CD5+ B cells. A small proportion of circulating B cells coexpresses CD19 and CD5; this specimen had 10% CD19+ B cells, 0.8% CD19+/CD5+ B cells, and 65% CD5+ T cells, as indicated in the bottom left contour plot. Because of the low frequency of CD5+ B cells, we acquired 500 000 or more CD19+ B cells, which in this case contained 7.6% CD5+ B cells (middle contour plot). This allows us to determine CXCR5 expression levels on a sufficient number of CD19+/CD5+ B cells in gate R1. The right-hand histogram overlay displays the CXCR5 expression on CD5+ B cells in R1 (gray histogram), compared with the respective isotype control (white histogram), and the CXCR5 MFIR is displayed next to the CXCR5 histogram.
High-level CXCR5 surface expression on CLL cells and normal CD5+ B cells. The top panel displays overlay histograms that depict the relative red fluorescence intensity after staining of CLL B cells, CXCR5-positive T cells (TFH cells), or neoplastic B cells from a patient with multiple myeloma with PE-labeled anti-CXCR5 mAbs (dark-gray histograms) or PE-conjugated isotype controls (light-gray histograms). CLL cells were identified by gating on the CD19-expressing cells, TFH cells by gating on the CD5+ and CXCR5-positive and CD19− lymphocytes, whereas multiple myeloma cells were gated by costaining with anti-CD38 and anti-CD138 mAbs. The CXCR5 mean fluorescence intensity ratio (MFIR) values are displayed next to each CXCR5 histogram. Compared with other B-cell neoplasias, TFH cells, or CD5− B cells, CLL B cells and normal CD5+ B cells consistently expressed higher levels of surface CXCR5, and multiple myeloma cells did not express surface CXCR5. The right-hand side box in the top panel displays a bar diagram that depicts the mean (± SEM) CXCR5 MFIRs for TFH cells (n = 3), normal CD5− B cells (n = 11), normal CD5+ B cells (n = 3), CLL B cells (n = 49), prolymphocytic leukemia cells (PLL, n = 3), follicular lymphoma cells (FCL, n = 2), mantle cell lymphoma cells (MCL, n = 2), immunocytoma (IC, n = 1), and multiple myeloma (MM, n = 3). The bottom panel displays contour plots that depict the staining and gating of buffy coat cells to determine CXCR5 expression on normal CD5+ B cells. A small proportion of circulating B cells coexpresses CD19 and CD5; this specimen had 10% CD19+ B cells, 0.8% CD19+/CD5+ B cells, and 65% CD5+ T cells, as indicated in the bottom left contour plot. Because of the low frequency of CD5+ B cells, we acquired 500 000 or more CD19+ B cells, which in this case contained 7.6% CD5+ B cells (middle contour plot). This allows us to determine CXCR5 expression levels on a sufficient number of CD19+/CD5+ B cells in gate R1. The right-hand histogram overlay displays the CXCR5 expression on CD5+ B cells in R1 (gray histogram), compared with the respective isotype control (white histogram), and the CXCR5 MFIR is displayed next to the CXCR5 histogram.
CXCR5 expression in mutated, CD38 CLL and nonmutated, CD38+ CLL
| CLL sample no. . | IgVH % deviation . | % CD38+ . | Isotype control . | Mean CXCR5 . | CXCR5 MFIR . |
|---|---|---|---|---|---|
| Mutated, CD38− | |||||
| ––––1 | 8.7 | 2 | 2.1 | 249.0 | 120.3 |
| ––––2 | 6.1 | 1 | 2.7 | 190.0 | 71.7 |
| ––––3 | 8.8 | 0.4 | 2.8 | 371.0 | 131.1 |
| ––––4 | 5.1 | 1 | 2.1 | 255.0 | 123.8 |
| ––––5 | 4.8 | 0.7 | 2.4 | 279.0 | 118.2 |
| ––––6 | 7.8 | 0.8 | 2.7 | 198.0 | 73.1 |
| ––––Mean | — | — | 2.4 | 257.0 | 106.4 |
| ––––STDV | — | — | 0.3 | 65.6 | 26.7 |
| Nonmutated, CD38+ | |||||
| ––––7 | 0.7 | 97 | 2.3 | 285.0 | 123.4 |
| ––––8 | 0 | 54 | 2.3 | 129.0 | 56.6 |
| ––––9 | 0 | 21.3 | 2.2 | 401.0 | 186.5 |
| ––––10 | 0.7 | 62 | 3.3 | 297.0 | 91.1 |
| ––––11 | 0 | 94 | 2.5 | 212.0 | 86.2 |
| ––––Mean | — | — | 2.5 | 264.8 | 108.8 |
| ––––STDV | — | — | 0.4 | 101.5 | 49.5 |
| CLL sample no. . | IgVH % deviation . | % CD38+ . | Isotype control . | Mean CXCR5 . | CXCR5 MFIR . |
|---|---|---|---|---|---|
| Mutated, CD38− | |||||
| ––––1 | 8.7 | 2 | 2.1 | 249.0 | 120.3 |
| ––––2 | 6.1 | 1 | 2.7 | 190.0 | 71.7 |
| ––––3 | 8.8 | 0.4 | 2.8 | 371.0 | 131.1 |
| ––––4 | 5.1 | 1 | 2.1 | 255.0 | 123.8 |
| ––––5 | 4.8 | 0.7 | 2.4 | 279.0 | 118.2 |
| ––––6 | 7.8 | 0.8 | 2.7 | 198.0 | 73.1 |
| ––––Mean | — | — | 2.4 | 257.0 | 106.4 |
| ––––STDV | — | — | 0.3 | 65.6 | 26.7 |
| Nonmutated, CD38+ | |||||
| ––––7 | 0.7 | 97 | 2.3 | 285.0 | 123.4 |
| ––––8 | 0 | 54 | 2.3 | 129.0 | 56.6 |
| ––––9 | 0 | 21.3 | 2.2 | 401.0 | 186.5 |
| ––––10 | 0.7 | 62 | 3.3 | 297.0 | 91.1 |
| ––––11 | 0 | 94 | 2.5 | 212.0 | 86.2 |
| ––––Mean | — | — | 2.5 | 264.8 | 108.8 |
| ––––STDV | — | — | 0.4 | 101.5 | 49.5 |
The boldface numbers represent the mean and standard deviation of the fluorescence intensities for the isotype controls, CXCR5, and CXCR5 MFIR.
— indicates not applicable; STDV, standard deviation.
CXCL13 induces time- and dose-dependent CXCR5 receptor endocytosis
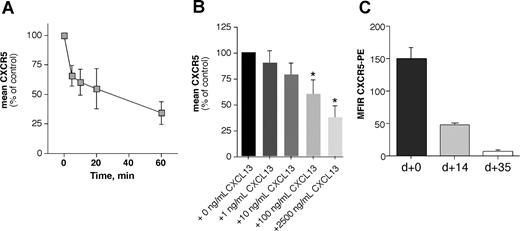
Binding of a chemokine to its receptor leads to internalization of the receptor-ligand complex with subsequent activation of intracellular signal cascades.45 This is a characteristic for all chemokine receptors and may allow for continuous sampling of chemoattractants, permitting the cells to follow a chemotactic gradient. Figure 2A displays the mean (± SD) relative CXCR5 fluorescence of 5 different CLL samples incubated for various times with 1 μg/mL CXCL13. Figure 2B shows the mean relative fluorescence of B CLL cells from 6 different patients stained with anti-CXCR5 mAbs after incubation with increasing concentrations of CXCL13 or medium alone. We found that CXCL13 induces a time- and dose-dependent downmodulation of CXCR5 on CLL B cells in the endocytosis assay (Figure 2A,B).
CXCR5 receptor eudocytosis in CLL cells. CXCL13 induces CXCR5 receptor endocytosis in a time-dependent (A) and dose-dependent (B) fashion, and coculture with NLCs induces CXCR5 down-regulation on CLL B cells (C). (A) CXCR5 expression was determined before and after incubation of CLL cells with 1 μg/mL CXCL13 at various time points; the different time points are displayed on the horizontal axis. The vertical axis displays the mean (± STDV, n = 5) relative CXCR5 expression on the CLL cells compared with the CXCR5 expression before addition of CXCL13. (B) CLL cells were exposed to increasing concentrations of CXCL13, as displayed on the horizontal axis. Then, CLL cells were stained with anti-CXCR5 antibodies, and surface CXCR5 expression was detected by flow cytometry. Increasing concentrations of CXCL13 induced down-regulation of surface CXCR5 that was significantly lower than CXCR5 expression of controls that were not exposed to CXCL13 at higher concentrations, as indicated by the asterisks. Displayed are the mean (± SEM) relative CXCR5 expression levels from 6 different patients compared with the CXCR5 levels of the untreated controls, as indicated on the horizontal axis. (C) CXCR5 surface expression was determined at initiation of NLC cocultures (n = 13), and subsequently aliquots of CLL cells were removed from NLC cocultures to determine whether this coculture system affects CXCR5 levels. After 14 days (n = 9) and 35 days (n = 8), CLL cells displayed significantly lower CXCR5 MFIRs than the respective CLL samples at the initiation of the cultures, suggesting CXCR5 endocytosis by CXCL13 that is secreted by NLCs.
CXCR5 receptor eudocytosis in CLL cells. CXCL13 induces CXCR5 receptor endocytosis in a time-dependent (A) and dose-dependent (B) fashion, and coculture with NLCs induces CXCR5 down-regulation on CLL B cells (C). (A) CXCR5 expression was determined before and after incubation of CLL cells with 1 μg/mL CXCL13 at various time points; the different time points are displayed on the horizontal axis. The vertical axis displays the mean (± STDV, n = 5) relative CXCR5 expression on the CLL cells compared with the CXCR5 expression before addition of CXCL13. (B) CLL cells were exposed to increasing concentrations of CXCL13, as displayed on the horizontal axis. Then, CLL cells were stained with anti-CXCR5 antibodies, and surface CXCR5 expression was detected by flow cytometry. Increasing concentrations of CXCL13 induced down-regulation of surface CXCR5 that was significantly lower than CXCR5 expression of controls that were not exposed to CXCL13 at higher concentrations, as indicated by the asterisks. Displayed are the mean (± SEM) relative CXCR5 expression levels from 6 different patients compared with the CXCR5 levels of the untreated controls, as indicated on the horizontal axis. (C) CXCR5 surface expression was determined at initiation of NLC cocultures (n = 13), and subsequently aliquots of CLL cells were removed from NLC cocultures to determine whether this coculture system affects CXCR5 levels. After 14 days (n = 9) and 35 days (n = 8), CLL cells displayed significantly lower CXCR5 MFIRs than the respective CLL samples at the initiation of the cultures, suggesting CXCR5 endocytosis by CXCL13 that is secreted by NLCs.
Moreover, we examined for CXCR5 expression in CLL-NLC cocultures. While CLL cells retained the expression of B-cell surface antigens such as CD19, there was a marked reduction in staining for surface CXCR5 after 14 and 35 days in NLC cultures (Figure 2C). After 14 days, the CXCR5 MFIR of CLL B cells in NLC cultures was 46.3 (± 0.9, mean ± SEM, n = 9) and 5.91 (± 1.37, n = 8) after 35 days, and therefore significantly lower than the CXCR5 MFIR of the respective CLL B cells at the time of initiation of NLC cultures (CXCR5 MFIR: 148.8 ± 18.8, n = 13), indicating that CLL cells undergo CXCR5 endocytosis in NLC cocultures due to CXCL13 secretion by NLCs. Previously, we noticed that coculture with NLCs also down-regulates CXCR4 expression on CLL cells due to SDF-1 expression by the NLCs.5
CXCL13 induces actin polymerization in CLL B cells
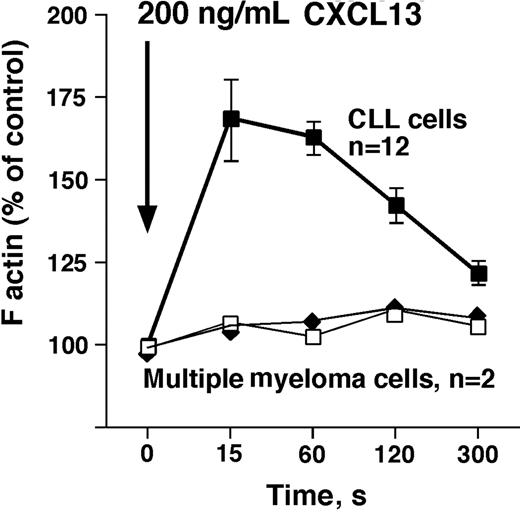
We detected a robust, transient increase in F-actin within 15 seconds after exposure of CLL cells (n = 12) to CXCL13, followed by a subsequent depolymerization, as shown in Figure 3. In contrast, myeloma cells lack CXCR5 surface expression, and did not display an increase in F-actin after stimulation with CXCL13 (n = 2, Figure 3).
CXCL13 induces actin polymerization in CLL B cells, but not in multiple myeloma cells. Intracellular F-actin was measured using FITC-labeled phalloidin after addition of 200 ng/mL CXCL13 at time 0 second, as indicated by the arrow. Results are displayed as percentage of intracellular F-actin relative to that prior to the addition of CXCL13, as indicated on the vertical axis. ■ represent data points that are the mean (± SD) relative F-actin content of CLL cell samples from 12 different patients at the time points displayed on the horizontal axis. □ and ◆ represent F-actin content in multiple myeloma cells from 2 different patients after stimulation with CXCL13. In contrast to CLL cells, myeloma cells did not display a transient increase in F-actin that is a characteristic cellular response to stimulation with a chemokine.
CXCL13 induces actin polymerization in CLL B cells, but not in multiple myeloma cells. Intracellular F-actin was measured using FITC-labeled phalloidin after addition of 200 ng/mL CXCL13 at time 0 second, as indicated by the arrow. Results are displayed as percentage of intracellular F-actin relative to that prior to the addition of CXCL13, as indicated on the vertical axis. ■ represent data points that are the mean (± SD) relative F-actin content of CLL cell samples from 12 different patients at the time points displayed on the horizontal axis. □ and ◆ represent F-actin content in multiple myeloma cells from 2 different patients after stimulation with CXCL13. In contrast to CLL cells, myeloma cells did not display a transient increase in F-actin that is a characteristic cellular response to stimulation with a chemokine.
Chemotaxis of CLL cells in response to CXCL13
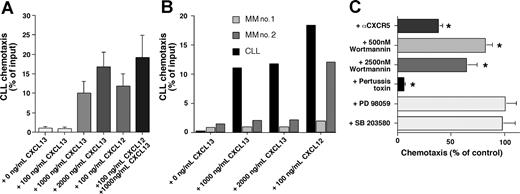
Figure 4A depicts the chemotactic response of CLL cells to increasing concentrations of CXCL13 and to 100 ng/mL CXCL12. Immunophenotyping of the transmigrated cells demonstrated that the majority of migrated CLL PBMCs were CLL B cells, based on CD19/CD5 expression of the migrated cells; 97.8% (± 2.1%) of the migrated cells displayed CD19/CD5 expression (n = 8). The maximum migration of CLL B cells at the concentrations tested was 16.7% (± 4%) of input cells (mean ± SEM, n = 16) with 2000 ng/mL CXCL13. The combination of 100 ng/mL CXCL12 and 1000 ng/mL CXCL13 induced migration of 19% (± 6%) of input CLL cells (mean ± SEM, n = 16), which was significantly higher than the chemotaxis to either chemoattractant alone (100 ng/mL SDF-1: 11.7% ± 3.4%, n = 16, 1000 ng/mL CXCL13: 9.9% ± 3.3%, n = 16), suggesting an additive effect of CXCL12 and CXCL13 in the chemotaxis assay. In contrast to CLL cells (Figure 5B black bars), multiple myeloma cells did not migrate to CXCL13, whereas one myeloma cell sample displayed a strong chemotaxis response to CXCL12 (Figure 4B MM no. 2).
CXCL13 induces chemotaxis of CLL B cells, but not of multiple myeloma cells. CLL cell chemotaxis is pertussis-toxin sensitive and wortmannin sensitive. (A) CLL cell chemotaxis was measured toward increasing concentrations of CXCL13, and/or 100 ng/mL CXCL12, as indicated on the horizontal axis. The bars represent relative proportion of input CLL cells that had migrated within 2 hours in response to the chemokine(s), as indicated on the vertical axis, and are the mean (± SD) from 16 different patients. (B) In contrast to CLL B cells from a representative patient (▄), multiple myeloma cells from 2 different patients ( and
and  ) did not display chemotaxis to the different concentrations of CXCL13 that are indicated on the horizontal axis. However, myeloma cells from one patient (MM no. 2) displayed robust chemotaxis to 100 ng/mL CXCL12. (C) To determine signaling pathways involved in CLL cell chemotaxis to CXCL13, CLL cells were preincubated with the reagents that are displayed on the vertical axis before being assayed for chemotaxis to 1000 ng/mL CXCL13. The bars depict the mean (± SEM) relative chemotaxis of CLL cells from 8 different patients after treatment with the various agents compared with untreated controls (100%). Anti-CXCR5 mAbs, wortmannin, and pertussis toxin significantly inhibited CLL cell chemotaxis to CXCL13, as indicated by the asterisks.
) did not display chemotaxis to the different concentrations of CXCL13 that are indicated on the horizontal axis. However, myeloma cells from one patient (MM no. 2) displayed robust chemotaxis to 100 ng/mL CXCL12. (C) To determine signaling pathways involved in CLL cell chemotaxis to CXCL13, CLL cells were preincubated with the reagents that are displayed on the vertical axis before being assayed for chemotaxis to 1000 ng/mL CXCL13. The bars depict the mean (± SEM) relative chemotaxis of CLL cells from 8 different patients after treatment with the various agents compared with untreated controls (100%). Anti-CXCR5 mAbs, wortmannin, and pertussis toxin significantly inhibited CLL cell chemotaxis to CXCL13, as indicated by the asterisks.
CXCL13 induces chemotaxis of CLL B cells, but not of multiple myeloma cells. CLL cell chemotaxis is pertussis-toxin sensitive and wortmannin sensitive. (A) CLL cell chemotaxis was measured toward increasing concentrations of CXCL13, and/or 100 ng/mL CXCL12, as indicated on the horizontal axis. The bars represent relative proportion of input CLL cells that had migrated within 2 hours in response to the chemokine(s), as indicated on the vertical axis, and are the mean (± SD) from 16 different patients. (B) In contrast to CLL B cells from a representative patient (▄), multiple myeloma cells from 2 different patients ( and
and  ) did not display chemotaxis to the different concentrations of CXCL13 that are indicated on the horizontal axis. However, myeloma cells from one patient (MM no. 2) displayed robust chemotaxis to 100 ng/mL CXCL12. (C) To determine signaling pathways involved in CLL cell chemotaxis to CXCL13, CLL cells were preincubated with the reagents that are displayed on the vertical axis before being assayed for chemotaxis to 1000 ng/mL CXCL13. The bars depict the mean (± SEM) relative chemotaxis of CLL cells from 8 different patients after treatment with the various agents compared with untreated controls (100%). Anti-CXCR5 mAbs, wortmannin, and pertussis toxin significantly inhibited CLL cell chemotaxis to CXCL13, as indicated by the asterisks.
) did not display chemotaxis to the different concentrations of CXCL13 that are indicated on the horizontal axis. However, myeloma cells from one patient (MM no. 2) displayed robust chemotaxis to 100 ng/mL CXCL12. (C) To determine signaling pathways involved in CLL cell chemotaxis to CXCL13, CLL cells were preincubated with the reagents that are displayed on the vertical axis before being assayed for chemotaxis to 1000 ng/mL CXCL13. The bars depict the mean (± SEM) relative chemotaxis of CLL cells from 8 different patients after treatment with the various agents compared with untreated controls (100%). Anti-CXCR5 mAbs, wortmannin, and pertussis toxin significantly inhibited CLL cell chemotaxis to CXCL13, as indicated by the asterisks.
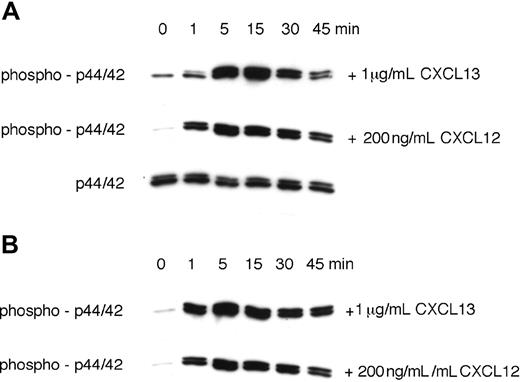
CXCL13 stimulated prolonged p44/42 MAP kinase activation in CLL B cells. Activation of p44/42 MAP kinase was determined by Western blot after stimulation with 1 μg/mL CXCL13 or 200 ng/mL CXCL12, as indicated on the right-hand side. To determine the time course of p44/42 MAPK activation, CLL cell lysates were obtained at the indicated time points and probed with anti–phospho-p44/42 mAbs. To confirm equal loading of protein, blots were reprobed with an antibody to the nonphosphorylated p44/42 MAPK. Panels A and B display MAP kinase activation in CLL cells from 2 different patients.
CXCL13 stimulated prolonged p44/42 MAP kinase activation in CLL B cells. Activation of p44/42 MAP kinase was determined by Western blot after stimulation with 1 μg/mL CXCL13 or 200 ng/mL CXCL12, as indicated on the right-hand side. To determine the time course of p44/42 MAPK activation, CLL cell lysates were obtained at the indicated time points and probed with anti–phospho-p44/42 mAbs. To confirm equal loading of protein, blots were reprobed with an antibody to the nonphosphorylated p44/42 MAPK. Panels A and B display MAP kinase activation in CLL cells from 2 different patients.
Chemotaxis of CLL cells was significantly inhibited by preincubation of the input cells with anti-CXCR5 mAbs to levels that were 37.8% (± 4.4%, mean ± SEM, n = 10, P < .01) of respective controls, demonstrating that a specific interaction between CXCL13 and CXCR5 was necessary for chemotaxis (Figure 4C). CXCR5 is the only receptor known to bind CXCL13, and therefore we suggest that the failure to achieve 100% inhibition with the anti-CXCR5 mAb may reflect the competition of relatively high concentrations of the chemokine (eg, 1000 ng/mL) with anti-CXCR5 mAbs during a 2-hour assay. Internalization and recycling of CXCR5 receptors after anti-CXCR5 mAb binding or partial dissociation of this antibody at the physiologic temperatures of the chemotaxis assay may also play a role in this context. Chemotaxis to CXCL13 was also significantly inhibited by pertussis toxin to levels that were 5.5% (± 1.3%, mean ± SEM, n = 8) of the chemotaxis of the untreated control samples, indicating that this activity was dependent on signaling through a Gi protein(s). Furthermore, pretreatment with wortmannin, a broadly active inhibitor of PI3 kinase, significantly inhibited CXCL13-induced migration of CLL B cells to 81.5% (± 7%, mean ± SEM, n = 8, P < .01) at 500 nM and to 63.7% (± 10.2%, mean ± SEM, n = 8, P < .01) at 2500 nM. In contrast, PD98059, a selective p44/42 MAP kinase inhibitor, and SB203580, a selective p38 MAP kinase inhibitor, did not inhibit CLL cell chemotaxis to CXCL13 (Figure 4C).
CXCL13 induces prolonged activation of p44/42 MAPK
We and others have shown that stimulation of chemokine receptors results in the transient activation of the p44/42 mitogen-activated protein (MAP) kinase (ERK1/2).5,46 Because CXCL12 was described to induce sustained p44/42 MAPK activation,46 we compared p44/42 MAPK activation in CLL cells in response to 1 mg/mL CXCL13 or 200 ng/mL CXCL12. Figure 5A,B display immunoblots from 2 CLL samples that depict a robust, prolonged p44/42 MAPK activation in response to both CXCL12 and CXCL13.
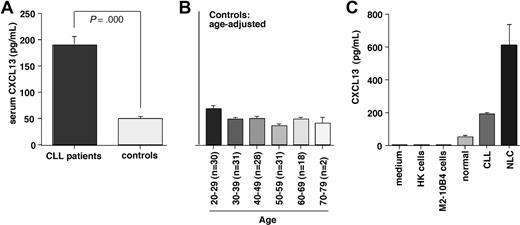
CXCL13 serum levels and correlation with β2 microglobulin
We found significantly higher CXCL13 levels in serum from CLL patients compared with healthy volunteers. The mean (± SEM) CXCL13 level in CLL patients was 187.9 (± 17.2 pg/mL, n = 100) compared with 48.4 (± 2.6 pg/mL, n = 140, P < .001) in the healthy control group. Figure 6A displays mean CXCL13 levels in CLL patients and controls; Figure 6B displays the mean CXCL13 levels in healthy volunteers in an age-adjusted fashion. Moreover, we correlated CXCL13 levels in CLL patients with clinical stage of the disease, serum LDH, white blood cell count, and β2 microglobulin. We found a significant positive correlation between CXCL13 and β2 microglobulin with a correlation coefficient r = 0.32 and P less than .01 (n = 81).
CXCL13 serum levels in CLL patients and healthy controls, and in supernatants from NLC cultures. (A) Serum samples from CLL patients (n = 100) and healthy controls (n = 140) were assayed for CXCL13 protein by ELISA. Displayed are the mean (± SEM) CXCL13 levels, as indicated on the vertical axis. CLL patients had significantly higher CXCL13 serum levels than controls. (B) To determine whether age may affect CXCL13 levels and partially account for higher CXCL13 levels in CLL patients, we analyzed CXCL13 levels in the control population in an age-adjusted fashion. Displayed are the mean (± SEM) CXCL13 levels (vertical axis) in each of the age groups, and the numbers of samples tested in each age group, as displayed on the horizontal axis. We did not observe a trend for higher CXCL13 in older control subjects. (C) Comparison of CXCL13 levels in cell culture medium, conditioned medium from HK follicular dendritic cells, M2–10B4 marrow stromal cells, serum from healthy controls or CLL patients (same data as in Figure 5A), or supernatants from NLC cultures. In contrast to HK and M2–10B4 cell supernatants, NLC supernatants displayed high levels of CXCL13.
CXCL13 serum levels in CLL patients and healthy controls, and in supernatants from NLC cultures. (A) Serum samples from CLL patients (n = 100) and healthy controls (n = 140) were assayed for CXCL13 protein by ELISA. Displayed are the mean (± SEM) CXCL13 levels, as indicated on the vertical axis. CLL patients had significantly higher CXCL13 serum levels than controls. (B) To determine whether age may affect CXCL13 levels and partially account for higher CXCL13 levels in CLL patients, we analyzed CXCL13 levels in the control population in an age-adjusted fashion. Displayed are the mean (± SEM) CXCL13 levels (vertical axis) in each of the age groups, and the numbers of samples tested in each age group, as displayed on the horizontal axis. We did not observe a trend for higher CXCL13 in older control subjects. (C) Comparison of CXCL13 levels in cell culture medium, conditioned medium from HK follicular dendritic cells, M2–10B4 marrow stromal cells, serum from healthy controls or CLL patients (same data as in Figure 5A), or supernatants from NLC cultures. In contrast to HK and M2–10B4 cell supernatants, NLC supernatants displayed high levels of CXCL13.
CXCL13 expression in supernatants
To determine the possible origin of CXCL13 levels in CLL patients, we assayed protein concentrations of CXCL13 in conditioned medium from marrow stromal cells, follicular dendritic cells, and NLCs by ELISA (Figure 6C). We detected only minimal CXCL13 concentrations in cell culture medium (0.89 pg/mL, n = 1), conditioned medium from marrow stromal cells (1.67 ± 0.11 pg/mL, mean ± SEM, n = 2), and conditioned medium from follicular dendritic cells (0.22 ± 0 pg/mL, mean ± SEM, n = 2). In contrast, conditioned medium from NLCs displayed high levels of CXCL13 (609.9 ± 129.8 pg/mL, mean ± SEM, n = 4).
Nurselike cells express CXCL12 and CXCL13 mRNA
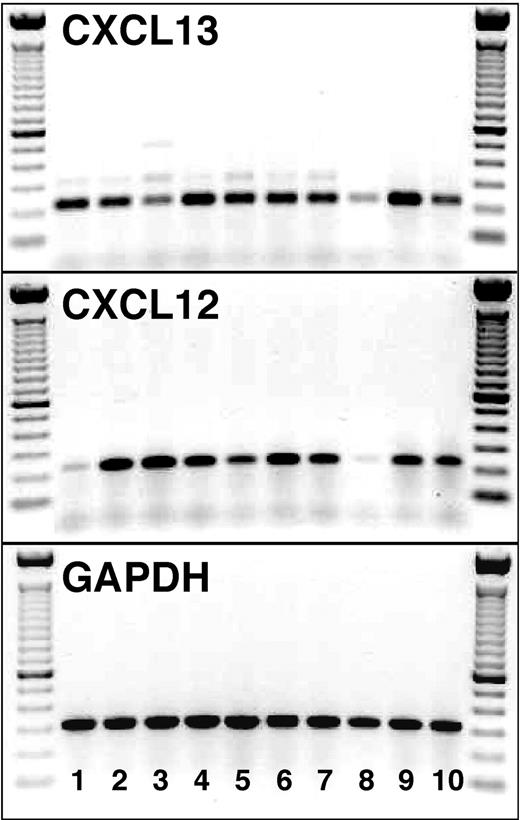
cDNA from purified NLCs from 10 patients with CLL was examined for CXCL12 and CXCL13 expression. For each sample, we obtained specific bands for both chemokine cDNAs (233 bp for CXCL12 and 225 bp for CXCL13, Figure 7). In contrast, we did not detect any CXCL12 expression in purified CLL B cells (n = 3), whereas 1 of 3 patients showed a weak expression of CXCL13 at day 1, and 2 patients showed an expression of different intensity at day 14 of the incubation (data not shown).
CXCL13 and CXCL12 mRNA expression by NLCs. cDNA from purified NLCs from 10 patients with CLL (lanes 1-10) was examined for the expression of CXCL13 (top box) and CXCL12 (middle box) mRNA by RT-PCR. The specific PCR fragments (225 bp for CXCL13 and 233 bp for CXCL12) are visible in each of the 10 NLC samples. The bottom box displays PCR fragments of the expected size using GAPDH primers for the same NLC samples. On the left- and right-hand side of each of the boxes, the separation of the 100-bp marker DNA is displayed.
CXCL13 and CXCL12 mRNA expression by NLCs. cDNA from purified NLCs from 10 patients with CLL (lanes 1-10) was examined for the expression of CXCL13 (top box) and CXCL12 (middle box) mRNA by RT-PCR. The specific PCR fragments (225 bp for CXCL13 and 233 bp for CXCL12) are visible in each of the 10 NLC samples. The bottom box displays PCR fragments of the expected size using GAPDH primers for the same NLC samples. On the left- and right-hand side of each of the boxes, the separation of the 100-bp marker DNA is displayed.
CXCL13 immunohistochemistry
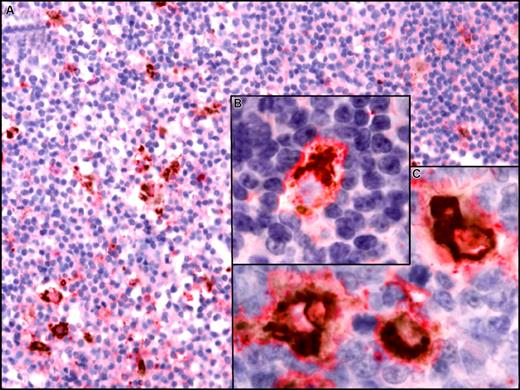
Using a DAB/permanent red double-stain system, we detected coexpression of CXCL13 by CD68+ macrophages in lymph nodes from patients with CLL (Figure 8). In contrast, we did not detect CXCL13 expression by CD21+ follicular deudritic cells (FDCs) (data not shown).
CXCL13 expression in situ by CD68+ macrophages in lymph nodes from CLL patients. Paraffin-embedded sections of lymph nodes from CLL patients were incubation with anti-CD68 and anti-CXCL13 mAbs. For simultaneous detection of CD68 and CXCL13, a biotin-free system was used that is based on peroxidase with DAB as chromogen (brown) for CD68 detection and alkaline phosphatase with permanent red chromogenic substrate for detection of CXCL13. Images were visualized using a Zeiss Axiovert 135 microscope, C-Apochromat objectives, an AxioCam ICc3 camera, and Axioplan 2 imaging software (all from Carl Zeiss, Jena, Germany) and processed using Canvas 9 software (ACD Systems, Victoria, BC). (A) A lymph node section at low magnification (200×) that depicts CD68+ cells scattered throughout the displayed section that coexpress CXCL13. (B,C) Higher magnification that depicts coexpression of CD68 (brown, perinuclear) and CXCL13 (red) at 630×.
CXCL13 expression in situ by CD68+ macrophages in lymph nodes from CLL patients. Paraffin-embedded sections of lymph nodes from CLL patients were incubation with anti-CD68 and anti-CXCL13 mAbs. For simultaneous detection of CD68 and CXCL13, a biotin-free system was used that is based on peroxidase with DAB as chromogen (brown) for CD68 detection and alkaline phosphatase with permanent red chromogenic substrate for detection of CXCL13. Images were visualized using a Zeiss Axiovert 135 microscope, C-Apochromat objectives, an AxioCam ICc3 camera, and Axioplan 2 imaging software (all from Carl Zeiss, Jena, Germany) and processed using Canvas 9 software (ACD Systems, Victoria, BC). (A) A lymph node section at low magnification (200×) that depicts CD68+ cells scattered throughout the displayed section that coexpress CXCL13. (B,C) Higher magnification that depicts coexpression of CD68 (brown, perinuclear) and CXCL13 (red) at 630×.
Discussion
During the past few years, it has increasingly been recognized that signals from the microenvironment make pivotal contributions to the progression of hematopoietic and epithelial malignancies. Cross talk between tumor cells and nonmalignant accessory cells (mesenchymal fibroblasts, macrophages, endothelial cells, and others) confers survival and drug-resistance signals that are likely to be involved in minimal residual disease (MRD) and relapses commonly seen in the treatment of patients with various cancers. A particularly important role of the microenvironment in B-cell neoplasias is suggested by recent studies that demonstrated correlations between the survival of follicular lymphoma patients and molecular features of the nonmalignant cells in the lymphoma microenvironment (lymphocytes and monocytes/macrophages, dendritic cells)47 or the content of lymphoma-associated macrophages.8 CLL cells are highly dependent on their microenvironment for survival as they rapidly undergo spontaneous apoptosis when removed from the patient and placed into conventional culture conditions. This apoptosis can be prevented by coculture with monocyte-derived CD68+ nurselike cells5 or other accessory cells, such as marrow stromal cells or follicular dendritic cells.48,49 NLCs are present in secondary lymphoid tissues from CLL patients,6 and therefore NLCs can be considered an in vitro model for lymphoma-associated macrophages described in situ as an important part of the lymphoma microenvironment.8,50
Here, we provide evidence that CXCL13 expression by NLCs plays a role in cross talk between CLL cells and accessory cells of monocyte lineage (NLCs, CD68+ cells). CLL B cells express high levels of functional CXCR5 chemokine receptors. In contrast, neoplastic cells from patients with multiple myeloma, a B-cell malignancy that typically does not involve secondary lymphatic tissues, did not express surface CXCR5 and did not respond to stimulation with the CXCR5 ligand CXCL13 (Figures 1,3,4). CXCR5 expression on CLL cells was similar to its expression on normal CD5+ B cells, and significantly higher compared with CD5− B cells, follicular helper T cells (TFH cells) or neoplastic B cells from patients with other B-cell malignancies (Figure 1). Given the similarities between CLL and murine B1 cells, higher CXCR5 levels on CLL cells and normal CD5+ B cells compared with normal CD5− B cells recapitulate the CXCR5 overexpression that previously was reported for B1 cells compared with B2 cells in the murine system.27,28
Furthermore, we noticed CXCL13 mRNA expression by NLCs, and CXCL13 protein expression in supernatants from NLCs and in situ by CD68+ cells in lymph nodes from CLL patients. This observation highlights that cross talk via chemokines (CXCL12, CXCL13) between CD68+ cells and CLL cells is an integral part of the microenvironment in CLL, supporting the concept that accessory cells (and particularly macrophages) contribute to cancer progression and treatment outcome, as demonstrated in follicular lymphoma8,47 and epithelial cancers (reviewed in Condeelis and Pollard51 ).
Initially, high CXCL13 expression was noticed in human spleen and lymph nodes by Northern blot analysis,16 and the nonlymphoid stromal cells, in particularly FDCs within B-cell follicles of secondary lymphoid tissues were considered the principal source of CXCL13.52 However, more recently, macrophages within the peritoneal cavity28 or at sites of inflammation33 have been recognized to secrete CXCL13, whereas FDC-like cells do not secrete CXCL13 in vitro.53 Our detection of CXCL13 mRNA and protein secretion by NLCs, but lack of CXCL13 in supernatants of the HK FDC line (Figure 6C), therefore is in accordance with these previous studies and suggests that CXCL13 secretion by monocyte-lineage cells is involved not only in lymphoid neogenesis associated with chronic inflammation/autoimmunity, as suggested by Carlsen et al,33 but also in lymphadenopathy in CLL and possibly other B-cell lymphomas.
Elevated CXCL13 protein levels have been reported in serum from human immunodeficiency virus (HIV) patients54 and CSF from patients with neuroborreliosis55 and multiple sclerosis.56 These data and our observation that serum from CLL patients contains elevated CXCL13 levels (Figure 6A) indicate that chronic inflammation and/or lymphoid neogenesis (lymphadenopathy in CLL) is associated with local or systemic elevations of CXCL13.
Up-regulation of CXCR5 that occurs during B-cell development57 is critical for achieving competence for B-cell recirculation through lymphoid follicles and may play a role in maintaining B-cell longevity, as suggested earlier.52 Consequently, the high CXCR5 levels on CLL cells are likely to be important for recirculation of CLL cells within lymphoid tissues, where CLL cells receive survival signals from contact with NLCs through distinct receptors such as CXCR5, CXCR4, the BCR, CD38, CD40, and other cognate interactions. The notion that CXCR5 signaling may not only induce migration, but also have a CLL cell survival-supporting function is suggested by our observation that CXCL13 induces a prolonged p44/42 MAP kinase (ERK1/2) activation (Figure 5). Prolonged MAPK activation has been considered a unique feature of CXCL12 involved in lymphocyte homeostasis.46 We reported earlier that CLL cells display prolonged p44/42 MAPK activation in response to CXCL12,5 suggesting a role in CLL cell maintenance, although the importance of MAPK signaling for CLL cell survival remains controversial.9 Here, we demonstrated prolonged MAPK activation in CLL B cells in response to CXCL13 (Figure 5), which has not previously been reported to our knowledge, and it would be interesting to determine whether this signaling response is a general response in CXCR5+ T and/or B lymphocytes or restricted to CLL cells.
Upon further differentiation toward plasma cells, CXCR5 is down-regulated and responsiveness to CXCL13 is lost, whereas responsiveness to CXCL12 is increased, allowing for plasma cell lodgment within the marrow.58 Therefore, lack of CXCR5 expression by multiple myeloma cells and their unresponsiveness to CXCL13 (Figures 1,3,4B) is in accordance with an earlier study,59 reflects the developmental B-cell stage, and demonstrates that chemokine receptor expression pattern are retained by the neoplastic B cells.
Collectively, this study suggests that CXCR5 allows for CLL cell trafficking and migration to CXCL13-secreting CD68+ cells in lymphoid tissues, where CLL cells receive prosurvival signals and are partially protected from the effects of cytotoxic therapies. Because of this function in leukemia-microenvironment interaction, and also because of its high expression levels and almost exclusive expression on (CLL) B cells, CXCR5 represents an attractive novel therapeutic target in patients with CLL that may also be applicable in various autoimmune diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Leukemia Research Foundation New Investigator Award (J.A.B.), a Deutsche José Carreras Leukämiestiftung grant DJCLS R02/08 (J.A.B.), and a CLL Global Research Foundation grant (J.A.B.).
We are grateful to B. Weinhold, M. Henneberg, M. Krome, M. Quiroga, U. Habig-Buchwald, R. Fröhlich, M. Riedl, C. Ströhlein, and R. Lapushin for expert technical assistance, and to Dr K. Zirlik and Dr G. Zilow for providing CLL cell and control serum samples.
Authorship
Contribution: A.B. analyzed CXCR5 expression and function; M.N. performed RT-PCR for CXCL12 and CXCL13; A.S.-G. provided tissue samples and performed and analyzed immunohistochemistry; W.G.W. and M.J.K. provided patients' samples, analyzed data, and reviewed the paper; and J.A.B. designed the research, supervised the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan A. Burger, Department of Leukemia, Unit 428, The University of Texas M. D. Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: jaburger@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal