Nef is a multifunctional pathogenetic protein of HIV-1, the interaction of which with Hck, a Src tyrosine kinase highly expressed in macrophages, has been shown to be responsible for the development of AIDS. However, how the Nef-Hck interaction leads to the functional aberration of macrophages is poorly understood. We recently showed that Nef markedly inhibited the activity of macrophage colony-stimulating factor (M-CSF), a primary cytokine for macrophages. Here, we show that the inhibitory effect of Nef is due to the Hck-dependent down-regulation of the cell surface expression of M-CSF receptor Fms. In the presence of Hck, Nef induced the accumulation of an immature under–N-glycosylated Fms at the Golgi, thereby down-regulating Fms. The activation of Hck by the direct interaction with Nef was indispensable for the down-regulation. Unexpectedly, the accumulation of the active Hck at the Golgi where Nef prelocalized was likely to be another critical determinant of the function of Nef, because the expression of the constitutive-active forms of Hck alone did not fully down-regulate Fms. These results suggest that Nef perturbs the intracellular maturation and the trafficking of nascent Fms, through a unique mechanism that required both the activation of Hck and the aberrant spatial regulation of the active Hck.
Introduction
HIV-1 infections lead to the development of AIDS by causing progressive degeneration of the immune system.1,–3 The main cellular targets of HIV-1 are CD4+ T cells and macrophages, and the depletion of CD4+ T cells caused by an infection is suggested to account for many aspects of the pathogenesis of HIV-1.1,–3 Meanwhile, a number of studies have revealed the functional aberration of HIV-1–infected macrophages.4,5 Infected macrophages showed an altered profile of the production of cytokine/chemokines4 or migratory capacity,5 which might contribute to the uncontrolled homeostasis of the immune system. Indeed, functional analyses of HIV-1 Nef protein have revealed that macrophages as well as CD4+ T cells play an important role in the development of AIDS.
Nef is a 25- to 30-kDa protein with no enzymatic activity encoded by the HIV-1 genome.6,7 Studies of HIV-1–infected patients have clearly demonstrated Nef to be a critical determinant of the development of AIDS: HIV-1 strains without an intact Nef gene were frequently isolated from nonprogressive long-term survivors.8,9 Subsequent study of HIV-1 transgenic mice confirmed the pathogenetic activity of Nef: targeted expression of the entire coding sequence of HIV-1 in CD4+ T cells and macrophages caused a severe AIDS-like disease in mice, which was completely abolished by the disruption of the Nef gene.10 Importantly, only an amino acid substitution in the proline-rich (PxxP) motifs of Nef was sufficient to protect mice from the development of AIDS-like disease.11 A number of studies have revealed that Nef interacts with a subset of cellular Src family tyrosine kinases, via the PxxP motifs.12,,–15 The Nef PxxP motifs had an affinity for the Src homology (SH3) domain of Hck, Lyn, and possibly c-Src, but not of Fgr, Fyn, Lck, and Yes.12,,–15 In particular, the interaction between the Nef PxxP motifs and the Hck SH3 domain was likely to be important, because the interaction caused the activation of Hck.13,–15 Indeed, a study with HIV-1 transgenic mice clearly demonstrated the importance of the Nef-Hck interaction for the development of AIDS: the appearance of the AIDS-like disease was significantly delayed when the HIV-1 transgenic mice expressing an intact Nef gene were crossed with an hck−/− background.11 Given that Hck is expressed in macrophages but not in CD4+ T cells,16 the finding indicates that the Nef-Hck interaction in macrophages is at least in part responsible for the development of AIDS. However, little is known of the molecular mechanisms by which the Nef-Hck interaction contributes to the functional aberration of macrophages and the development of AIDS. The fact that Src kinases including Hck have both positive and negative roles in cell signaling pathways16,,–19 makes it difficult to predict the functional consequences of the Nef-Hck interaction.
A well-characterized function of Nef is the down-regulation of the cell surface expression of CD46,7,20 or major histocompatibility complex class I (MHC I).6,7,21,–23 Nef accelerates the endocytosis of CD4,20 the receptor for HIV-1,1,–3 which allows an efficient viral release from the host cells.6,7 Nef reduces the level of the surface expression of MHC I through multiple mechanisms,21,–23 which diminishes the recognition of the infected cells by cytotoxic T cells.6,7 However, these hallmark functions of Nef may not fully account for the functional significance of the Nef-Hck interaction, because the down-regulation of CD4 or MHC I occurs even in the absence of Hck (ie, in CD4+ T cells).20,,–23 Meanwhile, we and others have recently identified the functions of Nef that are dependent on Hck.24,–26 Drakesmith et al demonstrated that Nef down-regulated the surface expression of HFE, an iron homeostasis regulator expressed on macrophages, which was abolished by a dominant-negative Hck.24 Briggs et al demonstrated that Nef mimicked the cell growth–promoting activity of granulocyte-macrophage colony-stimulating factor (GM-CSF), a cytokine that supports the proliferation and differentiation of monocyte/macrophages,27 possibly through a mechanism that required Hck and the Stat3 transcription factor.25 Nef might contribute to the survival of macrophages by mimicking GM-CSF receptor pathways, allowing long-term viral replication.25 In contrast to the latter finding, we demonstrated that Nef inhibited the growth of human myeloid leukemia TF-1-fms cells mediated by macrophage colony-stimulating factor (M-CSF),26 another cytokine essential for the proliferation and differentiation of monocytes/macrophages.28 The growth inhibition of the cells correlated well with the impaired activation of the M-CSF receptor Fms,26 which is a tyrosine kinase encoded by the proto-oncogene c-fms.28 Impaired activation of Fms was also observed in human embryonic 293 cells coexpressing Nef and Hck, but not in cells expressing Nef alone or Hck alone.26 Thus, these data indicated that Nef inhibited the activation of Fms through a mechanism that required Hck.
The functions of macrophages are distinctly regulated by M-CSF and GM-CSF,27,28 as evidenced by the marked difference in the morphology of macrophages derived from these cytokines.29 Moreover, these macrophages showed different profiles of the production of chemokines/cytokines.29 Thus, it is possible that Nef affects the functions of macrophages by differently modulating the activities of M-CSF and GM-CSF, contributing to the uncontrolled immune system. However, little is known of the molecular mechanisms by which Nef differently modulates the activities of these cytokines, through the common target Hck. In this study, we therefore attempted to clarify how the Nef-Hck interaction caused the impaired activation of Fms.
Methods
Hematopoietic cell lines and culture conditions
Human myeloid leukemia TF-1 cells30 were maintained with RPMI1640 medium supplemented with 10% FCS and 2 ng/mL recombinant human GM-CSF (rhGM-CSF; PeproTech, Rocky Hill, NJ). TF-1-fms cells,31 which were obtained by introducing the plasmid pCEF-c-fms encoding the human c-fms gene into the TF-1 cells, were maintained with RPMI1640–10% FCS in the presence of 100 ng/mL rhM-CSF (a gift from Morinaga Milk Industry, Kanagawa, Japan) and 200 μg/mL G418 (Calbiochem, Darmstadt, Germany). TF-1-fms-Nef-ER cells26 were obtained by introducing pEBB-Nef-ER-IRES-puro32 into TF-1-fms cells, and maintained in the presence of rhM-CSF, G418, and 1.5 μg/mL puromycin (Sigma, St Louis, MO). The plasmid encoded the Nef-ER fusion protein composed of Nef (derived from the NL4–3 strain of HIV-1) and the hormone-binding domain of the murine estrogen receptor (ER).32 In this system, Nef was basally inactive but it was induced to function by the estrogen analog, 4-hydroxytamoxifen (4-HT; Sigma).32 We also established TF-1 cells expressing the Nef-ER fusion protein (TF-1-Nef-ER) by using the same plasmid. The transfection was performed with Lipofectin reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's recommendations. Transfected cells were selected in media containing rhGM-CSF and puromycin, followed by limiting dilution to isolate stable clones. The expression of Nef-ER in these clones was determined by Western blotting26 with anti-Nef rabbit antiserum obtained through the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD).33 The cell growth was determined by colorimetric assay with MTT reagent (Sigma), and the absorbance of each culture was measured at 595 nm with a microplate reader (Thermo Electron, Vantaa, Finland). The expression of Fms on TF-1-fms-Nef-ER cells and that of GM-CSF receptors on TF-1-Nef-ER cells was analyzed on a FACSCalibur using Cell Quest Software (Becton Dickinson, Mountain View, CA).26 Anti-Fms rat monoclonal IgG (clone 12-2D6; Zymed, South San Francisco, CA) was labeled with FITC using Fluorescein Labeling Kit-NH2 (Dojindo, Kumamoto, Japan). FITC-labeled anti–GM-CSF receptor α chain (clone 4H1) and PE-labeled anti–GM-CSF β chain (clone 1C1) were purchased from eBioscience (San Diego, CA).
Macrophages and nucleofection
Human peripheral blood samples were collected from adults donors after informed consent was obtained in accordance with the Declaration of Helsinki and based on a protocol approved by the Institutional Review Board of the Faculty of Medical and Pharmaceutical Sciences, Kumamoto University. Monocytes were enriched from peripheral blood mononuclear cells by adherence to dishes for 1 hour. Macrophages were prepared by culturing the monocytes with RPMI1640 medium supplemented with 15% FCS and 100 ng/mL rhM-CSF for 5 to 7 days. The nucleofection with the Human Macrophage Nucleofector Kit and the Nucleofector II device (Amaxa, Cologne, Germany) was performed according to the manufacturer's recommendations. In brief, 5 × 105 macrophages were nucleofected with 5 μg plasmid and then cultured with Macrophage-SFM medium (Gibco, Grand Island, NY) supplemented with 15% FCS and 10 ng/mL rhGM-CSF for 8 to 12 hours. The nucleofected macrophages were cultured with GM-CSF, because M-CSF caused the down-regulation of Fms (Figure S2B,C). To identify the Nef-expressing macrophages, we used the pRc/CMV-CD8-Nef plasmid34 encoding Nef (derived from the SF2 strain of HIV-1) fused to the extracellular/transmembrane regions of CD8. As a control, we used the plasmid encoding only those regions of CD8 (pRc/CMV-CD8).34 The nucleofected macrophages were detached from the culture dishes using the enzyme-free cell dissociation buffer (Gibco), and then subjected to flow cytometric analysis on a FACSCalibur. Labeled antibodies used were PE-labeled anti-Fms (clone 3-4A4; Santa Cruz Biotechnology, Santa Cruz, CA), APC-labeled anti-CD8 (clone DK25; Dako, Glostrup, Denmark), and PE-labeled anti-CD4 (clone S3.5; Caltag, Burlingame, CA).
293 cell lines, transfection, and plasmids
Human embryonic kidney 293 cells (Invitrogen) were maintained with DME medium supplemented with 10% FCS. We also used 293 cells stably expressing Fms, both Fms and Hck, or CD4. 293-Fms cells were established by transfecting pCEF-c-fms31 followed by the enrichment of Fmshigh cells with a JSAN cell sorter (Bay bioscience, Kobe, Japan). 293-Fms/Hck cells were established by further transfecting a human Hck expression plasmid into the 293-Fms cells. For this purpose, Hck cDNA35 cloned in the vector pIRES2-EGFP (Clontech, Mountain View, CA) was subcloned into pIRES-bleo3 (Clontech). An Hckhigh clone was isolated from the transfected cells by Western blotting. 293-CD4 cells were established by transfecting pEneoMOS-CD436 followed by the enrichment of CD4high cells by the sorting. These cells were maintained with media containing 200 μg/mL G418 or 200 μg/mL phleomycin D1 (Invitrogen), or both. Transient transfection experiments with these 293 cell lines were performed essentially as described previously.26 In brief, cells grown on a 12-well tissue culture plate were transfected with a total of 1.6 μg plasmid using LipofectAMINE2000 reagent (Invitrogen).
The transient expression of Fms was achieved with pCEF-c-fms. The transient expression of Hck was mostly achieved with Hck cDNA cloned in pcDNA3.1 (Invitrogen), except for the flow cytometric analysis in which Hck cDNA cloned in pIRES2-EGFP was used (Figure 2A). Based on an earlier report,14 we also prepared constitutive-active (YF and AxxA) and kinase-dead (KE) forms of Hck by using QuikChange II Site-Directed Mutagenesis Kits (Stratagene, La Jolla, CA). The transient expression of Nef was achieved mostly with pRc/CMV-CD8-Nef,34 the Nef of which was derived from the SF2 strain of HIV-1. In a selected experiment (Figure 4A), we used Nef of the NL4-3 strain, as the mutants used in the analysis were derived from the strain. WL/AA, LL/AA, and AxxA mutants were provided by A. Adachi (University of Tokushima, Tokushima, Japan) and subcloned into the vector pRc/CMV-CD8. The M20A mutant37 was also subcloned into this vector.
Western blotting, flow cytometry, and immunofluorescence with 293 cells
The preparation of total cell lysate and Western blotting were performed essentially as described.26,38 In a selected experiment (Figure 2C), a monolayer of transfected 293 cells was treated with trypsin or control PBS buffer for 3 minutes at room temperature immediately prior to the lysis. Total cell lysate was also subjected to a lectin pull-down assay,39 using wheat germ agglutinin (WGA)–agarose and concanavalin A (Con A)–agarose (both from Wako, Osaka, Japan). Alternatively, total cell lysate was treated with either endo-β-N-acetylglucosaminidase H (Endo-H) or peptide-N-glycosidase F (PNGase F) (both from Roche, Mannheim, Germany), according to the manufacturer's recommendations. Primary antibodies used were as follows: anti–N-terminal portion of Fms (H-300; Santa Cruz Biotechnology), anti–C-terminal portion of Fms (C-20; Santa Cruz Biotechnology), anti-Nef rabbit antiserum,33 anti-Hck (clone 18; Transduction Laboratories, Lexington, KY), and anti-ERK (K-23; Santa Cruz Biotechnology).
The transfected cells were detached from the culture dishes and subjected to a flow cytometric analysis with anti–Fms-PE, anti–CD4-PE, or anti–CD8-APC as above. For immunostaining, cells were directly fixed in 2% paraformaldehyde, permeabilized with ethanol, and stained with primary antibodies for 12 hours followed by labeled secondary antibodies.40,41 The primary antibodies used were as follows: anti-Fms rat IgG (clone 3-4A4-E4; Abcam, Cambridge, MA), anti-GM130 mouse IgG (Transduction Laboratories), anti-CD8 rabbit IgG (H-160; Santa Cruz Biotechnology), and rabbit IgG specific for Hck phosphorylated at Tyr411 (Santa Cruz Biotechnology). The labeled secondary antibodies used were as follows: anti–rat IgG-AlexaFluo488, anti–mouse IgG-AlexaFluo568, and anti–rabbit IgG-AlexaFluo488 (Molecular Probes, Eugene, OR). Nuclei were stained with DAPI (Molecular Probes). The fluorescent signals were visualized with a BZ-8000 fluorescence microscope (Keyence, Osaka, Japan) equipped with Plan-Fluor ELWD 20×/0.45 objective lenses (Nikon, Tokyo, Japan). Image processing was performed using BZ-Analyzer (Keyence) and Adobe Photoshop software (Adobe Systems, San Jose, CA).
Results
Nef selectively inhibits M-CSF–dependent growth and down-regulates Fms
In this study, we initially attempted to confirm the stimulatory effect of Nef on GM-CSF reported by another group,25 using the same system in which we found the inhibitory effect on M-CSF.26 We previously established human myeloid TF-1-fms cells expressing a conditionally active Nef-ER fusion protein.26,32 Although TF-1-fms was an M-CSF–dependent clone derived from GM-CSF–dependent TF-1 cells,30,31 TF-1-fms cells lost their growth response to GM-CSF due to long-term maintenance with M-CSF.42 Thus, we also established TF-1 clones expressing the Nef-ER fusion proteins, the level of which was comparable with that in the pre-established TF-1-fms-Nef-ER clone (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). The inducible activation of Nef by the estrogen analog 4-HT was verified by the down-regulation of CD4 expression (data not shown). As shown (Figure S1A,B) and consistent with the results of the other group,25 the activation of Nef did not inhibit but enhanced the GM-CSF–dependent growth of TF-1-Nef-ER cells, albeit slightly. However, the activation of Nef markedly inhibited the M-CSF–dependent growth of TF-1-fms-Nef-ER cells (Figure S1A,C). These results confirmed that Nef did not actively induce the death of these cells but selectively inhibited the activity of M-CSF.
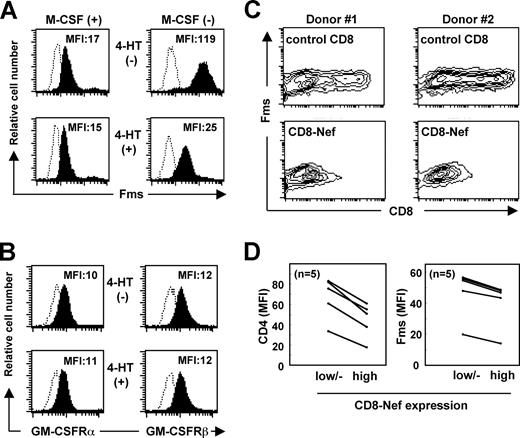
Next, we carefully examined whether Nef down-regulated the surface expression of Fms, as a possible mechanism for the selective inhibitory effect of Nef on the activity of M-CSF. In a previous study in which TF-1-fms-Nef-ER cells cultured under M-CSF–containing conditions were used, we failed to observe an obvious down-regulation of Fms expression by Nef.26 However, the effect of Nef might have been underestimated under such conditions, because M-CSF itself down-regulated the expression by inducing the internalization/degradation of Fms.43 Indeed, the addition of M-CSF caused the down-regulation of Fms in both TF-1-fms-Nef-ER cells (Figure S2A) and primary macrophages (Figure S2B) in a dose-dependent manner and an obvious effect of Nef on the surface level of Fms was not detected under such conditions (Figure 1A left panels). However, under the M-CSF–free Fms-high conditions, a significant reduction in the surface expression of Fms was observed in the Nef-active TF-1-fms-Nef-ER cells (Figure 1A right panels). The surface expression of CD29 (integrin β1), CD33, and CD54 (ICAM-1) was unaffected by the same treatment (data not shown). Furthermore, such down-regulation was not observed with the α chain and β chain of GM-CSF receptors (Figure 1B). Thus, the inhibitory effect of Nef on the activity of M-CSF but not of GM-CSF was likely to be due to the selective down-regulation of Fms expression.
Nef inhibits surface expression of Fms. (A) In the left histograms, TF-1-fms-Nef-ER cells were precultured with M-CSF–containing media in the absence (upper) or presence (lower) of 0.1 mM 4-HT for 24 hours. In the right histograms, TF-1-fms-Nef-ER cells were precultured with M-CSF–free media in the absence (top) or presence (bottom) of 0.1 μM 4-HT for 12 hours. The expression of Fms on these cells was analyzed by flow cytometry with PE-labeled anti-Fms antibody. The mean fluorescence intensity (MFI) of Fms expression is indicated. (B) TF-1-Nef ER cells were precultured with GM-CSF–free media in the absence (top) or presence (bottom) of 0.1 μM 4-HT for 12 hours. The surface expression of GM-CSF receptors was analyzed with FITC-labeled anti-α chain (left) and PE-labeled anti-β chain (right) antibodies. The MFI of GM-CSF receptor expression is indicated. (C) Macrophages were nucleofected with the control CD8 plasmid or CD8-Nef plasmid and then costained with APC-labeled anti-CD8 and PE-labeled anti-Fms. Results with macrophages obtained from 2 different donors are shown as contour plots. (D) As in panel C, the nucleofected macrophages were costained with APC-labeled anti-CD8 and PE-labeled anti-Fms, or with APC-labeled anti-CD8 and PE-labeled anti-CD4. The MFI of the expression of Fms or CD4 in the populations of CD8low/−, CD8high, CD8-Neflow/−, or CD8-Nefhigh was analyzed. The results with macrophages obtained from 5 different donors are summarized.
Nef inhibits surface expression of Fms. (A) In the left histograms, TF-1-fms-Nef-ER cells were precultured with M-CSF–containing media in the absence (upper) or presence (lower) of 0.1 mM 4-HT for 24 hours. In the right histograms, TF-1-fms-Nef-ER cells were precultured with M-CSF–free media in the absence (top) or presence (bottom) of 0.1 μM 4-HT for 12 hours. The expression of Fms on these cells was analyzed by flow cytometry with PE-labeled anti-Fms antibody. The mean fluorescence intensity (MFI) of Fms expression is indicated. (B) TF-1-Nef ER cells were precultured with GM-CSF–free media in the absence (top) or presence (bottom) of 0.1 μM 4-HT for 12 hours. The surface expression of GM-CSF receptors was analyzed with FITC-labeled anti-α chain (left) and PE-labeled anti-β chain (right) antibodies. The MFI of GM-CSF receptor expression is indicated. (C) Macrophages were nucleofected with the control CD8 plasmid or CD8-Nef plasmid and then costained with APC-labeled anti-CD8 and PE-labeled anti-Fms. Results with macrophages obtained from 2 different donors are shown as contour plots. (D) As in panel C, the nucleofected macrophages were costained with APC-labeled anti-CD8 and PE-labeled anti-Fms, or with APC-labeled anti-CD8 and PE-labeled anti-CD4. The MFI of the expression of Fms or CD4 in the populations of CD8low/−, CD8high, CD8-Neflow/−, or CD8-Nefhigh was analyzed. The results with macrophages obtained from 5 different donors are summarized.
The novel function of Nef was further confirmed by nucleofecting Nef into human primary macrophages (Figure 1C,D). The purity of the macrophage preparations was usually more than 95% and 85% when assessed by the expression of CD14 and Fms, respectively (Figure S3). We used the CD8-Nef plasmid encoding Nef fused to the extracellular/transmembrane regions of CD834 to identify Nef-positive macrophages. The nucleofection of the control CD8 plasmid encoding only those regions of CD8 did not affect the expression of Fms (Figure 1C “control CD8” panels). In contrast, in the CD8-Nef–nucleofected macrophages, the Fmshigh population was reduced as the expression of CD8-Nef increased (Figure 1C “CD8-Nef” panels). Such down-regulation of Fms as well as CD4 in the CD8-Nefhigh population was reproducibly observed with macrophages derived from different donors (Figure 1D). The supernatant obtained from macrophages nucleofected with the CD8-Nef plasmid did not affect the level of Fms in TF-1-fms cells (data not shown), suggesting that production of M-CSF, if any occurred, was not involved in the Nef-induced down-regulation of Fms in macrophages.
Down-regulation of Fms by Nef is Hck-dependent and due to inhibition of intracellular maturation/trafficking of Fms
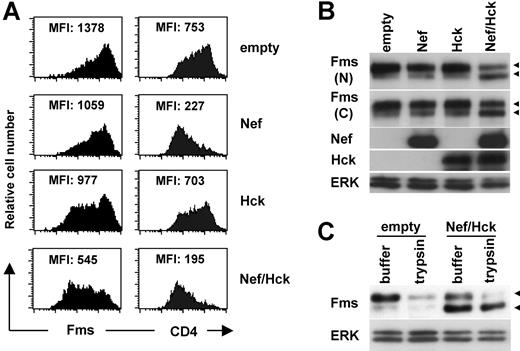
As both TF-1-fms cells26 and macrophages16 endogenously expressed Hck, it was possible that Hck was involved in the down-regulation of Fms caused by Nef. To examine this possibility and clarify the molecular mechanisms by which Nef down-regulated Fms, we next performed a transfection experiment using human 293 cells. As shown (Figure 2A left panels), the cotransfection of Nef and Hck markedly reduced the surface expression of Fms, although the transfection of Nef alone or Hck alone was effective to a certain degree. This was in contrast with the finding that the transfection of Nef alone was almost sufficient to reduce the surface expression of CD4 (Figure 2A right panels). The reduced surface expression of Fms was confirmed by Western blotting. As shown (Figure 2B), the amount of Fms species with a molecular weight of 150 kDa (gp150Fms, upper arrowhead) in the cells coexpressing Nef/Hck was obviously less than that in cells expressing Nef alone or Hck alone. Indeed, gp150Fms was the cell surface form of Fms, because the treatment of the cell surface with trypsin resulted in the loss of gp150Fms (Figure 2C). The trypsin-resistant gp150Fms might represent an intracellular pool of mature Fms that would be rapidly inserted into the plasma membrane. Interestingly, in parallel with the decrease in the expression of gp150Fms, an increase in the expression of a lower molecular weight species (130 kDa, lower arrowheads) was observed in the cells coexpressing Nef/Hck (Figure 2C). The 130-kDa species was a Fms-related product, because the 2 antibodies against the different portions of Fms (the N-terminus and C-terminus) detected the species (herein referred to as gp130Fms). In contrast to gp150Fms, gp130Fms was an intracellular form of Fms, because it was unaffected by the trypsin treatment (Figure 2C). Thus, the down-regulation of Fms observed in TF-1-fms-Nef-ER cells and macrophages was reproducible in 293 cells cotransfected with Nef and Hck, and associated with the increase of the intracellular gp130Fms.
Nef reduces surface expression of Fms in 293 cells and increases intracellular gp130Fms, in the presence of Hck. (A) In the left panels, parental 293 cells were transfected with the Fms plasmid, alone or in combination with the plasmid for Nef (CD8-Nef) or Hck (IRES-EGFP), and then stained with PE-labeled anti-Fms. In the right panels, 293 cells stably expressing CD4 were transfected with the indicated plasmids and stained with PE-labeled anti-CD4. These cells were costained with APC-labeled anti-CD8, and the data for cells positive for both CD8 and EGFP are shown. The MFI of the expression of Fms or CD4 is indicated. (B) As in panel A, parental 293 cells were transfected with the Fms plasmid, alone or in combination with the plasmid for Nef or Hck. Total cell lysate was prepared and subjected to Western blotting with antibodies against the N-terminal portion of Fms (N), the C-terminal portion of Fms (C), Hck, Nef, or ERK. (C) 293 cells stably expressing Fms were cotransfected with Nef and Hck (Nef/Hck), or transfected with empty vectors (empty), and then treated with trypsin or control buffer. Total cell lysate was prepared and subjected to Western blotting with antibodies against the C-terminal portion of Fms or ERK. (B,C) The ERK blot is a loading control. The ◀ indicate the position of gp150Fms or gp130Fms.
Nef reduces surface expression of Fms in 293 cells and increases intracellular gp130Fms, in the presence of Hck. (A) In the left panels, parental 293 cells were transfected with the Fms plasmid, alone or in combination with the plasmid for Nef (CD8-Nef) or Hck (IRES-EGFP), and then stained with PE-labeled anti-Fms. In the right panels, 293 cells stably expressing CD4 were transfected with the indicated plasmids and stained with PE-labeled anti-CD4. These cells were costained with APC-labeled anti-CD8, and the data for cells positive for both CD8 and EGFP are shown. The MFI of the expression of Fms or CD4 is indicated. (B) As in panel A, parental 293 cells were transfected with the Fms plasmid, alone or in combination with the plasmid for Nef or Hck. Total cell lysate was prepared and subjected to Western blotting with antibodies against the N-terminal portion of Fms (N), the C-terminal portion of Fms (C), Hck, Nef, or ERK. (C) 293 cells stably expressing Fms were cotransfected with Nef and Hck (Nef/Hck), or transfected with empty vectors (empty), and then treated with trypsin or control buffer. Total cell lysate was prepared and subjected to Western blotting with antibodies against the C-terminal portion of Fms or ERK. (B,C) The ERK blot is a loading control. The ◀ indicate the position of gp150Fms or gp130Fms.
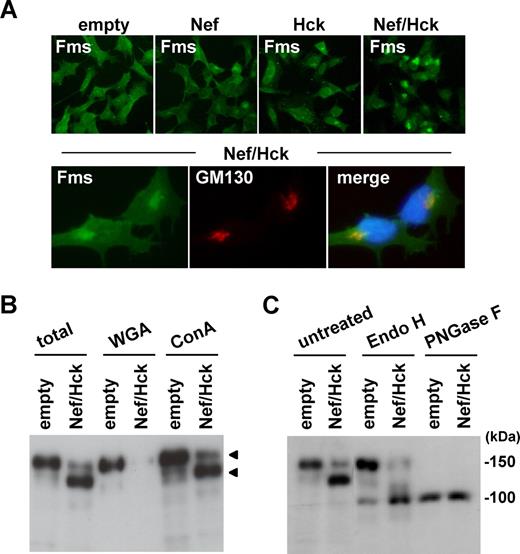
To further characterize the intracellular gp130Fms that appeared in the cells coexpressing Nef/Hck, we next performed immunofluorescence microscopy. As shown (Figure 3A top panels; Figure S4 top panels), the pattern of Fms staining in the coexpressing cells was quite different from that in cells expressing Nef alone or Hck alone. In a significant proportion of the cells coexpressing Nef/Hck, intense staining of Fms was detected in a perinuclear compartment (Figure 3A top Nef/Hck panel), which largely overlapped the signal for GM130, a marker for the Golgi apparatus44 (Figure 3A bottom panels) and that for Vti1a, another Golgi marker45 (Figure S4). Such intense staining of Fms in the perinuclear compartment was also detected in a few cells transfected with Nef alone or Hck alone (Figure 4A; Figure S4), which overlapped the signal for GM130 (Figure S4). Thus, it was highly likely that gp130Fms appeared in the coexpressing cells predominantly localized to the Golgi. As the N-glycosylation of many glycoproteins including Fms is known to be intimately linked with intracellular trafficking,46,,,–50 we then analyzed the state of the N-glycosylation of gp130Fms. For this purpose, we used 2 lectins, WGA and Con A, which recognize sialic acid and mannose, respectively.39 As shown (Figure 3B), both gp150Fms and gp130Fms bound to Con A, whereas only gp150Fms bound to WGA, indicating that gp150Fms was modified with both mannose and sialic acid, but gp130Fms was not modified with sialic acid. Indeed, gp150Fms and gp130Fms showed similar electrophoretic mobility following the complete digestion of oligosaccharide groups by PNGase F, whereas only gp130Fms was sensitive to Endo-H, which selectively cleaves high-mannose type oligosaccharides (Figure 3C). These results suggested that the difference in their sizes was due to a difference in the N-glycosylation. Given that nascent Fms polypeptides are modified initially with mannose at the endoplasmic reticulum and terminally with sialic acid at the Golgi,46,,–49 our results strongly suggested that the Nef/Hck-dependent accumulation of gp130Fms at the Golgi was due to the perturbation of intracellular N-glycosylation and/or trafficking of nascent Fms.
gp130Fms appearing in 293 cells coexpressing Nef/Hck is Golgi-localized underglycosylated Fms. (A) 293 cells stably expressing Fms were transfected with the control CD8 plasmid (empty) or CD8-Nef plasmid (Nef). Similarly, 293 cells stably coexpressing Fms and Hck were transfected with the control CD8 plasmid (Hck) or CD8-Nef plasmid (Nef/Hck). These cells were stained with anti-Fms antibody (top panels). In the bottom panels, 293 cells stably coexpressing Fms and Hck were transfected with CD8-Nef and costained with anti-Fms antibody (green), anti-GM130 antibody (red), and DAPI (blue). (B) 293 cells stably expressing Fms were cotransfected with Nef and Hck (Nef/Hck), or transfected with empty vectors (empty). The total cell lysate was subjected to Fms Western blotting directly (total) or after pull down with WGA-agarose or Con A–agarose. The arrowheads indicate the position of gp150Fms or gp130Fms. (C) Total cell lysate prepared as in panel B was subjected to Fms Western blotting directly (untreated) or after treatment with endo-β-N-acetylglucosaminidase H (Endo H) or peptide-N-glycosidase F (PNGase F).
gp130Fms appearing in 293 cells coexpressing Nef/Hck is Golgi-localized underglycosylated Fms. (A) 293 cells stably expressing Fms were transfected with the control CD8 plasmid (empty) or CD8-Nef plasmid (Nef). Similarly, 293 cells stably coexpressing Fms and Hck were transfected with the control CD8 plasmid (Hck) or CD8-Nef plasmid (Nef/Hck). These cells were stained with anti-Fms antibody (top panels). In the bottom panels, 293 cells stably coexpressing Fms and Hck were transfected with CD8-Nef and costained with anti-Fms antibody (green), anti-GM130 antibody (red), and DAPI (blue). (B) 293 cells stably expressing Fms were cotransfected with Nef and Hck (Nef/Hck), or transfected with empty vectors (empty). The total cell lysate was subjected to Fms Western blotting directly (total) or after pull down with WGA-agarose or Con A–agarose. The arrowheads indicate the position of gp150Fms or gp130Fms. (C) Total cell lysate prepared as in panel B was subjected to Fms Western blotting directly (untreated) or after treatment with endo-β-N-acetylglucosaminidase H (Endo H) or peptide-N-glycosidase F (PNGase F).
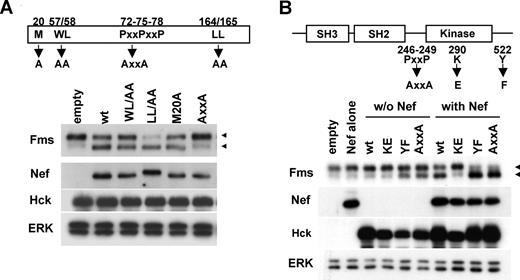
Activation of Hck by Nef is essential but not sufficient for accumulation of gp130Fms. (A) The Nef mutants used (M20A, WL/AA, AxxA, and LL/AA) are schematically shown. All the constructs are CD8-Nef chimeras. 293 cells stably expressing Fms were cotransfected with wild-type Hck and the plasmid indicated, and then analyzed for the expression of Fms, Nef, Hck, or ERK by Western blotting. (B) Schematic representations of Hck and the mutants used. KE is the kinase-dead form, whereas AxxA and YF are the constitutive-active forms.14 293 cells stably expressing Fms were transfected with empty vectors (empty), Nef plasmid (Nef), or the indicated Hck plasmid (“w/o Nef” lanes), or cotransfected with wild-type Nef and the indicated Hck plasmid (“with Nef” lanes). Then, the transfected cells were analyzed for the expression of Fms, Nef, Hck, or ERK by Western blotting. (A,B) The ERK blot is a loading control. The ◀ indicate the position of gp150Fms or gp130Fms.
Activation of Hck by Nef is essential but not sufficient for accumulation of gp130Fms. (A) The Nef mutants used (M20A, WL/AA, AxxA, and LL/AA) are schematically shown. All the constructs are CD8-Nef chimeras. 293 cells stably expressing Fms were cotransfected with wild-type Hck and the plasmid indicated, and then analyzed for the expression of Fms, Nef, Hck, or ERK by Western blotting. (B) Schematic representations of Hck and the mutants used. KE is the kinase-dead form, whereas AxxA and YF are the constitutive-active forms.14 293 cells stably expressing Fms were transfected with empty vectors (empty), Nef plasmid (Nef), or the indicated Hck plasmid (“w/o Nef” lanes), or cotransfected with wild-type Nef and the indicated Hck plasmid (“with Nef” lanes). Then, the transfected cells were analyzed for the expression of Fms, Nef, Hck, or ERK by Western blotting. (A,B) The ERK blot is a loading control. The ◀ indicate the position of gp150Fms or gp130Fms.
Down-regulation of Fms by Nef is dependent on activation of Hck and spatial regulation of active Hck
We next attempted to clarify the role of Hck in the down-regulation of Fms expression by Nef. Initially, we examined whether the direct interaction with Hck (Figure S5) was required for the function of Nef, using Nef mutants. As shown (Figure 4A), the AxxA mutant defective in the interaction with Hck12 failed to down-regulate Fms, that is, the decrease of gp150Fms and the concomitant increase of gp130Fms. In contrast, the other 3 mutants still down-regulated Fms (Figure 4A). The WL/AA and LL/AA mutants, and the M20A mutant were shown to be defective in the down-regulation of CD4 and MHC I, respectively,24,37 which was confirmed in our experimental system (data not shown). These results suggested that the down-regulation of Fms by Nef was mechanistically different from that of CD4 or MHC I, and dependent on the direct interaction with Hck. Thus, we next examined whether the activation of Hck by Nef was necessary and sufficient for the down-regulation of Fms, using Hck mutants. As shown (Figure 4B), Nef failed to down-regulate Fms when cotransfected with the kinase-dead KE mutant, but almost completely down-regulated Fms when cotransfected with the YF or AxxA mutant, both of which were the constitutive-active form. However, it should be noted that the transfection of these constitutive-active forms of Hck alone was not necessarily sufficient to achieve the full down-regulation of Fms (Figure 4B, see YF, AxxA, wt + Nef, YF + Nef, and AxxA + Nef lanes). These results clearly indicated that the activation of Hck was necessary but not sufficient for the Nef/Hck-induced down-regulation of Fms.
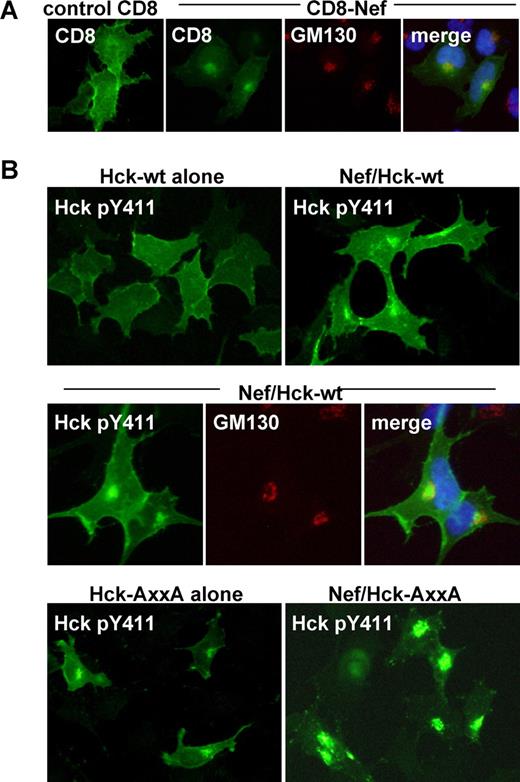
It has been shown that Nef distributes to the Golgi as well as the plasma membrane.22,24 Indeed, intense signal of the CD8-Nef chimera was detected in the perinuclear compartment, which overlapped the signal for GM130 (Figure 5A). Thus, it was possible that the activation of Hck at the Golgi or the recruitment of the active Hck to the Golgi was another factor necessary for Nef to down-regulate Fms. To explore this possibility, we examined whether the active Hck in the Nef-expressing cells indeed localized to the Golgi and its existence at the Golgi correlated with the down-regulation of Fms. To detect the active Hck, we stained cells with the antibody specific for Hck phosphorylated at Tyr411, which was the major autophosphorylation site.14 As shown (Figure 5B), an intense signal for the active Hck was indeed detected in the perinuclear compartment, in cells coexpressing Nef and wild-type Hck but not in cells expressing wild-type Hck alone (top panels), which largely overlapped the signal for GM130 (middle panels). Such colocalization of Nef and active Hck in the perinuclear compartment was also observed in macrophages nucleofected with the CD8-Nef plasmid (Figure S6). Moreover, the constitutive-active AxxA Hck tended to localize to the perinuclear compartment when expressed alone, and almost exclusively localized to the perinuclear compartment when coexpressed with Nef (Figure 5B bottom panels). Thus, the degree to which the active Hck accumulated at the Golgi correlated well with the observed down-regulation of Fms (Figure 4B). Taken together, these results suggest that the novel function of Nef (ie, the down-regulation of Fms expression by perturbing the maturation/trafficking of nascent Fms) is dependent on both the activation of Hck and the spatial regulation of the active Hck.
Nef induces Golgi localization of active Hck. (A) Parental 293 cells were transfected with the control CD8 plasmid or CD8-Nef plasmid, and then stained with anti-CD8 antibody (green), anti-GM130 antibody (red), or DAPI (blue). (B) In the top panels, parental 293 cells were transfected with wild-type Hck, or cotransfected with wild-type Hck and Nef, and then stained with the antibody specific for active Hck (ie, Hck phosphorylated at Tyr411). In the middle panels, parental 293 cells cotransfected with wild-type Hck and Nef were costained with anti-Hck pTyr411 antibody (green), anti-GM130 antibody (red), and DAPI (blue). In the bottom panels, parental 293 cells were transfected with the constitutive-active AxxA Hck (see Figure 4B), or cotransfected with the AxxA Hck and wild-type Nef, and then stained with anti-Hck pTyr411 antibody. See “Western blotting, flow cytometry, and immunofluorescence with 293 cells” for image acquisition information.
Nef induces Golgi localization of active Hck. (A) Parental 293 cells were transfected with the control CD8 plasmid or CD8-Nef plasmid, and then stained with anti-CD8 antibody (green), anti-GM130 antibody (red), or DAPI (blue). (B) In the top panels, parental 293 cells were transfected with wild-type Hck, or cotransfected with wild-type Hck and Nef, and then stained with the antibody specific for active Hck (ie, Hck phosphorylated at Tyr411). In the middle panels, parental 293 cells cotransfected with wild-type Hck and Nef were costained with anti-Hck pTyr411 antibody (green), anti-GM130 antibody (red), and DAPI (blue). In the bottom panels, parental 293 cells were transfected with the constitutive-active AxxA Hck (see Figure 4B), or cotransfected with the AxxA Hck and wild-type Nef, and then stained with anti-Hck pTyr411 antibody. See “Western blotting, flow cytometry, and immunofluorescence with 293 cells” for image acquisition information.
Discussion
In this study, we showed for the first time that Nef down-regulated the expression of Fms (Figures 1,2). The down-regulation was due to perturbation of the intracellular trafficking of nascent Fms (Figure 3), and likely to be a cause of the inhibitory effect of Nef on the activity of M-CSF because neither the activity of GM-CSF nor the cell surface expression of GM-CSF receptors was inhibited by Nef (Figure 1). Importantly, the present study strongly suggested that the down-regulation of Fms expression by Nef was due to a previously unreported mechanism that depended on both the activation of Hck and the aberrant spatial regulation of the active Hck (Figures 4,5).
The Nef-induced down-regulation of Fms was obviously mechanistically different from that of CD4 or MHC I in its dependence on Hck (Figures 2A,3A)6,7,20,,–23 but appeared to resemble that of HFE. The Nef-induced down-regulation of HFE was abolished by either a mutation in the PxxP motifs of Nef or the overexpression of the dominant-negative Hck.24 However, how Hck was involved in the Nef-induced down-regulation of HFE remains to be analyzed.24 Interestingly, the YxxA motif in the cytoplasmic tail of HFE (342YVLA) was shown to be required for Nef to down-regulate HFE.24 The tyrosine-based YxxA motif was conserved in the kinase domain of Fms (873YQMA, GenBank accession number P07333). However, when coexpressed with Hck, Nef also down-regulated a Fms mutant lacking the motif prepared by introducing the stop codon at 873Y (data not shown). Thus, the mechanism for the Nef/Hck-induced down-regulation of Fms was likely to be somewhat different from that of HFE. Our earlier experiment revealed that gp130Fms was tyrosine phosphorylated in cells coexpressing Nef and Hck.26 However, the ligand-independent tyrosine phosphorylation of Fms was not a direct cause of the down-regulation of Fms, because Nef also down-regulated a Fms mutant lacking the entire intracellular region when coexpressed with Hck (Figure S7).
The Nef/Hck-induced down-regulation of Fms was associated with the accumulation of the immature Fms at the Golgi (Figure 3). The experiment with Hck mutants clearly demonstrated that the activation of Hck was indispensable for the down-regulation of Fms (Figure 4B). The finding that Nef failed to down-regulate Fms when coexpressed with Lyn or Fgr (data not shown) further supported the conclusion, because Hck was the only Src kinase activated by Nef among Src kinases highly expressed in macrophages (ie, Hck, Lyn, and Fgr).13,,–16 However, to our surprise, the activation of Hck was not the sole determinant of the down-regulation of Fms, because the expression of the constitutive-active Hck (YF or AxxA) alone was insufficient to fully achieve the down-regulation (Figure 4B). Our finding that the degree to which the active Hck accumulated at the Golgi correlated well with that of the down-regulation of Fms (Figures 4B,5B) strongly suggested that Nef down-regulated Fms through both the activation of Hck and the accumulation of the active Hck at the Golgi. The idea may answer why Hck, the downstream effector molecule important for the Fms signaling pathways,38,50,,–53 is involved in the down-regulation of Fms by Nef.
A significant pool of Nef has been shown to localize to the Golgi.22,24 Indeed, the CD8-Nef chimera used in this study localized to the Golgi as well as the plasma membrane (Figure 5A). This was not due to the fusion of the region of CD8 to the N-terminus of Nef, because the Nef-EGFP chimera, in which EGFP was fused to the C-terminus of Nef, also localized to the Golgi (data not shown). Thus, it was likely that the interaction with the Golgi-resident Nef or the recruitment of the active Hck led to the accumulation of the active Hck at the Golgi. However, it is unclear how this accumulation leads to a block of the intracellular trafficking of Fms in the same compartment. A plausible possibility might be direct interaction of the active Hck with Fms at the Golgi. Indeed, our earlier coimmunoprecipitation experiment revealed the formation of a molecular complex between Hck and Fms.26 Meanwhile, it is known that the tyrosine located in the juxtamembrane domain of Fms (Y561 in human and Y559 in murine) serves as a binding site for Src kinases including Hck when the residue is autophosphorylated.51,,–54 However, when coexpressed with Hck, Nef also down-regulated a Fms mutant in which the tyrosine residue was replaced with phenylalanine (data not shown). Thus, the active Hck at the Golgi may interact with Fms via unidentified site(s) or form complexes with Fms indirectly. Another possibility might be an alteration of the Golgi structure caused by the accumulation of the active Hck at the compartment. Recent studies revealed that Src kinases including Hck were present on the Golgi membrane as well as the plasma membrane.55,–57 The importance of the Golgi-localized Src kinases for the maintenance of the Golgi structure was clearly demonstrated by the finding that SYF fibroblasts lacking the 3 ubiquitous Src kinases (Src, Yes, and Fyn) exhibited an aberrant morphology of the Golgi with collapsed stacks and bloated cisternae.58 Interestingly, it was also demonstrated that the exogenous expression of the constitutive-active Src (E378G) in the SYF cells affected the distribution of some if not all Golgi-specific proteins.58 Thus, it is possible that the accumulation of the active Hck affects the structure of the Golgi and thereby perturbs the trafficking of Fms.
A study with HIV-1 transgenic mice has clearly proved the importance of the interaction of Nef with Hck in macrophages for the development of AIDS.11 Nevertheless, the functional consequences of the Nef/Hck interaction are not fully understood. The activation of Hck induced by the direct interaction with Nef is basically thought to cause the activation of macrophages, which may favor the replication of HIV-1. Indeed, Komuro et al demonstrated that the expression of Hck at a high level in macrophages correlated well with high titer replication of HIV-1.59 Moreover, Briggs et al raised the possibility that the Nef-Hck interaction caused the activation of the Stat3 transcription factor, thereby mimicking the signaling pathway of the GM-CSF receptor.25 However, the present study revealed that the Nef/Hck interaction also played a negative role: the molecular interaction caused the down-regulation of Fms and inhibition of the activity of M-CSF, which is likely to be due to the aberrant spatial regulation of the active Hck. The differential modulation of the activities of GM-CSF and M-CSF by Nef may alter the profile of production of cytokine/chemokines in HIV-1–infected macrophages, contributing to the development of AIDS. Future studies will clarify whether small compounds specifically targeting the Nef-Hck interaction prevent the progression of the disease. Moreover, a detailed mechanistic analysis of the unique function of Nef will help us to understand how Fms and Src kinases tightly regulate the signaling pathways and functions of macrophages.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Y. Endo for secretarial assistance.
This work was supported in part by Health and Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan (S.O., S.S.).
Authorship
Contribution: M.H. and S.S. were responsible for the overall experimental work and design; Y.Y. and H.A., for DNA cloning; R.H., for Western blotting; H.H., for flow cytometry; N.S., for immunofluorescence; K.M. and S.O., for project planning and data analysis.
M.H. and S.S. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Seiji Okada, Division of Hematopoiesis, Center for AIDS Research, Kumamoto University, Honjo 2-2-1, Kumamoto-city, Kumamoto 860-0811, Japan; e-mail: okadas@gpo.kumamoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal