FCRL1 (Fc receptor–like 1) is a cell-surface membrane protein belonging to FCRL family and is preferentially expressed on B cells. To evaluate FcRL1 as an immunotherapy target for B-cell malignancies, we prepared anti-FCRL1 mAbs without cross-reactivity to other FCRL family proteins and analyzed FCRL1 protein expression on malignant cells from patients and on B-cell lines. Frequent FCRL1 expression was observed by flow cytometry on 12 B-cell non-Hodgkin lymphoma (B-NHL) cell lines and many patient samples: 12 of 14 chronic lymphocytic leukemia (CLL), 7 of 7 follicular lymphoma (FL), 13 of 17 hairy cell leukemia (HCL), and 2 of 3 mantle cell lymphoma (MCL). Two recombinant immunotoxins, E3(Fv)-PE38 and E9(Fv)-PE38, were constructed. Both immunotoxins bound to FCRL1-positive cells with similar affinities (3.4 and 3.2 nM) and were cytotoxic to cell lines, but E9(Fv)-PE38 was 4- to 20-fold more cytotoxic than E3(Fv)-PE38. The concentrations that inhibited response by 50% (IC50s) of E9(Fv)-PE38 on 11 different FCRL1-positive cell lines ranged from 1.0 ng/mL to 90 ng/mL and correlated with the FCRL1 expression levels. Our results suggest that anti-FCRL1 immunotoxin E9(Fv)-PE38 exhibits remarkably specific cytotoxicity and merits further evaluation for the treatment of FCRL1-positive malignancies, including CLL, HCL, FL, MCL, and other B-NHL.
Introduction
Fc receptor–like (FCRL) genes (alternative names, FcRH, IRTA, IFGP, SPAP) belong to a large family of lymphocyte receptors located at human chromosome 1q21-23.1,2 FCRL1-6 genes encode type I transmembrane glycoproteins with 3 to 9 extracellular Ig domains and cytoplasmic tails containing immunoreceptor tyrosine–based inhibition and/or activation motifs.1 FCRL1-5 are preferentially expressed by B cells, while FCRL6 is mainly expressed by natural killer (NK) cells and T cells.1 The potential of FCRL1-5 to deliver activating and/or inhibitory signals suggests that these receptors can play a role in regulating cellular differentiation and modulating the initiation and termination of B-cell responses.1
Because of their preferential expression on B-lineage cells, we have been studying FCRL1-5 proteins for their use in immunodiagnostics and in immunotherapy. We have shown that the FCRL5 (FcRH5, IRTA2) protein and its soluble alternative splicing product are expressed in multiple myeloma, chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), and hairy cell leukemia (HCL).3,4 Other groups have reported the expression of FCRL family in several hematologic malignancies, such as FCRL1-5 in CLL5 and FCRL4 (FcRH4, IRTA1) in mucosa-associated lymphoid tissue lymphoma6 and diffuse large B-cell lymphoma.7
FCRL1 (FcRH1, IRTA5) has 3 extracellular immunoglobulin-like domains and 2 intracellular immunoreceptor tyrosine–based activation-like motifs.8,9 FCRL1 protein expression has been examined in different stages of B-cell development.5,10 RNA level of FCRL1 expression was found in some Burkitt lymphoma cell lines.8 Protein level expression of FCRL1 in CLL at a much higher level than other FCRL members has been reported.5 Thus FCRL1 appears to be a highly promising one among FCRL members as the new immunotherapy target antigen for B-cell–derived leukemia and lymphoma.
Aiming at developing a new immunotherapy of cancers, we have been focusing on immunotoxins, composed of potent protein toxins connected to monoclonal antibodies (mAbs) that bind to cancer cells.11,12 Our main strategy is the use of a 38-kDa fragment of Pseudomonas exotoxin A (PE38) fused to the Fvs fragment of mAbs to produce recombinant immunotoxins. This strategy has been successfully used to target antigens on plasma membrane such as CD22, CD25, CD30, CD123, and mesothelin.13,,,–17 Several immunotoxins have undergone clinical evaluation.13,17,–19 The most successful of these is BL22 that targets CD22 on B-cell malignancies and has produced a very high complete remission rate in chemotherapy-resistant relapsed HCL.18,19
To explore the potential of FCRL1 as an immunotherapy target, we prepared mAbs against FCRL1. One difficulty to make the mAbs was the substantial homology between the same subtypes of Ig domains of FCRL1-6 (up to 86% identity). There are some epitopes shared by FCRL1-6 extracellular domains evidenced by the presence of many cross-reactive anti-FCRL5 and anti-FCRL2 mAbs with other FCRL proteins3 (and T.I., S.N., and I.P., unpublished data, June 2005). In this study, we developed mAbs that react specifically with FCRL1 but not with the other 5 homologous FCRL proteins. Flow cytometric analysis showed FCRL1 expression in B-cell non-Hodgkin lymphoma (B-NHL) cell lines, and malignant cells from patients with CLL, follicular lymphoma (FL), HCL, and MCL. Then 2 recombi-nant immunotoxins E3(Fv)-PE38 and E9(Fv)-PE38 were con-structed, and their cell-binding ability and cytotoxic activity to FCRL1-positive cell lines were evaluated. Our data show that E9(Fv)-PE38 specifically kills cells expressing FCRL1 and merits further evaluation for the treatment of FCRL1-positive malignancies.
Methods
Cells
BL2 was kindly provided by Ms Susan Buhl (Albert Einstein College of Medicine, Bronx, NY). AS283A was kindly provided by Dr Giovanna Tosato (National Cancer Institute [NCI], National Institutes of Health [NIH], Bethesda, MD). REC1 was kindly provided by Dr Thomas Licht (Technische Universitat Munchen, Munich, Germany). NU-DUL-1, SU-DHL-6, and OCI-Ly7 were kindly provided by Dr Louis M. Staudt (NCI, NIH). BL60, BL74, BL104, and KEM I were kindly provided by Dr Riccardo Dalla-Favera (Columbia University, New York, NY). Ramos and Daudi were obtained from ATCC (Manassas, VA). DOHH2, CRO-AP2, and Granta519 were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). DOHH2, NU-DUL-1, SU-DHL-6, and Ramos are grown in RPMI 1640 medium with 10% heat-inactivated FBS. Daudi and CRO-AP2 are grown in RPMI 1640 medium with 20% heat-inactivated FBS. Granta519 is grown in DMEM medium with 10% heat-inactivated FBS. AS283A, BL2, BL60, BL74, BL104, KEM I, OCI-Ly7, and REC1 are grown in IMDM medium with 10% heat-inactivated FBS.
The blood cells from CLL, MCL, and HCL patients and the lymph node cells from FL patients were obtained via an approved protocol at the NCI, NIH. Buffy coats of healthy donors were obtained from the Department of Transfusion Medicine (NIH) according to a protocol approved by NIH. Informed consent was obtained in accordance with the Declaration of Helsinki.
FCRL plasmids and recombinant proteins
The FCRL1-5 cDNAs were obtained by reverse-transcription–polymerase chain reaction (RT-PCR) using human spleen cDNA as template.6 The FCRL6 cDNA was obtained by RT-PCR from normal human peripheral mononuclear cells (PBMCs) and the sequence matched to the reference DNA sequence with accession number NM001004310 in the NCBI database.20 For FCRL antigens in immunization and screening, full-length cDNA of each FCRL was subcloned into the pcDNA3 vector (Invitrogen, Carlsbad, CA).6 For immunoreactions, extracellular domains of FCRL1-6 were also expressed as Fc-fusion proteins in the supernatants of 293T cells as described previously.6
Production and characterization of the mAbs
Six-week-old female Balb/C mice were immunized 4 times with 20 μg FCRL1 expression plasmid DNA as described previously.21 The mice were boosted with 293T cells transfected with the same FCRL1 plasmid, and 3 days later, the spleen cells were fused with SP2/0-neo myeloma cells. The hybridomas were screened for secretion of specific mAbs in an enzyme-linked immunosorbent assay (ELISA), based on reactivity with FCRL1-Fc. After multiple rounds of cell cloning by limiting dilution, the established hybridomas were grown to a high density to harvest the mAbs from cell culture supernatants.
The cross-reactivity of the anti-FCRL1 mAbs with other FCRL family proteins was tested in an ELISA using FCRL-Fc fusion protein and in a flow cytometry analysis using 293T cells transfected with the pcDNA3-derived plasmids encoding each FCRL protein. The isotypes of the mAbs were determined by mouse mAb isotyping reagents (ISO2; Sigma, St Louis, MO). IgG concentrations in the culture supernatants were determined by a sandwich ELISA.22 Affinities (dissociation constant, Kd) of the anti-FCRL1 mAbs to FCRL1-Fc were determined by ELISA as described previously.23
An indirect ELISA using FCRL1-human Fc fusion protein was performed for the screening of hybridomas. Microtiter plates (MaxiSorp; Nalge Nunc, Rochester, NY) were coated with 100 ng per 50 μL/well goat anti–human IgG in PBS for 2 hours at room temperature. Next, 50 ng per 100 μL/well FCRL1-Fc in blocking buffer (25% DMEM, 5% FBS, 25 mM HEPES, 0.5% bovine serum albumin, 0.1% sodium azide in PBS) was added to each well and incubated for 2 hours at room temperature. After washing with PBS containing 0.05% Tween 20, 50 μL of the hybridoma supernatants was added and incubated for 2 hours at room temperature. After washing, the bound mAbs were detected by a 2-hour incubation with horseradish peroxidase–labeled goat anti–mouse IgG (Jackson Immuno-Research, West Grove, PA) followed by tetramethylbenzidine substrate (Pierce, Rockford, IL).
The classification of the mAbs into epitope groups was conducted by the mutual competition of all possible pairs of the anti-FCRL1 mAbs (7 × 7 = 49) in a label-free competitive ELISA as previously described.24
Flow cytometric analysis of FCRL1 expression
Normal human PBMCs were isolated from the buffy coat of a healthy donor by a Ficoll-Paque density gradient. The PBMCs were stained by a mixture of FITC-CD14, PerCP-Cy5.5-CD3, APC-CD56, Pacific blue–CD19 (BD Biosciences, San Jose, CA), PE-conjugated anti-FCRL1 E3 mAb (E3-PE; custom made by Molecular Probes, Eugene, OR) or PE-conjugated isotype control IgG1 (Sigma). The stained cells (250 000 events) were analyzed by a LSRII flow cytometer (BD Biosciences). B-NHL cell lines were incubated with E3-PE or isotype IgG1-PE (Sigma) and analyzed by a FACSCalibur flow cytometer (BD Biosciences).
Whole blood from patients with CLL, MCL, and HCL and lymph node biopsies from patients with FL were stained as described with a panel of antibodies containing E3-PE and other disease-specific antibodies.25,26 The antibody panels were chosen based on the number of cells, previous histologic diagnosis, and available clinical history. The presence of tumor cells was determined using an extensive panel of antibodies and confirmed by detection of light chain restriction in cells with the appropriate tumor-specific immunophenotype. FCRL1 expression by the tumor cells was measured using a cocktail of CD20-FITC/E3-PE/CD19-PerCPCy5.5/CD5-APC in CLL and MCL cases, CD20-FITC/E3-PE/CD19-PerCPCy5.5/CD10-APC in FL cases, and CD103-FITC/E3-PE/CD20-PerCP/CD11c-APC in HCL cases. Four-color cytometry was performed with a FACSCalibur flow cytometer (BD Biosciences) as described.25
Construction, purification, and binding ability of single-chain Fv (scFv)–PE38 immunotoxin
Total cellular RNAs were isolated from E3 and E9 hybridoma cells using a RNeasy mini kit (Qiagen, Valencia, CA). VH and VL cDNAs of the mAbs were obtained by a rapid amplification of CDNA ends (RACE) method using the SMART RACE cDNA amplification kit (Clontech, Mountain View, CA) as described.27 The cDNAs were used as template for PCR reactions for VH and VL. The PCR products were cloned into the pCR4-TOPO vector using a TOPO TA cloning kit (Invitrogen). Five independent clones for each chain were sequenced to exclude the possibility of PCR error. The obtained sequences were new and not reported in GenBank. The VH and VL were assembled into a scFv and fused to PE38. The scFv-PE38 immunotoxins were produced by refolding of inclusion body protein and purified by 2 ion chromatography steps followed by a sizing column as previously described.27
To test the binding ability of E3(Fv)-PE38 and E9(Fv)-PE38 immunotoxins, Ramos cells were incubated with different concentrations of immunotoxins for 1 hour on ice, followed by a 1-hour incubation at 4°C with rabbit anti-PE38 polyclonal antibody produced in our laboratory. Then cells were incubated with PE-conjugated goat F(ab′)2 anti–rabbit IgG (BioSource, Camarillo, CA) at 4°C for 30 minutes and analyzed by a FACSCalibur flow cytometer (BD Biosciences).
Cytotoxicity assay
Cytotoxicity on cell lines was measured by a cell viability assay. Cells were seeded into 96-well plates at 3 × 104 of cells/well. Serial dilutions of immunotoxins in 0.2% human serum albumin were added to the cells, at final concentrations from 0.1 to 1000 ng/mL in 150 μL. After incubation for 72 hours at 37°C, 10 μL WST-8 (Dojindo Molecular Technologies, Gaithersburg, MD) was added to each well, and the cells were incubated for 4 to 6 hours at 37°C. The absorbance of the sample at 450 nm was measured with a reference wavelength of 650 nm. Cytotoxicity is defined by IC50, concentration that gave a 50% decrease on cell viability, which is midway between the level of viability in the absence of toxin and that in the presence of 10 μg/mL cycloheximide (as a positive control). All experiments were performed in triplicate wells. For stability assays, 20 ng/mL E9(Fv)-PE38 in 0.2% human serum albumin was incubated at 4°C or 37°C from 0 to 8 hours prior to a cytotoxicity assay on Ramos cells.
Results
Anti-FCRL1 mAb production and characteristics
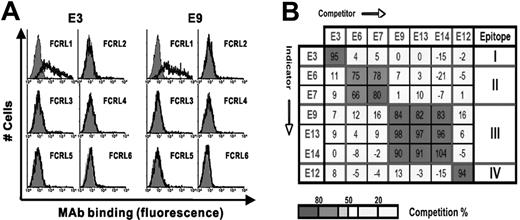
We produced 7 stable hybridomas secreting mAbs (6 IgG1s and one IgG2a) that react with the extracellular domain of FCRL1. The characteristics of the mAbs are summarized in Table 1A. Their affinity to FCRL1-Fc fusion proteins by ELISA assay are 4.4 to 6.8 nM. All the mAbs reacted with full-length FCRL1 expressed by 293T cells transfected with a FCRL1 expression plasmid. Notably, they were not reactive to FCRL2-6 proteins either in ELISA using each FCRL Fc-fusion protein or in fluorescence-activated cell sorting (FACS) using each FCRL protein expressed on 293T cells. The reactivity data of 2 representative mAbs E3 and E9 are shown in Figure 1A. Figure 1B shows that that the epitopes of this panel of mAbs can be classified into 4 topographic groups by a mutual competition assay. We conclude that the panel of mAbs specifically recognizes 4 distinct epitopes present on the native form of the FCRL1 extracellular domain. We conjugated mAb E3 with phycoerythrin (E3-PE) for use in flow cytometry.
Characterization of anti-FCRL1 mAbs
| Name . | Isotope . | Affinity of Kd (nM) . | Topographical epitope . |
|---|---|---|---|
| E3 | γ1 × κ | 4.2 | I |
| E6 | γ1 × κ | 6.2 | II |
| E7 | γ1 × κ | 5.5 | II |
| E9 | γ1 × κ | 5.1 | III |
| E12 | γ1 × κ | 6.8 | IV |
| E13 | γ1 × κ | 5.4 | III |
| E14 | γ2a × κ | 6.1 | III |
| Name . | Isotope . | Affinity of Kd (nM) . | Topographical epitope . |
|---|---|---|---|
| E3 | γ1 × κ | 4.2 | I |
| E6 | γ1 × κ | 6.2 | II |
| E7 | γ1 × κ | 5.5 | II |
| E9 | γ1 × κ | 5.1 | III |
| E12 | γ1 × κ | 6.8 | IV |
| E13 | γ1 × κ | 5.4 | III |
| E14 | γ2a × κ | 6.1 | III |
Reactivity to FcRL1 was positive in all cases. Cross-reactivity to FcRL2-6 in ELISA and FACS was negative in all cases.
Characterization of anti-FCRL1 mAbs. (A) Assessment of cross-reactivity of the representative mAbs E3 and E9 in FACS using 293T cells transfected with each FCRL expression plasmid. Expression of each FCRL on the cells was confirmed by anti-FCRL mAb (data not shown). Cells were stained with E3 or E9 mAb (solid line) or isotype mAb (gray histogram). (B) Topographic epitope mapping of anti-FCRL1 mAbs by mutual competition. The binding of indicator mAbs (listed in rows) to FCRL1-Fc in the presence of more than 100-fold excess amounts of competitor mAbs (listed in columns). Strengths of competition are shown as percentages in each box, which are shaded according to the key at the bottom of the figure.
Characterization of anti-FCRL1 mAbs. (A) Assessment of cross-reactivity of the representative mAbs E3 and E9 in FACS using 293T cells transfected with each FCRL expression plasmid. Expression of each FCRL on the cells was confirmed by anti-FCRL mAb (data not shown). Cells were stained with E3 or E9 mAb (solid line) or isotype mAb (gray histogram). (B) Topographic epitope mapping of anti-FCRL1 mAbs by mutual competition. The binding of indicator mAbs (listed in rows) to FCRL1-Fc in the presence of more than 100-fold excess amounts of competitor mAbs (listed in columns). Strengths of competition are shown as percentages in each box, which are shaded according to the key at the bottom of the figure.
Expression of FCRL1 on normal human PBMCs and human B-cell lines
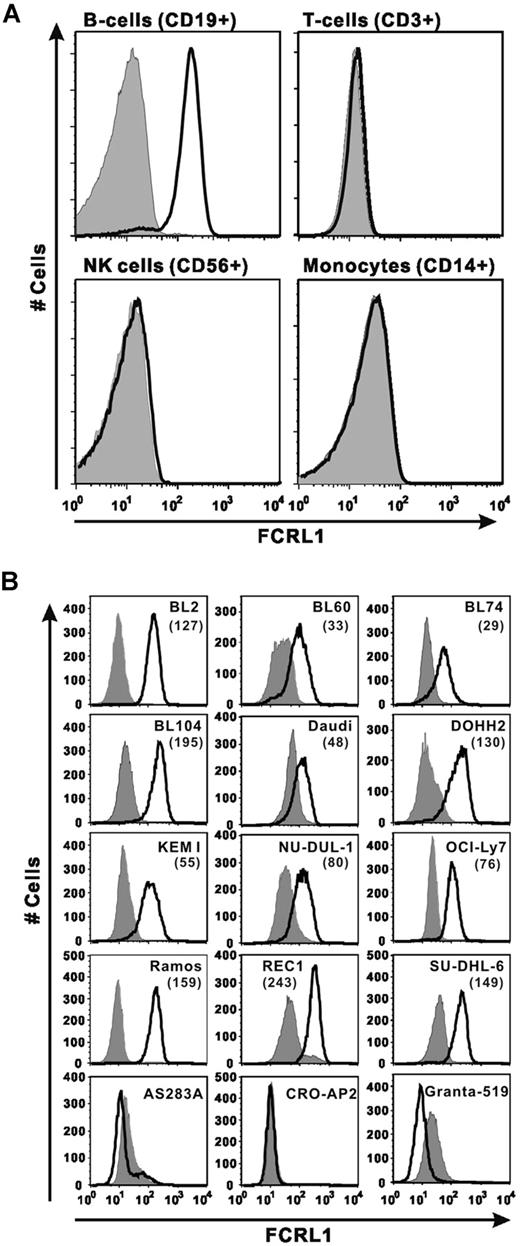
To test mAb reactivity to endogenous FCRL1 antigen, we first confirmed the reactivity of our mAb with normal PBMCs in human blood. As shown in Figure 2A, PBMCs stained with a B-cell marker (CD19+) were strongly and uniformly stained by E3-PE. This result indicates that the anti-FCRL1 mAb reacts with the native form of endogenous FCRL1 expressed on normal B cells. In contrast, no binding of the anti-FCRL1 mAb was observed with T cells (CD3+), NK cells (CD56+), or monocytes (CD14+). This result also indicates that there is no cross-reactivity of the anti-FCRL1 mAb with other cell surface markers on the other cell types.
FCRL1 expression on B cells in human PBMCs and B-NHL cell lines. (A) PBMCs from a healthy donor were stained with marker mAb combination of anti–CD19-Pacific blue, anti–CD3-PerCP-Cy5.5, anti–CD14-FITC, and anti–CD56-APC, anti-FCRL1 E3-PE, or isotype IgG1-PE and analyzed with a LSRII flow cytometer. Each panel shows the overlay of cells from indicated gates stained with E3-PE (solid line) or isotype IgG-PE (gray histogram). (B) B-cell lines were stained with E3-PE (solid line) or isotype IgG1-PE (gray histogram). The MFIs subtracted by the value of isotype control are shown in parentheses.
FCRL1 expression on B cells in human PBMCs and B-NHL cell lines. (A) PBMCs from a healthy donor were stained with marker mAb combination of anti–CD19-Pacific blue, anti–CD3-PerCP-Cy5.5, anti–CD14-FITC, and anti–CD56-APC, anti-FCRL1 E3-PE, or isotype IgG1-PE and analyzed with a LSRII flow cytometer. Each panel shows the overlay of cells from indicated gates stained with E3-PE (solid line) or isotype IgG-PE (gray histogram). (B) B-cell lines were stained with E3-PE (solid line) or isotype IgG1-PE (gray histogram). The MFIs subtracted by the value of isotype control are shown in parentheses.
Next we surveyed 15 human Burkitt lymphoma and other B-NHL cell lines for FCRL1 expression. We detected FCRL1 expression in 12 of 15 B-cell lines tested (Figure 2B). The presence or absence of FCRL1 mRNA in some of the stained cell lines or nonstained cell lines was confirmed by RT-PCR (data no shown). The FCRL1 expression level varied approximately 10-fold in the positive cell lines assessed by median fluorescence intensity (MFI). REC1 has the highest FCRL1 expression (MFI = 243) and BL74 has the lowest level (MFI = 29). Eight cell lines showed relatively strong FCRL1 expression (MFI > 70).
FCRL1 expression on patients with various hematologic malignancies: summary of the survey
| Disease . | Sample numbers . | |
|---|---|---|
| Total . | Positive (%) . | |
| CLL | 14 | 12 (86) |
| FL | 7 | 7 (100) |
| HCL | 17 | 13 (76) |
| MCL | 3 | 2 (67) |
| Disease . | Sample numbers . | |
|---|---|---|
| Total . | Positive (%) . | |
| CLL | 14 | 12 (86) |
| FL | 7 | 7 (100) |
| HCL | 17 | 13 (76) |
| MCL | 3 | 2 (67) |
Expression of FCRL1 on patients' malignant cells
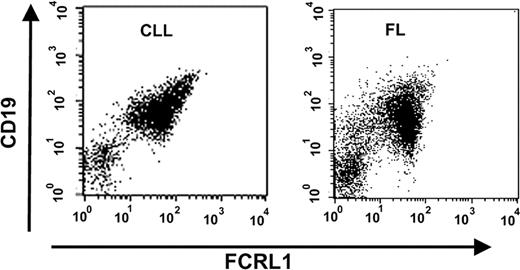
Because the expression of FCRL1 in malignant cells is of great importance for validating the potential of FCRL1 as an immunotherapy target, we examined FCRL1 expression on malignant cells from many patients with CLL, FL, HCL, and MCL using an anti-FCRL1 E3-PE antibody. As summarized in Table 3, 86% of CLL patients (12/14), 100% of FL patients (7/7), 76% of HCL patients (13/17), and 67% of MCL patients (2/3) were found to be FCRL1 positive with 100% positivity.26 Figure 3 shows representative FACS analyses of peripheral blood cells from a CLL patient and lymph node cells from a FL patient. In each sample, the malignant cells were identified as the major population by demonstration of a monoclonal (kappa/lambda light chain–restricted) B-cell population (expressing CD19, CD20, and CD22) expressing tumor-specific antigens. Staining by E3-PE in the identified tumor cells allowed determination of cell surface expression of FCRL1. In a comparison with standard PE fluorescence beads, the number of antibody-binding sites per cell of malignant cells from CLL patients ranged from 384 to 1527 (median: 1186) and was usually higher than the normal B-cell counterpart from the same patients (data not shown).
Cytotoxicity of E9(Fv)-PE38 on B-NHL cell lines
| Cell lines . | IC50, ng/mL . | MFI . |
|---|---|---|
| Burkitt lymphoma | ||
| BL2 | 19 | 127 |
| BL60 | 30 | 33 |
| BL74 | 30 | 29 |
| BL104 | 8.0 | 195 |
| Daudi | 90 | 48 |
| KEM I | 600 | 55 |
| Ramos | 16 | 159 |
| DLBCL | ||
| OCI-Ly7 | 50 | 76 |
| REC1 | 1.0 | 243 |
| SU-DHL-6 | 18 | 149 |
| Other B-NHL | ||
| DOHH2 | 8.2 | 130 |
| NU-DUL-1 | 30 | 80 |
| AS283A | >1000 | — |
| CRO-AP2 | >1000 | — |
| Granta-519 | >1000 | — |
| Cell lines . | IC50, ng/mL . | MFI . |
|---|---|---|
| Burkitt lymphoma | ||
| BL2 | 19 | 127 |
| BL60 | 30 | 33 |
| BL74 | 30 | 29 |
| BL104 | 8.0 | 195 |
| Daudi | 90 | 48 |
| KEM I | 600 | 55 |
| Ramos | 16 | 159 |
| DLBCL | ||
| OCI-Ly7 | 50 | 76 |
| REC1 | 1.0 | 243 |
| SU-DHL-6 | 18 | 149 |
| Other B-NHL | ||
| DOHH2 | 8.2 | 130 |
| NU-DUL-1 | 30 | 80 |
| AS283A | >1000 | — |
| CRO-AP2 | >1000 | — |
| Granta-519 | >1000 | — |
MFI indicates median fluorescence intensity; DLBCL, diffuse large B-cell lymphoma; B-NHL, B-cell non-Hodgkin lymphoma; and —, not applicable.
FCRL1 expression on patients with various hematologic malignancies. Representative FACS analysis. Peripheral blood cells from a patient with CLL and lymph node cells from a patient with FL were analyzed by FACS with PerCP-CD19 and anti-FCRL1 E3-PE. Cells gated for lymphocytes were shown in the dot plots with CD19 (y-axis)– and FCRL1 (x-axis)–positive staining.
FCRL1 expression on patients with various hematologic malignancies. Representative FACS analysis. Peripheral blood cells from a patient with CLL and lymph node cells from a patient with FL were analyzed by FACS with PerCP-CD19 and anti-FCRL1 E3-PE. Cells gated for lymphocytes were shown in the dot plots with CD19 (y-axis)– and FCRL1 (x-axis)–positive staining.
Preparation and binding ability of anti-FCRL1 scFv-PE38 immunotoxins
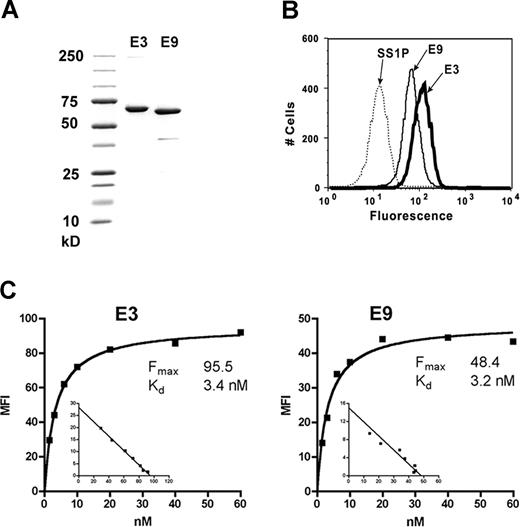
Because our data indicated the potential use of FCRL1 as a target for immunotherapy of B-cell malignancies, we made specific immunotoxins against FCRL1. Two mAbs with the highest affinities, E3 and E9 (binding to epitopes I and III, respectively), were chosen to construct scFv-PE38 immunotoxins. Both antibodies were IgG1 and showed no antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity on cell lines (data not shown). Each immunotoxin was expressed in E coli as inclusion bodies, refolded, and purified to near 95% homogeneity. Figure 4A shows an sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the final purified immunotoxins. The yields of pure monomeric immunotoxin ranged from 3% to 4%, within the usual range on many other immunotoxins made in our laboratory.
Purified anti-FCRL1 immunotoxins and their binding abilities. (A) Purified E3(Fv)-PE38 and E9(Fv)-PE38 on SDS-PAGE. (B) Binding of 60 nM E3(Fv)-PE38 and E9(Fv)-PE38 to Ramos cells. SS1P, an antimesothelin immunotoxin, was used as negative control. (C) Apparent affinities of E3(Fv)-PE38 and E9(Fv)-PE38 on Ramos cells. Binding saturation curve and nonlinear re-gression analysis were conducted with GraphPad Prism (GraphPad Software, San Diego, CA).
Purified anti-FCRL1 immunotoxins and their binding abilities. (A) Purified E3(Fv)-PE38 and E9(Fv)-PE38 on SDS-PAGE. (B) Binding of 60 nM E3(Fv)-PE38 and E9(Fv)-PE38 to Ramos cells. SS1P, an antimesothelin immunotoxin, was used as negative control. (C) Apparent affinities of E3(Fv)-PE38 and E9(Fv)-PE38 on Ramos cells. Binding saturation curve and nonlinear re-gression analysis were conducted with GraphPad Prism (GraphPad Software, San Diego, CA).
Purified immunotoxins were incubated with Ramos cells to test their binding to FCRL1 by flow cytometry. SS1P, an immunotoxin targeting mesothelin,17 which is not expressed on the surface of Ramos cells, was used as a negative control. Significant specific binding of E3(Fv)-PE38 and E9(Fv)-PE38 to Ramos cells was observed (Figure 4B). The apparent affinities of E3(Fv)-PE38 and E9(Fv)-PE38 with FCRL1 on Ramos cells are 3.4 and 3.2 nM, respectively (Figure 4C). These high-affinity values are comparable with immunotoxins that have been evaluated in clinical trials. No binding of E3(Fv)-PE38 and E9(Fv)-PE38 to FCRL1-negative cell lines, CRO-AP2, AS283A, or Granta519, was detected (data not shown).
Cytotoxicity and stability of anti-FCRL1 immunotoxins
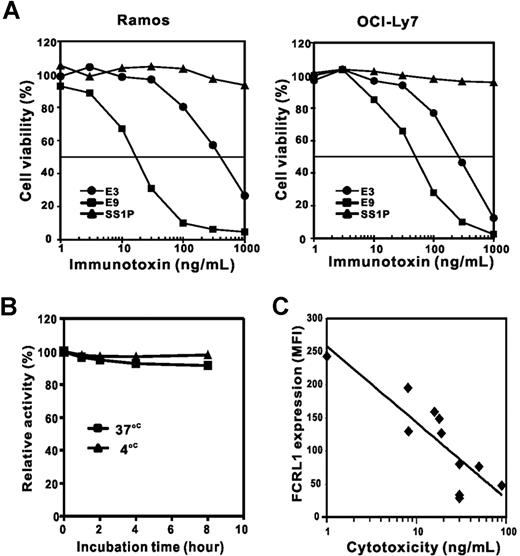
The cytotoxicity of E3(Fv)-PE38 and E9(Fv)-PE38 was tested on 2 FCRL1-expressing cell lines, Ramos and OCI-Ly7 (Figure 5A). E9(Fv)-PE38 had a higher activity on Ramos and OCI-Ly7 (IC50s = 18 and 50 ng/mL, respectively) than E3(Fv)-PE38 (IC50s = 400 and 220 ng/mL, respectively). The negative immunotoxin control, SS1P, showed no cell killing activity, indicating that the cytotoxicity of E3(Fv)-PE38 and E9(Fv)-PE38 depends on specific FCRL1 binding. E3(Fv)-PE38 and E9(Fv)-PE38 showed no cytotoxicity on FCRL1-negative cells, CRO-AP2, AS283A, or Granta519 (IC50s > 1000 ng/mL).
Cytotoxic activity of anti-FCRL1 immunotoxins. (A) Cytotoxicity of E3(Fv)-PE38 and E9(Fv)-PE38 was conducted on Ramos and OCI-Ly7 cells by cell viability assay. (B) Stability of E9(Fv)-PE38. Immunotoxin (20 ng/mL) was incubated at 4°C (▴) or 37°C (■) for 1, 2, 4, or 8 hours prior to treatment of Ramos cells. The activity at time zero is set as 100%. (C) The correlation between cytotoxicity (x-axis, by IC50s) and FCRL1 expression level (y-axis, by MFIs). The Pearson correlation coefficient (r) is 0.859 (P < .001).
Cytotoxic activity of anti-FCRL1 immunotoxins. (A) Cytotoxicity of E3(Fv)-PE38 and E9(Fv)-PE38 was conducted on Ramos and OCI-Ly7 cells by cell viability assay. (B) Stability of E9(Fv)-PE38. Immunotoxin (20 ng/mL) was incubated at 4°C (▴) or 37°C (■) for 1, 2, 4, or 8 hours prior to treatment of Ramos cells. The activity at time zero is set as 100%. (C) The correlation between cytotoxicity (x-axis, by IC50s) and FCRL1 expression level (y-axis, by MFIs). The Pearson correlation coefficient (r) is 0.859 (P < .001).
For the application to patients, the stability of immunotoxins at physiological temperature is important. To examine the stability of E9(Fv)-PE38, it was incubated for up to 8 hours at 37°C and the cytotoxicity was examined (Figure 5B). Even after an 8-hour incubation, E9(Fv)-PE38 retained more than 90% of its cell killing activity, indicating E9(Fv)-PE38 is stable in this condition.
B-NHL cell lines with different levels of FCRL1 expression were also tested with E9(Fv)-PE38 (Table 3). Among the tested cell lines, REC1, with the highest level of FCRL1, is the most sensitive cell line (IC50 = 1 ng/mL). With the exception of KEM I (IC50 = 600 ng/mL), the cytotoxicities are well correlated with the expression levels of FCRL1 (Figure 5C). The FCRL1 expression level is likely to be an important factor in determining the susceptibility of cells to the anti-FCRL1 immunotoxin.
Discussion
In this study, we demonstrate that FCRL1 is a promising target for the immunotherapy of B-cell malignancies. By use of our FCRL1-specific cross-reactivity–free mAbs, we confirmed the frequent expression of FCRL1 on CLL and in addition found frequent FCRL1 expression in FL and other B-cell malignancies. We succeeded in making potent immunotoxins targeting FCRL1-positive cells and showed a relationship between the immunotoxin activity and the FCRL1 expression level on cell lines.
The FCRL1 expression profile on different B-cell lines in our study is consistent with previous reports: mRNA expression of FCRL1 was reported with BL60, BL74, BL104, Daudi, Ramos, and KEM I cell lines.8 Our survey additionally revealed a wide expression of FCRL1 in many other B-cell lines tested, some of which do not express other FCRL proteins shown in previous studies.3,8,9 The wide expression of FCRL1 makes it a good target for immunotherapy. In fact, we found the FCRL1 expression on all of FL cells tested (7/7) in this study. FL is the second most common subtype of B-NHL and does not express FCRL5 (0/25 specimens) (unpublished data). FL is considered to be derived from B cells in germinal center of secondary lymphoid organs,28 where FCRL1 is expressed and FCRL5 is not expressed.9
The human FCRL1 gene is located at chromosome region 1q21 where genome abnormalities including translocations frequently occur in various B-cell malignancies.29,–31 For FCRL5 (IRTA2), the expression levels are on average 10-fold higher in 1q21 abnormal cell lines.8,32 In this study, high expression of FCRL1 expression on CLL cells was noticed. It is likely that some abnormality in the genome up-regulates FCRL1 expression in B-cell malignancies. However, among 12 FCRL1-positive cell lines tested, only BL104 is abnormal at 1q21. The abnormality in 1q21 may not be the major factor to inducing high expression of FCRL1 in B-cell malignancies. Further studies on the association of FCRL1 expression and 1q21 abnormality are needed.
The cytotoxicity level of E9(Fv)-PE38 (1.0-90.0 ng/mL) for FCRL1-positive cell lines is comparable with other immunotoxins in clinical trials.13,17,–19 The stability of immunotoxins is important for clinical use. In this study, we found that E9(Fv)-PE38 retained more than 90% activity after incubation for 8 hours at 37°C, which is necessary for clinical application.
Our comparison of the 2 anti-FCRL1 immunotoxins, E3(Fv)-PE38 and E9(Fv)-PE38, indicates that both immunotoxins bind to FCRL1-positive cells with a similar affinity and specificity, but E9(Fv)-PE38 is 4- to 20-fold more cytotoxic than E3(Fv)-PE38. These 2 immunotoxins bind to different epitopes (Figure 1B) and may be internalized at different rates or processed differently with the cells. We recently observed a similar phenomenon for anti-CD123 immunotoxins that target different epitopes on CD123.16 A panel of anti-FCRL1 mAbs against 4 different epitopes was produced in this study. We plan to evaluate targeting other FCRL1 epitopes in further studies.
Anti-FCRL1 immunotoxins will kill both malignant cells and normal B cells with FCRL1 expression. But B stem cells and pro/pre-B cells have no or very low expression of FCRL1,5,10 which will allow healthy B cells to regenerate after treatment and return to normal levels. FCRL1 has 2 ITAM-like motifs and has the potential to serve as an activating coreceptor on B cells. There is concern that agent-targeting immunomodulatory receptors may cause adverse effects, such as in the case of anti-CD28 mAb TGN1412.33 However, in contrast to mAbs, immunotoxins against FCRL1 are monovalent and do not cross-link FCRL1. Thus the possibility of anti-FCRL1 immunotoxins triggering downstream activating signals by FCRL1 is very low. Like the other immunotoxins currently used in clinical trials, the safety of anti-FCRL1 immunotoxins will need to be evaluated in nonhuman primates.
Overall, our data show that FCRL1 has the potential to be an excellent therapeutic target for treatment of CLL, HCL, FL, MCL, and other B-NHL diseases. E9(Fv)-PE38 has specific and strong cytotoxic effects on FCRL1-expressing cell lines and provides a possible new therapy for FCRL1-positive malignances.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research.
National Institutes of Health
Authorship
Contribution: X.D. cloned Fv, made immunotoxins and tested them, and wrote and revised the paper; S.N. and T.I. made mAbs and tested them; M.S.-S. analyzed cells from patients for expression; I.P. conceived the project, provided supervision throughout, and wrote and revised the paper.
X.D. and S.N. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ira Pastan, Laboratory of Molecular Biology, National Cancer Institute, 37 Convent Dr, Rm 5106, Bethesda, MD 20892-4264; e-mail: pastani@mail.nih.gov.