B-cell chronic lymphocytic leukemia (B-CLL) progression is frequently accompanied by clinical lymphadenopathy, and the CCL21 chemokine may play an important role in this process. Indeed, CCR7 (the CCL21 receptor), as well as matrix metalloproteinase-9 (MMP-9), are overexpressed in infiltrating B-CLL cells. We have studied whether MMP-9 is regulated by CCL21 and participates in CCL21-dependent migration. CCL21 significantly increased B-CLL MMP-9 production, measured by gelatin zymography. This was inhibited by blocking extracellular signal-regulated kinase-1/2 (ERK1/2) activity or by cell transfection with CCR7-siRNA. Accordingly, CCL21/CCR7 interaction activated the ERK1/2/c-Fos pathway and increased MMP-9 mRNA. CCL21-driven B-CLL cell migration through Matrigel or human umbilical vein endothelial cells (HUVEC) was blocked by anti-CCR7 antibodies, CCR7-siRNA transfection, or the ERK1/2 inhibitor U0126, as well as by anti-MMP-9 antibodies or tissue inhibitor of metalloproteinase 1 (TIMP-1). These results strongly suggest that MMP-9 is involved in B-CLL nodal infiltration and expand the roles of MMP-9 and CCR7 in B-CLL progression. Both molecules could thus constitute therapeutic targets for this disease.
Introduction
As B-cell chronic lymphocytic leukemia (B-CLL) progresses, malignant cells extravasate and infiltrate lymphoid tissues.1,2 CCR7, the receptor for the CCL19 and CCL21 chemokines,3,4 plays an important role in B-CLL cell entry into lymph nodes, and CCR7 overexpression correlates with the presence of lymphadenopathy.5,6 Another important contributor to B-CLL cell migration may be matrix metalloproteinase-9 (MMP-9),7,–9 which is produced by B-CLL cells10,–12 and overexpressed when these cells infiltrate tissues with a diffuse pattern.11
We showed recently that MMP-9 is up-regulated by α4β1 integrin or CXCR4 (the CXCL12 chemokine receptor) engagement12 and plays a crucial role in B-CLL cell migration through basement membranes and human umbilical vein endothelial cells (HUVEC).12 To further understand the role of MMP-9 in B-CLL progression, we have now studied whether MMP-9 is regulated by CCR7 ligation and/or involved in CCR7-dependent migration.
Methods
Approval was obtained from the review boards of the Hospital Clínico Universitario, Valencia, and the Hospital Universitario Puerta de Hierro, Madrid, for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Patients and cells
CD5+ B-lymphocytes were purified from peripheral blood samples from 6 patients with B-CLL as reported previously.12 HUVECs were provided by Dr M. L. Botella (Centro de Investigaciones Biológicas, Madrid, Spain) and cultured as described previously.12
Additional materials and methods can be found in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results and discussion
To determine whether MMP-9 was regulated by CCR7 ligation, B-CLL cells were incubated with or without CCL21; after 24 hours, the conditioned media were analyzed by gelatin zymography. Figure 1A shows that CCL21 significantly increased (P ≤ .01) MMP-9 secretion compared with untreated cells. As previously reported for B-CLL cells,11,12 MMP-9 was present as a 92-kDa form. The CCL21 effect was completely blocked by inhibiting CCR7 or ERK1/2 signaling with pertussis toxin (PT) or U0126, respectively, while inhibiting phosphatidylinositol 3-kinase (PI3-K), p38 mitogen-activated protein kinase (MAPK), protein kinase C, or Src kinases, had no effect (Figure 1A). To study whether CCL21 activated ERK1/2, cells were incubated with CCL21 and, at various times, they were lysed and lysates analyzed by Western blotting. CCL21 induced ERK1/2 phosphorylation, which peaked after 10 minutes of exposure (Figure 1B) and persisted for at least 24 hours (not shown). This effect was specifically inhibited by PT and U0126 (Figure 1B). Further analysis of these samples revealed that CCL21 also activated the ERK1/2 effector c-fos13 (Figure 1C), suggesting that CCL21/CCR7 may regulate MMP-9 at various levels including gene transcription. Indeed, CCL21 significantly increased MMP-9 mRNA levels measured after 6 to 16 hours of exposure, then declining after 24 hours (Figure S1).
CCL21 up-regulates MMP-9 via ERK1/2 signaling. (A) B-CLL cells were treated or not with 1000 ng/mL CCL21 in the presence or absence of the indicated inhibitors (PT, 200 ng/mL; Wortmannin (Wmn), 30 nmol/L; the rest, 5 μmol/L). After 24 hours, the conditioned media was analyzed by gelatin zymography. (B) Western blots of lysates of B-CLL cells treated or not (10 minutes) with CCL21, and with or without the indicated inhibitors. (C) Reverse transcription-polymerase chain reaction analysis of c-fos in the same samples shown in (B). Vertical lines in (A) and (C) have been inserted to indicate a repositioned gel lane. (D) Flow cytometric analysis of CCR7 expression in untransfected (continuous lines), control siRNA- (dotted lines) or CCR7 siRNA-transfected (shaded areas) cells. (E) Gelatin zymographic analysis of the conditioned media of untransfected or siRNA-transfected cells treated or not with CXCL12 or CCL21 for 24 hours. Error bars represent SD. *P ≤ .05; **P ≤ .01.
CCL21 up-regulates MMP-9 via ERK1/2 signaling. (A) B-CLL cells were treated or not with 1000 ng/mL CCL21 in the presence or absence of the indicated inhibitors (PT, 200 ng/mL; Wortmannin (Wmn), 30 nmol/L; the rest, 5 μmol/L). After 24 hours, the conditioned media was analyzed by gelatin zymography. (B) Western blots of lysates of B-CLL cells treated or not (10 minutes) with CCL21, and with or without the indicated inhibitors. (C) Reverse transcription-polymerase chain reaction analysis of c-fos in the same samples shown in (B). Vertical lines in (A) and (C) have been inserted to indicate a repositioned gel lane. (D) Flow cytometric analysis of CCR7 expression in untransfected (continuous lines), control siRNA- (dotted lines) or CCR7 siRNA-transfected (shaded areas) cells. (E) Gelatin zymographic analysis of the conditioned media of untransfected or siRNA-transfected cells treated or not with CXCL12 or CCL21 for 24 hours. Error bars represent SD. *P ≤ .05; **P ≤ .01.
To further establish that the CCL21/CCR7 interaction was responsible for MMP-9 up-regulation, we studied the effect of CCL21 on siRNA transfected cells. Transfection with control siRNA did not affect CCR7 surface expression (Figure 1D) or MMP-9 induction by CCL21 (Figure 1E), indicating that MMP-9 production was not impaired by the procedure. However, CCR7 gene silencing significantly inhibited CCR7 expression (Figure 1D), and CCL21-induced MMP-9 up-regulation (Figure 1E), confirming the specificity of the effect. Moreover, CXCL12, another chemokine that up-regulates MMP-9 upon binding to CXCR4,12 enhanced MMP-9 levels on CCR7 siRNA-cells (Figure 1E), indicating that both axes, CCL21/CCR7 and CXCL12/CXCR4, functioned independently from each other. Indeed, simultaneous treatment with CCL21 and CXCL12 did not have an additive effect on MMP-9 production (Figure 1E). This probably reflects the physiologic situation in which the 2 chemokines are associated with different tissues4 yet may provide a continuous stimulus for MMP-9 production.
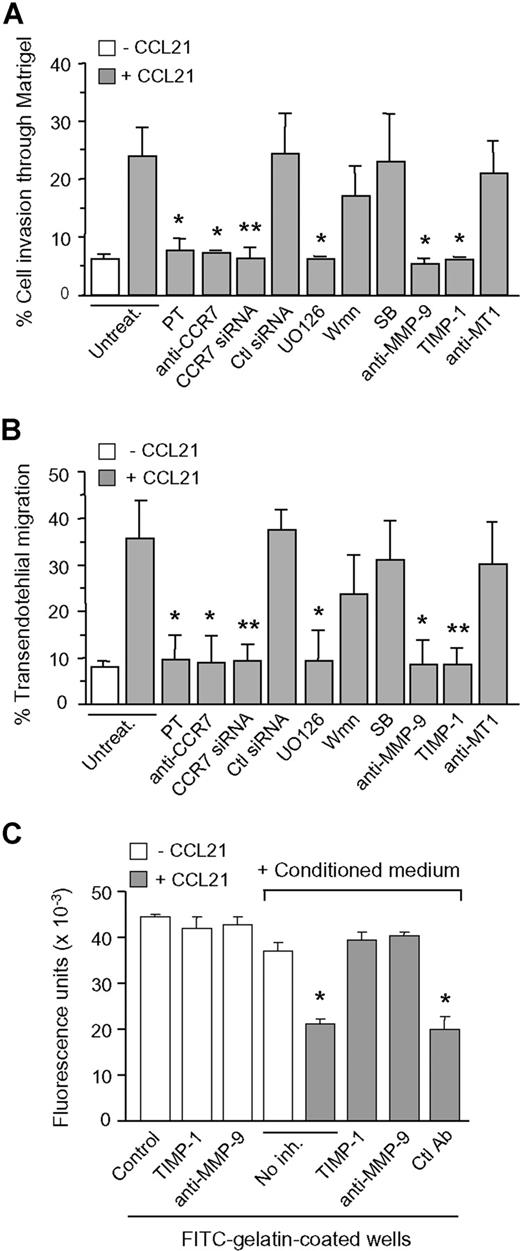
We next studied whether MMP-9 was involved in CCL21-induced cell migration. B-CLL cells migrated through Matrigel or HUVEC in response to CCL21, and this was completely inhibited by PT, anti-CCR7 antibodies (Abs), or CCR7 siRNA transfection (Figure 2A,B). U0126 also blocked cell migration in both systems, whereas PI3-K or p38 MAPK inhibitors had no significant effect. It is noteworthy that anti-MMP-9 Abs or the MMP-9–specific inhibitor known as tissue inhibitor of metalloproteinase 1 (TIMP-1), but not control anti-MT1-MMP Abs, inhibited cell migration (Figure 2A,B). To determine whether MMP-9 enzymatic activity was involved in this effect, we incubated the conditioned media of untreated or CCL21-treated B-CLL cells with FITC-gelatin and measured the fluorescence after 24 hours. Incubation with the conditioned media of untreated cells had a minor effect, but the conditioned media of CCL21-treated cells significantly decreased the fluorescence (Figure 2C). This was reversed by anti-MMP-9 Abs or TIMP-1, whereas these compounds had no effect in the absence of conditioned media (Figure 2C). These results indicated that secreted MMP-9 had catalytic activity, that this activity was clearly increased upon CCL21 treatment (which enhances MMP-9 production), and that its role in cell migration probably involves the degradation of the matrix and/or basement membrane. Despite this functional assay, the mature 85-kDa MMP-9 form was not detected in the B-CLL conditioned media. Similar observations have been reported previously for other cell types.14 Although the reason for this remains unclear, one possibility is that mature MMP-9 is produced at levels that are undetectable yet sufficient for focalized activity. MMP-9 activation most likely occurs at the pericellular space, where several proteases, including plasmin, may be present. MMP-9 could also be activated by nonproteolytic mechanisms (conformational changes, oxidative modifications)14 thus resulting in an active 92-kDa enzyme.
Role of MMP-9 in CCL21/CCR7-driven B-CLL cell migration. B-CLL cells with or without previous incubation with the indicated inhibitors or transfected with siRNAs were added to Transwell filters coated with Matrigel (A) or HUVEC (B). CCL21 (1000 ng/mL) was added to the medium in the bottom chamber, except for the control. After 24 hours, migrated cells were counted by flow cytometry. Values represent the percentage of total cells added. (C) The conditioned media of untreated or CCL21-treated B-CLL cells was added to FITC-gelatin-coated wells in the presence or absence of the indicated inhibitors (TIMP-1, 5 nmol/L; Abs, 10 μg/mL). TIMP-1 or anti-MMP-9 Ab alone were also added to the wells. After 24 hours, the fluorescence was quantitated. Values are the average of 2 different patients with duplicate determinations. *P ≤ .05; **P ≤ .01.
Role of MMP-9 in CCL21/CCR7-driven B-CLL cell migration. B-CLL cells with or without previous incubation with the indicated inhibitors or transfected with siRNAs were added to Transwell filters coated with Matrigel (A) or HUVEC (B). CCL21 (1000 ng/mL) was added to the medium in the bottom chamber, except for the control. After 24 hours, migrated cells were counted by flow cytometry. Values represent the percentage of total cells added. (C) The conditioned media of untreated or CCL21-treated B-CLL cells was added to FITC-gelatin-coated wells in the presence or absence of the indicated inhibitors (TIMP-1, 5 nmol/L; Abs, 10 μg/mL). TIMP-1 or anti-MMP-9 Ab alone were also added to the wells. After 24 hours, the fluorescence was quantitated. Values are the average of 2 different patients with duplicate determinations. *P ≤ .05; **P ≤ .01.
Our results therefore establish a novel role for the CCL21/CCR7 axis as a MMP-9 regulator and clearly show that MMP-9 is an important mediator in CCL21-driven cell migration. CCL21 is expressed in high endothelial venules, a major route for lymphocyte homing to lymphoid tissues,15,–17 and lack of CCR7 impairs B-cell entry into lymph nodes.18 Besides its role in B-CLL nodal infiltration, CCR7 may also induce B-CLL cell survival,19 thus contributing in various ways to B-CLL progression. Our present findings expand the CCR7 roles in B-CLL and strongly suggest that MMP-9 is involved in B-CLL lymph node infiltration. MMP-9 and/or CCR7 could therefore constitute targets for treatment of this malignancy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mercedes Hernández del Cerro for excellent technical assistance.
This work was supported by grants PI060400 (to A.G.P.) and PI061637 (to M.J.T.) from the Ministerio de Sanidad y Consumo, and by the Fundación de Investigación Médica Mutua Madrileña (FMM; to A.G.P.). J.R.M. was supported by FMM and the Fundación Ramón Areces.
Authorship
Contribution: J.R-M. performed research and designed some experiments. M.J.T. and J.A.G-M. contributed patient samples. A.G-P. designed and supervised research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angeles García-Pardo, Departamento de Fisiopatología Celular y Molecular, Centro de Investigaciones Biológicas, CSIC, Ramiro de Maeztu 9, 28040 Madrid, Spain; e-mail: agarciapardo@cib.csic.es.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal