In this study, we have identified a unique combinatorial effect of the chemokines KC/MIP-2 and the cytokine granulocyte colony-stimulating factor (G-CSF) with respect to the rapid mobilization of neutrophils from the bone marrow in a model of acute peritonitis. At 2 hours following an intraperitoneal injection of thioglycollate, there was a 4.5-fold increase in blood neutrophil numbers, which was inhibited 84% and 72% by prior administration of blocking mAbs against either the chemokines KC/MIP-2 or G-CSF, respectively. An intraperitoneal injection of G-CSF acted remotely to stimulate neutrophil mobilization, but did not elicit recruitment into the peritoneum. Further, in vitro G-CSF was neither chemotactic nor chemokinetic for murine neutrophils, and had no priming effect on chemotaxis stimulated by chemokines. Here, we show that, in vitro and in vivo, G-CSF induces neutrophil mobilization by disrupting their SDF-1α–mediated retention in the bone marrow. Using an in situ perfusion system of the mouse femoral bone marrow to directly assess mobilization, KC and G-CSF mobilized 6.8 × 106 and 5.4 × 106 neutrophils, respectively, while the infusion of KC and G-CSF together mobilized 19.5 × 106 neutrophils, indicating that these factors act cooperatively with respect to neutrophil mobilization.
Introduction
A blood neutrophilia is a characteristic feature of infections and inflammatory disorders, due initially to the rapid mobilization of neutrophils from the bone marrow reserve. The factors and mechanisms regulating the mobilization of neutrophils from the bone marrow during inflammation are, however, unknown
Chemokines are generated locally at sites of inflammation,1,–3 and orchestrate the local recruitment of neutrophils from the blood into tissues by promoting neutrophil adhesion to the endothelium and transmigration into tissues.4,–6 Thus, in a number of inflammatory models, blockade of CXC chemokines with neutralizing mAbs, or CXCR1/2 receptor antagonism, has been shown to effectively reduce neutrophil recruitment to the site of inflammation.7,,–10 More recently, a role for the chemokine receptor CCR2 and the chemokines MCP-1 and MCP-3 has been described in the mobilization of monocytes from the bone marrow during inflammation,11,,,–15 suggesting that chemokines generated at sites of inflammation may act remotely to mobilize leukocytes from the bone marrow.
The cytokine granulocyte colony-stimulating factor (G-CSF) is critically involved in granulopoiesis under homeostatic conditions; mice with genetic deletion of either G-CSF or G-CSF receptor (G-CSFR) having markedly reduced numbers of neutrophils in their blood and bone marrow.16,17 Chronic treatment of mice or humans with G-CSF over 4 to 5 days results in an increase in circulating numbers of neutrophils, thought to be due to both increased granulopoiesis and an indirect effect of G-CSF in promoting neutrophil mobilization.18,–20 Mechanistically chronic treatment with G-CSF is thought to stimulate neutrophil mobilization either by reducing SDF-1α levels in the bone marrow or by down-regulating their expression of CXCR4.21,,,–25 The importance of G-CSF in neutrophil biology during acute and chronic inflammatory reactions is not completely understood. Most of the publications investigating the activities of G-CSF during inflammation postulate a preferential role in promoting granulopoiesis26,27 and mobilization of neutrophils, specifically in the chronic phase of the inflammation.17,28,29 Conflicting data are reported with respect to the direct role of G-CSF in recruiting neutrophils from the blood into the inflamed tissue.27,30 Interestingly, it has been shown that a single intravenous injection of G-CSF leads to a rapid increase in circulating neutrophil numbers in both mice and humans, suggesting that G-CSF could potentially rapidly mobilize neutrophils from the bone marrow.31,32
Previous studies have documented significant increases in blood levels of chemokines, G-CSF, and granulocyte-macrophage CSF (GM-CSF) in animal models of acute inflammation and in patients with infections,17,26,28,33,–35 suggesting that these chemokines and cytokines may potentially stimulate mobilization of neutrophils from the bone marrow during inflammatory reactions. In this study, using a murine model of acute peritonitis, we have determined that the rapid mobilization of neutrophils from the bone marrow is driven by the coordinated actions of the CXC chemokines, KC and MIP-2, and the cytokine G-CSF acting via distinct mechanisms.
Methods
Animals
Female Balb/c mice were purchased from Harlan (Oxford, United Kingdom). Mice were used at the age of 6 to 8 weeks. United Kingdom Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act of 1986 were strictly observed.
Reagents
General laboratory chemicals and cell-culture reagents were purchased from either Life Technologies (Paisley, United Kingdom) or Sigma-Aldrich (Poole, United Kingdom). Recombinant murine chemokines/cytokines KC/CXCL1, MIP-2/CXCL2, G-CSF, and SDF-1α/CXCL12 were from PeproTech EC (London, United Kingdom). The PE-conjugated rat anti–mouse Ly-6G/Ly6C (Gr-1) mAb (clone RB6–8C5; IgG2b), the PE-conjugated rat IgG2b mAb (clone A95-1) isotype control, and the rat anti–mouse CD16/32 (Fcγ III/II) mAb (clone 2.4G2) were obtained from BD Biosciences (Oxford, United Kingdom). The mAbs rat anti–mouse KC (clone 48415.111; IgG2a), rat anti–mouse MIP-2 (clone 40605; IgG2b), rat anti–mouse G-CSF (clone 67604; IgG1), rat anti–mouse GM-CSF (clone MP122E9; IgG2a), and the rat IgG1 (clone 43414), IgG2a, (clone 5444.11), and IgG2b (clone 141945) isotype controls were obtained from R&D Systems (Abingdon, United Kingdom). The CXCR4 antagonist AMD3100 was obtained from Sigma-Aldrich (Poole, United Kingdom). For differential staining of leukocytes in bloodsmears, the KWIK-DIFF stain kit was used (Thermo Electron, Pittsburgh, PA).
Isolation of murine neutrophils
Mice were killed, femurs and tibias were cut out, and the muscles were removed. The femoral head and condoyles were removed and the bone marrow was flushed with 2 mL Hanks balanced salt solution (HBSS) buffer (Ca2+/Mg2+-free HBSS, 30 mM HEPES, and 15 mM EDTA). Cells were centrifuged (300g for 10 minutes) and resuspended in 1 mL HBSS buffer. The cell suspension was added on top of a Histopaque gradient (Histopaque 1119 and Histopaque 1077-1) and centrifuged for 30 minutes at 700g. The neutrophil-containing lower cell layer was collected and washed twice with HBSS buffer.
Isolation of leukocytes from bone marrow, peritoneal cavity, and blood
To isolate leukocytes from bone marrow, femurs were removed and the bone marrow was harvested by flushing with 2 mL HBSS buffer, as described. Erythrocytes were removed using hypotonic shock, and the leukocytes were resuspended in HBSS buffer. Peritoneal leukocytes were collected by flushing the peritoneal cavity with 3 mL of ice-cold phosphate-buffered saline (PBS; Ca2+/Mg2+-free). Erythrocytes were removed by hypotonic shock, and the peritoneal leukocytes were resuspended in fluorescence-activated cell sorter (FACS) buffer. Blood samples of 10 μL were collected from the mouse tail. Total leukocyte numbers in bone marrow, peritoneal cavity, or blood were determined by light microscopy using a Neubauer hematocytometer (Hawksley, Lancing, United Kingdom).
Flow cytometric identification of neutrophils
For flow cytometric analysis, cells were resuspended in FACS buffer (PBS, 0.5% bovine serum albumin [BSA], and 0.1% NaN3). To examine the amount of neutrophils in perfusate, peritoneal cavity, or bone marrow, surface expression of the granulocyte marker Ly-6G/Ly6C (Gr-1) was determined. Isolated leukocytes (5 × 105/100 μL FACS buffer) were preincubated with murine Fc block for 10 minutes and then stained with anti–Ly-6G/Ly6C (Gr-1)–PE (20 μg/mL) or a PE-labeled isotype-matched control mAb for 20 minutes. The samples were run on a FACSCalibur flow cytometer (BD Biosciences, Oxford, United Kingdom) and analyzed using CellQuest (BD Biosciences) software. Neutrophils were identified as Gr-1high–positive cells and the characteristic high side-scatter profile.
In vivo mobilization of neutrophils into the blood or peritoneal cavity
To determine mobilization of neutrophils from the bone marrow into the blood, Balb/c mice were injected with 100 μL of KC, MIP-2, G-CSF (660 nM each), or PBS via the tail vein. After 2 hours, blood and bone marrow samples were collected. To analyze in vivo mobilization of neutrophils into the peritoneal cavity, Balb/c mice were injected with 200 μL MIP-2, G-CSF (330 nM each), or PBS intraperitoneally. Blood samples and peritoneal leukocytes were collected after 2 hours. The content of neutrophils in the bone marrow and the peritoneal cavity were determined by flow cytometry. Differential blood cell counts were determined in blood smears by KWIK-DIFF stain. Total leukocyte numbers in bone marrow, peritoneal cavity, or blood were determined by light microscopy using a Neubauer hematocytometer.
Thioglycollate-induced peritonitis
Peritonitis was induced by intraperitoneal injection of 500 μL 3% autoclaved thioglycollate broth (Sigma-Aldrich) as described before.35,36 After 2 hours, mice were killed, and peritoneal leukocytes, bone marrow leukocytes, and blood samples were collected. The neutrophil content in the peritoneal cavity, bone marrow, and blood was determined by flow cytometry or differential blood cell counts. In some experiments, mice were pretreated with neutralizing or blocking agents. The blocking antibodies anti-KC (50 μg) and anti–MIP-2 (20 μg); anti–G-CSF (150 μg) and anti–GM-CSF (50 μg); the control antibodies IgG1 (150 μg), IgG2A (50 μg), and IgG2B (20 μg); and the CXCR4 antagonist AMD3100 (100 μg), as well as anti–G-CSF (150 μg) in combination with AMD3100 (100 μg) were injected intraperitoneally in 500 μL PBS 20 minutes before the administration of thioglycollate. Doses of neutralizing antibodies were used according to previous studies.33,37,38
In situ perfusion of the femoral bone marrow
The femoral bone marrow was perfused as previously described.39,40 Briefly, mice were anesthetized, and polyethylene cannulae were inserted into the femoral artery and vein. Modified Krebs-Ringer bicarbonate buffer was used, with the following composition: 10 mM D-glucose, 2.5 mM CaCl2, 0.49 mM MgCl2 × 6H2O, 4.56 mM KCl, 120 mM NaCl, 0.7 mM Na2HPO4, 1.5 mM NaH2PO4, and 24 mM NaHCO3, supplemented with Ficoll T-70 4% and BSA 0.1% and gassed with 95% O2, 5% CO2. The perfusion buffer at 37°C was infused at a rate of 0.1 mL/minute via the arterial cannula and removed from the venous cannula using a Minipulse peristaltic pump (Anachem, Luton, United Kingdom). The vasculature was perfused for an initial 5 minutes to remove blood and then perfused for a further 60 minutes. A total of 400 μL of chemokine or/and cytokine were infused directly into the femoral artery over a period of 10 minutes at a rate of 0.04 mL/minute using an infusion/withdrawal pump (Harvard Instruments, Edenbridge, United Kingdom). The final concentration of chemokine/cytokine was calculated assuming a total volume of 1.4 mL over the 10-minute infusion period. It should be noted that the final concentrations were not measured and are calculated assuming the factors are confined to the perfusion volume. G-CSF (15 nM) was infused directly after the initial 5-minute washout period. In another set of experiments, KC (15 nM) was infused after 35 minutes of perfusion. In a combined G-CSF/KC experiment, G-CSF was infused directly after 5 minutes of perfusion, and KC was infused additionally after 35 minutes of perfusion of the femoral bone marrow. The perfusate was collected onto ice and centrifuged (200g for 10 minutes at 4°C). Erythrocytes were lysed using hypotonic shock. The total number of mobilized leukocytes was counted by light microscopy using a Neubauer hematocytometer. The neutrophil content in perfusate was determined by flow cytometry.
Neutrophil chemotaxis assay
Purified neutrophils were resuspended at a concentration of 5 × 106/mL in chemotaxis assay buffer (RPMI 1640, 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin), and their chemotactic behavior was ascertained, as described previously.41,42 In detail, 20 μL of the neutrophil suspension was placed on top of a Neuroprobe ChemoTx chemotaxis plate (Receptor Technologies, Adderbury, United Kingdom). The bottom well contained 31 μL assay buffer with or without chemokine. Chemokines were used at concentrations as indicated. In some experiments, the cells were pretreated at 37°C with chemokine or cytokine before being placed in the chemotaxis assay. Assay chambers were incubated for 60 minutes at 37°C. The number of neutrophils that migrated into the bottom chamber was determined by a FACSCalibur flow cytometer (BD Biosciences), with relative cell counts obtained by acquiring events for a set time period of 30 seconds.
Statistics
Statistical significance was determined using the 1-way analysis of variance (ANOVA) test followed by the Dunnett multiple comparison test assuming equal variance.
Results
Role of KC, MIP-2, G-CSF, and GM-CSF in acute peritonitis
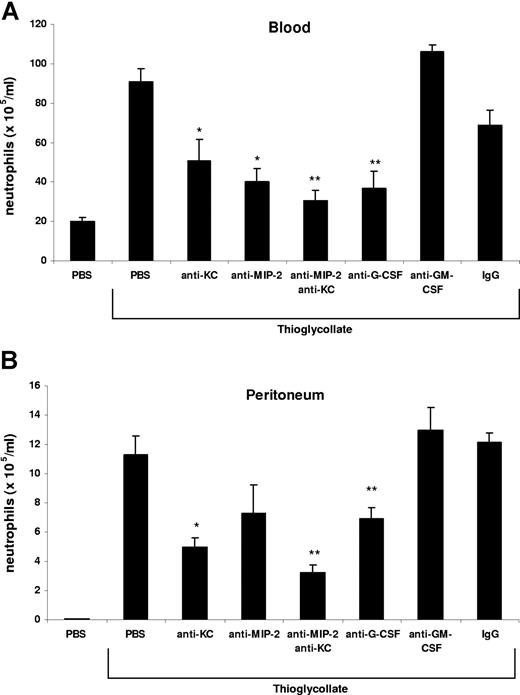
Neutrophils are rapidly mobilized from the bone marrow into the blood during acute inflammatory reactions. In this study, we have used an acute model of thioglycollate-induced peritonitis to examine the factors that stimulate the rapid mobilization of neutrophils from the bone marrow and their recruitment to the site of inflammation. Intraperitoneal injection of 3% thioglycollate into Balb/c mice induced a 4.5-fold increase in circulating neutrophil numbers and a concomitant 200-fold increase in peritoneal neutrophil numbers after 2 hours (Figure 1). Administration of blocking antibodies against either KC or MIP-2 alone inhibited the rise in circulating neutrophil numbers by 45% and 55%, respectively, as well as neutrophil recruitment into the peritoneal cavity by 56% and 35%, respectively. While neutralization of both KC and MIP-2 significantly inhibited the rise in circulating neutrophil numbers by 84% and neutrophil recruitment into the peritoneal cavity by 71%, blockade of G-CSF also inhibited the increase in blood neutrophils by 72% and their recruitment into the peritoneal cavity by 40%. In contrast, blockade of GM-CSF had no effect on neutrophil numbers in the blood or peritoneal cavity (Figure 1). In these experiments, we observed most effective inhibition, with respect to both blood and peritoneal numbers of neutrophils, by neutralizing both chemokines. This is consistent with previous studies that have shown that MIP-2 and KC are functionally redundant and that it is necessary to neutralize both chemokines to reduce neutrophil accumulation at the site of inflammation.37
KC/MIP-2 and G-CSF but not GM-CSF contribute to blood and tissue neutrophilia in TG-induced peritonitis. Neutralizing antibodies against KC (50 μg), MIP-2 (20 μg), KC and MIP-2, G-CSF (150 μg), GM-CSF (50 μg), or rat isotype-control mAb (mixture of control isotype IgGs) were administered intraperitoneally 20 minutes before intraperitoneal injection of 3% thioglycollate (TG). The number of neutrophils per milliliter of blood (A) and the number of neutrophils per milliliter of peritoneal lavage fluid (B) were determined 2 hours following TG injection. Results are expressed as means plus or minus SEM. Data from 6 independent experiments are combined (n = 4-15 mice). **P < .01 (1-way ANOVA and Dunnett test).
KC/MIP-2 and G-CSF but not GM-CSF contribute to blood and tissue neutrophilia in TG-induced peritonitis. Neutralizing antibodies against KC (50 μg), MIP-2 (20 μg), KC and MIP-2, G-CSF (150 μg), GM-CSF (50 μg), or rat isotype-control mAb (mixture of control isotype IgGs) were administered intraperitoneally 20 minutes before intraperitoneal injection of 3% thioglycollate (TG). The number of neutrophils per milliliter of blood (A) and the number of neutrophils per milliliter of peritoneal lavage fluid (B) were determined 2 hours following TG injection. Results are expressed as means plus or minus SEM. Data from 6 independent experiments are combined (n = 4-15 mice). **P < .01 (1-way ANOVA and Dunnett test).
Effect of KC, MIP-2, and G-CSF on neutrophil mobilization and recruitment
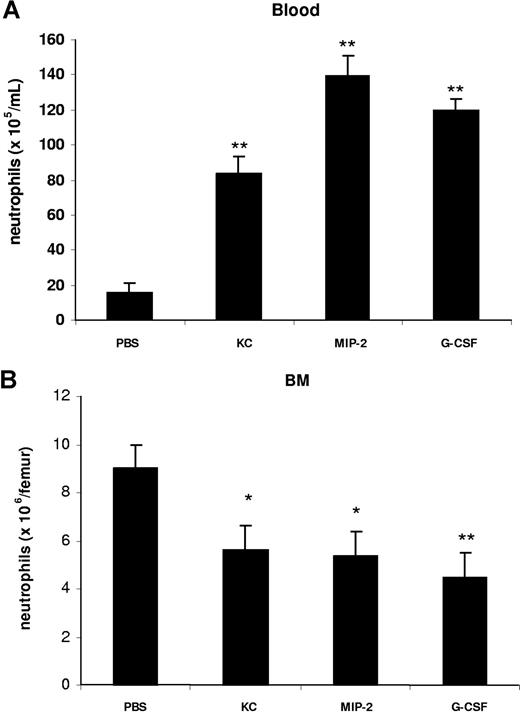
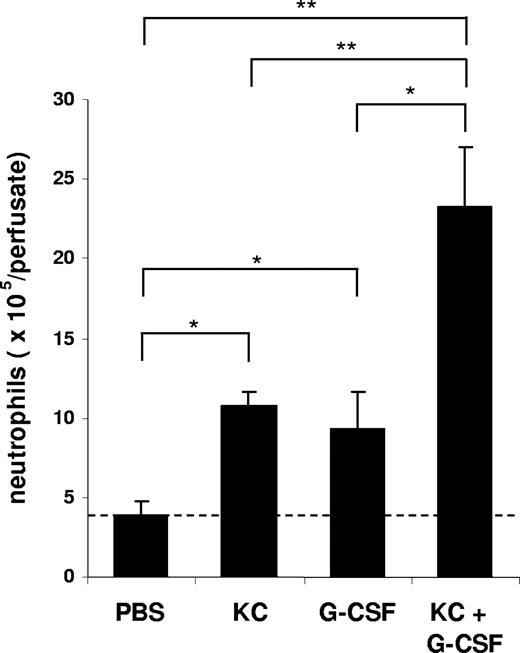
To characterize the effect of KC, MIP-2, and G-CSF on neutrophil mobilization from the bone marrow, we injected 100 μL of optimal concentrations of KC, MIP-2, or G-CSF intravenously into the blood. At 2 hours after a single injection of each factor, we observed a significant increase in circulating neutrophil numbers in the blood and a concomitant reduction of neutrophil numbers in the bone marrow (Figure 2). To specifically address the question whether chemokines and G-CSF could act cooperatively at the level of the bone marrow to mobilize neutrophils, we used an in situ perfusion system of the mouse femoral bone marrow. For these experiments, we used low concentrations of the chemokine and cytokine to enable us to detect synergistic effects of these 2 factors with respect to mobilization, as we have previously observed with the chemokine eotaxin and the cytokine IL-5 regarding eosinophil mobilization.43 In these experiments, subtracting basal release of neutrophils (3.8 × 106), KC, and G-CSF mobilized 6.8 × 106 and 5.4 × 106 neutrophils, respectively, while the infusion of KC and G-CSF together mobilized 19.5 × 106 neutrophils, indicating that these factors acted cooperatively with respect to neutrophil mobilization (Figure 3).
Intravenous administration of KC, MIP-2, and G-CSF stimulates the mobilization of neutrophils from the bone marrow into the blood. Mice injected intravenously with either 100 μL of PBS, KC, MIP-2, or G-CSF (660 nM each). The number of neutrophils per milliliter of blood (A) and the number of bone marrow neutrophils (per femur) (B) were determined 2 hours following injection. Results are expressed as means plus or minus SEM (n = 4 mice). *P < .05; **P < .01 (1-way ANOVA and Dunnett test).
Intravenous administration of KC, MIP-2, and G-CSF stimulates the mobilization of neutrophils from the bone marrow into the blood. Mice injected intravenously with either 100 μL of PBS, KC, MIP-2, or G-CSF (660 nM each). The number of neutrophils per milliliter of blood (A) and the number of bone marrow neutrophils (per femur) (B) were determined 2 hours following injection. Results are expressed as means plus or minus SEM (n = 4 mice). *P < .05; **P < .01 (1-way ANOVA and Dunnett test).
Mobilization of neutrophils from the perfused femoral bone marrow in mice following the infusion of KC and G-CSF alone or in combination. The number of neutrophils (Gr-1high) mobilized from the bone marrow after infusion of PBS, G-CSF (15 nM), KC (15 nM), or G-CSF and KC are shown. Results are expressed as means plus or minus SEM (n = 4 mice). *P < .05; **P < .01 (1-way ANOVA and Dunnett test).
Mobilization of neutrophils from the perfused femoral bone marrow in mice following the infusion of KC and G-CSF alone or in combination. The number of neutrophils (Gr-1high) mobilized from the bone marrow after infusion of PBS, G-CSF (15 nM), KC (15 nM), or G-CSF and KC are shown. Results are expressed as means plus or minus SEM (n = 4 mice). *P < .05; **P < .01 (1-way ANOVA and Dunnett test).
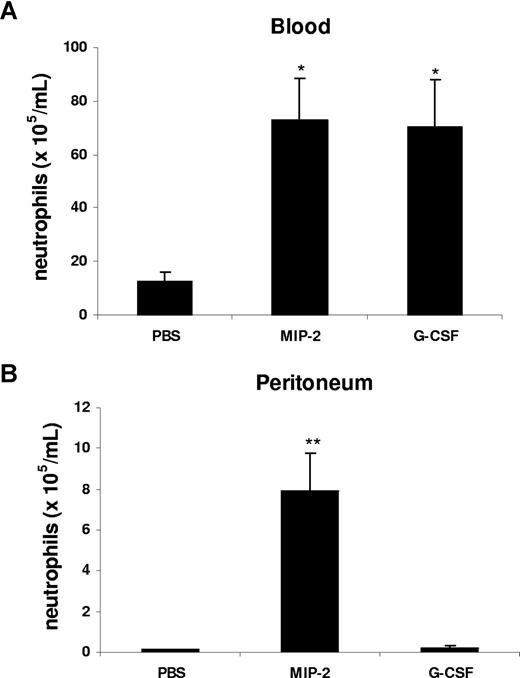
Our data suggest that in the peritonitis model, CXC chemokines and cytokines are generated locally at the site of inflammation, but act systemically to mobilize neutrophils. To model this in vivo, we injected G-CSF or MIP-2 intraperitoneally and examined the effect on mobilization and local recruitment. Interestingly, intraperitoneal administration of MIP-2 led to a significant increase of circulating neutrophil numbers and promoted neutrophil recruitment into the peritoneal cavity, while G-CSF was only effective in acting remotely to stimulate neutrophil mobilization into the blood, but did not stimulate local recruitment into the peritoneum (Figure 4).
G-CSF and MIP-2 both mobilize neutrophils from the bone marrow, while MIP-2 but not G-CSF stimulates recruitment into tissue. Mice were injected intraperitoneally with either 200 μL of PBS, MIP-2, or G-CSF (330 nM each). The number of neutrophils per milliliter of blood (A) and the number of neutrophils per milliliter of peritoneal lavage fluid (B) were determined 2 hours following injection. Results are expressed as means plus or minus SEM (n = 3 mice). *P < .05; **P < .01 (1-way ANOVA and Dunnett test).
G-CSF and MIP-2 both mobilize neutrophils from the bone marrow, while MIP-2 but not G-CSF stimulates recruitment into tissue. Mice were injected intraperitoneally with either 200 μL of PBS, MIP-2, or G-CSF (330 nM each). The number of neutrophils per milliliter of blood (A) and the number of neutrophils per milliliter of peritoneal lavage fluid (B) were determined 2 hours following injection. Results are expressed as means plus or minus SEM (n = 3 mice). *P < .05; **P < .01 (1-way ANOVA and Dunnett test).
The effect of G-CSF on neutrophils is neither chemotactic nor chemokinetic
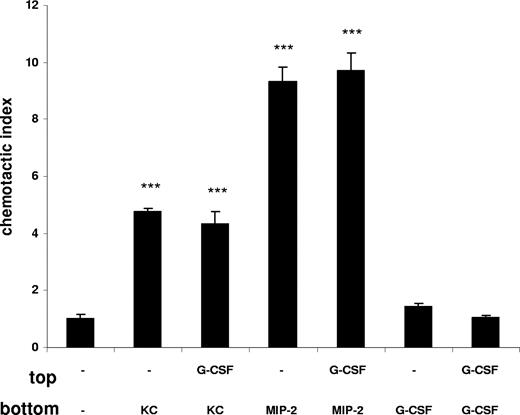
To understand further the mechanism whereby G-CSF promotes neutrophil mobilization during acute inflammation, we performed in vitro chemotaxis assays with freshly purified murine bone marrow neutrophils. As shown in Figure 5, neutrophils migrate well toward the chemokines KC and MIP-2, whereas no migration toward G-CSF was detectable (Figure 5). In addition, we wanted to examine whether G-CSF has a chemokinetic or priming effect on neutrophils, enhancing their chemotactic behavior. Our results show that direct treatment of neutrophils with G-CSF does not affect their chemotaxis toward KC or MIP-2 (Figure 5). Further, when neutrophils were preincubated for 30 minutes with G-CSF, the response to chemokines was not enhanced (data not shown), indicating that G-CSF did not prime murine neutrophils. Finally, addition of G-CSF to both the upper and lower wells did not stimulate neutrophil migration, indicating that G-CSF was not chemokinetic for murine neutrophils. These data suggest that the effect of G-CSF on murine neutrophils is neither chemotactic nor chemokinetic, and that G-CSF has no priming effect with respect to chemokine-stimulated chemotaxis.
G-CSF does not stimulate the chemotaxis or chemokinesis of murine neutrophils. Freshly isolated murine bone marrow neutrophils were used in a chemotaxis assay. Neutrophils were placed on top of a chemotaxis plate in the presence or absence of G-CSF (10 nM). KC (1 nM), MIP-2 (1 nM), or G-CSF (10 nM) were added to the bottom chamber as indicated. Migration of neutrophils is expressed as the mean number of cells counted per bottom well, mean plus or minus SD. A representative figure of 3 independent experiments is shown (n = 3). ***P < .001 (1-way ANOVA and Dunnett test).
G-CSF does not stimulate the chemotaxis or chemokinesis of murine neutrophils. Freshly isolated murine bone marrow neutrophils were used in a chemotaxis assay. Neutrophils were placed on top of a chemotaxis plate in the presence or absence of G-CSF (10 nM). KC (1 nM), MIP-2 (1 nM), or G-CSF (10 nM) were added to the bottom chamber as indicated. Migration of neutrophils is expressed as the mean number of cells counted per bottom well, mean plus or minus SD. A representative figure of 3 independent experiments is shown (n = 3). ***P < .001 (1-way ANOVA and Dunnett test).
G-CSF disrupts the SDF-1α/CXCR4 chemokine axis during neutrophil mobilization
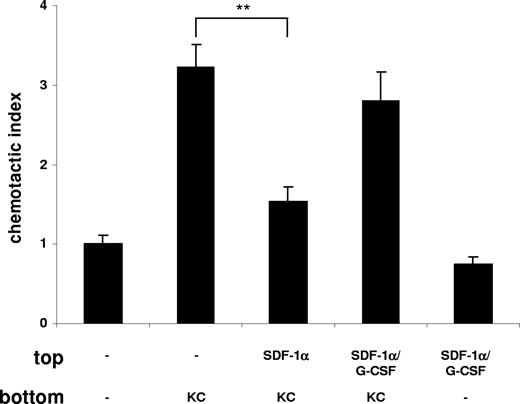
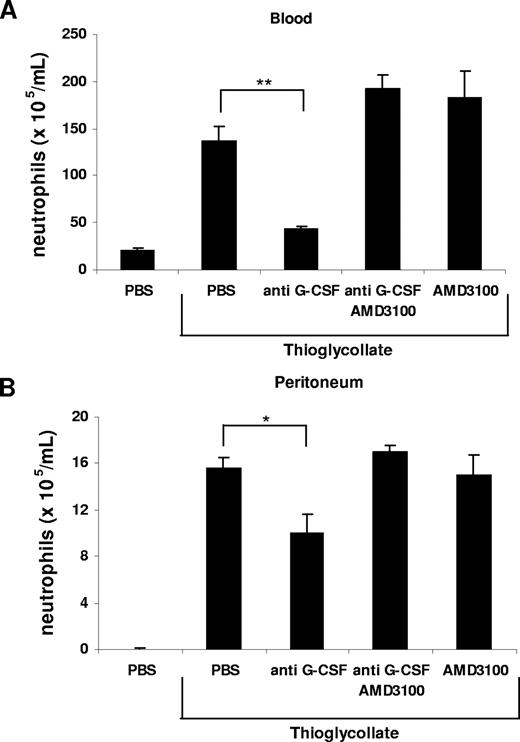
It was previously postulated that chronic treatment with G-CSF may promote neutrophil mobilization from the bone marrow by disrupting the SDF-1α/CXCR4 chemokine axis.20,21,23,24,40 To investigate whether G-CSF interfered with the SDF-1α/CXCR4 retention signal, we performed another in vitro chemotaxis assay. We mimicked the retention of neutrophils by SDF-1α in the bone marrow by pretreatment of freshly isolated murine bone marrow neutrophils with SDF-1α, which resulted in a significant inhibition of their migration toward KC (Figure 6) as previously reported.40 This inhibitory effect of SDF-1α on neutrophil chemotaxis could be abrogated by addition of G-CSF, reconstituting the migration toward KC (Figure 6). To demonstrate the effect of G-CSF on the SDF-1α/CXCR4 chemokine axis in vivo, we reverted to the thioglycollate-induced peritonitis model. Remarkably, we could reverse the inhibition of neutrophil mobilization from the bone marrow and their recruitment into the peritoneal cavity, caused by administration of the G-CSF–neutralizing mAb, by directly blocking CXCR4 with the antagonist AMD3100 (Figure 7). We conclude from these data that G-CSF contributes to neutrophil mobilization by disrupting the SDF-1α/CXCR4 retention signal under acute inflammatory conditions.
G-CSF blocks the retention of neutrophils by SDF-1α. Freshly isolated murine bone marrow neutrophils were used in a chemotaxis assay and then placed on top of the chemotaxis plate in the presence or absence of G-CSF (10 nM) as indicated. Neutrophils were preincubated in the presence or absence of SDF-1α (50 nM) for 15 minutes at 37°C. KC (1 nM) was added to the bottom chamber as indicated. Migration of neutrophils is expressed as the mean number of cells counted per bottom well, mean plus or minus SD. A representative figure of 4 independent experiments is shown (n = 3). **P < .01 (1-way ANOVA and Dunnett test).
G-CSF blocks the retention of neutrophils by SDF-1α. Freshly isolated murine bone marrow neutrophils were used in a chemotaxis assay and then placed on top of the chemotaxis plate in the presence or absence of G-CSF (10 nM) as indicated. Neutrophils were preincubated in the presence or absence of SDF-1α (50 nM) for 15 minutes at 37°C. KC (1 nM) was added to the bottom chamber as indicated. Migration of neutrophils is expressed as the mean number of cells counted per bottom well, mean plus or minus SD. A representative figure of 4 independent experiments is shown (n = 3). **P < .01 (1-way ANOVA and Dunnett test).
Inhibition of neutrophil mobilization by blockade of G-CSF is abrogated by administration of AMD3100. Anti–G-CSF–blocking antibody (150 μg) and AMD3100 (100 μg) alone or in combination were administered intraperitoneally 20 minutes before intraperitoneal injection of 3% TG. The number of neutrophils per milliliter of blood (A) and the number of neutrophils per milliliter of peritoneal lavage fluid (B) were determined 2 hours following TG injection. Results are expressed as means plus or minus SEM (n = 3). *P < .05; **P < .005 (1-way ANOVA and Dunnett test).
Inhibition of neutrophil mobilization by blockade of G-CSF is abrogated by administration of AMD3100. Anti–G-CSF–blocking antibody (150 μg) and AMD3100 (100 μg) alone or in combination were administered intraperitoneally 20 minutes before intraperitoneal injection of 3% TG. The number of neutrophils per milliliter of blood (A) and the number of neutrophils per milliliter of peritoneal lavage fluid (B) were determined 2 hours following TG injection. Results are expressed as means plus or minus SEM (n = 3). *P < .05; **P < .005 (1-way ANOVA and Dunnett test).
Discussion
Mobilization of neutrophils from the bone marrow is the first step in their trafficking to sites of infection or inflammation. In this study, we have identified a unique combinatorial effect of the ELR + CXC chemokines KC/MIP-2 and the cytokine G-CSF with respect to the rapid mobilization of neutrophils from the bone marrow in a model of acute peritonitis. While it is well documented that ELR + CXC chemokines play an important role in the recruitment of neutrophils from the blood into tissues during inflammation,7,28,35,44 this is the first report that ELR + CXC chemokines contribute to neutrophil mobilization from the bone marrow during acute inflammatory reactions.
Interestingly, we show that at these early time points (2 hours), G-CSF also plays a critical role in mobilizing neutrophils from the bone marrow. In contrast, GM-CSF, a cytokine required for emergency granulopoiesis in response to infection and inflammation, a response that occurs over several days,45,46 is not required for the rapid neutrophil mobilization (Figure 1A). We have previously shown that the cytokine IL-5 drives the mobilization of eosinophils from the bone marrow in models of allergic airway inflammation.39,47 Others have recently shown that chemokines play a central role in mobilizing monocytes from the bone marrow in models of chronic inflammation.11,,,–15 This is therefore the first demonstration that chemokines, in combination with a cytokine, are required for leukocyte mobilization during a specific inflammatory reaction.
To directly assess whether locally generated chemokines and G-CSF can act remotely to mobilize neutrophils from the bone marrow, we examined the effect of a single intraperitoneal injection of these factors on neutrophil recruitment into the blood and tissue. The results shown in Figure 4 indicate that MIP-2 acts both systemically to increase circulating neutrophil numbers and locally to promote neutrophil recruitment into the tissue. In this regard, it is interesting that the same chemokine can stimulate mobilization and recruitment, as it suggests that neutrophils are not desensitized by the initial mobilizing response to the chemokine. This may reflect the low concentrations of chemokines that actually promote these responses in vivo as compared with high levels of CXCR2 expression on mature neutrophils.40 In contrast, G-CSF although administered intraperitoneally did not stimulate the local recruitment of neutrophils into the peritoneum, but rather acted systemically to stimulate neutrophil mobilization.
The lack of effect of G-CSF with respect to local recruitment from the blood into the peritoneum suggested to us that G-CSF was not chemotactic for murine neutrophils. In fact, in vitro chemotaxis assays showed that G-CSF was neither chemotactic nor chemokinetic for murine bone marrow neutrophils (Figure 5). It should be noted that previous results have been reported with respect to the chemokinetic/chemotactic effects of human G-CSF on human peripheral blood neutrophils.48,,–51 As we can find no reports of effects of G-CSF stimulating the migration of murine neutrophils, there may be a species difference in this context. Finally, we were not able to show a priming effect of G-CSF with respect to chemokine-stimulated chemotaxis (Figure 5), again an effect reported with human neutrophils.52,53
Mature neutrophils in the bone marrow reserve are present in the hematopoietic compartment, and mobilization is dependent on their migration across the bone marrow endothelium into the bone marrow sinuses. We believe that chemokines in the blood create a gradient across the bone marrow sinusoidal endothelium, thereby stimulating neutrophil migration out of the bone marrow into the blood. Indeed, using an in situ perfusion system of the femoral bone marrow, we have shown directly that infusing the ELR plus CXC chemokines KC (CXCL1) and MIP-2 (CXCL2) into the bone marrow vasculature stimulates the rapid mobilization of neutrophils.40,54 In addition, we have previously shown that the cytokine IL-5, which stimulates eosinophil chemokinesis, mobilizes eosinophils from the bone marrow in vivo.39 Furthermore, we have shown a synergistic effect between the chemokinetic effect of IL-5 and the chemotactic effect of the chemokine eotaxin, with respect to eosinophil migration in vitro and mobilization in situ.43 In contrast, in this study we found that G-CSF alone or in combination with chemokines does not stimulate neutrophil migration in vitro (Figure 5). Thus, we believe that G-CSF acts in a distinct manner to the chemokines to mobilize neutrophils from the bone marrow.
SDF-1α is expressed constitutively in the bone marrow, and numerous lines of evidence support the hypothesis that the SDF-1α/CXCR4 chemokine axis plays an important role in the retention of neutrophils within the bone marrow.40,55,–57 Chronic treatment of mice with G-CSF over 4 to 5 days stimulates neutrophil granulopoiesis and mobilization. It has been proposed that mobilization may be due to a reduction in the levels of SDF-1α in the bone marrow,20,23 inhibition of CXCR4 expression,21,25 or cleavage of CXCR4 from the cell surface.21,24 The role of proteases in this response has recently been excluded.21,58 In this study, experiments performed in vitro show for the first time that G-CSF can rapidly inhibit the retention signal delivered by SDF-1α (Figure 6). G-CSF can therefore stimulate the acute mobilization of neutrophils from the bone marrow by disrupting the CXCR4/SDF-1α chemokine axis in the same way that the CXCR4 antagonist induces a rapid blood neutrophilia.40,59 Indeed, this mechanism of G-CSF–stimulated mobilization is further supported by the data presented in Figure 7 showing that the CXCR4 antagonist reverses the inhibition of neutrophil mobilization from the bone marrow caused by administration of the G-CSF–neutralizing mAb.
The fact that in the model of peritonitis blockade of either the chemokines or G-CSF alone resulted in a greater than 70% inhibition of blood neutrophilia suggests that G-CSF and the chemokines may act in a coordinated manner with respect to mobilization. To assess this directly, we used an in situ perfusion system of the mouse femoral bone marrow. We show here that while neutrophils were mobilized in response to direct infusion of G-CSF and KC alone, a more than additive effect was observed with respect to mobilization when KC and G-CSF were infused together (Figure 3), suggesting that these factors may indeed act in a coordinated fashion in vivo. It has been shown that transgenic mice, which carry a targeted (knock-in) mutation of their G-CSFR such that the cytoplasmic (signaling) domain of the G-CSFR is replaced with that of the erythropoietin receptor (EpoR), exhibit near-normal levels of mature neutrophils in the bone marrow, while treatment with G-CSF fails to induce significant mobilization of neutrophils to the blood. In accordance with our data, these GEpoR mice show normal chemokine-stimulated mobilization of neutrophils from the bone marrow, indicating that the chemokine-induced mobilization of neutrophils is indeed G-CSF–independent.20,60
Taken together, our data suggest a new paradigm for the acute mobilization of neutrophils from the bone marrow, requiring the coordinated actions of both G-CSF and the CXC chemokines KC and MIP-2 acting via distinct mechanisms. Thus, under acute inflammatory conditions, systemically acting chemokines promote mobilization by stimulating the chemotaxis of neutrophils across the bone marrow sinusoidal endothelium, a process facilitated by G-CSF that acts by blocking the retention signal delivered by bone marrow–derived SDF-1α.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the European Community INNOCHEM (LSHB-CT-2005-518167), the Wellcome Trust (project grant no. 073677/Z/03/Z), and the British Heart Foundation (project grant no. PG/05/-92).
Wellcome Trust
Authorship
Contribution: A.M.W., S.C.P., and R.C.F. performed experiments; A.M.W. and S.M.R. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sara M. Rankin, Leukocyte Biology Section, National Heart and Lung Institute, Imperial College London, Sir Alexander Fleming Building, South Kensington Campus, Exhibition Road, London SW7 2AZ, United Kingdom; e-mail: s.rankin@imperial.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal