Abstract
Although chronic lymphocytic leukemia (CLL) has remained incurable with standard treatments, newer therapeutic approaches, such as chemoimmunotherapy or stem cell transplantation, bear the potential for prolonged survival. We estimated trends in age-specific 5- and 10-year absolute and relative survival of CLL patients in the United States between 1980-1984 and 2000-2004 from the 1973 to 2004 database of the Surveillance, Epidemiology, and End Results Program. Period analysis was used to disclose recent developments with minimum delay. Overall, 5- and 10-year absolute survival from diagnosis increased from 54.2% to 60.2% (+6 percentage points; P < .0001) and from 27.8% to 34.8% (+7 percentage points; P < .0001), respectively. Despite a strong age gradient in prognosis, increases in 5-year absolute and relative survival over time were rather homogeneous across age groups. In contrast, increases in 10-year absolute and relative survival close to or well above 10% units were observed for all patients younger than 80 years of age at diagnosis compared with no increase at all for older patients. Long-term survival expectations of patients with CLL have substantially improved over the past 2 decades except for patients 80 years of age or older at the time of diagnosis. Future studies are needed to confirm and expand our findings.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in adults in the Western world and predominantly affects the elderly. Although CLL remains incurable with standard treatments, important progress in treatment, which classically is given only when the disease has become symptomatic, has been made in recent years. The aim of this study was to monitor progress in long-term survival of patients diagnosed with CLL from the early 1980s to the early 21st century using novel techniques of period analysis to define variation in relative survival over time.1,2
Methods
All data presented in this paper are derived from the 1973-2004 limited-use database of the Surveillance, Epidemiology, and End Results (SEER) Program of the United States National Cancer Institute issued in April 2007.3 Data included in the 1973 to 2004 SEER database are from population-based cancer registries in the states of Connecticut, New Mexico, Utah, Iowa, and Hawaii, and the metropolitan area of Atlanta, Detroit, Seattle-Puget Sound, and San Francisco–Oakland, which, together, cover a population of approximately 30 million people. Geographic areas were selected for inclusion in the SEER program based on their ability to operate and maintain a high-quality population-based cancer reporting system and for their epidemiologically significant population subgroups.
Overall, 20 847 patients 15 years of age or older with a first diagnosis of CLL (and no previous cancer diagnosis) between 1973-2004, who have been followed for vital status until the end of 2004, were included in the dataset. After exclusion of 24 patients (0.12%) who were reported by autopsy only and 332 patients (1.59%) who were reported by death certificate only, there remained 20 491 patients (98.29%) for the survival analysis.
Five- and 10-year survival was calculated for the calendar periods 1980-1984, 1985-1989, 1990-1994, 1995-1999, and 2000-2004 using the period analysis methodology.1 Furthermore, we tested for statistical significance of trends in 5- and 10-year survival between 1980-1984 and 2000-2004 by a recently described modeling approach.2 All analyses were carried out separately for the following 4 major age groups: 15 to 59, 60 to 69, 70 to 79, and 80 and older, as well as for men and women.
With period analysis, first proposed by Brenner and Gefeller in 1996,4 only survival experience during the period of interest is included in the analysis. This is achieved by left truncation of observations at the beginning of the period in addition to right censoring at its end. It has been shown by extensive empirical evaluation that period analysis provides more up-to-date long-term survival estimates than traditional “cohort-based” survival analysis and quite closely predicts long-term survival expectations of cancer patients diagnosed within the period of interest.5-8 The method has been included recently in major statistical software packages, including the SEER*Stat package released by the National Cancer Institute,9 to facilitate more up-to-date cancer survival estimation.
According to standard practice in population-based cancer survival analysis, relative survival was calculated in addition to absolute (observed) survival. Relative survival reflects survival of cancer patients compared with survival of the general population. It is calculated as the ratio of absolute survival of cancer patients divided by the expected survival of a group of persons of the corresponding sex, age, and race in the general population.10,11 Estimates of expected survival were derived according to the so-called Ederer II method12 using US sex-, age-, and race-specific life tables.13
For most malignancies, relative survival in subsequent years among patients who have already survived the first years after diagnosis, called “conditional relative survival rates,” increases with time from diagnosis and approaches 100% in the longer run if most of the surviving cancer patients can be considered to be cured. However, CLL remains incurable in most cases. We therefore assessed conditional relative survival in the subsequent 10 years among patients with CLL who had survived the first years after diagnosis. This analysis was restricted to the calendar period 2000-2004 because the time series of data included in the SEER database is too short to provide analogous calculations for the earlier calendar periods. Furthermore, we restricted this part of the analysis to patients younger than 60 years of age and those 60 to 69 at the time of diagnosis because very long-term survival expectations are rather low even in the general population at higher ages.
Results
Numbers of cases by age group, sex, and calendar period are shown in Table 1. More than half of the cases were 70 years of age or older at the time of diagnosis. CLL was most commonly diagnosed in the 70 to 79 age group, with approximately 30% of cases occurring in this age group. Overall numbers of cases increased by approximately 20% between 1980-1984 and 2000-2004. The increase was most pronounced (> 40%) in age groups 15 to 59 years of age and 80 and older, whereas a more modest increase (+19%), and no increase at all was seen in age groups 70 to 79 and 60 to 69 years, respectively. Approximately 60% of cases were men, and this proportion remained fairly stable over time.
Numbers of patients with CLL by age group, sex, and calendar period
| Age, sex . | Calendar period . | Total . | ||||
|---|---|---|---|---|---|---|
| 1980-1984 . | 1985-1989 . | 1990-1994 . | 1995-1999 . | 2000-2004 . | ||
| All | 3642 | 3968 | 4236 | 4191 | 4454 | 20 491 |
| 15-59 | 670 | 762 | 812 | 928 | 965 | 4137 |
| 60-69 | 1079 | 1115 | 1164 | 956 | 1055 | 5369 |
| 70-79 | 1096 | 1252 | 1311 | 1343 | 1303 | 6305 |
| 80+ | 797 | 839 | 949 | 964 | 1131 | 4680 |
| Men | 2177 | 2355 | 2451 | 2492 | 2645 | 12 120 |
| Women | 1465 | 1613 | 1785 | 1699 | 1809 | 8371 |
| Age, sex . | Calendar period . | Total . | ||||
|---|---|---|---|---|---|---|
| 1980-1984 . | 1985-1989 . | 1990-1994 . | 1995-1999 . | 2000-2004 . | ||
| All | 3642 | 3968 | 4236 | 4191 | 4454 | 20 491 |
| 15-59 | 670 | 762 | 812 | 928 | 965 | 4137 |
| 60-69 | 1079 | 1115 | 1164 | 956 | 1055 | 5369 |
| 70-79 | 1096 | 1252 | 1311 | 1343 | 1303 | 6305 |
| 80+ | 797 | 839 | 949 | 964 | 1131 | 4680 |
| Men | 2177 | 2355 | 2451 | 2492 | 2645 | 12 120 |
| Women | 1465 | 1613 | 1785 | 1699 | 1809 | 8371 |
In 1980-1984, 54.2% of all patients were still alive 5 years after diagnosis (Table 2 upper block). This proportion increased to 60.2% in 2000-2004 (P < .001). There was a strong age gradient in 5-year survival that slightly diminished over time because of stronger improvements in the 3 older age groups than in the youngest age group. In contrast, in 10-year survival, the largest increase (+12.4 percentage points) was seen in the 15 to 59 age group; still, rather substantial increases close to 10% units were seen in age groups 60 to 69 and 70 to 79, but no improvement at all was seen among the oldest patients, for whom 10-year survival remained as low as 6% (Table 2 second block). The still significant P value for this age group is explained by the fact that this P value refers to the entire 10-year follow-up period, and thus still reflects the nonpersistent improvement in the early years after diagnosis.
Five- and 10-year estimates of absolute and relative survival of patients with CLL by age group, sex, and calendar period
| Age, sex . | Calendar period . | Increase* . | P† . | |||
|---|---|---|---|---|---|---|
| 1980-1984 . | 2000-2004 . | |||||
| PE . | SE . | PE . | SE . | |||
| 5-year absolute survival | ||||||
| All | 54.2 | 0.9 | 60.2 | 0.8 | +6.0 | <.0001 |
| 15-59 | 76.6 | 1.6 | 81.0 | 1.3 | +4.4 | <.001 |
| 60-69 | 66.4 | 1.5 | 74.5 | 1.4 | +8.1 | <.0001 |
| 70-79 | 49.8 | 1.6 | 58.5 | 1.4 | +8.7 | <.0001 |
| 80+ | 24.0 | 1.6 | 30.0 | 1.5 | +6.0 | <.001 |
| Men | 51.7 | 1.1 | 59.7 | 1.0 | +8.0 | <.001 |
| Women | 57.6 | 1.3 | 60.9 | 1.2 | +3.3 | .008 |
| 10-year absolute survival | ||||||
| All | 27.8 | 0.9 | 34.8 | 0.7 | +7.0 | <.001 |
| 15-59 | 46.5 | 2.3 | 58.9 | 1.7 | +12.4 | <.001 |
| 60-69 | 38.1 | 1.9 | 48.1 | 1.6 | +10.0 | <.001 |
| 70-79 | 20.8 | 1.6 | 30.0 | 1.3 | +9.2 | <.001 |
| 80+ | 6.7 | 1.1 | 6.4 | 0.8 | −0.3 | .011 |
| Men | 24.5 | 1.2 | 33.6 | 1.0 | +9.1 | <.001 |
| Women | 32.2 | 1.5 | 36.4 | 1.2 | +4.2 | .019 |
| 5-year relative survival | ||||||
| All | 69.2 | 1.1 | 75.2 | 0.9 | +6.0 | <.001 |
| 15-59 | 80.8 | 1.7 | 84.1 | 1.3 | +3.3 | .004 |
| 60-69 | 75.8 | 1.8 | 82.7 | 1.6 | +6.9 | .003 |
| 70-79 | 66.3 | 2.1 | 74.3 | 1.8 | +8.0 | <.001 |
| 80+ | 48.4 | 3.1 | 56.9 | 2.8 | +8.5 | .008 |
| Men | 67.2 | 1.5 | 74.4 | 1.2 | +7.2 | <.001 |
| Women | 71.9 | 1.6 | 76.4 | 1.5 | +4.5 | .002 |
| 10-year relative survival | ||||||
| All | 46.0 | 1.5 | 55.4 | 1.2 | +9.4 | <.001 |
| 15-59 | 53.1 | 2.6 | 64.5 | 1.8 | +11.4 | <.001 |
| 60-69 | 52.6 | 2.6 | 62.7 | 2.1 | +10.1 | .021 |
| 70-79 | 42.1 | 3.2 | 55.0 | 2.3 | +12.9 | <.001 |
| 80+ | 32.7 | 5.0 | 29.6 | 3.6 | −3.1 | .058 |
| Men | 42.0 | 2.0 | 53.5 | 1.5 | +11.5 | <.001 |
| Women | 51.2 | 2.3 | 58.1 | 1.8 | +6.9 | .003 |
| Age, sex . | Calendar period . | Increase* . | P† . | |||
|---|---|---|---|---|---|---|
| 1980-1984 . | 2000-2004 . | |||||
| PE . | SE . | PE . | SE . | |||
| 5-year absolute survival | ||||||
| All | 54.2 | 0.9 | 60.2 | 0.8 | +6.0 | <.0001 |
| 15-59 | 76.6 | 1.6 | 81.0 | 1.3 | +4.4 | <.001 |
| 60-69 | 66.4 | 1.5 | 74.5 | 1.4 | +8.1 | <.0001 |
| 70-79 | 49.8 | 1.6 | 58.5 | 1.4 | +8.7 | <.0001 |
| 80+ | 24.0 | 1.6 | 30.0 | 1.5 | +6.0 | <.001 |
| Men | 51.7 | 1.1 | 59.7 | 1.0 | +8.0 | <.001 |
| Women | 57.6 | 1.3 | 60.9 | 1.2 | +3.3 | .008 |
| 10-year absolute survival | ||||||
| All | 27.8 | 0.9 | 34.8 | 0.7 | +7.0 | <.001 |
| 15-59 | 46.5 | 2.3 | 58.9 | 1.7 | +12.4 | <.001 |
| 60-69 | 38.1 | 1.9 | 48.1 | 1.6 | +10.0 | <.001 |
| 70-79 | 20.8 | 1.6 | 30.0 | 1.3 | +9.2 | <.001 |
| 80+ | 6.7 | 1.1 | 6.4 | 0.8 | −0.3 | .011 |
| Men | 24.5 | 1.2 | 33.6 | 1.0 | +9.1 | <.001 |
| Women | 32.2 | 1.5 | 36.4 | 1.2 | +4.2 | .019 |
| 5-year relative survival | ||||||
| All | 69.2 | 1.1 | 75.2 | 0.9 | +6.0 | <.001 |
| 15-59 | 80.8 | 1.7 | 84.1 | 1.3 | +3.3 | .004 |
| 60-69 | 75.8 | 1.8 | 82.7 | 1.6 | +6.9 | .003 |
| 70-79 | 66.3 | 2.1 | 74.3 | 1.8 | +8.0 | <.001 |
| 80+ | 48.4 | 3.1 | 56.9 | 2.8 | +8.5 | .008 |
| Men | 67.2 | 1.5 | 74.4 | 1.2 | +7.2 | <.001 |
| Women | 71.9 | 1.6 | 76.4 | 1.5 | +4.5 | .002 |
| 10-year relative survival | ||||||
| All | 46.0 | 1.5 | 55.4 | 1.2 | +9.4 | <.001 |
| 15-59 | 53.1 | 2.6 | 64.5 | 1.8 | +11.4 | <.001 |
| 60-69 | 52.6 | 2.6 | 62.7 | 2.1 | +10.1 | .021 |
| 70-79 | 42.1 | 3.2 | 55.0 | 2.3 | +12.9 | <.001 |
| 80+ | 32.7 | 5.0 | 29.6 | 3.6 | −3.1 | .058 |
| Men | 42.0 | 2.0 | 53.5 | 1.5 | +11.5 | <.001 |
| Women | 51.2 | 2.3 | 58.1 | 1.8 | +6.9 | .003 |
PE indicates point estimate.
Increase from 1980-1984 to 2000-2004 in percentage points.
P value for trend from calendar period 1980-1984 to calendar period 2000-2004.
Given the high rates of mortality from other causes at older ages, relative survival rates provide a better picture of progress against excess mortality attributable to CLL over time. Even when looking at relative survival, 10-year survival rates are substantially lower than 5-year survival rates in all age groups, which reflects the frequent occurrence of late deaths attributable to CLL and the general lack of cure for patients with CLL (Table 2 blocks 3 and 4). However, the gap between 5- and 10-year relative survival has diminished substantially because of particularly pronounced improvement (+11.4 percentage points) in the latter in the 15 to 59 age group, whereas the opposite is true for the 80 and older age group. Increased survival in the first 5 years after diagnosis could not be maintained in the longer run among these patients. Relative survival was almost as good and showed similar trends in the 60 to 69 age group compared with the 15 to 59 age group. Relative survival remained somewhat lower in the 70 to 79 age group, even though improvement in 10-year relative survival was strongest in this age group (+12.9 percentage points).
Both 5-year absolute and 5-year relative survival, and especially 10-year absolute and 10-year relative survival, were substantially higher among women than among men in 1980-1984. Survival increased over time among both men and women. As a result of stronger increases in men than among women, the gender differences decreased over time.
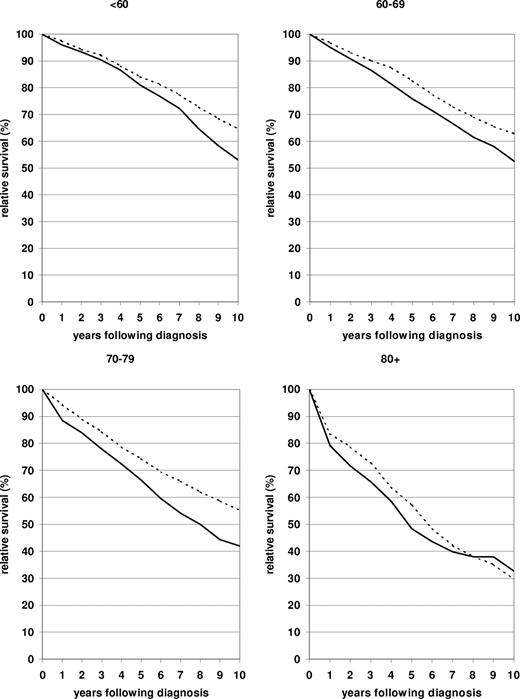
A more comprehensive picture of the relative survival curves more than 10 years after diagnosis in 2000-2004 compared with 1980-1984 in the 4 age groups is shown in Figure 1. The lack of flattening of the relative survival curves indicates the long-term persistence of excess mortality for this malignancy. Nevertheless, marked improvements were achieved throughout 10 years after diagnosis in the younger than 60, 60 to 69, and 70 to 79 age groups, whereas the moderate improvement seen in the 80 and older age group up to 5 years after diagnosis decreased and eventually was lost entirely in the longer run.
Ten-year relative survival curves of patients with CLL by major age group. Period estimates for 1980-1984 ( ) and 2000-2004 (
) and 2000-2004 ( ).
).
Ten-year relative survival curves of patients with CLL by major age group. Period estimates for 1980-1984 ( ) and 2000-2004 (
) and 2000-2004 ( ).
).
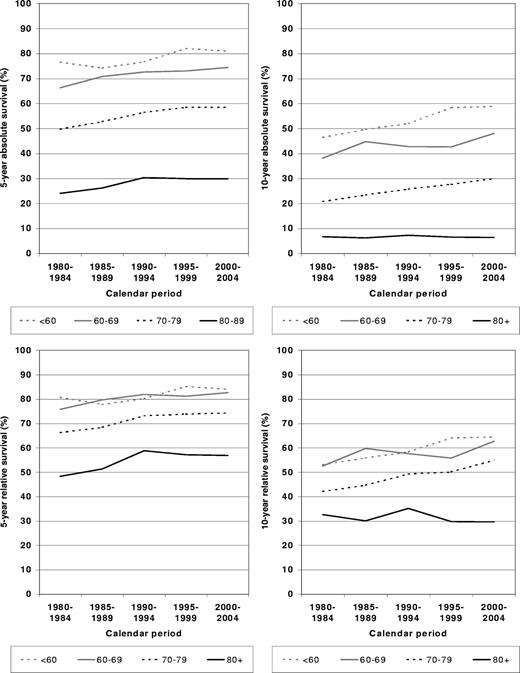
To address the question of in which periods the largest improvements were achieved, 5- and 10-year absolute and relative survival rates are shown by calendar period in Figure 2. In patients diagnosed younger than 60 years of age, the largest improvements were seen between 1990-1994 and 1995-1999. Patients 60 to 69 years of age at diagnosis seem to have caught up with this development and closed the gap in terms of relative survival in 2000-2004. A rather steady upward trend over time was observed for both absolute and relative survival for the 70 to 79 age group. In the 80 and older age group, 5-year absolute and relative survival increased between 1980-1984 and 1990-1994, but this increase did not continue in the later periods, and it was not maintained beyond 5 years after diagnosis.
Period estimates of 5- and 10-year absolute and relative survival of patients with CLL by major age groups in defined calendar periods between calendar period 1980-1984 and calendar period 2000-2004.
Period estimates of 5- and 10-year absolute and relative survival of patients with CLL by major age groups in defined calendar periods between calendar period 1980-1984 and calendar period 2000-2004.
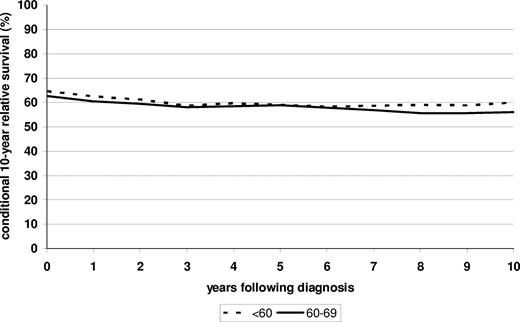
Despite improvements in prognosis over time, conditional relative survival in subsequent 10 years continued to slowly decrease (from levels slightly above 60% to levels slightly below 60%) rather than increase within 10 years after diagnosis in the 2000-2004 period, among both patients younger than 60 and patients 60 to 69 years of age at the time of diagnosis (Figure 3).
Conditional survival in subsequent 10 years among patients younger than 60 and 60 to 69 years of age at diagnosis who have already survived 0 to 10 years after diagnosis. Period analysis is for the 2000-2004 period.
Conditional survival in subsequent 10 years among patients younger than 60 and 60 to 69 years of age at diagnosis who have already survived 0 to 10 years after diagnosis. Period analysis is for the 2000-2004 period.
Discussion
This first application of period analysis to age-specific long-term survival of patients with CLL discloses slow but steady improvement in both 5- and 10-year survival of patients diagnosed with CLL younger than 80 years of age between 1980-1984 and 2000-2004. In the oldest age group (80 and older), improvement is also seen in the first 5 years after diagnosis but not in the longer run. In 2000-2004, 10-year relative survival close to 65% was achieved for patients younger than 70 years of age, and 10-year relative survival of 55% was reached in the 70 to 79 age group. Increases in survival were stronger in men than in women, thereby reducing sex differences in prognosis that have been consistently observed in the past.14 Despite increased survival from diagnosis, conditional relative survival in the subsequent 10 years remained at levels around 60% even in 2000-2004 and did not increase with time from diagnosis among patients who had survived the first years after diagnosis, indicating that cures are still rare.
Overall, trends were very similar for absolute and relative survival rates. The consistently somewhat stronger improvement in 10-year compared with 5-year survival in all but the oldest age groups indicates improvement also in the later phases or recurrent disease. Because relative survival rates specifically reflect excess mortality attributable to CLL, improvement in long-term survival expectation is not explained by enhanced management of comorbidity. Earlier detection of CLL could have contributed to longer survival. No specific screening measures for CLL have been used in the periods of investigation, but a trend toward earlier diagnosis has been shown by research groups in the United States as well as in Europe.15-17 Furthermore, increased awareness and modern technologies (eg, cytofluorometry) may have contributed to better identification of CLL. In the past, lymphomas in leukemic phase may often have been misdiagnosed as CLL. However, advances in therapy are likely to have contributed to the observed improvement in prognosis as well.
The diagnosis of CLL does not necessarily convey the necessity of treatment. However, treatment has classically been indicated in symptomatic or progressive disease according to criteria defined by stage of disease.18 Treatment of CLL has been markedly improved by highly effective new drugs and drug combinations. Until the late 1980s, only chlorambucil- and cyclophosphamide-containing regimens had shown relatively good activity in CLL but rarely led to complete remissions.18,19 The purine analog fludarabine emerged rapidly as the standard second-line treatment in CLL in the 1990s and recently has also become an established first-line treatment option in CLL. Its use in monotherapy or combinational therapy resulted in higher response and event-free survival, but increased overall survival has been difficult to document, partly because of subsequently administered additional therapies.20 Newer therapeutic approaches include autologous and allogenic stem cell transplantation, the latter being potentially curative but limited in use by high treatment-associated morbidity and mortality,21 and antibodies, such as rituximab and alemtuzumab.22-24 Improvements in supportive care, including better use of prophylactic antibiotics and antiviral medications, may have contributed to the improvements seen in survival as well, especially among older patients.
Despite steady improvement of survival within 10 years after diagnosis among patients diagnosed at younger ages, patients who had already survived the first years after diagnosis continued to have high excess mortality in subsequent years compared with the general population in the 2000-2004 period. In contrast to most other malignancies, conditional long-term relative survival in subsequent years still did not increase with time from diagnosis in 2000-2004. This pattern indicates the persistent lack of cure for patients with CLL despite improvements in survival within 10 years after diagnosis.
Several limitations of our analysis that relied on routinely collected cancer registry data deserve careful consideration. There are indications that CLL is underreported in cancer registries or that reporting is delayed because a large proportion of patients do not require treatment at the time of diagnosis.25,26 Selective underreporting of patients not requiring treatment or delayed reporting may have led to some underestimation of true survival from diagnosis, especially in more recent years, which may have led to underestimation of increases in survival.
Because of the lack of data on treatment modalities and stem cell transplantation, it is not possible to directly link increases in survival to specific therapeutic innovations. Furthermore, no distinction could be made according to stage of disease or cytogenetic profile, which have been shown to have high prognostic relevance.27,28 In contrast, our analysis provides a reflection of the net impact of the efficacy of management of the disease on the population level. Although we cannot comment on the effects of specific treatments on survival, our data suggest that current treatments for CLL can affect the course of the disease in addition to ameliorating symptoms.
Although the period analysis methodology used in our study provides more up-to-date long-term survival estimates than traditional “cohort-based” survival analysis, even the period estimates tend to be somewhat too pessimistic in case of ongoing improvement in prognosis,5,6 as one would expect from ongoing therapeutic progress in CLL. In particular, our analysis, which is restricted to currently available data up to 2004, cannot possibly reflect further progress made in more recent years.
Despite these limitations, our analysis provides compelling evidence of increased long-term survival expectations of patients with CLL in the early 21st century. Future population-based studies are needed to confirm and expand our findings. Randomized clinical trials should be conducted to expand our understanding of underlying mechanisms with the overall aim to improve treatment decisions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The work of Dianne Pulte was supported by a faculty research visit grant from the German Academic Exchange Service and a grant from the Cornell CLL Research Center. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Authorship
Contribution: H.B. designed and carried out the analysis and drafted the paper. A.G. and D.P. critically reviewed and contributed to finalizing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hermann Brenner, MD, MPH, Division of Clinical Epidemiology & Aging Research, German Cancer Research Center, Bergheimer Strasse 20, D-69115 Heidelberg, Germany; e-mail: h.brenner@dkfz-heidelberg.de.