Abstract
The calcitonin receptor-like receptor (crlr) is a major endothelial cell receptor for adrenomedullin, a peptide vasodilator involved in cardiovascular development, homeostasis, and disease. Here, we used the zebrafish (Danio rerio) model to characterize the role of crlr in vascular development. Crlr is expressed within somites from the 4- to the 13-somite stage and by arterial progenitors and axial vessels during zebrafish development. Loss of crlr results in profound alterations in vascular development and angiogenesis, including atrophic trunk dorsal aorta and interruption of anterior aortic bifurcation, delay in intersomitic vessel development, and lack of blood circulation. Remarkably, crlr morphants are characterized by the loss of arterial endothelial cell identity in dorsal aorta, as shown by the lack of expression of the arterial markers ephrin-B2a, DeltaC, and notch5. Down-regulation of crlr affects vascular endothelial growth factor (vegf) expression, whereas vegf overexpression is sufficient to rescue arterial differentiation in crlr morphants. Finally, genetic and biochemical evidences indicate that somitic crlr expression is under the control of sonic hedgehog. These data demonstrate that crlr plays a nonredundant role in arterial differentiation, representing a novel element of the sonic hedgehog–vegf-notch signaling cascade that controls arterial/venous fate.
Introduction
The calcitonin receptor-like receptor (crlr) is a promiscuous 7-transmembrane G protein–coupled receptor (GPCR) that binds various ligands by forming complexes with different receptor activity-modifying proteins (RAMPs).1 The interaction of crlr protein with RAMP-2 or RAMP-3 leads to the formation of heterodimeric complexes representing the functional receptors for adrenomedullin, a peptide vasodilator involved in cadiovascular stresses and tumor angiogenesis.2 crlr is highly expressed in endothelium,3 and crlr-null mice die in utero at midgestation with hydrops fetalis and developmental abnormalities in cardiovascular tissues.4 Remarkably, adrenomedullin-null mice show a similar cardiovascular phenotype that provides compelling genetic and in vivo evidence that crlr is the primary receptor through which adrenomedullin acts during embryonic development.4 In vertebrates, the large midline artery and vein are the first blood vessels to develop via a vasculogenic process that involves migration, differentiation, and assembly of the vascular endothelial growth factor (vegf) receptor-2 (kdr)+ angioblasts from the lateral posterior mesoderm.5 Molecular differences exist between arterial and venous endothelial cells before the onset of circulation and the activation of complex genetic pathways specifies artery and vein identity, leading to the expression of specific arterial and venous markers (eg, ephrin-B2a and ephB4, respectively).6 Genetic alterations of arterial/venous specification are hypothesized to be responsible for congenital hereditary arteriopathies. Indeed, mutations of notch pathway components involved in arterial specification are associated with congenital defects of the cardio-vascular system.7
The zebrafish (Danio rerio) has emerged as an useful tool for the genetic analysis of development processes and as a model for human genetic diseases.8 The optical transparency and ability to survive for 3 to 4 days without functioning circulation make the zebrafish embryo especially amenable for vascular biology studies.9 Experimental evidences in zebrafish have shown that a signaling pathway controlled by sonic hedgehog (shh), vegf, and notch drive arterial/venous cell fate determination.10 Suppression of shh activity leads to vegf down-regulation and prevents arterial differentiation that can be rescued by vegf overexpression, thus demonstrating that shh acts upstream of vegf. On the other hand, vegf is unable to rescue artery gene marker expression in embryos lacking notch function, whereas exogenous notch activity can induce arterial differentiation in the absence of vegf. Thus, a shh-vegf-notch signaling cascade is responsible for inducing arterial differentiation.11 However, other factors may contribute to the arterial differentiation process (reviewed in Lawson and Weinstein12 ).
Recently, the activation of an adrenomedullin signaling pathway in coordination with vegf and notch activity has been implicated in the differentiation of arterial endothelial cells from kdr+ vascular precursors in murine embryonic stem cell cultures.13 In the present study, we performed the characterization and functional analysis of the adrenomedullin receptor crlr during vascular development in zebrafish. Crlr is expressed within the somites in early and midsomitogenesis and in angioblasts and blood vessels of the zebrafish embryo. Embryos lacking crlr activity show profound alterations in vascular development and angiogenesis, characterized by arterial malformations, defects in intersomitic vessel (ISV) sprouting and organization, and lack of blood circulation. In association with these morphologic defects, crlr knockdown causes the down-regulation of vegf and notch5 expression and prevents arterial differentiation. Genetic and biochemical evidences indicate that crlr is a shh-regulated element that controls arterial differentiation by acting upstream of vegf. The results demonstrate for the first time that crlr plays a nonredundant role in arterial development.
Methods
Zebrafish stocks
Molecular cloning of zebrafish crlr
A BLAT search17 on the University of California Santa Cruz Genome Bioinformatics Site (http://genome.ucsc.edu) using the human crlr amino acid sequence as a query identifies 2 homolog genes in the zebrafish genome present on chromosome 9 (crlr-1; expressed sequence tag [EST] zgc:100872) and chromosome 6 (crlr-2, EST LOC567131), respectively. Real-time reverse transcription–polymerase chain reaction (RT-PCR) analy-sis was performed using the following primer sets: zebrafish crlr-1 (forward: 5′-AGCTGCTGGACGATTTGTCT-3′, reverse: 5′-TCCAGTGCCTTCTCAAAACC-3′); and zebrafish crlr-2 (forward: 5′-CACAATCCTCTGCTGCTTCA-3′, reverse 5′- CGCTGTTTTTTGTGATCGT-3′). The PCR fragment with the full-length coding sequence of crlr-1 was cloned into the TOPO vector (Invitrogen, Carlsbad, CA).
The deduced amino acid sequence of zebrafish crlr-1 was aligned with Mus musculus and Homo sapiens crlr protein sequences using the clustaw alignment algorithm of Vector NTI Advance 10.1.1 software (Invitrogen). Structural features of amino acid sequences of zebrafish crlr protein were predicted using ExPASy Proteomics tools (http://ca.expasy.org/tools/).
Whole-mount in situ hybridization
Digoxigenin- and fluorescin-labeled RNA probes were transcribed from linear cDNA constructs (Roche Applied Science, Indianapolis, IN). Whole-mount in situ hybridization (ISH) was performed as described.18
Double fluorescence ISH was performed using the TSA Cyanine 3 and 5, TMR, Fluorescein Evaluation Kit (PerkinElmer, Waltham, MA). For sectioning, fixed embryos were dehydrated in an ethanol series, cleared in toluene, and paraffin embedded for 2 hours.
Morpholino-mediated knockdown of crlr and chemical treatment
Antisense morpholino (MO) oligonucleotides (Gene Tools, Corvallis, OR) crlr-MO1 (5′-AGCTCGCTGTCATCTTCTTTGGCAT-3′) and crlr-MO2 (5′-TGTACTAATGTGTTGTGTCTCTACCTC-3′) were directed against the 5′ untranslated region (UTR) spanning the crlr-1 ATG start codon and the predicted splice donor site at the end of exon 2 of the immature crlr-1 mRNA, respectively. Std-MO (5′-CCTCTTACCTCAGTTACAATTTATA-3′) was used as a control. Routinely, MOs were microinjected in 4.0 nL volume into 1- to 4-cell stage embryos at the concentration of 0.2 mM for each MO unless specified otherwise. A subset of embryos was coinjected with 0.2 mM of crlr-MO1 or std-MO and murine crlr mRNA (50 ng μL−1, kindly provided by W. Born, University of Zurich, Switzerland) or vegf121 mRNA11 (2 ng μL−1, kindly provided by N. Lawson, University of Massachusetts, Worcester, MA).
Fertilized eggs were treated in E3 buffer containing DMSO as a vehicle, 50 μM cyclopamine, or 2.5 μg/mL GS4012 (Calbiochem, San Diego, CA) and allowed to develop at 28.5°C.
Microangiography
Tetramethylrhodamine isothiocyanate (TRITC)–dextran (molecular weight 2.0 × 106; Invitrogen) was dissolved in double distilled water at 20 mg/mL and microinjected into the sinus venosus/cardinal vein of zebrafish embryos at 50 hpf as described.19
Microscopy
Embryos were mounted in agarose-coated dishes and photographed under an epifluorescence Leica MZ16 F stereomicroscope (1× Plan Apo objective, NA 0.141) equipped with DFC480 digital camera and ICM50 software version 2.8.1 (all from Leica, Wetzlar, Germany). Confocal images were acquired with a Leica TCS SP2 confocal laser microscope (20×/0.7 NA, HC Plan Apo objective), and confocal stacks were assembled using Imaris software version 6.0 (Bitplane, Zurich, Switzerland).
Quantitative RT-PCR analysis
Total RNA was extracted from 40 embryos per group and treated with DNase using the RNeasy Micro kit (Qiagen, Valencia, CA). RT products were amplified in the real-time PCR reaction with Bio-Rad iCycler iQ Real-Time PCR Detection System using an iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. Primer sequences were as follows: ephrin-b2a (forward: 5′-cccatttcccccaaagacta-3′, reverse: 5′-cttccccatgaggagatgc-3′); flt4 (forward: 5′-actggtcgacttcaacactgg-3′, reverse: 5′-atccaggacgttacgcagag-3′); β-actin (forward: 5′-cgtgacatcaaggagaagct-3′, reverse: 5′-tcgtggataccgcaagattc-3′); vegf (forward: 5′-tgctcctgcaaattcacacaa-3′, reverse: 5′-atcttggcttttcacatctgcaa-3′); kdra (forward: 5′-tggagttccagcacccttta-3′, reverse: 5′-cgtccttcttcaccctttca-3′); and shh (forward: 5′-aaagcccacattcattgctc-3′, reverse: 5′-atccaggacgtt-acgcagag -3′).
Data in triplicate were normalized for zebrafish β-actin expression and represent the percentage change in morphant embryos relative to controls. All the experiments were repeated 4 times with similar results.
Results
Molecular cloning and sequence analysis of zebrafish crlr
A BLAT search17 on the UCSC Genome Bioinformatics Site using the human crlr amino acid sequence as a query identifies 2 homolog genes in the zebrafish genome present on chromosome 9 (crlr-1) and chromosome 6 (crlr-2), respectively. Ensembl analysis (zebrafish assembly version 7, Sanger Institute, Cambridge, United Kingdom) of exon-intron organization and syntenic neighboring genomic contest of both genes reveals that zebrafish crlr-1 represents the homolog of the human crlr gene present on chromosome 2. Moreover, RT-PCR expression analysis of both genes at different stages of development (from 0 to 3 dpf) reveals that crlr-1 is expressed throughout zebrafish development, whereas crlr-2 is expressed only in maternal stage (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). On this basis, we focused our study on the expression and function of crlr-1, hereafter named crlr, during zebrafish development.
The full-length 1.49-kb zebrafish crlr transcript (GenBank20 accession no. NM_001004010) was cloned from the total RNA isolated from zebrafish embryos at 24 hpf. The cDNA encodes for a putative 472–amino acid protein with the characteristic hormone receptor domain and the 7-transmembrane class-B GPCR domain present in crlr homologs in other species (Figure S1B). Protein sequence comparison revealed that zebrafish crlr protein is highly homologous to human and murine crlr proteins, with 78% to 79% and 92% to 94% similarity in the hormone receptor and GPCR domains, respectively. Also, cysteine residues essential for a proper adrenomedullin interaction21 are conserved in the extracellular amino-terminal domain of zebrafish crlr (Figure S1B).
Temporal and spatial expression pattern of crlr
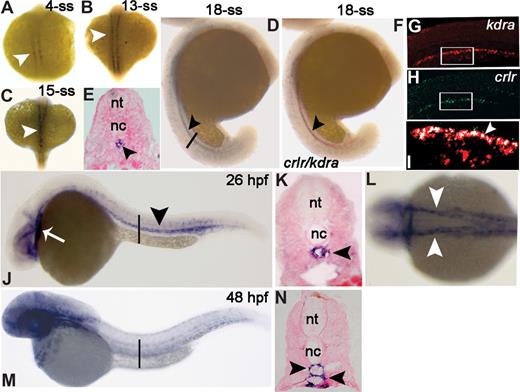
We analyzed the expression pattern of the crlr gene in early zebrafish embryos by whole-mount ISH. No expression was observed prior to the 1-somite stage (1-ss; S.N., unpublished observations, February 2007). From the 4-ss, crlr is expressed within the medial aspect of the newly formed somites immediately adjacent to the notochord (Figure 1A). Somite expression is maintained until the 13-ss (Figure 1B), being lost thereafter. At the 15- to 18-ss, a stripe of crlr+ cells is apparent in the midline and extends through the trunk and posterior part of the embryo (Figure 1C,D), likely representing the endothelial precursor cells migrated to the future axial vascolature region under the nothocord (Figure 1E). To assess this hypothesis, crlr expression was analyzed by double ISH with the early endothelial marker vegf receptor-1 kdra, mainly expressed in dorsal aorta (DA) progenitor cells.22 At 18-ss, crlr stains in the same field of kdra+ endothelial cells proximal to the notochord (Figure 1F). Confocal laser double fluorescence ISH with kdra (Figure 1G) and crlr (Figure 1H) probes confirmed that the most dorsal kdra+ DA angioblasts coexpress crlr (Figure 1I).
Zebrafish crlr is expressed in somites, endothelial cells, and their precursors. Crlr expression was analyzed in zebrafish embryos by ISH at the indicated developmental stages. Embryos are anterior to the left and lateral to the top, except in panels A-C and L that represent dorsal views. (E,K,N) The transverse sections through the trunk are highlighted as vertical black bars in panels D, J, and M, respectively. At 4-ss (A) and 13-ss (B) crlr is expressed within the somites (white arrowheads). At 15-ss, white arrowhead points to crlr expression along a midline through the trunk (C). At 18-ss (D,E) crlr is expressed in the midline under the notochord (black arrowhead). At this stage, double ISH (F) shows the expression of crlr (blue) by kdra+ angioblasts (red). Confocal laser microscopy shows a detail of the tail region of 18-ss embryo after double fluorescence ISH for kdra (red in panel G) and crlr (green in panel H); the area in white boxes are merged in panel I, where white arrowheads point to the layer of crlr+/kdra+ DA angioblasts close to the notochord (merged signal in white). (J) At 26 hpf, crlr is expressed by blood vessels of the head, in the heart tube (white arrow), and in DA of the trunk (black arrowhead). (K) Cross-section of a 26-hpf embryo, confirming crlr expression in DA (black arrowhead). At this stage, crlr is expressed also by the right- and left-lateral DA (panel L; white arrowheads). At 48 hpf, lateral view (M) and cross-section (N, black arrowheads) demonstrate crlr expression in both DA and PCV.
Zebrafish crlr is expressed in somites, endothelial cells, and their precursors. Crlr expression was analyzed in zebrafish embryos by ISH at the indicated developmental stages. Embryos are anterior to the left and lateral to the top, except in panels A-C and L that represent dorsal views. (E,K,N) The transverse sections through the trunk are highlighted as vertical black bars in panels D, J, and M, respectively. At 4-ss (A) and 13-ss (B) crlr is expressed within the somites (white arrowheads). At 15-ss, white arrowhead points to crlr expression along a midline through the trunk (C). At 18-ss (D,E) crlr is expressed in the midline under the notochord (black arrowhead). At this stage, double ISH (F) shows the expression of crlr (blue) by kdra+ angioblasts (red). Confocal laser microscopy shows a detail of the tail region of 18-ss embryo after double fluorescence ISH for kdra (red in panel G) and crlr (green in panel H); the area in white boxes are merged in panel I, where white arrowheads point to the layer of crlr+/kdra+ DA angioblasts close to the notochord (merged signal in white). (J) At 26 hpf, crlr is expressed by blood vessels of the head, in the heart tube (white arrow), and in DA of the trunk (black arrowhead). (K) Cross-section of a 26-hpf embryo, confirming crlr expression in DA (black arrowhead). At this stage, crlr is expressed also by the right- and left-lateral DA (panel L; white arrowheads). At 48 hpf, lateral view (M) and cross-section (N, black arrowheads) demonstrate crlr expression in both DA and PCV.
At 26 hpf, crlr expression is maintained in the axial vasculature of the trunk (Figure 1J); cross-sections at this level showed that crlr marks the main medial DA (Figure 1K). Also, crlr is expressed in the right- and left-lateral DA (Figure 1L) and rostrally in the primitive internal carotid arteries (Figure 1J). We also observed crlr expression in heart tube at this stage (Figure 1J). At 48 hpf, crlr is expressed both in the DA and in the posterior cardinal vein (PCV) of the trunk (Figure 1M,N), and it is widely expressed in the vasculature of the head and in the pectoral fin mesoderm (S.N., unpublished observations, February 2007).
MO knockdown of crlr function results in vascular defects
To assess the functional role of crlr in zebrafish vascular development we used an antisense MO knockdown approach.23 To this purpose, a MO was designed directed against the 5′ UTR spanning the crlr ATG start codon to inhibit protein translation (crlr-MO1). tg(fli1:EGFP)y1 transgenic zebrafish embryos in which EGFP expression is driven by the promoter of the panendothelial marker fli-1 were injected at the 1- to 4-cell stage with 0.8 pmol/embryo of crlr-MO1 or of control std-MO, and the development of GFP-labeled blood vessels was directly observed in live embryos.
Whole-mount microscopic analysis of 28-hpf crlr morphants revealed no obvious morphological abnormalities, with crlr-MO1–injected embryos appearing slightly thinner than control embryos (Figure 2A,B). However, crlr morphants showed defects in vascular morphology and circulatory patterns. Indeed, crlr-MO1–injected embryos showed no apparent blood circulation and displayed a disorganized trunk vessel plexus with a remarkable reduction in the size of DA (Figure 2D). At variance, embryos injected with control MO exhibited normal blood circulation and vasculature morphology, as indicated by fully lumenized well-defined trunk vessels (Figure 2C).
Crlr knockdown results in vascular defects. Vascular morphology in tg(fli1:EGFP)y1 embryos injected with std-MO (control in panels A,C,E,F,I, and K) or crlr-MO1 (morphant in panels B,D,G,H,J, and L). Morphologic appearance of control (A) and crlr morphant (B) embryos at 28 hpf (head and trunk regions are highlighted by black and red boxes, respectively). Differential interference contrast images of the trunk region (lateral view) of live zebrafish embryos injected with std-MO (C) or crlr-MO1 (D), the lumen of DA and PCV are indicated by red and white bars, respectively; note the abnormal size of DA and the presence of cells with endothelial morphology (red arrow) in proximity to the notochord in crlr morphant (D). Confocal laser microscopy showing details of the head (E,G) and trunk (F,H) regions (lateral view) in std-MO– and crlr-MO1–injected tg(fli1:EGFP)y1 embryos (DA [red bar], PVC [white bar]; white arrow points to poorly developed ISVs in crlr morphant). Crlr knockdown causes also an interruption of the vascular tube at the bifurcation of the DA. Dorsal view of the lateral dorsal aortae (red arrows) and of cardinal vein branches (white arroweads) in control (I) and crlr-MO1–injected (L) tg(fli1:EGFP)y1 embryos; lumens of the lateral dorsal aortae and cardinal vein branches are indicated by red and white bars, respectively. Note the poor lumenization and formation of lateral aortae branches in crlr morphant compared with the posterior cardinal vein. Microangiography performed at 50 hpf shows that blood circulation is confined to the head and heart regions (white arrow) in crlr morphants (L) when compared with control embryos (K). White arrowhead in panel L points to the interruption of the blood flow at the level of bifurcation of the DA.
Crlr knockdown results in vascular defects. Vascular morphology in tg(fli1:EGFP)y1 embryos injected with std-MO (control in panels A,C,E,F,I, and K) or crlr-MO1 (morphant in panels B,D,G,H,J, and L). Morphologic appearance of control (A) and crlr morphant (B) embryos at 28 hpf (head and trunk regions are highlighted by black and red boxes, respectively). Differential interference contrast images of the trunk region (lateral view) of live zebrafish embryos injected with std-MO (C) or crlr-MO1 (D), the lumen of DA and PCV are indicated by red and white bars, respectively; note the abnormal size of DA and the presence of cells with endothelial morphology (red arrow) in proximity to the notochord in crlr morphant (D). Confocal laser microscopy showing details of the head (E,G) and trunk (F,H) regions (lateral view) in std-MO– and crlr-MO1–injected tg(fli1:EGFP)y1 embryos (DA [red bar], PVC [white bar]; white arrow points to poorly developed ISVs in crlr morphant). Crlr knockdown causes also an interruption of the vascular tube at the bifurcation of the DA. Dorsal view of the lateral dorsal aortae (red arrows) and of cardinal vein branches (white arroweads) in control (I) and crlr-MO1–injected (L) tg(fli1:EGFP)y1 embryos; lumens of the lateral dorsal aortae and cardinal vein branches are indicated by red and white bars, respectively. Note the poor lumenization and formation of lateral aortae branches in crlr morphant compared with the posterior cardinal vein. Microangiography performed at 50 hpf shows that blood circulation is confined to the head and heart regions (white arrow) in crlr morphants (L) when compared with control embryos (K). White arrowhead in panel L points to the interruption of the blood flow at the level of bifurcation of the DA.
Confocal laser microscopy confirmed the presence of a disorganized trunk vessel plexus and of poorly defined boundaries between the DA and PVC in tg(fli1:EGFP)y1 embryos injected with crlr-MO1 when compared with embryos injected with std-MO (Figure 2F,H). Moreover, crlr knockdown caused a delay in ISV development (Figure 2H). Indeed, only a few ISV sprouts were detectable in crlr morphants at 28 hpf, whereas std-MO–injected embryos showed normally developed ISVs that, sprouted bilaterally from the DA, have migrated dorsally between the somites, assuming a typical T shape (Figure 2F). As development continues, ISVs do not follow the normal stereotypical intersomitic pathway in crlr morphants (S.N., unpublished observations, March 2007). Moreover, dorsal view of crlr-MO1–injected tg(fli1:EGFP)y1 embryos showed that lateral dorsal aortae are thin and fail to lumenize, with an evident interruption at the level of the anterior aortic bifurcation (Figure 2J) when compared with the lumenized lateral dorsal aortae of control embryos (Figure 2I). In contrast, PCV branches are similar in control and crlr morphants (Figure 2I,J). Microangiography was performed at 50 hpf to further assess the impact of aortic dysmorphogenesis on the integrity of the circulation in crlr-MO1–injected embryos. Crlr morphants were characterized by the lack of blood circulation that was occasionally limited to the circulation of the trunk as a consequence by the blockade of the blood flow at the level of the anterior aortic bifurcation (Figure 2K,L). Also, blood pooling was observed in the cardiac region (Figure 2L). At variance with the observed alterations in the trunk vasculature, 28-hpf crlr morphants showed no evident alterations in the vasculature of the head when compared with control embryos (Figure 2E,G). The vascular defects observed in crlr-MO1–injected embryos are summarized in Table 1.
Summary of the vascular defects in crlr morphant embryos
| Lack of blood circulation . | Alterations in ISV development . | Alterations in trunk DA development . | Interruption at the anterior DA bifurcation . | crlr-MO1, % of injected embryos± SD . | crlr-MO2, % of injected embryos± SD . |
|---|---|---|---|---|---|
| − | − | − | − | 14 ± 2 | 35 ± 7 |
| + | − | + | − | 21 ± 6 | 21 ± 4 |
| + | − | + | + | 20 ± 4 | 26 ± 10 |
| + | + | + | + | 45 ± 4 | 9 ± 1 |
| Lack of blood circulation . | Alterations in ISV development . | Alterations in trunk DA development . | Interruption at the anterior DA bifurcation . | crlr-MO1, % of injected embryos± SD . | crlr-MO2, % of injected embryos± SD . |
|---|---|---|---|---|---|
| − | − | − | − | 14 ± 2 | 35 ± 7 |
| + | − | + | − | 21 ± 6 | 21 ± 4 |
| + | − | + | + | 20 ± 4 | 26 ± 10 |
| + | + | + | + | 45 ± 4 | 9 ± 1 |
One- to 4-cell stage zebrafish VEGFR2:G-RCFP embryos were injected with 0.8 pmol crlr-MO1 (n = 34) or crlr-MO2 (n = 25), and vascular defects were assessed at 28 hpf. Data are expressed as percentage of morphants showing the indicated vascular defect(s). The results are the mean plus or minus SD of two independent experiments. No vascular defects were observed in std-MO–injected embryos (n = 32). − indicates absence of the phenotypic defect; +, presence of the phenotypic defect.
To better characterize the defects in the developing vasculature, crlr morphants were analyzed for fli-1 expression by ISH. At 8-ss, the expression of panendothelial fli-1, together with that of notochord no-tail24 and of nascent somite myoD,25 were not affected in crlr morphants, thus indicating the lack of alterations in the vascular and nonvascular differentiation programs prior to this time of development (Figure S2). In keeping with the data obtained with tg(fli1:EGFP)y1 embryos, the fli-1 transcript was abundantly expressed in the axial vasculature of both control and crlr morphants at 28 hpf, indicating that endothelial cell migration and localization is not defective in the embryos lacking crlr activity. However, also in this case, a reduction in the angiogenic development of fli-1+ ISVs was observed in crlr morphants when compared with controls (Figure S2).
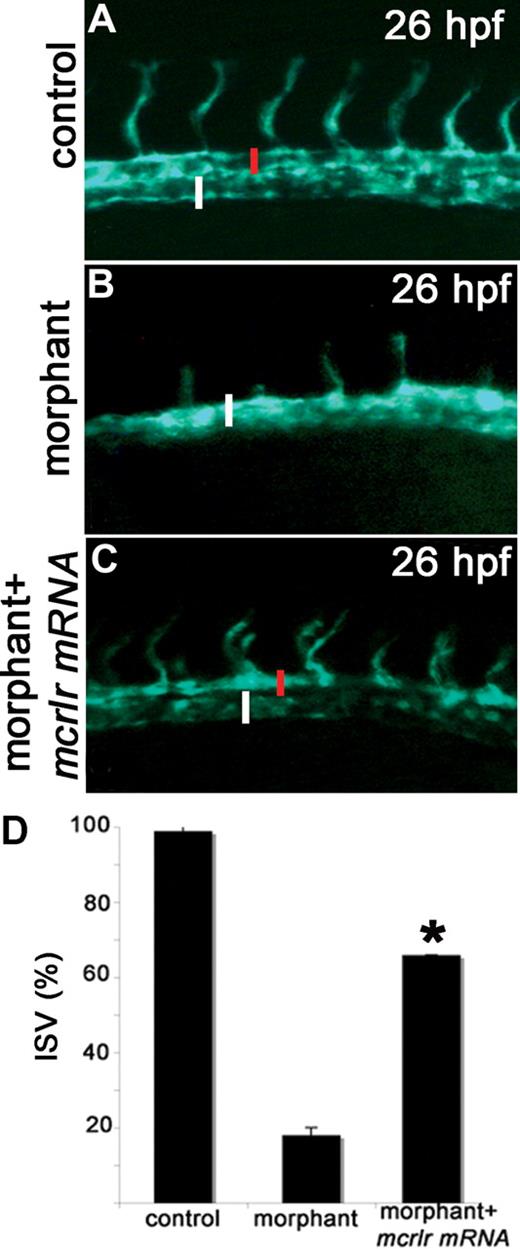
To confirm the specificity of the vascular defects in crlr morphants, crlr-MO1 was coinjected with a MO-resistant form of the murine crlr mRNA. In 2 independent experiments, analysis of the embryos at 28 hpf showed the ability of the murine transcript to rescue the crlr-knockdown phenotype as assessed by the presence of normally developed ISVs in 66% of rescued embryos (10 of 15 embryos in both experiments) compared with 16% of crlr morphants (2 of 15 embryos and 3 out of 15 embryos in the 2 experiments, respectively; P < .02, Student t test; Figure 3).
Murine crlr overexpression rescues the vascular phenotype in zebrafish crlr morphants. Murine crlr mRNA was injected in VEGFR2:G-RCFP crlr morphants, and axial vasculature of the trunk was examined under an epifluorescence microscope at 26 hpf. The reduction in size of DA (red bar) and the delay in ISV development observed in crlr morphants (B) are rescued by murine crlr overexpression (C). (A) control VEGFR2:G-RCFP embryo. PCV is indicated by the white bar. The percentage of embryos showing normal ISV development in the different experimental groups is shown in panel D. Data are the mean plus or minus SD of 2 independent experiments with 15 embryos per group. *Statistically different from the “morphant” group; P < .02, Student t test.
Murine crlr overexpression rescues the vascular phenotype in zebrafish crlr morphants. Murine crlr mRNA was injected in VEGFR2:G-RCFP crlr morphants, and axial vasculature of the trunk was examined under an epifluorescence microscope at 26 hpf. The reduction in size of DA (red bar) and the delay in ISV development observed in crlr morphants (B) are rescued by murine crlr overexpression (C). (A) control VEGFR2:G-RCFP embryo. PCV is indicated by the white bar. The percentage of embryos showing normal ISV development in the different experimental groups is shown in panel D. Data are the mean plus or minus SD of 2 independent experiments with 15 embryos per group. *Statistically different from the “morphant” group; P < .02, Student t test.
To further confirm that the phenotype observed in crlr-MO1–injected embryos is specifically related with crlr knockdown, embryos were injected with a different crlr-specific MO targeted against the splice donor site at the end of exon 2 of the immature crlr mRNA (crlr-MO2). Injection of crlr-MO2 caused vascular defects similar to those caused by crlr-MO1 even though with a lower frequency (Table 1) due to the non-complete blockade of crlr mRNA maturation (S.N., unpublished observations, May 2007).
Taken together, these findings indicate a nonredundant vascular function for crlr in vivo. In particular, crlr appears to play a major role in aortic vessel development.
Arterial endothelial markers are lost in crlr morphants
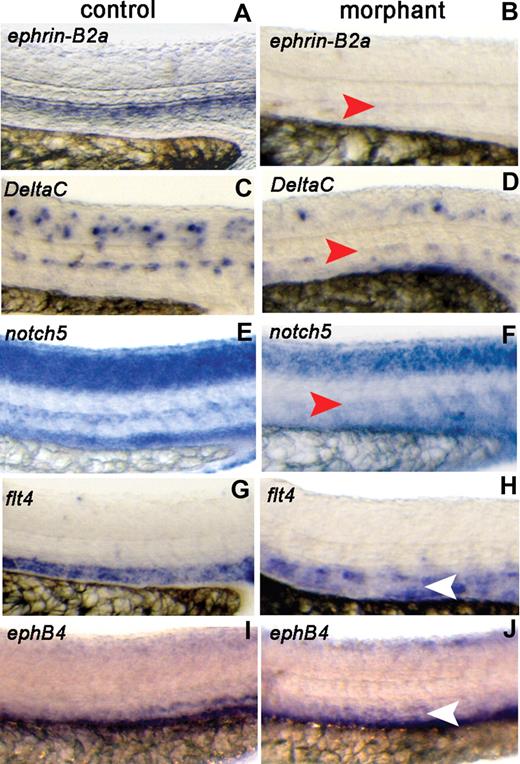
To assess whether crlr plays a specific role in arterial differentiation during vascular development in zebrafish, we examined the expression of arterial- and venous-specific markers11 in axial vessels of the trunk in std-MO– and crlr-MO1–injected embryos at 26 hpf. When compared with std-MO–injected embryos, clrl morphants show a specific loss of the expression of the arterial genes ephrin-B2a, DeltaC, and notch5 in the DA (Figure 4A-F), whereas their expression in other districts of the embryo was not significantly affected (Figure S3A). To determine the effects on venous cell fate, we assayed for expression of flt4 which is initially expressed in both arterial and venous primordial cells and becomes restricted to venous vessels by 24 hpf.26 Embryos injected with std-MO and crlr-MO1 showed the typical down-regulation of flt4 in the DA and its normal expression in PCV (Figure 4G,H). Also, the expression of the venous marker ephB4 was not affected in the PCV of crlr-MO1–injected embryos (Figure 4I,J). In keepingwith these observations, real-time RT-PCR analysis showed a significant decrease of ephrin-B2a expression, but not of flt4 expression, in crlr morphants when compared with control embryos (Figure S3B).
Crlr knockdown affects arterial gene expression. Std-MO–injected embryos (control) and crlr-MO1–injected embryos (morphant) were analyzed at 26 hpf for the expression of the indicated arterial and venous markers by ISH. Lateral view of the trunk region is shown (DA, red arrowheads; PCV, white arrowheads). Crlr morphants were characterized by lack of expression of ephrin-B2a (panel B; 13 of 17 embryos examined), DeltaC (panel D; 13 of 19 embryos examined), and notch5 (panel F; 10 of 13 embryos examined). In contrast, venous markers flt4 (H) and ephB4 (J) were expressed in 7 of 10 and in 11 of 12 crlr morphants, respectively. Arterial and venous markers were instead expressed in all the control embryos examined (ranging between 10 and 16 embryos per gene investigated).
Crlr knockdown affects arterial gene expression. Std-MO–injected embryos (control) and crlr-MO1–injected embryos (morphant) were analyzed at 26 hpf for the expression of the indicated arterial and venous markers by ISH. Lateral view of the trunk region is shown (DA, red arrowheads; PCV, white arrowheads). Crlr morphants were characterized by lack of expression of ephrin-B2a (panel B; 13 of 17 embryos examined), DeltaC (panel D; 13 of 19 embryos examined), and notch5 (panel F; 10 of 13 embryos examined). In contrast, venous markers flt4 (H) and ephB4 (J) were expressed in 7 of 10 and in 11 of 12 crlr morphants, respectively. Arterial and venous markers were instead expressed in all the control embryos examined (ranging between 10 and 16 embryos per gene investigated).
Consistent with the morphologic vascular defects, these data demonstrate that crlr has a function in zebrafish arterial differentiation and development.
Crlr genetically interacts with the vegf pathway during arterial differentiation
Angioblasts within the zebrafish lateral mesoderm are restricted to arterial or venous fate during mid-somitogenesis.10,27 At this stage, shh in the notochord modulates the somitic expression of vegf which, in turn, regulates the arterial differentiation of the first flk-1+ angioblasts migrating toward the midline.11,28 As shown, crlr is also expressed within the somites at this stage, suggesting that impaired arterial differentiation in zebrafish embryo lacking crlr activity might be the consequence of defects in the shh/vegf genetic pathway.
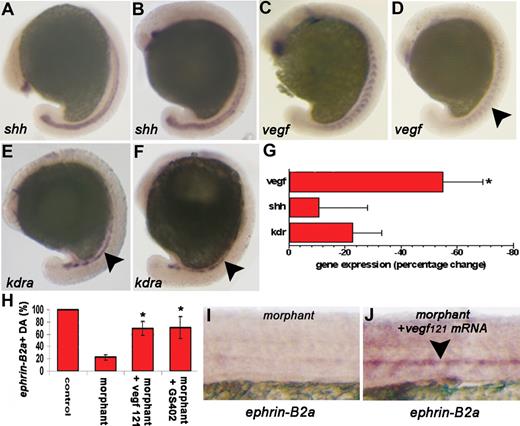
To assess this hypothesis, we investigated the expression of shh, vegf, and kdra in 15-ss embryos injected with crlr-MO1 or std-MO. Whole-mount ISH and real time RT-PCR analysis of crlr morphants shows a significant and reproducible down-regulation of vegf expression within the somites, with no significant changes in shh expression in the notochord (Figure 5A-G). Also, kdra+ angioblasts migrating toward the midline were normally present at this time of development in crlr morphants when compared with control embryos (Figure 5E,F). The concomitant down-regulation of ephrin-B2a and vegf activity in embryos lacking crlr raises the possibility that vegf may provide a signal for arterial differentiation downstream of crlr. On this basis, to assess whether exogenous vegf is sufficient to rescue ephrin-B2a expression in the absence of crlr activity, crlr morphants were injected with the mRNA encoding for zebrafish vegf121 and assayed for the expression of ephrin-B2a at 26 hpf. As shown in Figure 5H-J, the injection of 5 pg/embryo of vegf121 mRNA rescued the expression of ephrin-B2a in DA, whereas no ephrin-B2a stain was detectable in the trunk of crlr morphants injected with control GFP mRNA. A similar rescue was observed also after treatment of crlr-MO1–injected embryos with GS4012, a potent vegf inducer29 (Figure 5H). These results indicate that vegf overexpression is sufficient to rescue arterial differentiation in the absence of crlr activity and suggest that the loss of ephrin-B2a expression in crlr morphants is due to the down-regulation of vegf activity downstream of crlr.
Crlr acts upstream of vegf in modulating arterial differentiation in zebrafish. Std-MO–injected embryos (A,C,E) and crlr-MO1–injected embryos (B,D,F) were analyzed at 15-ss for shh, vegf, and kdra expression by ISH (panels A-F; embryos are anterior to the left and lateral to the top). No changes in shh notochord expression (panel B; n = 15) and in endothelial progenitor kdra expression (panel F; n = 13) were observed in crlr morphants when compared with controls (A,E). Note the proper expression and localization of the kdra staining in crlr morphant embryos (black arrowhead in panel F) when compared with control embryos (black arrowhead in panel E). In contrast, somitic vegf expression was reduced in 20 of 32 morphants examined (panel D; black arrowhead). No changes in gene expression were instead observed in control std-MO–injected embryos when compared with uninjected embryos (n = 25, data not shown). (G) Total RNA was extracted at 15-ss from 40 embryos per group, and shh, vegf, and kdra expression were evaluated by real-time RT-PCR. Data in triplicate were normalized for zebrafish β-actin expression and represent the percentage change in morphant embryos relative to controls. Similar results were obtained in 4 independent experiments (*P < .05 vs controls; Student t test). (H) ephrin-B2a expression was assessed at 26 hpf in the DA of the trunk of GFP mRNA–injected control embryos (n = 30), crlr morphants (n = 25), vegf121 mRNA–injected crlr morphants (n = 25), and GS402-treated crlr morphants (n = 25) and data were expressed as percentage of positive embryos. The results are the mean plus or minus SD of 3 independent experiments (*P < .05 vs crlr morphants, Student t test). (I,J) representative images of the trunk of crlr morphant (I) and vegf121 mRNA-injected crlr morphant (J) embryos showing the ability of vegf overexpression to rescue ephrin-B2a expression in the DA of crlr morphants (black arrowhead).
Crlr acts upstream of vegf in modulating arterial differentiation in zebrafish. Std-MO–injected embryos (A,C,E) and crlr-MO1–injected embryos (B,D,F) were analyzed at 15-ss for shh, vegf, and kdra expression by ISH (panels A-F; embryos are anterior to the left and lateral to the top). No changes in shh notochord expression (panel B; n = 15) and in endothelial progenitor kdra expression (panel F; n = 13) were observed in crlr morphants when compared with controls (A,E). Note the proper expression and localization of the kdra staining in crlr morphant embryos (black arrowhead in panel F) when compared with control embryos (black arrowhead in panel E). In contrast, somitic vegf expression was reduced in 20 of 32 morphants examined (panel D; black arrowhead). No changes in gene expression were instead observed in control std-MO–injected embryos when compared with uninjected embryos (n = 25, data not shown). (G) Total RNA was extracted at 15-ss from 40 embryos per group, and shh, vegf, and kdra expression were evaluated by real-time RT-PCR. Data in triplicate were normalized for zebrafish β-actin expression and represent the percentage change in morphant embryos relative to controls. Similar results were obtained in 4 independent experiments (*P < .05 vs controls; Student t test). (H) ephrin-B2a expression was assessed at 26 hpf in the DA of the trunk of GFP mRNA–injected control embryos (n = 30), crlr morphants (n = 25), vegf121 mRNA–injected crlr morphants (n = 25), and GS402-treated crlr morphants (n = 25) and data were expressed as percentage of positive embryos. The results are the mean plus or minus SD of 3 independent experiments (*P < .05 vs crlr morphants, Student t test). (I,J) representative images of the trunk of crlr morphant (I) and vegf121 mRNA-injected crlr morphant (J) embryos showing the ability of vegf overexpression to rescue ephrin-B2a expression in the DA of crlr morphants (black arrowhead).
Shh provides a signal upstream of vegf to modulate arterial differentiation.11 On this basis, we investigated crlr transcript levels in embryos lacking shh activity. Embryos carrying a null mutation for sonic-you (syut4 mutants) and wild-type embryos treated with 25 μM cyclopamine, a potent inhibitor of shh signaling,30 fail to express somitic crlr at 10-ss (Figure S4). These results demonstrate that shh modulates crlr somite expression in a putative shh/crlr/vegf pathway. In keeping with this hypothesis, shh overexpression fails to induce vegf up-regulation and causes only a limited ephrin-B2a expression in crlr morphants when compared with shh-overexpressing control embryos (Figure S5).
Discussion
In this study, we report the characterization and functional analysis of crlr, a major adrenomedullin receptor, in zebrafish. Previous studies in murine models have implicated the adrenomedullin/crlr system in cardiovascular development, homeostasis, and disease.31 Here we show that crlr has a nonredundant function in zebrafish arterial differentiation and development.
In zebrafish, crlr is expressed in early and mid-somitogenesis within the medial aspect of the newly formed somites immediately adjacent to the notochord. Then, crlr is expressed in kdra+ angioblasts that will coalesce into the presumptive DA. At later stages, crlr is expressed by the endothelium of the head and of axial vasculature, first in DA (at 26 hpf) and then also in PCV (at 48 hpf). Consistent with this expression pattern, knockdown of crlr activity by specific MOs results in profound effects on the vasculature of the zebrafish embryo, characterized by delay and alterations in ISV development, dysmorphogenesis of DA of the trunk and interruption at the level of the anterior aortic bifurcation, and lack of blood circulation. Defects in aortic bifurcation have been observed also in embryos lacking vegf or phospolipase C gamma-1 activity32 and in gridlock zebrafish mutants.33 However, similar to that observed in notch mutants,22 suppression of crlr function in zebrafish does not affect gridlock expression (S.N., unpublished observations, May 2007). Interestingly, only minor, if any, defects in the vasculature of the head were observed in crlr morphants, suggesting that crlr function is redundant for head vascularization or that the remaining amount of crlr protein activity may be sufficient for normal head vessel development.
The vascular defects in the trunk of crlr morphants are associated with an impairment of aortic endothelial cell differentiation, as shown by the lack of expression of the specific arterial markers ephrin-B2a, DeltaC, and notch5. Despite the loss of artery-specific gene expression, the DA is present in its proper location, which indicates a defect in the differentiation of aortic endothelial cells, rather than a defect of angioblasts to migrate and form this vessel structure. Indeed, kdra expression is normal at 10-ss (S.N., unpublished observations, April 2007), and kdra+ angioblasts are properly located under the notochord at 15-ss, indicating that the formation and migration of DA progenitors are crlr independent. Moreover, the expression patterns of the panendothelial marker fli-1 and of the kdr promoter-driven GFP are not affected by suppression of crlr activity. Finally, the effect of crlr knockdown appears to be limited to artery specification, with no effect being observed by crlr-MO injection on the expression of the venous genes flt4 and ephB4 in PCV.
Arterial differentiation in zebrafish is under the control of a shh-vegf-notch pathway.11 Our data indicate that crlr affects this pathway by modulating vegf activity. Indeed, suppression of crlr function causes a significant, even though not complete inhibition of somitic vegf expression, with no effects on shh expression. This is in keeping with the vegf down-regulation observed in mice knocked out for the crlr ligand adrenomedullin.34 vegf MO experiments have shown that the penetrance of the vascular phenotypes associated with the lack of vegf activity is strictly dose dependent.35 Accordingly, the vascular defects observed in crlr morphants, including alterations in ISV development and DA maturation, resemble the least severe phenotypic alterations detectable in zebrafish embryos injected with low doses of vegf MO and in heterozygous vegfa mutant mice.36 In keeping with these observations, vegf overexpression is sufficient to rescue arterial differentiation in crlr morphants.
Shh plays a key role in arterial differentiation by acting upstream of vegf.11
Here, we show that also crlr is a shh-modulated gene in zebrafish. Indeed, inhibition of shh function, as it occurs in syut4 mutants or in cyclopamine-treated embryos, results in a complete down-regulation of somitic crlr expression. Also, in keeping with a putative shh/crlr/vegf pathway, shh overexpression fails to induce vegf up-regulation in crlr morphants. However, crlr overexpression does not rescue vegf signaling and arterial differentiation in cyclopamine-treated embryos and in syut4 mutants lacking shh activity (S.N., unpublished observations, June 2007 and January 2008), indicating that crlr is necessary but not sufficient to drive arterial differentiation.
Although our observations indicate that somitic crlr modulates vegf expression to regulate arterial differentiation, we cannot rule out a direct role for endothelial crlr in DA formation and angiogenesis at later stages of development, as also suggested by its expression in specified DA progenitors as well as in the endothelium of axial vessels. Indeed, crlr morphants show a significant delay in ISV development. Relevant to this point is the observation that alterations of vascular homeostasis, including arteriosclerosis and tumor angiogenesis, are associated with alterations in the adrenomedullin/receptor system.2 Accordingly, crlr down-regulation following crlr-MO1 injection strongly inhibits tumor angiogenesis when zebrafish embryos are grafted with proangiogenic mammalian tumor cells37,38 (Figure S6). Finally, it is interesting to note that the homolog of the human adrenomedullin receptor (admr), a gene encoding for a protein distinct from crlr, has been shown to be expressed in the endothelium of developing zebrafish.39 Even though the biological function of this receptor has not been elucidated, it will be of importance to establish its relationship, if any, with crlr in mediating the biological activity of adrenomedullin during development and vascular pathologies.
In vitro observations had shown that adrenomedullin signaling acts synergistically with vegf to induce in vitro arterial differentiation in murine embryonic stem cell cultures.13 Here, we demonstrate that the major adrenomedullin receptor crlr is a novel element of the genetic cascade that controls arterial differentiation in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to thank F. Cotelli (University of Milan) for helpful discussion and criticisms, S. Mitola (University of Brescia) for her advice in fluorescence microscopy, and N. Lawson (University of Massachusetts) for the vegf expression vector and criticisms.
This work was supported by grants from Istituto Superiore di Sanità (Oncotechnological Program), Ministero dell'Istruzione, Università e Ricerca (Centro di Eccellenza per l'Innovazione Diagnostica e Terapeutica, Cofin projects), Associazione Italiana per la Ricerca sul Cancro, Fondazione Berlucchi, and NOBEL Project Cariplo to M.P.
Authorship
Contribution: S.N. and M.P. designed research; S.N., C.T., L.G., and G.S. performed research; S.N. and M.P. analyzed the data; and S.N. and M.P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Presta, General Pathology, Department of Biomedical Sciences and Biotechnology, University of Brescia, Viale Europa 11, 25123 Brescia, Italy; e-mail: presta@med.unibs.it.

![Figure 2. Crlr knockdown results in vascular defects. Vascular morphology in tg(fli1:EGFP)y1 embryos injected with std-MO (control in panels A,C,E,F,I, and K) or crlr-MO1 (morphant in panels B,D,G,H,J, and L). Morphologic appearance of control (A) and crlr morphant (B) embryos at 28 hpf (head and trunk regions are highlighted by black and red boxes, respectively). Differential interference contrast images of the trunk region (lateral view) of live zebrafish embryos injected with std-MO (C) or crlr-MO1 (D), the lumen of DA and PCV are indicated by red and white bars, respectively; note the abnormal size of DA and the presence of cells with endothelial morphology (red arrow) in proximity to the notochord in crlr morphant (D). Confocal laser microscopy showing details of the head (E,G) and trunk (F,H) regions (lateral view) in std-MO– and crlr-MO1–injected tg(fli1:EGFP)y1 embryos (DA [red bar], PVC [white bar]; white arrow points to poorly developed ISVs in crlr morphant). Crlr knockdown causes also an interruption of the vascular tube at the bifurcation of the DA. Dorsal view of the lateral dorsal aortae (red arrows) and of cardinal vein branches (white arroweads) in control (I) and crlr-MO1–injected (L) tg(fli1:EGFP)y1 embryos; lumens of the lateral dorsal aortae and cardinal vein branches are indicated by red and white bars, respectively. Note the poor lumenization and formation of lateral aortae branches in crlr morphant compared with the posterior cardinal vein. Microangiography performed at 50 hpf shows that blood circulation is confined to the head and heart regions (white arrow) in crlr morphants (L) when compared with control embryos (K). White arrowhead in panel L points to the interruption of the blood flow at the level of bifurcation of the DA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/10/10.1182_blood-2007-10-118166/6/m_zh80100819440002.jpeg?Expires=1769150534&Signature=uVkh0zXn45gQ0sh~7MiZM5FfdIe0Hhv42kuQRRJmbCduJmbKjImIKcgZXXqHtl1CwpwoHg71sRutYctkX7M6rOD5OlH55Yt8ckG8cWIzgaCOm1vAZegOZlWzaMmw5-iRyVZVhd6ejZ9js9nS1ku7YfpMIqr4iDMev3Edc3tGUtGRr4Lv7D8TbiYfBqcB0d3ApKMKAlaOp7oop58sz4~Qj7TL9njm32Jcw05CS-kkzMofRKL2FnpDkV5kW~3Y2NTm-wfrgIm4w-JigmRm0dQv8qG9TQVSO-OR8T~p4JnY-9l9hPZodWfxxhkR1lw2YRa63~jpZ1smLR3vdKeNgBrO9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)