Abstract
We examined the chronic lymphocytic leukemia (CLL) cells of 2457 patients evaluated by the CLL Research Consortium (CRC) and found that 63 (2.6%) expressed immunoglobulin (Ig) encoded by the Ig heavy-chain-variable-region gene (IGHV), IGHV3-21. We identified the amino acid sequence DANGMDV (motif-1) or DPSFYSSSWTLFDY (motif-2) in the Ig heavy-chain (IgH) third complementarity-determining region (HCDR3) of IgH, respectively, used by 25 or 3 cases. The IgH with HCDR3 motif-1 or motif-2, respectively, was paired with Ig light chains (IgL) encoded by IGLV3-21 or IGKV3-20, suggesting that these Ig had been selected for binding to conventional antigen(s). Cases that had HCDR3 motif-1 had a median time from diagnosis to initial therapy comparable with that of cases without a defined HCDR3 motif, as did cases that used mutated IGHV3-21 (n = 27) versus unmutated IGHV3-21 (n = 30). Of 7 examined cases that used Ig encoded by IGHV3-21/IGLV3-21, we found that 5 had a functionally rearranged IGKV allele that apparently had incurred antigendriven somatic mutations and subsequent rearrangement with KDE. This study reveals that CLL cells expressing IGHV3-21/IGLV3-21 most likely were derived from B cells that had experienced somatic mutation and germinal-center maturation in an apparent antigen-driven immune response before undergoing Ig-receptor editing and after germinal-center leukemogenic selection.
Introduction
The relative risk for early disease progression in chronic lymphocytic leukemia (CLL) generally is associated with the mutational status of the immunoglobulin heavy chain variable region genes (IGHV) expressed by the leukemia B cells. Whereas patients with CLL cells that use IGHV genes that have undergone somatic mutations typically have an indolent clinical course, patients with leukemia cells that express unmutated (U) IGHV generally experience relatively rapid disease progression.1-6 To be considered unmutated, the IGHV used by the leukemia cells should have 98% or more nucleic acid sequence homology with a known germline IGHV,4,7-9 although thresholds as low as 97% nucleic acid sequence homology have been proposed.10,11
An apparent exemption to this generalization is the subgroup of patients with CLL cells that use IGHV3-21. Tobin et al12 initially reported that patients with CLL cells that express IGHV3-21 have relatively aggressive disease, even when the expressed IGHV3-21 had evidence for somatic mutation.13,14 However, such mutated (M) IGHV3-21 frequently had higher percent homology to the germline IGHV3-21 (eg, > 97%) than did the M-IGHV of other CLL cases that do not use IGHV3-21, which on average have less than 94% homology to any known germline IGHV gene.3,4,15 This led to speculation that there might be genetic polymorphism in IGHV3-21 and that use of different IGHV3-21 alleles only gave the appearance that such Ig had undergone somatic mutation.16 However, Tobin et al14 analyzed the germline IGHV3-21 genes of patients with CLL cells that used M-IGHV3-21 and found that genetic polymorphism could not account for the base substitutions observed in the expressed IGHV3-21 genes, indicating that they indeed had incurred somatic mutations.13,14 However, it still was is not resolved whether CLL cells expressing such M-IGHV3-21 were derived from B cells that had experienced maturation in the germinal center, where B cells can undergo Ig somatic hypermutation during the immune response to antigen.
Furthermore, the prevalence of CLL cases that use IGHV3-21 in the United States is uncertain. Although initially reported that 31 of 265 patients in a Scandinavian study had CLL cells that used IGHV3-21 (12%), more recent studies of patients mostly from southern and central Europe found that less than 3% of all patients used this IGHV.14,17,18 This has generated speculation that the frequency of patients with CLL cells that use IGHV3-21 might vary geographically or among different ethnic groups.
For these reasons, we examined the frequency and clinical behavior of cases that used IGHV3-21 among a large cohort (n = 2457) of CLL patients evaluated in the United States by the CLL Research Consortium (CRC). In addition, we examined the productively and nonproductively rearranged immunoglobulin kappa light chain variable region genes (IGKV) of such cases to interrogate the B-cell ontogeny of CLL cells that use IGHV3-21.
Methods
Patients
The study evaluated blood samples from 2457 CLL patients followed by the CRC. After informed consent, blood was collected from patients who satisfied diagnostic and immunophenotypic criteria for CLL. Institutional review board approval was obtained from Moores Cancer Center, Division of Hematology/Oncology, Department of Medicine, University of California, San Diego, and the CRC for these studies for the procurement of the samples in all cases, in accordance with the Declaration of Helsinki.
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Hypaque 1077 (Sigma-Aldrich, St Louis, MO), washed twice, and analyzed directly or suspended in fetal calf serum containing 10% dimethylsulfoxide (Sigma-Aldrich) for storage in liquid nitrogen. All samples contained more than 90% CLL B cells as assessed by flow cytometric analyses and the isotype of the expressed immunoglobulin light chains (IgL) was determined by flow cytometry, as described.19
IGHV, IGKV, and IGLV gene analyses
Total cellular RNA was isolated from 5 × 106 CLL B cells using RNeasy reagents (Qiagen, Valencia, CA), as per the manufacturer's instructions. First-strand cDNA was synthesized from one-third of the total purified RNA using an oligo-dT primer and Superscript II RT (Invitrogen, Carlsbad, CA). The remaining RNA was removed with RNase H and the cDNA purified using QIAquik purification columns (Qiagen). The purified cDNA was poly-dG-tailed using deoxyguanosine triphosphate and terminal deoxytransferase (Roche, Indianapolis, IN). The IGHV gene expressed by the CLL B cells was determined by reverse transcription-polymerase chain reaction (RT-PCR) enzyme-linked immunosorbant assay (ELISA) technique.20,21 The cDNA from each sample was amplified using IGHV, IGKV, or IGLV family-specific primers for the sense strand of the gene of interest and antisense IGHM, IGKC, or IGLC consensus primers, respectively. The PCR products were size selected by electrophoresis in 2% agarose containing 0.5 g/mL ethidium bromide (Invitrogen). The expected products were excised and purified using QIAquik purification columns (Qiagen). Most PCR products were sequenced directly, although in several cases, amplified products were cloned into pGEM-T (Promega, Madison, WI) and analyzed as described.22
Nucleic acid sequence analyses were conducted using the fluorescence-dideoxy-chain-termination method and an Applied Biosystems 377 automated nucleic acid sequence analyzer (ABI, Foster City, CA). Nucleotide sequences were analyzed using DNASTAR (DNASTAR, Madison, WI) and compared with the sequences deposited in the V BASE, IMGT,23,24 and BLAST sequence databases. The IGHV gene expression of each of all cases was determined. Somatic mutations were identified by comparison with the most homologous germline IGHV, IGKV, or IGLV gene. We determined the mutational status by determining the number of nucleotide differences between the 5′ end of framework 1 (FW1) and the 3′ end of FW3 and dividing this by the number of IGHV nucleotides. IGHV, IGKV, or IGLV genes, with 98% or more homology with the corresponding germline IGHV, IGKV, or IGLV sequence were considered unmutated. For insertions/duplications or deletions, each inserted/duplicated or deleted sequence was registered as being only one mutation, regardless of the number of extra or missing nucleotides.25 Silent (S) mutations are defined as changes to the nucleic acid sequence that do not change the amino acid sequence of a protein, whereas replacement (R) mutations cause changes in the amino acid sequence of the encoded protein.26
The method of Corbett et al was used to assign IGHD genes of the longer gene families (D2 and D3), and 7 consecutive nucleotides were used for the shorter IGHD gene families.27 Ig heavy chain (IgH) third complementarity-determining region (HCDR3) was determined by the method of Kabat et al,28 and defined by the number of amino acids between codon 94 at the end of FW3 and the conserved Trp of position 102 at the beginning of FW4. The Ig light chain (IgL) CDR3 (LCDR3) was defined by the number of amino acids between codon 88 at the end of FW3 and the conserved Phe of position 97 at the beginning of FW4.
The LCDR3 isoelectric point (pI) was calculated based on the LCDR3 amino acid sequences between codon 88 at the end of FW3 and the conserved Phe of position 97 at the beginning of FW4. Cluster analysis of all sequence was performed using MegAlign (DNASTAR).
KDE analysis
High-molecular-weight DNA was isolated from PBMC with QIAmp DNA Blood Kit (Qiagen). IGKV-to-IGKJ, IGKJ-C-intron-to-KDE and IGKV-to-KDE rearrangements were amplified by PCR on genomic DNA using the appropriate primers.29 When a case had 2 IGKV rearrangements with IGKV-KDE or VK-JK-KDE, the case was considered to have rearranged both IGK alleles.30 Nucleotide sequences were analyzed using DNASTAR (DNASTAR) and compared with the sequences deposited in the V BASE, IMGT,23,24 and BLAST sequence databases.
Statistical analysis
Complete clinical data were available for 57 patients with CLL cells found to express the IGHV3-21 gene. The time from diagnosis of CLL to initial therapy was evaluated using Kaplan-Meier curves, the log-rank test, and the Cox Proportional Hazards Regression model (Breslow method for ties). Fisher exact test was used to compare proportions of the various molecular features. Mutation distribution between CDRs and FWs was evaluated by multinomial distribution models.31 Statistical power considerations were based on a log-rank test at alpha = 0.05. Statistical analyses were performed using GraphPad Prism software and R (version 2.3.1). P values less than .05 were considered statistically significant. All P values are 2-sided, and there is no correction for multiple comparisons.
Results
Molecular features of IGHV3-21 cases used in CLL
Among 2457 CLL patients, we found 63 cases that had CLL cells that expressed functional IGHV3-21 (2.6%). Thirty-three cases (52%) expressed U-IGHV with greater than 98% nucleic acid sequence homology to the germ line IGHV3-21. The remaining 30 cases expressed M-IGHV with homology to the germ line IGHV3-21 of less than 98%, ranging from 91.3% to 97.6% (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The M-IGHV3-21 used by such cases had on average fewer base substitutions than that of other IGHV expressed in CLL that are considered somatically mutated. A statistically significant difference was observed between the average percent germline-sequence homology of M-IGHV3-21 (n = 30) and that of M-IGHV (n = 143) used by CLL cases in a previously described cohort of 307 patients (study 1),3 which were not selected for use of IGHV3-21 (mean ± SD, 95.5% ± 1.9% vs 92.4% ± 3.2%, P < .001). The proportion of cases using M-IGHV with 96% to 98% nucleic acid sequence homology to the respective germline gene was significantly higher in the cases that used IGHV3-21 than in the cases that used other IGHV, 50% (15 of 30) versus 12% (17 of 143), respectively (P < .001).
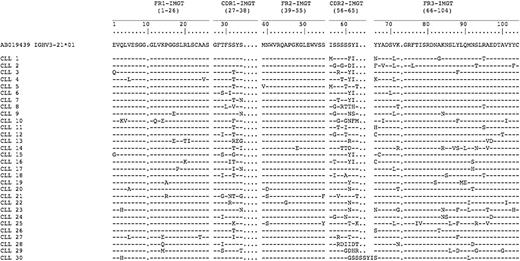
We noted that the M-IGHV3-21 had R mutations clustered in the CDRs (Figure 1). Similar to what was observed by Belessi et al,32 we frequently observed deletions or insertions in HCDR2 codon 61 (9 of 30, 30%) or R mutations in the same codon (13 of 30, 43%). In addition, we observed R mutations in the FW3 in 7 of these cases (23%) at codon 88, which most commonly reflected A → T transversions, resulting in a substitution of one aromatic amino acid (Y) for that of another (F).
Amino acid sequences of IGHV3-21 mutated cases. Capital letters indicate amino acid changes resulting from R mutations. Hyphens indicate homology at that position to the reference IGHV3-21 amino acid sequence (accession number AB019439). Listed on the left is the designation for each CLL sample.
Amino acid sequences of IGHV3-21 mutated cases. Capital letters indicate amino acid changes resulting from R mutations. Hyphens indicate homology at that position to the reference IGHV3-21 amino acid sequence (accession number AB019439). Listed on the left is the designation for each CLL sample.
The most common IGHJ segment used by these cases was IGHJ6 (34 of 63 cases, 54%), followed by IGHJ4 (19 of 63 cases, 30%). On the other hand, a known IGHD segment could be identified in only 36 of the 63 productive IGHV3-21 rearrangements. IGHD3-9 was the most commonly used segment, being detected in 5 rearrangements. A specific IGHD segment could not be assigned in 27 cases. Nevertheless, 25 of these 27 cases had highly similar amino acid sequences in the IgH CDR3 (HCDR3) that defined a characteristic motif, namely, DANGMDV. This HCDR3 motif is analogous to the HCDR3 motif described by Tobin et al,12,14 which we designate as motif 1. Of the 25 cases with motif 1 HCDR3, 13 (13 of 25, 52%) expressed M-IGHV3-21 and 12 (12 of 25, 48%) expressed U-IGHV3-21 (Table 1).
Ig gene characteristics of IGHV3-21–expressing patients
| . | IGHV3-21 mutation status . | Paired light chain variable region . | |||
|---|---|---|---|---|---|
| Mutated . | Unmutated . | IGLV3-21 . | IGKV3-20 . | Other . | |
| HCDR3 motif 1 (N = 25) | 13 | 12 | 24 | 0 | 1 |
| HCDR3 motif 2 (N = 3) | 0 | 3 | 0 | 3 | 0 |
| No HCDR3 motif (N = 35) | 17 | 18 | 7 | 4 | 24 |
| . | IGHV3-21 mutation status . | Paired light chain variable region . | |||
|---|---|---|---|---|---|
| Mutated . | Unmutated . | IGLV3-21 . | IGKV3-20 . | Other . | |
| HCDR3 motif 1 (N = 25) | 13 | 12 | 24 | 0 | 1 |
| HCDR3 motif 2 (N = 3) | 0 | 3 | 0 | 3 | 0 |
| No HCDR3 motif (N = 35) | 17 | 18 | 7 | 4 | 24 |
Three of the 63 cases (4.8%) had an IgH encoded by U-IGHV3-21 and a previously unrecognized stereotypic HCDR3 amino acid motif (DPSFYSSSWTLFDY), which we designate as motif 2 (Table 1). We could not identify a shared HCDR3 motif among the other 35 cases that used IGHV3-21. Of these 35 cases, 17 (17 of 35, 49%) used M-IGHV3-21-encoded IgH, whereas 18 (18 of 35, 51%) expressed U-IGHV3-21-encoded IgH (Table 1).
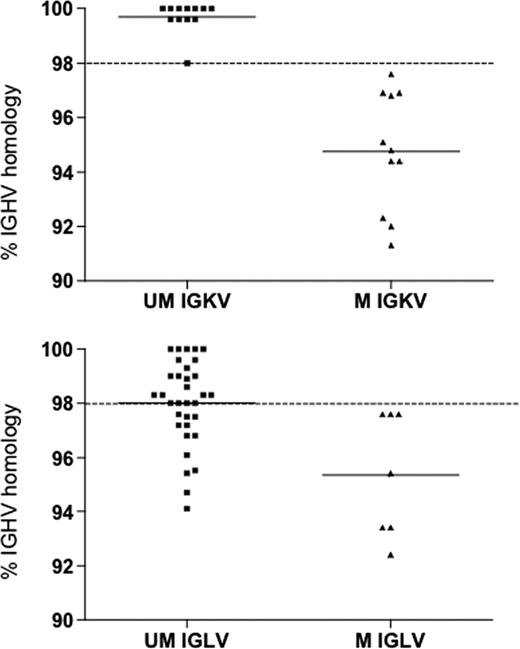
Of the 63 IGHV3-21 cases detected, 40 (63.5%) expressed lambda IgL and 23 (36.5%) used kappa IgL. Among the cases that expressed kappa IgL, IGKV3-20 was the most frequently used (7 of 23, 30%), followed by IGKV1-D8 (3 of 23, 13%) and IGKV1-5 (3 of 23, 13%). Among the 40 cases that expressed lambda IgL, most used IGLV3-21 or IGLV2-14, which accounted for 77% (31 of 40) or 7% (3 of 40) of these cases, respectively. Of the 31 cases that expressed IGLV3-21, 24 had IgH with the HCDR3 motif 1 (Table 1), whereas the other 7 cases had an IgH chain without a shared HCDR3 motif. Twelve used IgH encoded by M-IGHV3-21 and an IgL encoded by an U-IGLV3-21 (Figure 2), 9 cases of which used IgH with the stereotypic HCDR3 motif 1 (Table S1). On the other hand, each of the cases that used an IgH with the HCDR3 motif 2 (n = 3) expressed a kappa IgL encoded by IGKV3-20 (Table 1).
Comparison of the heavy chain mutational status in IGHV3-21 expressing IGKV or IGLV genes. The lines indicate the mean percent of IGHV homology in the UM IGKV or IGLV (mean percentage IGHV homology, 99.7% and 98%, respectively) and in the M IGKV or IGLV (mean percentage IGHV homology, 94.8% and 95.3%, respectively).
Comparison of the heavy chain mutational status in IGHV3-21 expressing IGKV or IGLV genes. The lines indicate the mean percent of IGHV homology in the UM IGKV or IGLV (mean percentage IGHV homology, 99.7% and 98%, respectively) and in the M IGKV or IGLV (mean percentage IGHV homology, 94.8% and 95.3%, respectively).
Clinical features of IGHV3-21 CLL cases
We compared the time from diagnosis to first treatment for the 57 cases that used IGHV3-21 for whom clinical data were available. The median follow-up was 5.4 years. Thirty-two of 57 (56.2%) patients had received therapy at the time of the sample collection (Table 2). The median time from diagnosis to first treatment for all patients was 5.7 years. This is not significantly different from the 6.6 years noted for all patients within the previously described CLL cohort of 307 patients by the CRC (study 1),3 who were not selected for use of IGHV3-21 (P = .08).
Genetic characterization and clinical course of IGHV3-21–expressing patients and of all patients within the previously described CLL cohort of 307 patients (study 1)3
| Patient characteristics . | IGHV3-21 . | Study 1* . |
|---|---|---|
| No. of patients | 63 | 307 |
| Median age, y | 59 | 52 |
| Age range, y | 39-79 | 30-77 |
| Male | 43 (68%) | 201 (65%) |
| Female | 20 (32%) | 106 (35%) |
| M IGHV | 30 (48%) | 143 (47%) |
| U IGHV | 33 (52%) | 164 (53%) |
| No. of patients with TTT and VH data available | 57 | 307 |
| M IGHV | 27 | 143 |
| Median time from diagnosis to first therapy, y | 5.7 | 9.2 |
| U IGHV | 30 | 164 |
| Median time from diagnosis to first therapy, y | 6.0 | 3.4 |
| No. of patients with TTT and sample collection data available | 57 | 307 |
| No. of nontreated patients | 25 (44%) | 223 (73%) |
| Median time from diagnosis to follow-up, y | 4.5 | 4.5 |
| Therapy after sample collection | 14 (24%) | 69 (31%) |
| Median time from diagnosis to first therapy, y | 3.0 | 4.0 |
| Therapy before sample collection | 18 (32%) | 84 (27%) |
| Median time from diagnosis to sample collection, y | 3.7 | 3.8 |
| Median time from diagnosis to first therapy, y | 1.5 | 1.8 |
| Patient characteristics . | IGHV3-21 . | Study 1* . |
|---|---|---|
| No. of patients | 63 | 307 |
| Median age, y | 59 | 52 |
| Age range, y | 39-79 | 30-77 |
| Male | 43 (68%) | 201 (65%) |
| Female | 20 (32%) | 106 (35%) |
| M IGHV | 30 (48%) | 143 (47%) |
| U IGHV | 33 (52%) | 164 (53%) |
| No. of patients with TTT and VH data available | 57 | 307 |
| M IGHV | 27 | 143 |
| Median time from diagnosis to first therapy, y | 5.7 | 9.2 |
| U IGHV | 30 | 164 |
| Median time from diagnosis to first therapy, y | 6.0 | 3.4 |
| No. of patients with TTT and sample collection data available | 57 | 307 |
| No. of nontreated patients | 25 (44%) | 223 (73%) |
| Median time from diagnosis to follow-up, y | 4.5 | 4.5 |
| Therapy after sample collection | 14 (24%) | 69 (31%) |
| Median time from diagnosis to first therapy, y | 3.0 | 4.0 |
| Therapy before sample collection | 18 (32%) | 84 (27%) |
| Median time from diagnosis to sample collection, y | 3.7 | 3.8 |
| Median time from diagnosis to first therapy, y | 1.5 | 1.8 |
Data are from Rassenti et al.3
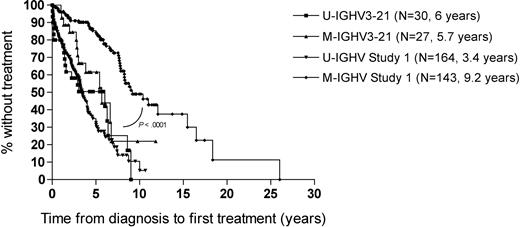
We did not observe a significant difference between the median time from diagnosis to first treatment between cases that used U-IGHV3-21 (n = 30) versus M-IGHV3-21 genes (n = 27) (6.0 years and 5.7 years, respectively, hazards ratio (HR) = 0.63, P = .2). The median time from diagnosis to initial treatment for all IGHV3-21 cases (5.7 years) appeared longer than that of patients with CLL cells that used other U-IGHV (3.4 years) in a previously described cohort of 307 patients,3 but this difference was not statistically significant (HR = 1.4, P = .1). Moreover, the median time from diagnosis to first treatment of the patients with CLL cells that expressed M-IGHV3-21 (5.7 years) was significantly shorter than that of patients with CLL cells that used M-IGHV other than IGHV3-21 (9.2 years, HR = 0.36, P = .001), but longer than the 3.4 years noted for patients with CLL cells that had U-IGHV other than IGHV3-21, in this previous cohort (HR = 1.79, P = .048) (Figure 3). The median time from diagnosis to first treatment of the patients with CLL cells that used U-IGHV3-21 (6.0 years) also appeared longer than that of patients with CLL cells that used U-IGHV other than IGHV3-21 in this previously described cohort, but the difference was not statistically significant (HR = 1.1, P = .6).
Comparison between median time from diagnosis to first treatment of study 1 cohort (N = 307)† and IGHV3-21 (N = 57) using cutoff = 98%. †Data are from Rassenti et al.3
Comparison between median time from diagnosis to first treatment of study 1 cohort (N = 307)† and IGHV3-21 (N = 57) using cutoff = 98%. †Data are from Rassenti et al.3
Prior studies suggested that patients with CLL cells that use IGHV3-21–encoded IgH with HCDR3 motif 1 have a worse prognosis than do patients with CLL cells that use IGHV3-21–encoded IgH without a defined motif.17,33 However, other studies failed to observe such a difference in prognosis between these subgroups of patients.18,34 In the current study, the patients who had CLL cells with IGHV3-21–encoded IgH with HCDR3 motif 1 actually appeared to have a longer median time from diagnosis to first treatment (6.7 years, n = 22) than did patients with IGHV3-21–encoded IgH without a defined HCDR3 motif (5.4 years, n = 32). However, this difference was not statistically significant (P = .2). Moreover, we did not discern a significant difference in the median times from diagnosis to initial therapy between cases that used IgH with or without a defined HCDR3 motif among the subgroups of patients with CLL cells that used either U-IGHV3-21 or M-IGHV3-21. As such, it does not appear that patients with CLL cells that use IGHV3-21–encoded IgH with HCDR3 motif 1 necessarily have more aggressive disease than do patients with CLL cells that use IGHV3-21–encoded IgH without a defined motif.
KDE analysis
We examined for rearranged IGKV genes in 7 cases with CLL cells that expressed antibodies with IGHV and IGLV encoded by a M-IGHV3-21 and an U-IGLV3-21, respectively. We found 5 (71%) cases each had one nonfunctional IGKV rearrangement and one functional VK-JK rearrangement that apparently underwent subsequent rearrangement with KDE (Table 3). The IGKV of each of these cases had somatic mutations with germline–nucleic-acid-sequence homology ranging from 93.6% to 97.6% (mean, 96.1%). Silent mutations were clustered in the Ig variable-region FW, whereas replacement mutations were clustered in the CDRs (Figure 4). On the other hand, all 9 IGKV genes that were nonproductively rearranged in the leukemia cells of this patient subgroup had 100% homology with a known germline IGKV gene. Two cases each had 2 nonfunctional IGKV rearrangements with either IGKV-IGKJ or IGKV-KDE, none of which was apparently expressed and then aborted via receptor editing.
KDE analysis results in IGHV3-21/IGLV3-21 discordant cases and IGHV1-69/IGLV3-09
| Sample ID . | IGK allele configuration . | IGKV . | IGJK . | Frame . | % IGKV homology . | FR, P . | CDR, P . | Replacement mutation . | Silent mutation . | % IGHV homology . | % IGLV homology . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR . | CDR . | FR . | CDR . | ||||||||||
| CLL1 IGHV3-21/IGLV3-21 | VK-JK-KDE | IGKV1-39 | IGJK3 | + | 96.1 | 0.009 | 0.026 | 3 | 4 | 4 | 0 | 95.4 | 98.9 |
| IGKV-KDE | IGKV2D-29 | − | 100 | ||||||||||
| CLL2 IGHV3-21/IGLV3-21 | VK-JK-KDE | IGKV1-17 | IGJK4 | + | 93.6 | 0.001 | 0.013 | 5 | 6 | 6 | 1 | 94.7 | 99.6 |
| IGKV-KDE | IGKV2-30 | − | 100 | ||||||||||
| CLL3 IGHV3-21/IGLV3-21 | VK-JK-KDE | IGKV1-39 | IGJK5 | + | 96.4 | 0.003 | 0.017 | 2 | 4 | 4 | 0 | 96.8 | 99.3 |
| IGKV-KDE | IGKV2-28 | − | 100 | ||||||||||
| CLL4 IGHV3-21/IGLV3-21 | VK-JK-KDE | IGKV1-9 | IGJK4 | + | 96.8 | 0.009 | 0.012 | 2 | 3 | 4 | 0 | 96.8 | 98.9 |
| IGKV-KDE | IGKV1-33 | − | 100 | ||||||||||
| CLL5 IGHV3-21/IGLV3-21 | VK-JK-KDE | IGKV3-11 | IGJK5 | + | 97.6 | 0.041 | 0.031 | 2 | 3 | 2 | 0 | 97.2 | 98.3 |
| IGKV-KDE | IGKV1-33 | − | 100 | ||||||||||
| CLL6 IGHV3-21/IGLV3-21 | IGKV-J | IGKV1-33 | IGJK2 | − | 100 | 97.5 | 98.6 | ||||||
| IGKV-KDE | IGKV1-27 | − | 100 | ||||||||||
| CLL7 IGHV3-21/IGLV3-21 | IGKV-J | IGKV2-18 | IGJK4 | − | 100 | 97.5 | 98.3 | ||||||
| IGKV-KDE | IGKV1-27 | − | 100 | ||||||||||
| CLL1 IGHV1-69/IGLV3-09 | IGKV-KDE | IGKV1-16 | − | 100 | 100 | 100 | |||||||
| IGKV-KDE | IGKV3-15 | − | 100 | ||||||||||
| CLL2 IGHV1-69/IGLV3-09 | IGKV-J | IGKV1-27 | IGJK4 | − | 100 | 99.3 | 100 | ||||||
| IGKV-KDE | IGKV3-7 | − | 100 | ||||||||||
| CLL3 IGHV1-69/IGLV3-09 | IGKV-KDE | IGKV1-9 | − | 100 | 100 | 100 | |||||||
| IGKV-KDE | IGKV4-1 | − | 100 | ||||||||||
| CLL4 IGHV1-69/IGLV3-09 | IGKV-J | IGKV1-33 | IGJK3 | − | 100 | 100 | 100 | ||||||
| IGKV-KDE | IGKV3-11 | − | 100 | ||||||||||
| Sample ID . | IGK allele configuration . | IGKV . | IGJK . | Frame . | % IGKV homology . | FR, P . | CDR, P . | Replacement mutation . | Silent mutation . | % IGHV homology . | % IGLV homology . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR . | CDR . | FR . | CDR . | ||||||||||
| CLL1 IGHV3-21/IGLV3-21 | VK-JK-KDE | IGKV1-39 | IGJK3 | + | 96.1 | 0.009 | 0.026 | 3 | 4 | 4 | 0 | 95.4 | 98.9 |
| IGKV-KDE | IGKV2D-29 | − | 100 | ||||||||||
| CLL2 IGHV3-21/IGLV3-21 | VK-JK-KDE | IGKV1-17 | IGJK4 | + | 93.6 | 0.001 | 0.013 | 5 | 6 | 6 | 1 | 94.7 | 99.6 |
| IGKV-KDE | IGKV2-30 | − | 100 | ||||||||||
| CLL3 IGHV3-21/IGLV3-21 | VK-JK-KDE | IGKV1-39 | IGJK5 | + | 96.4 | 0.003 | 0.017 | 2 | 4 | 4 | 0 | 96.8 | 99.3 |
| IGKV-KDE | IGKV2-28 | − | 100 | ||||||||||
| CLL4 IGHV3-21/IGLV3-21 | VK-JK-KDE | IGKV1-9 | IGJK4 | + | 96.8 | 0.009 | 0.012 | 2 | 3 | 4 | 0 | 96.8 | 98.9 |
| IGKV-KDE | IGKV1-33 | − | 100 | ||||||||||
| CLL5 IGHV3-21/IGLV3-21 | VK-JK-KDE | IGKV3-11 | IGJK5 | + | 97.6 | 0.041 | 0.031 | 2 | 3 | 2 | 0 | 97.2 | 98.3 |
| IGKV-KDE | IGKV1-33 | − | 100 | ||||||||||
| CLL6 IGHV3-21/IGLV3-21 | IGKV-J | IGKV1-33 | IGJK2 | − | 100 | 97.5 | 98.6 | ||||||
| IGKV-KDE | IGKV1-27 | − | 100 | ||||||||||
| CLL7 IGHV3-21/IGLV3-21 | IGKV-J | IGKV2-18 | IGJK4 | − | 100 | 97.5 | 98.3 | ||||||
| IGKV-KDE | IGKV1-27 | − | 100 | ||||||||||
| CLL1 IGHV1-69/IGLV3-09 | IGKV-KDE | IGKV1-16 | − | 100 | 100 | 100 | |||||||
| IGKV-KDE | IGKV3-15 | − | 100 | ||||||||||
| CLL2 IGHV1-69/IGLV3-09 | IGKV-J | IGKV1-27 | IGJK4 | − | 100 | 99.3 | 100 | ||||||
| IGKV-KDE | IGKV3-7 | − | 100 | ||||||||||
| CLL3 IGHV1-69/IGLV3-09 | IGKV-KDE | IGKV1-9 | − | 100 | 100 | 100 | |||||||
| IGKV-KDE | IGKV4-1 | − | 100 | ||||||||||
| CLL4 IGHV1-69/IGLV3-09 | IGKV-J | IGKV1-33 | IGJK3 | − | 100 | 100 | 100 | ||||||
| IGKV-KDE | IGKV3-11 | − | 100 | ||||||||||
IGKV amino acid sequences of 5 cases with CLL cells expressing M-IGHV3-21 with HCDR3 motif1, U-IGLV3-21, and in-frame IGKV-J-KDE rearrangements. Capital letters indicate amino acid changes resulting from R mutations. Hyphens indicate homology at that position to the respective reference IGKV amino acid sequence. Listed on the left is the designation for each CLL sample.
IGKV amino acid sequences of 5 cases with CLL cells expressing M-IGHV3-21 with HCDR3 motif1, U-IGLV3-21, and in-frame IGKV-J-KDE rearrangements. Capital letters indicate amino acid changes resulting from R mutations. Hyphens indicate homology at that position to the respective reference IGKV amino acid sequence. Listed on the left is the designation for each CLL sample.
None of the IGKV genes involved in the VK-JK-KDE rearrangements was expressed in the κ-expressing CLL cells that used M-IGHV3-21. Among the 23 cases with CLL cells with IgH encoded by IGHV3-21 and κ IgL, the upstream IGJK genes IGJK1 and IGJK2 were the most commonly used, being observed in 9 (39%) and 8 (35%) cases, respectively, whereas downstream IGJK genes IGJK4 and IGJK5 were observed only in 1 (4%) and 2 (9%) cases, respectively. Among the 5 VK-JK-KDE rearrangements, 4 of the 5 cases (80%) rearranged with downstream IGJK genes either IGJK4 or IGJK5 (40% for both).
We calculated the LCDR3 pI values for the amino acid sequences found between codon 88 at the end of FW3 and the conserved Phe at position 97 located at the beginning of FW4. A statistically significant difference was observed between the mean LCDR3 pI value for the 5 VK-JK-KDE rearrangements (12.5) and the mean LCDR3 pI of IGLV3-21–encoded IgL expressed by the CLL cells of these 5 patients (4.4, P < .001).
We examined for IGKV-KDE rearrangements in 4 cases of λ-IgL expressing CLL cells that used the U-IGHV1-69 and U-IGLV3-09 genes that also had stereotyped HCDR3 and LCDR3. The HCDR3 and LCDR3 of these patients have been previously characterized and described as CLL69-C.35 Each of these cases had CLL cells that expressed 2 nonfunctional IGKV alleles. These alleles had IGKV genes that were 100% homologous to the respective IGKV germline sequences and had rearranged with either KDE or IGJK (Table 3).
Discussion
In this study we examined the CLL cells of a large cohort of patients followed by the CRC in the United States for expression of IGHV3-21. Although initially reported that this IGHV was used by the CLL cells of more than 10% of patients in Scandinavia,14 the frequency of cases that use IGHV3-21 in our large cohort (2.6%) was more in line with the frequency observed in cases from southern and central Europe (2.9%).17 The patients who enrolled in the CRC study were not selected, but presented from diverse regions of the United States to CRC sites in multiple regions across the country. Conceivably, the initial study involving patients from Scandinavia had a less diverse population of patients. Further studies are required to define whether patients of Scandinavian ancestry have a higher use frequency of IGHV3-21 or whether geographic differences account for differences in use frequency. Nevertheless, this study indicates that patients who express this IGHV constitute only a relatively small subset of patients seen in major referral centers in the United States.
Primary structure analyses of the IgH and IgL variable regions provide evidence that the IGHV3-21–encoded Ig in CLL are selected for their ability to bind antigen. In addition to observing Ig with a stereotypic HCDR3 motif identified in earlier studies12,14 (called motif 1), we discerned another stereotypic HCDR3 motif in the IgH expressed by 3 unrelated cases of CLL, which we called motif 2. We found the HCDR3 with motif 1 or motif 2 determined the use of particular IgL. Whereas the IgH with motif 1 almost invariably were paired with lambda IgL encoded by U-IGLV3-21,14,17,18 the IgH with HCDR3 motif 2 were each paired with kappa IgL encoded by U-IGKV3-20. Moreover, because these IgH encoded by U-IGHV3-21 only differed from one another in the HCDR3, the nonstochastic association of IgL with IgH that had motif 1 or motif 2 appears exclusively predicated on the structure of the HCDR3. Similar nonstochastic pairing of IgH with particular IgL based exclusively on the HCDR3 was observed for Ig encoded by U-IGHV1-69 in CLL.35 Because the HCDR3 contributes substantially into the Ig antigen-binding pocket, these examples provide compelling evidence that these Ig were selected for their ability to bind a conventional antigen(s), rather than to a superantigen(s) that interacts with Ig in regions other than the HCDR3.
Prior studies noted that patients with CLL cells that use IGHV3-21 typically had a more aggressive clinical course than did patients with CLL cells that use M-IGHV, other than IGHV3-21. We found that the median time from diagnosis to initial therapy of patients who used M-IGHV3-21 (5.7 years, n = 27) was not significantly different from that of patients who used U-IGHV3-21 (6.0 years, n = 30), but significantly shorter than that of patients who used other M-IGHV in general (namely, 9.2 years, n = 143), as noted in an earlier study of 307 unselected CLL patients evaluated by the CRC.3 As such, the generalization that patients with CLL cells that use M-IGHV have more indolent disease does not appear to apply to cases that use IGHV3-21.
Many of the cases that used M-IGHV3-21 had only modest numbers of somatic mutations (mean ± SD, 95.5% ± 2%) that most often (9 of 12) had IgH with HCDR3 motif 1 paired with a lambda IgL encoded by U-IGLV3-21 with more than 98% sequence homology to the germ line IGLV3-21 (mean ± SD, 99% ± 0.6%). The modest numbers of mutations in the IgH and the lack of mutations in the IgL support the notion that such cases were derived from B cells that were selected in a compartment other than that of the germinal center, where B cells typically experience somatic hypermutation, often resulting in extensive mutations in the Ig variable region.36-38
In this regard, such CLL cases might have developmental histories similar to those of CLL cells that use U-IGHV. Despite also showing strong evidence for expression of restricted amino acid motifs in the HCDR3 and nonstochastic IgH and IgL pairing predicated on HCDR3 motifs, the Ig used by CLL cells that express IGHV1-69 typically have little or no somatic mutations. Such CLL cells most likely were derived from B cells that had not experienced somatic hypermutation and maturation within the germinal center. The possible common developmental histories of CLL cells that use marginally M-IGHV3-21 or U-IGHV1-69 potentially could provide a clue as to why such cases have more aggressive disease than do CLL cases that use other M-IGHV.
To interrogate the developmental histories of CLL cells that express such modestly mutated IGHV3-21, we evaluated the rearranged IGKV of cases that expressed M-IGHV3-21 together with U-IGLV3-21. Previous studies already demonstrated the presence of functional VK-JK rearrangements inactivated by secondary rearrangements with KDE in lambda IgL–expressing CLL cases that used IgH encoded by IGHV3-2118 or other IGHV genes.30,39 The current study is the first to examine for mutations in the VK-JK rearrangements that were inactivated via KDE rearrangement in CLL cells that expressed M-IGHV3-21 and U-IGLV3-21. We found that 5 of 7 cases examined had a functional IGKV rearranged with IGJK that apparently had undergone secondary Ig gene rearrangement with KDE. Analyses of the IGKV gene sequences revealed somatic mutations in these rearranged IGKV, resulting in their having only 93.6% to 97.6% homology with any known IGKV. In contrast, none of the IGKV rearranged in CLL cells that used U-IGHV1-69 together with U-IGLV3-09 had such productive VK-JK rearrangements. Interestingly, we found that the frequency of replacement mutations in the CDRs of the productively rearranged VK-JK genes of IGHV3-21 CLL was significantly higher than that noted for R mutations in the framework regions of these IGKV (mean R mutations in the CDRs: 4 of 45 bases (mean frequency, 8%), mean R mutations in FWs: 2.8 of 237 bases (mean frequency, 1.2%; P = .003). Because these CDRs encode the residues that line the pocket of the antigen binding site, such nonstochastic distribution of R versus S mutations argues that such sequences encoded antibodies that were selected in the germinal center in an antigen-driven immune response.19,35 Moreover, these substitutions suggest that the B cells from which these CLL cases were derived previously expressed kappa IgL during the course of such immune responses. Subsequent gene rearrangement with the KDE apparently aborted expression of these kappa IgL by these B cells, which then were rescued by the expression of the other kappa IgL allele or a lambda IgL. Such a process has been observed when the Ig has high-affinity anti-self reactivity that could lead to activation-induced cell death and deletion of such B cells unless the B cell could abort expression of its kappa IgL through KDE rearrangement and then express another IgL that, together with the expressed IgH, does not have such deleterious reactivity. This process has been designated as Ig receptor editing.40,41
In this study, we observed a shift of the LCDR3 pI value from a pI of 12.5 in the LCDR3 of the 5 VK-JK-KDE that apparently previously had functional VK-JK rearrangements to a pI of 4.4 in the LCDR3 of the subsequently expressed IgL encoded by IGLV3-21. Prior studies on Ig receptor editing of anti-DNA antibodies found a similar shift from high LCDR pIs to low LCDR pIs,42-45 which was found to neutralize the positively charged HCDRs that were more prone to DNA-binding. Conceivably, the Ig receptor editing observed in these cases of IGHV3-21/IGLV3-21 CLL similarly might have functioned to abort deleterious autoantibody activity. However, because of the observed nonstochastic pairing of IGLV encoded by IGLV3-21 with IGHV encoded by IGHV3-21 with conserved HCDR3 motifs, it appears that Ig receptor editing is not limited to negative selection but might also allow for generation of Ig that are selected during B-cell leukemogenesis.46-49
It is interesting to note that all 5 of the cases in which we observed such apparent Ig receptor editing had defective kappa IgL rearrangements involving the other kappa IgL allele and expressed IGLV3-21 with little or no somatic mutations. As such, even though these cases expressed Ig with evidence for antigen selection, the process of somatic hypermutation apparently did not have to operate on B cells to generate such Ig that then appeared selected during leukemogenesis. This argues that selection and transformation of CLL cells probably occurred in a post–germinal center B-cell compartment.
Collectively, these analyses show that despite sharing association with more aggressive disease, most CLL cases that use M-IGHV3-21/IGLV3-21 are distinctive from cases that use U-IGHV1-69 in their B-cell differentiation history. Even those cases that use IGHV3-21 with only modest numbers of somatic mutations (mean ± SD, 96.5% ± 1.1%) apparently were derived from B cells that had undergone germinal center somatic hypermutation in an apparent antigen-driven immune response before subsequent gene rearrangement and selection during leukemogenesis. Finally, these data, together with data from other studies on other human diseases,50-54 raise the possibility that Ig receptor editing, recreated in the periphery, might play a role in the generation of Ig selected in the development of lymphocyte neoplasia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work funded in part by National Institutes of Health grant PO1-CA81534 for the CLL Research Consortium and R37-CA49870 (T.J.K.).
National Institutes of Health
Authorship
Contribution: E.M.G. designed and performed research, analyzed data, and wrote the manuscript; S.J. analyzed the data, performed statistical analysis, and wrote the manuscript; G.F.W. and L.Z.R. performed research and analyzed data; M.J.K., W.G.W., J.G.G., J.R.B., K.R.R., J.C.B., and N.E.K. contributed patient samples and data; A.W.G. performed the database and bioinformatics analysis; T.J.K. designed the research, contributed patient samples, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas J. Kipps, Moores UCSD Cancer Center, 3855 Health Sciences Drive, #0820, La Jolla, CA 92093-0820; e-mail: tkipps@ucsd.edu.