Abstract
Fanconi anemia (FA) is a genetic disease characterized by congenital abnormalities, bone marrow failure, and cancer susceptibility. A total of 13 FA proteins are involved in regulating genome surveillance and chromosomal stability. The FA core complex, consisting of 8 FA proteins (A/B/C/E/F/G/L/M), is essential for the monoubiquitination of FANCD2 and FANCI. FANCM is a human ortholog of the archaeal DNA repair protein Hef, and it contains a DEAH helicase and a nuclease domain. Here, we examined the effect of FANCM expression on the integrity and localization of the FA core complex. FANCM was exclusively localized to chromatin fractions and underwent cell cycle–dependent phosphorylation and dephosphorylation. FANCM-depleted HeLa cells had an intact FA core complex but were defective in chromatin localization of the complex. Moreover, depletion of the FANCM binding partner, FAAP24, disrupted the chromatin association of FANCM and destabilized FANCM, leading to defective recruitment of the FA core complex to chromatin. Our results suggest that FANCM is an anchor required for recruitment of the FA core complex to chromatin, and that the FANCM/FAAP24 interaction is essential for this chromatin-loading activity. Dysregulated loading of the FA core complex accounts, at least in part, for the characteristic cellular and developmental abnormalities in FA.
Introduction
The 13 identified Fanconi anemia (FA) proteins cooperate in a common cellular pathway regulating the cellular response to DNA cross-linking agents, such as cisplatin (CDDP), diepoxybutane (DEB), and mitomycin C (MMC).1 Of these FA proteins, 8 (A, B, C, E, F, G, L, and M) are assembled into a core complex,2,3 which contains a ubiquitin E3 ligase activity (FANCL subunit)4 and a DNA translocase activity (FANCM).5 In response to DNA damage, or during S-phase progression, the FA core complex coordinately monoubiquitinates 2 downstream substrates, FANCD26,7 and FANCI.8-10 These monoubiquitinated proteins subsequently translocate to the chromatin where they are believed to interact with additional downstream FA proteins, including FANCJ/BRIP1,11-13 FANCD1/BRCA2,14 and FANCN/PALB2.15,16 These downstream proteins then regulate homologous recombination (HR) repair. Disruption of any of the proteins in the FA pathway accounts for the common cellular and clinical phenotype of FA patients.17 How the pathway participates in the process of DNA cross-link repair remains unknown.18 Some FA complementation groups also exhibit additional phenotypic variation, suggesting that some FA genes have functions outside a simple linear FA pathway.19-22
The FA core complex may have additional functions beyond the monoubiquitination of FANCD2 and FANCI.8 A FANCD2-Ub linear fusion protein complements the MMC hypersensitivity of Fancd2-deficient chicken cells, but fails to correct the phenotype of FA core complex–deficient cells.23 The FA core complex may therefore have additional activities, such as the recognition of specific DNA substrates, the regulation of the DNA replication machinery, and/or the monoubiquitination of additional (unknown) substrates. These additional functions may be explained, at least in part, by the presence of FA core subcomplexes with variable sizes and variable subcellular distributions.3
When a replication fork encounters an interstrand DNA cross-link during replication, the replication fork arrests near the lesion, resulting in aberrant DNA structures. These abnormal structures activate checkpoint and repair pathways. FA cells, carrying mutations in FA genes, are highly sensitive to DNA cross-linking agents, compared with other DNA-damaging agents, such as ionizing radiation (IR), ultraviolet (UV), and hydroxyurea (HU). This hypersensitivity suggests that some components of the FA core complex may be involved in detecting and binding the DNA lesions caused by treatment of DNA cross-linking agents.
The recently identified FANCM5 and FANCJ11-13 proteins suggest a direct involvement of FA proteins at sites of DNA repair. FANCM is homologous to the archaeal protein Hef (helicase-associated endonuclease for fork-structured DNA), and is a member of the XP-F superfamily.24 The N-terminal region of FANCM is able to bind to single-stranded DNA.25 Moreover, FANCM has a DNA-dependent ATPase activity, and it can dissociate DNA triple helices in vitro.5,25 FANCJ/BRIP1, which is thought to play a role downstream in the FA pathway, is a 5′ to 3′ DNA helicase with substrate specificity toward specific DNA duplexes (Y-shaped DNA). Also, in support of a role for FA proteins in the processing of DNA structures, a recent study demonstrated that recombinant FANCD2 has direct DNA binding activity in vitro.26 Ciccia et al27 reported that recombinant FANCM is directed to branched DNA structures by a novel FA core complex member, FAAP24. Consistent with these studies, some branched DNA structures activate FANCD2 monoubiquitination in vitro.28 Deficiency of either FANCM or FAAP24, but not FANCJ, inhibits FANCD2 monoubiquitination. These results suggest that the DNA-binding affinity of the FANCM/FAAP24 complex may regulate the ubiquitin ligase activity or the localization of the FA core complex.
Here, we show that FANCM recruits the FA core complex to chromatin but is not essential for the assembly of the FA core subunits. Cells depleted of FANCM by siRNA, or cells in which both FANCM alleles are mutated, are defective in the chromatin association of the FA core complex. In addition, we found that the interaction of the FANCM with FAAP24 is required for their stable association in chromatin.
Methods
Cell culture
HeLa cells were maintained in Dulbecco modified Eagle medium (DMEM) with 15% fetal calf serum (FCS). EUFA867 (FANCM-deficient lymphoblasts) and GM02254A.L (wild-type lymphoblasts) were maintained in RPMI-1640 medium with 15% FCS. For generation of FLAG-FANCL HeLa cell lines, pMMP-puro-Flag-FANCL construct was used for the retrovirus production.
Cell synchronization
For synchronization of cells in G1/S phase, HeLa cells were incubated with 2.5 mM thymidine for 18 hours twice with an intervening 10 hours of incubation in fresh medium without thymidine.29 For synchronization of cells in M phase, HeLa cells were incubated with 2.5 mM thymidine for 20 hours, and then incubated for 12 hours with 200 ng/mL nocodazole without thymidine. The cell-cycle synchronization was confirmed by flow cytometry after propidium iodide staining.
Cell extraction and fractionation
For whole-cell extracts, cell pellets were directly lysed with SDS sample buffer. For biochemical cell fractionation, cells were first lysed with 0.1% Triton X-100 CSK buffers (10 mM PIPES [pH 6.8], 100 mM NaCl, 300 mM sucrose, MgCl2, 1 mM EGTA, 1 mM EDTA, 1 mM PMSF, 1 μg/mL leupeptin, 1 μg/mL aprotinin, 50 mM NaF, 0.1 mM NaVO4, and 0.1% Triton X-100) for 5 minutes on ice. After centrifugation (1500g for 5 minutes), the supernatant was labeled “S.” The pellet was washed once more with the same buffer and labeled “P.”
Antibodies
Rabbit anti-FANCM was kindly provided by Dr Weidong Wang (National Institutes of Health, Baltimore, MD),5 and anti-FAAP24 was kindly provided by Dr Steve West (London Research Institute, London, United Kingdom).27 Rabbit anti-FANCA, anti-FANCG, anti-FANCE antibodies were described previously.30 Affinity-purified anti-FANCG antibody was generated for immunofluorescent (IF) studies. Other reagents used in this study were as follows: anti-FANCJ (Novus, Littleton, CO), anti-FANCD2, monoclonal anti-FLAG M2, M2-agrarose beads and α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA), CHK1-p345 (Cell Signaling Technology, Danvers, MA), MUS81 and MCM3 (Bethyl Laboratories, Montgomery, TX), RPA70 (EMD Biosciences, San Diego, CA), Histone H3 (Upstate Biotechnology, Charlottesville, VA), and Orc2 (BD Pharmingen, San Diego, CA).
In addition, a new anti-FANCM antibody was raised against amino acids 1190 to 1273 of FANCM. This polypeptide fused to maltose binding protein was expressed in Escherichia coli and purified as instructed in the manufacturer's protocol (New England Biolabs, Ipswich, MA), prior to antibody generation in New Zealand white rabbits.
Immunoprecipitation
Whole cells were lysed with 0.5% TX-300mCSK containing 300 mM NaCl for 30 minutes on ice. After centrifugation (15 294g for 20 minutes), the supernatant was diluted to 150 mM NaCl before immunoprecipitation. Cell extracts were incubated with 15 μL anti-FLAG M2 agarose overnight at 4°C on a rocker, and washed 3 times with 0.1% TX-150mCSK buffer. The precipitates were analyzed on 3% to 8% Tris-acetate SDS–polyacrylamide gel electrophoresis (PAGE; Invitrogen, Carlsbad, CA).
siRNA transfection
siRNAs were synthesized by Qiagen (Valencia, CA). The target sequences of LacZ, FANCM, FAAP24-1, FAAP24-2, FANCA, and UBE2T are aacgtacgcggaatacttcga, aagctcataaagctctcggaa, attttcgaggatggcttgaca, tgcattctttatgtcaccgaa, cagcgttgagatatcaaagat, and gatctaagttgcctaccttga, respectively. Control siRNA is designed and provided by Qiagen.
MMC survival assay
HeLa cells transfected with siRNAs were seeded in duplicate in 96-well microplates at a density of 400 cells/well in appropriate medium. MMC was added at a final concentration of 0 to 200 nM. Cells were then incubated at 37°C in a 5% CO2 incubator for 5 days, and cell survival was then determined by staining nucleic acids with a proprietary dye (CyQUANT; Molecular Probes, Eugene, OR) and subsequently analyzed by a fluorescence microplate reader (FL × 800; Bio-Tek Instruments, Winooski, VT) according to the manufacturer's protocol. Alternatively, HeLa cells were transfected with the indicated siRNAs for 48 hours, and then exposed to the indicated concentration of MMC for 5 days. Viable cells were counted by trypan blue exclusion.
Immunofluorescence staining
HeLa cells were transfected with siRNAs for 48 hours before seeding into 4-well culture slide at a density of 20 000 cells per well. Cells were pre-extracted with phosphate-buffered saline (PBS) containing 0.3% Triton X-100 for 2 minutes and then fixed with 3.7% paraformaldehyde for 10 minutes on ice. After blocking with 5% goat serum/0.1% NP-40/PBS, the cells were incubated with the affinity purified rabbit polyclonal anti-FANCG antibody (1:400 dilution) overnight at 4°C. The Alexa Fluor 488 goat anti–rabbit IgG (Molecular Probes) was used as a secondary antibody at 1:500 dilution. After incubation with secondary antibody, samples were washed and mounted in Vectashield mounting media with DAPI (Vector Laboratories, Burlingame, CA). Cells were observed with a 63× objective lens (NA 1.4, oil) on a Zeiss Axiovert 200 M microscope (Thornwood, NY) equipped with a CoolSnap HQ camera (Photometrics, Tucson, AZ). Images were acquired and analyzed using SlideBook 4.0 software (Intelligent Imaging Innovations, Denver, CO).
Phosphatase treatment
Cell extracts were incubated with 400 U of λ phosphatase at 30°C for 40 minutes and then analyzed by Western blotting.
Results
FANCM localizes to chromatin and is phosphorylated following DNA damage
To examine the cellular localization of FANCM, cells were lysed in a low-salt (100 mM NaCl) and detergent-containing buffer, yielding a soluble fraction (S) and an insoluble pellet (P; Figure 1A). The S fraction contained cytoplasmic and nucleoplasmic proteins, and the P fraction contained proteins stably bound to nuclear structure (eg, chromatin and nuclear matrix proteins). FANCM was exclusively detected in the P fractions, and cellular localization did not change following DNA damage with MMC or replication block with HU (Figure 1B). MMC and HU activated phosphorylation of the chromatin-bound FANCM, consistent with previous studies.5 Monoubiquitinated FANCD2 was found primarily in the P fraction, as previously described,31 and FANCA was found in both fractions.
FANCM is detected exclusively in the chromatin-containing fraction. (A) Experimental protocol for cell fractionation described in this study. (B) HeLa cells were treated with MMC (50 ng/mL for 17 hours) and HU (2 mM for 17 hours) and then fractionated into the cytosol and nucleoplasmic fraction (S) and the chromatin-containing fraction (P). (C) HeLa cells were fractionated into S and P as in Figure 1A. The pellet was further extracted with 1% Triton X-100 (lanes 5-8) or 250 mM NaCl (lanes 9-12). After centrifugation, the supernatant and pellet were analyzed by Western blotting. (D) Cell extracts were incubated with lambda phosphatase and immunoblotted for the indicated proteins.
FANCM is detected exclusively in the chromatin-containing fraction. (A) Experimental protocol for cell fractionation described in this study. (B) HeLa cells were treated with MMC (50 ng/mL for 17 hours) and HU (2 mM for 17 hours) and then fractionated into the cytosol and nucleoplasmic fraction (S) and the chromatin-containing fraction (P). (C) HeLa cells were fractionated into S and P as in Figure 1A. The pellet was further extracted with 1% Triton X-100 (lanes 5-8) or 250 mM NaCl (lanes 9-12). After centrifugation, the supernatant and pellet were analyzed by Western blotting. (D) Cell extracts were incubated with lambda phosphatase and immunoblotted for the indicated proteins.
The P fraction was further extracted with 1% Triton or 250 mM NaCl (Figure 1C). FANCM was insoluble in 1% Triton (Figure 1C lanes 5-8), whereas more than 70% of FANCM was soluble in 250 mM NaCl (Figure 1C lanes 9-12). After MMC treatment, highly phosphorylated FANCM remained in the pellet after 250 mM NaCl extraction. The FANCM mobility shifts were abolished by treatment with lambda phosphatase (Figure 1D, compare lanes 9-12). These results imply that FANCM stably associates with chromatin and that MMC activates the phosphorylation of FANCM, thus increasing its binding affinity for chromatin.
Phosphorylation of FANCM is dynamically regulated during the cell cycle
To detect possible changes in the association of FANCM with chromatin during the cell cycle (Figure 2A), HeLa cells were synchronized at the G1/S boundary by double thymidine block (Figure 2A lanes 1-7) or in M phase by nocodazole treatment (Figure 2A lanes 8-12). Cells were collected at the indicated time points and fractionated prior to immunoblotting. All FANCM was found in P fractions (chromatin fractions) and no significant changes in FANCM levels were detected during the cell cycle. Monoubiquitinated FANCD2 was found in the P fractions during S phase, consistent with previous studies.31 FANCG levels were found in S and P fractions throughout the cell cycle, although the levels in the chromatin fraction were decreased in mitosis (lanes 8-12), consistent with previous studies indicating that the FA core complex is excluded from condensed mitotic chromosmes.32
FANCM undergoes cell cycle–dependent phosphorylation. (A) Asynchronously growing cells (lane 1), cells arrested in G1/S phase with double thymidine blocking and released into S phase (lanes 2-7), or cells arrested in metaphase with nocodazole and released into G1 phase (lanes 8-12) were fractionated into S and P and immunoblotted for the indicated proteins. Asyn indicates asynchronous cells. Synchrony was monitored by flow cytometric analysis. The SDS-PAGE gel used here was 4% Tris-glycine. (B) Experimental protocol used to prepare cell extracts described in panels C and D. (C) Either exponentially growing Flag-FANCL HeLa cells (Asyn; lanes 1-4), cells released from double thymidine block for 3 hours (S phase; lanes 5-8), or cells arrested in mitotic phase with nocodazole (M phase; lanes 9-12) were fractionated into S100 and S300. As a negative control for immunoprecipitation with FLAG antibody, parental HeLa cells (lanes 13-16) were used. Each fraction was immunoprecipitated with FLAG-M2 agarose beads, and the precipitates were immunoblotted for the indicated proteins. (D) Cells were arrested in the G1/S phase by double thymidine block (lanes 1-4) and released into S phase (lanes 5-16). Alternatively, cells were arrested in mitotic phase with nocodazole (laness 17-20) and fractionated into S100 and S300. Proteins from each fraction were immunoprecipitated with FLAG-M2 agarose beads, and the precipitates were immunoblotted with the indicated antibodies. Note that for the Western blots of Flag-FANCL (fourth row), immunoprecipitation samples were exposed for a shorter time than for input samples. This is indicated by the vertical lines in between. This was necessitated by the fact that the intensity of IgG bands that appeared on immunoprecipitation samples was substantially greater than that of the input Flag-FANCL bands. Entire images come from the identical gel.
FANCM undergoes cell cycle–dependent phosphorylation. (A) Asynchronously growing cells (lane 1), cells arrested in G1/S phase with double thymidine blocking and released into S phase (lanes 2-7), or cells arrested in metaphase with nocodazole and released into G1 phase (lanes 8-12) were fractionated into S and P and immunoblotted for the indicated proteins. Asyn indicates asynchronous cells. Synchrony was monitored by flow cytometric analysis. The SDS-PAGE gel used here was 4% Tris-glycine. (B) Experimental protocol used to prepare cell extracts described in panels C and D. (C) Either exponentially growing Flag-FANCL HeLa cells (Asyn; lanes 1-4), cells released from double thymidine block for 3 hours (S phase; lanes 5-8), or cells arrested in mitotic phase with nocodazole (M phase; lanes 9-12) were fractionated into S100 and S300. As a negative control for immunoprecipitation with FLAG antibody, parental HeLa cells (lanes 13-16) were used. Each fraction was immunoprecipitated with FLAG-M2 agarose beads, and the precipitates were immunoblotted for the indicated proteins. (D) Cells were arrested in the G1/S phase by double thymidine block (lanes 1-4) and released into S phase (lanes 5-16). Alternatively, cells were arrested in mitotic phase with nocodazole (laness 17-20) and fractionated into S100 and S300. Proteins from each fraction were immunoprecipitated with FLAG-M2 agarose beads, and the precipitates were immunoblotted with the indicated antibodies. Note that for the Western blots of Flag-FANCL (fourth row), immunoprecipitation samples were exposed for a shorter time than for input samples. This is indicated by the vertical lines in between. This was necessitated by the fact that the intensity of IgG bands that appeared on immunoprecipitation samples was substantially greater than that of the input Flag-FANCL bands. Entire images come from the identical gel.
Mobility shifts of FANCM were observed throughout the cell cycle, and these mobility shifts were removed with lambda phosphatase (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These results suggest that all or most of the posttranslational modification of FANCM resulted from phosphorylation. We cannot rule out the possibility of other posttranslational modifications (ie, acetylations, methylations) that may not significantly alter the electrophoretic mobility of FANCM. FANCM was moderately phosphorylated during S phase (Figure 2A lanes 2-6), extensively phosphorylated during mitosis (Figure 2A lanes 7-10), and dephosphorylated after mitotic exit (Figure 2A lanes 11,12). FANCM phosphorylation reached maximum levels during mitosis when FANCD2 monoubiquitination was not detectable and when FANCG levels in chromatin were decreased. These results suggest that hyperphosphorylation of FANCM may negatively regulate the E3 ubiquitin ligase activity of the FA core complex in mitotic cells.
Chromatin localization of FA core complex is regulated in a cell cycle–dependent manner
To investigate the function of FANCM phosphorylation during cell cycle, we examined the assembly of the FA core complex in HeLa cells in either M phase or S phase (Figure 2B-D). For these studies, we used HeLa cells stably expressing an epitope-tagged Flag-FANCL protein. Cells were fractionated into S100 and S300 fractions (Figure 2B), and FA proteins were analyzed. FA core complex protein levels did not vary during cell cycle. Flag FANCL coimmunoprecipitated with FANCM, FANCG, and FANCA in S-phase cells (Figure 2C lanes 5-8); however, in mitotic cells (lanes 9-12), FANCM remained chromatin-bound while FANCG and FANCA still coimmunoprecipitated from the soluble fraction (Figure 2C). To examine the dynamic changes in the interaction between FANCM and remaining FA core proteins during cell cycle, cells were collected at various times after release from G1/S arrest (Figure 2D). At G1/S (Figure 2D lanes 1-4), late S (Figure 2D lanes 13-16), and G2/M (Figure 2D lanes 17-20) phases, an interaction among the FANCM-FANCA-FANCG-FANCL proteins was detected in the soluble fractions. In contrast, in early S (Figure 2D lanes 5-8) and mid-S (Figure 2D lanes 9-12) phases, the interaction was preferentially detected in the insoluble fraction. Taken together, these results confirm that the intact FA core complex is present throughout the cell cycle but is released from the chromatin-bound FANCM protein in mitosis.
FANCM is required for chromatin association of the FA core complex
We next asked whether the stability and nuclear localization of FA core complex is dependent on FANCM (Figure 3). EUFA867 (FANCM-deficient) lymphoblasts and wild-type lymphoblasts were fractionated, and the cellular localization of the FA core complex was determined by immunoblotting (Figure 3A). In EUFA867, the levels of FANCA and FANCG were reduced, and FANCD2 monoubiquitination was not detected. Importantly, FANCA was undetectable in the chromatin-containing fraction compared with wild-type cells. These results demonstrate that FANCM depletion affects the stability and chromatin association of the FA core complex.
The chromatin association of the FA core complex is impaired in FANCM-depleted cells. (A) EUFA867 lymphoblast and wild-type cells (GM02254A.L) were treated with MMC (150 ng/mL for 17 hours) and fractionated into S and P. α-tubulin and histone H3 are loading controls for S and P fractions, respectively. (B) HeLa cells transfected with the indicated siRNAs were analyzed by immunoblotting to insure FANCM or FANCA protein knockdown. (C) HeLa cells treated with the indicated siRNAs for 72 hours were treated with MMC (50 ng/mL for 17 hours). The whole-cell extracts were immunoblotted for the indicated proteins. The α-tubulin is a loading control for S fraction, and MCM3 was used as a loading control for P fraction. The crossreactive band are labeled with asterisks in panels A and C. (D) HeLa cells transfected with indicated siRNA for 72 hours were incubated without or with MMC (50 ng/mL for 17 hours) and then stained with anti-FANCG antibody, either without or with 0.3% Triton X-100 pre-extraction before fixation.
The chromatin association of the FA core complex is impaired in FANCM-depleted cells. (A) EUFA867 lymphoblast and wild-type cells (GM02254A.L) were treated with MMC (150 ng/mL for 17 hours) and fractionated into S and P. α-tubulin and histone H3 are loading controls for S and P fractions, respectively. (B) HeLa cells transfected with the indicated siRNAs were analyzed by immunoblotting to insure FANCM or FANCA protein knockdown. (C) HeLa cells treated with the indicated siRNAs for 72 hours were treated with MMC (50 ng/mL for 17 hours). The whole-cell extracts were immunoblotted for the indicated proteins. The α-tubulin is a loading control for S fraction, and MCM3 was used as a loading control for P fraction. The crossreactive band are labeled with asterisks in panels A and C. (D) HeLa cells transfected with indicated siRNA for 72 hours were incubated without or with MMC (50 ng/mL for 17 hours) and then stained with anti-FANCG antibody, either without or with 0.3% Triton X-100 pre-extraction before fixation.
We next depleted FANCM in HeLa cells by siRNA transfection (Figure 3B). FANCM siRNA efficiently depleted FANCM, and the depletion was sustained for 7 days (Figure S2). In FANCA-depleted HeLa cells, the FANCG level was strongly reduced, confirming that the direct association between FANCA and FANCG is required for their stability.33,34 In contrast, in FANCM-depleted HeLa cells, levels of FANCA and FANCG were not significantly reduced, but the chromatin localization of FANCA, FANCG, and FANCE was disrupted compared with control siRNA–treated cells (Figure 3B,C). There was no change in FANCJ expression levels or cellular localization following FANCM knockdown (Figure 3C). Also, siRNA knockdown of other FA proteins in the FA pathway, such as the E2 enzyme, UBE-2T,35 did not affect localization of the FA core with chromatin (Figure S3).
We next performed immunofluorescence with an anti-FANCG antibody to detect the FA core complex in chromatin (Figure 3D). For this purpose, we generated an affinity-purified anti-FANCG antibody suitable for detecting endogenous FANCG protein in HeLa cells. To our knowledge, this is the first anti-FANCG antibody capable of detecting endogenous FANCG protein by immunofluorescence, as opposed to overexpressed FANCG protein. In the absence of MMC exposure, very little FANCG nuclear staining was observed. Following MMC exposure, increased FANCG nuclear staining was observed; however, this staining did not depend on the presence of the FANCM protein, since siRNA to FANCM did not decrease this staining. We next performed a triton pre-extraction of nuclei, prior to staining with the anti-FANCG antibody. Triton pre-extraction removed FANCG protein that was soluble in the nucleus or was only loosely bound to chromatin. Interestingly, MMC activated the chromatin association of FANCG protein, and siRNA to FANCM decreased this chromatin association. These results further support the hypothesis that FANCM is required for recruitment of FANCG and perhaps other FA core complex proteins to chromatin.
FANCM is not required for assembly or stability of the nucleoplasmic FA core complex
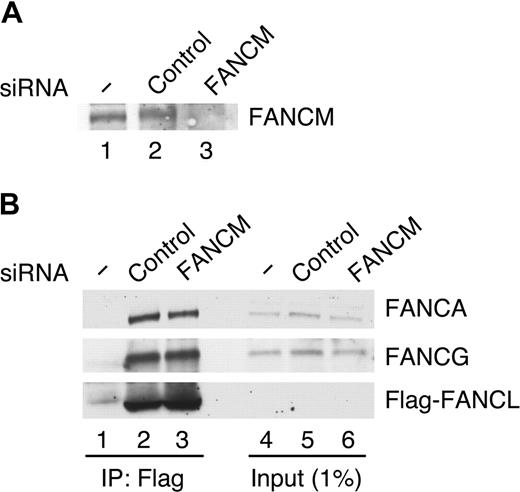
We further examined the assembly of the FA core complex in FANCM-depleted cells (Figure 4). To immunoprecipitate the FA core complex, we again used the stable HeLa cell line expressing Flag-FANCL. Cells transfected with control siRNA or FANCM siRNA were extracted with a high-salt-containing buffer and analyzed by immunoprecipitation (Figure 4A). The amount of FANCA and FANCG coimmunoprecipitated with Flag-FANCL did not differ significantly between control siRNA– and FANCM siRNA–treated cells (Figure 4B). These results demonstrate that FANCM is not essential for assembly of the FA core complex.
FANCM is not essential for the assembly of the FA core complex. (A) Parental HeLa cells (lane 1) and Flag-FANCL HeLa cells (lanes 2,3) transfected with indicated siRNAs for 72 hours were incubated with MMC (50 ng/mL for 17 hours) and harvested and immunoblotted for the indicted protein. (B) Cells treated as in panel A were extracted with 300 mM NaCl-CSK buffer and immunoprecipitated with FLAG-M2 agarose beads. The precipitates (lanes 1-3) and input (lanes 4-6) were immunoblotted for the indicated proteins.
FANCM is not essential for the assembly of the FA core complex. (A) Parental HeLa cells (lane 1) and Flag-FANCL HeLa cells (lanes 2,3) transfected with indicated siRNAs for 72 hours were incubated with MMC (50 ng/mL for 17 hours) and harvested and immunoblotted for the indicted protein. (B) Cells treated as in panel A were extracted with 300 mM NaCl-CSK buffer and immunoprecipitated with FLAG-M2 agarose beads. The precipitates (lanes 1-3) and input (lanes 4-6) were immunoblotted for the indicated proteins.
The chromatin localization of FAAP24 depends on FANCM
FAAP24 was recently identified as an integral member of the FA core complex.27 FANCM binds directly to FAAP24, and this interaction appears to be mediated by the carboxy terminal domain of FANCM.27 Moreover, FAAP24 exhibited direct DNA-binding activity, and showed preferential binding for branched chain DNA structures mimicking replication forks. Cellular depletion of either FANCM or FAAP24 reduces FANCD2 monoubiquitination. Taken together, these results suggest that FAAP24 (and FANCM) can anchor the FA core complex at stalled replication forks during S phase.
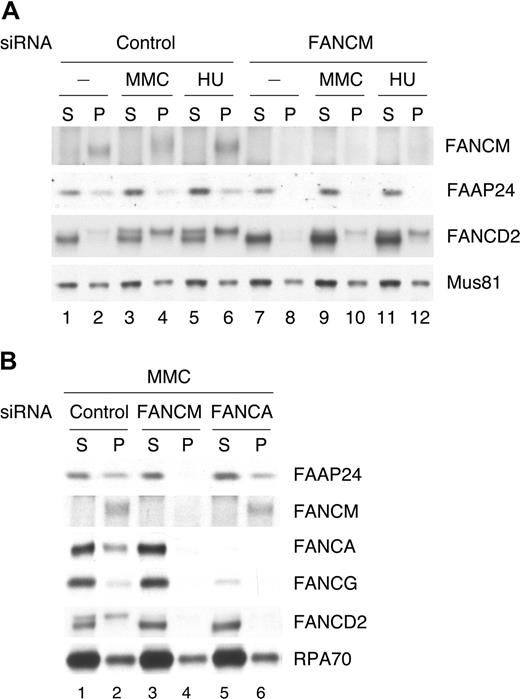
FAAP24 was detected in S and P fractions (Figure 5A). The abundance of FAAP24 was slightly decreased by FANCM depletion (Figure 5A lanes 7-12), and FANCM depletion disrupted the chromatin association of FAAP24 (Figure 5A lanes 8,10,12). In contrast, FANCA depletion did not affect the level or chromatin association of FAAP24, but the level of FANCG was significantly reduced (Figure 5B). These results demonstrate that chromatin-bound FAAP24 associates with FANCM directly, and its localization depends on FANCM but not on other FA core complex subunits.
The chromatin association of FAAP24 depends on FANCM. (A) HeLa cells transfected with control or FANCM siRNA for 72 hours were treated with MMC (50 ng/mL for 17 hours) or HU (2 mM for 17 hours) and harvested. Fractionated extracts were immunoblotted for the indicated proteins. Mus81 was used a loading control for both fractions. (B) HeLa cells transfected with the indicated siRNAs for 72 hours were treated with MMC (50 ng/mL for 17 hours) and harvested. Fractionated extracts were immunoblotted for the indicated proteins. RPA70 was used a loading control for both fractions.
The chromatin association of FAAP24 depends on FANCM. (A) HeLa cells transfected with control or FANCM siRNA for 72 hours were treated with MMC (50 ng/mL for 17 hours) or HU (2 mM for 17 hours) and harvested. Fractionated extracts were immunoblotted for the indicated proteins. Mus81 was used a loading control for both fractions. (B) HeLa cells transfected with the indicated siRNAs for 72 hours were treated with MMC (50 ng/mL for 17 hours) and harvested. Fractionated extracts were immunoblotted for the indicated proteins. RPA70 was used a loading control for both fractions.
FAAP24 depletion reduces the level of FANCM and impairs chromatin association of FANCM
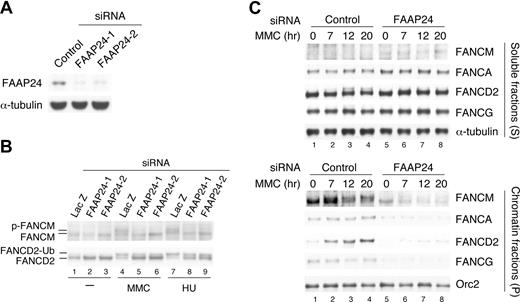
To examine the effect of FAAP24 depletion on FANCM, we used 2 different sets of FAAP24 siRNAs. HeLa cells transfected with siRNAs were fractionated and analyzed by Western blotting (Figure 6A). FAAP24 depletion moderately reduced the level of FANCM, and decreased the monoubiquitination of FANCD2 (Figure 6B). FAAP24 depletion also inhibited FANCM phosphorylation following DNA damage. FAAP24 siRNA also rendered the cells hypersensitive to MMC (Figure S4). Importantly, FAAP24 depletion reduced the level of FANCM in chromatin and disrupted chromatin association of FANCM, resulting in defective chromatin recruitment of FANCA and FANCG (Figure 6C). These results indicate that FAAP24 is required for stable chromatin association and stability of FANCM. The effects of various FA proteins on the chromatin association of the FA core complex are summarized in Figure 7A.
FANCM requires FAAP24 for its stable association in chromatin. (A) HeLa cells were transfected with indicated siRNAs for 72 hours, and then whole-cell extracts were immunoblotted. (B) HeLa cells transfected with indicated siRNAs for 72 hours were treated with MMC (50 ng/mL for 17 hours) or HU (2 mM for 17 hours), and whole-cell extracts were immunoblotted for the indicated proteins. (C) HeLa cells transfected with indicated siRNAs for 72 hours, and then incubated with MMC (50 ng/mL) for 7 hours, 12 hours, and 20 hours. Cells were then extracted and fractionated, and each fraction was immunoblotted for the indicated proteins.
FANCM requires FAAP24 for its stable association in chromatin. (A) HeLa cells were transfected with indicated siRNAs for 72 hours, and then whole-cell extracts were immunoblotted. (B) HeLa cells transfected with indicated siRNAs for 72 hours were treated with MMC (50 ng/mL for 17 hours) or HU (2 mM for 17 hours), and whole-cell extracts were immunoblotted for the indicated proteins. (C) HeLa cells transfected with indicated siRNAs for 72 hours, and then incubated with MMC (50 ng/mL) for 7 hours, 12 hours, and 20 hours. Cells were then extracted and fractionated, and each fraction was immunoblotted for the indicated proteins.
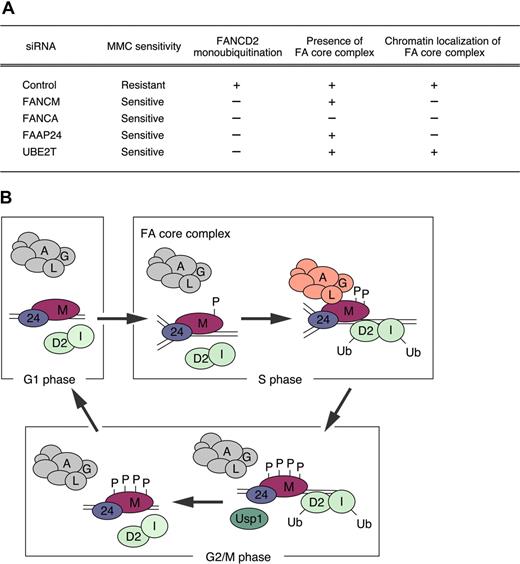
Model for the role of FANCM in regulating the FA pathway. (A) HeLa cells silenced for the indicated proteins by siRNA (columns) were analyzed for MMC sensitivity, FANCD2 monoubiquitination, presence of FA core complex, and chromatin localization of FA core complex (rows). + indicates indistinguishable from control siRNA–treated cells; and −, defective. (B) Schematic model describing the function of FANCM in the chromatin recruitment of the FA core complex. The FANCM-FAAP24 complex associates with chromatin throughout the cell cycle. Early in the cell cycle (G1 phase), the FA core (A/B/C/E/F/G/L complex) is assembled but does not associate with FANCM-FAAP24 complex in chromatin. In S phase, phosphorylated FANCM can recruit the FA core complex to chromatin, possibly to replication forks, and induce E3 ubiquitin ligase activity, resulting in monoubiquitination of FANCD2 and FANCI. In G2/M phase, hyperphosphorylated FANCM may promote the release of the FA core complex, and USP1 may deubiquitinate FANCD2 and FANCI monoubiquitination.
Model for the role of FANCM in regulating the FA pathway. (A) HeLa cells silenced for the indicated proteins by siRNA (columns) were analyzed for MMC sensitivity, FANCD2 monoubiquitination, presence of FA core complex, and chromatin localization of FA core complex (rows). + indicates indistinguishable from control siRNA–treated cells; and −, defective. (B) Schematic model describing the function of FANCM in the chromatin recruitment of the FA core complex. The FANCM-FAAP24 complex associates with chromatin throughout the cell cycle. Early in the cell cycle (G1 phase), the FA core (A/B/C/E/F/G/L complex) is assembled but does not associate with FANCM-FAAP24 complex in chromatin. In S phase, phosphorylated FANCM can recruit the FA core complex to chromatin, possibly to replication forks, and induce E3 ubiquitin ligase activity, resulting in monoubiquitination of FANCD2 and FANCI. In G2/M phase, hyperphosphorylated FANCM may promote the release of the FA core complex, and USP1 may deubiquitinate FANCD2 and FANCI monoubiquitination.
Discussion
Previous studies have suggested that FANCM is an essential component of the FA core complex. Lymphoblasts derived from a patient with FA-M (EUFA867) have decreased levels of other FA core complex proteins, such as FANCA and FANCG.5 In contrast, our results show that the FA core complex (A, B, C, E, F, G, and L) is stable and assembled, even when FANCM protein is depleted to undetectable levels by FANCM siRNA.
Taken together, our results indicate that FANCM depletion impairs the association of the FA core complex with chromatin but has no effect on the assembly of the FA core complex. We demonstrated that the chromatin recruitment of FA core complex requires chromatin-bound FANCM, but the assembly of FA core complex does not. There are conflicting reports regarding the role of FANCM in the core complex. FANCM-deficient human lymphoblasts (EUFA867) display reduced levels of FANCA and FANCG protein, compared with wild-type lymphoblasts. In contrast, in FANCM siRNA–treated cells, the levels of FA core complex proteins were not significantly different compared with control siRNA–treated cells. Also, the interaction between FANCA and FANCL is defective in EUFA867, whereas in FANCM siRNA–treated cells, the interaction between these 2 proteins does not differ. This discrepancy can be explained by the following possibilities. First, siRNA-mediated silencing of FANCM may display the acute defects of FANCM depletion, whereas a patient-derived FANCM-mutant lymphoblast cell line, EUFA867, may reflect developmental defects of FANCM deficiency. It should be noted that functional complementation of EUFA867 with FANCM cDNA has not been accomplished yet, suggesting that FANCM may not be the only gene-defective EUFA867. Second, the cellular localization of FANCM may be different between lymphoblasts and fibroblasts, possibly accounting for the different phenotypes resulting from FANCM depletion. Consistent with this possibility, when lymphoblasts such as GM02254A.L (wild-type cells) and EUFA121 (FA-F) were fractionated with the same procedure (Figure 1A), a significant amount of FANCM was detected in the soluble fraction. In contrast, a corrected FANCG mutant fibroblast line, EUFA326 + FANCG, exhibited a similar cellular localization of FANCM to HeLa cells (data not shown). Regardless of the mechanism, our results support a model of regulated interaction of the FA core complex with FANCM.
Previous studies have shown that a complex of FAAP24 and a C-terminal domain of FANCM binds to branched DNA molecules. These studies have led to a model in which the FAAP24 protein recruits the FA core complex to DNA.27 Our results demonstrate that FANCM is constitutively bound to chromatin (Figures 1, 2), and it is tethered to chromatin by the FAAP24 anchor protein. SiRNA disruption of FAAP24 leads to a release of FANCM protein from chromatin (Figure 6C). The FA core complex, in contrast, translocates in and out of the chromatin. These translocation events are highly regulated and depend on FANCM and perhaps on the phosphorylation state of FANCM. The FA core complex associates more strongly with chromatin after DNA damage and during the S phase of the cell cycle. The FA core complex is released from chromatin during mitosis. FA core complex binding to chromatin after DNA damage, or in S phase, depends on the presence of the FANCM protein.
How FANCM regulates the binding of the FA core complex to chromatin is unknown, and several models are possible. First, the FANCM protein may provide a direct “landing pad” for the FA core complex. The increased phosphorylation of FANCM during S phase may account for the direct binding of the FA core complex. Hyperphosphorylation of FANCM in mitosis may account for the release of the FA core complex. Second, the FANCM protein may translocate through replicating DNA during S phase, thus “opening” the chromatin and allowing the FA core complex to load. In this case, the ATPase activity of the FANCM protein, required for the translocase activity of the FANCM protein, may also be required for FA core complex loading.
Consistent with this latter model, FANCM appears to have homology and activity similar to members of the Snf2-family of SF2 helicases.36 Snf2-family proteins can translocate on double-stranded DNA but do not display strand displacement activity typical of a helicase. These proteins can translocate across complex DNA structures during S phase and can load protein complexes. Interestingly, one Snf2-family member, CSB, binds to chromatin and plays a critical role in transcription-coupled DNA repair. CSB recruits a complex of proteins, containing CSA and an E3 ligase,37-39 perhaps analogous to the E3 ligase of the FA core complex, ultimately leading to the regulated degradation of CSB. It will be interesting to determine whether the FA core complex binding to FANCM regulates the polyubiquitination and degradation of a subfraction of FANCM.
Several questions are raised regarding the function of FANCM in loading the FA core complex in chromatin. First, it remains unclear whether the FA core complex loads specifically at sites of stalled replication forks after DNA damage or more generally across a wider range of double-helical DNA. Second, it remains unknown whether the ATPase activity of FANCM is critical for core complex loading. Third, it remains unknown how FA core complex loading leads to DNA repair and resolution of DNA interstrand crosslinks.
The properties of FANCM protein described here lead us to propose a model of the FA pathway (summarized schematically in Figure 7B). During S phase, moderately phosphorylated FANCM, interacting with FAAP24, recruits the FA core complex to chromatin, promoting the accumulation and activation of FA core complex and resulting in FANCD2 monoubiquitination. In G2/M phase, FANCM is extensively phosphorylated by unknown kinases, thereby releasing the FA core complex from chromatin and “shutting off” the pathway. At the same stage, the deubiquitinating enzyme, USP1, is activated and deubiquitinates FANCD2 and FANCI. In the subsequent G1 phase, the dephosphorylated FANCM-FAAP24 complex remains in chromatin, and the FA core complex remains in the soluble nuclear fraction.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Weidong Wang for the human FA-M lymphoblast line (EUFA867) and Steve West for the anti-FAAP24 antibody. We also thank Carrie Ruit for technical assistance.
This work was supported by National Institutes of Health grants DK43889-15, HL52725-13, and U19A1067751-02.
National Institutes of Health
Authorship
J.M.K. designed and performed entire experiments, analyzed the data, and wrote the manuscript with the direction of A.D.D. Y.H.K. contributed the experiment for Figure 4. A.G. provided the stable cell line and affinity-purified antibody.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan D. D'Andrea, Department of Radiation Oncology, Division of Genomic Stability and DNA Repair, Dana-Farber Cancer Institute, Harvard Medical School, 44 Binney St, Boston, MA 02115; e-mail: alan_dandrea@dfci.harvard.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal