In this issue of Blood, Babich and colleagues disclose an elegant mechanism that serves to selectively release cargo from WPBs.
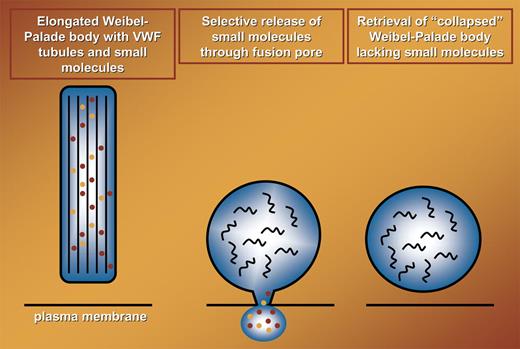
Weibel-Palade bodies (WPBs) are versatile storage organelles within endothelial cells that release their inflammatory and prothrombotic content into the circulation in response to a large number of agonists.1 Using real-time analysis of fluorescently tagged von Willebrand factor (VWF) and VWF propolypeptide, Babich et al nicely show that a proportion of WPBs transiently fuse with the plasma membrane during a “lingering kiss.” Fusion pores of approximately 12 nm in diameter are generated that facilitate the selective release of low-molecular-weight components like IL-8 from these organelles. An important physiological implication of these findings is that endothelial cells can selectively release proinflammatory cytokines like IL-8 and eotaxin-3 while retaining prothrombotic VWF. The selective release of small-core and membrane proteins comes at a cost, though; the elegantly shaped, cigarlike WPBs collapse into ordinary, circular vesicles.
In the original study by Weibel and Palade, the presence of tubular structures was noted.2 It has recently been shown that the tubules present within these organelles are hollow cylinders that are created by propolypeptide-guided condensation of VWF.3,4 The integrity of VWF-containing tubules that maintain the elongated form of WPBs is critically dependent on a low pH. The pH within WPBs during a lingering kiss increases considerably, and this most likely results in the so-called unfurling of tubules into long VWF filaments.3 Although this has not been addressed, it is likely that the increase in intracellular pH results in the rapid disappearance of VWF tubules (see figure). The morphological characteristics of these collapsed WPBs have not been determined. Therefore, it is presently unknown whether this class of WPBs can reassemble into rod-shaped structures, fuse with newly formed organelles, or exocytose following application of a more rigorous stimulus.
Selective release of small molecules (indicated in orange and red) during transient fusion of WPBs with the plasma membrane. During fusion elongated WPBs collapse into circular structures, designated collapsed WPBs. This most likely is due to the conversion of highly organized VWF tubules (thick black lines in left panel) into randomly organized VWF polymers (black curved lines in middle and right panels).
Selective release of small molecules (indicated in orange and red) during transient fusion of WPBs with the plasma membrane. During fusion elongated WPBs collapse into circular structures, designated collapsed WPBs. This most likely is due to the conversion of highly organized VWF tubules (thick black lines in left panel) into randomly organized VWF polymers (black curved lines in middle and right panels).
In their visually appealing article, Babich and coworkers show that lingering kisses account for 10% to 25% of all fusion events following stimulation with histamine. A large number of agonists can provoke release of WPBs. Clinically, this is exploited by the administration of desmopressin (DDAVP) to patients with von Willebrand disease or mild hemophilia A. Under these conditions, the release of WPBs is induced by cAMP-dependent signaling pathways that result in only modest activation of signaling molecules involved in regulation of WPB exocytosis.1 It will be interesting to determine whether, under these conditions, transient fusion of WPBs with the plasma membrane will occur at a higher frequency than observed following stimulation with histamine.
Overall, the findings of Babich et al highlight yet another fascinating aspect of the biology of the uniquely shaped WPB. Their study provides an excellent starting point to further explore the regulation and physiological significance of release of WPBs with different proinflammatory and vasoregulatory cargo.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal