In this issue of Blood, Saadoun and colleauges report changes in lymphocyte subsets in cryoglobulinemia patients prior to and after anti-CD 20 therapy.
Type II (mixed) cryoglobulin represents circulating immune complexes composed of a monoclonal IgM associated to a polyclonal IgG, which defines it as a rheumatoid factor.1 The cryoprecipitable immune complex can be found in the complete absence of clinical symptoms, but can deposit diffusely on endothelial surfaces, activate complement, and produce a systemic vasculitis syndrome. At the Mayo Clinic cryoglobulinemia accounts for 0.7% of monoclonal proteins seen over a 43-year period. However, only 29% of cryoglobulins were type II. Type I cryoglobulins are common incidental findings in patients with monoclonal gammopathy of uncertain significance and multiple myeloma; the majority are asymptomatic.
The presence of monoclonal IgM implies that there is a clonal proliferation of lympho-plasmacytic cells in the bone marrow. Some of these infiltrations are at a level where they can be defined as lymphoplasmacytic lymphoma, but often, specialized techniques are required to detect the presence of the clonal cells in the marrow. At the Second International Workshop on Waldenstrom Macroglobulinemia, mixed cryoglobulinemia was defined as a syndrome distinct from Waldenström macroglobulinemia, falling into the category of an IgM-related disorder without symptoms due to tumor mass or a significant marrow infiltration.2
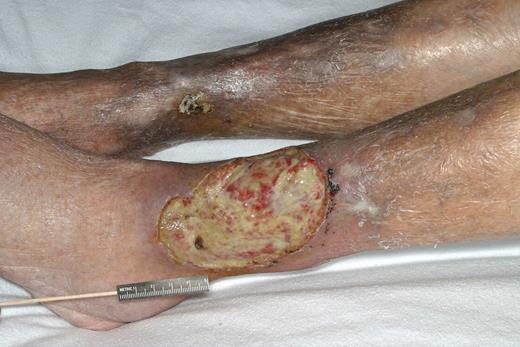
The immune complex was thought for many years to be triggered by an unknown antigen. However, in 1990, hepatitis C was recognized as an etiologic factor for type II cryoglobulinemia. The most common target organs for this immune complex vasculitis are the skin, nerves, kidney, and liver. Patients who manifest solely with cutaneous purpura may not require systemic therapy, as these are asymptomatic and are primarily of cosmetic concern. Renal involvement is the most urgent indication for intervention, because a glomerulopathy capable of producing nephrotic-range proteinuria and dialysis-dependent renal failure can occur. The typical renal lesion is membranoproliferative glomerulonephritis, and electron-dense deposits are found with intracapillary thrombi.3 The figure demonstrates a classic vasculitis ulcer associated with a type II cryoglobulin. In the presence of viral hepatitis, treatment with interferon plus ribavirin produces a response in over half of patients, but relapses are common.4 In severe vasculitis, corticosteroids are required because responses to interferon are delayed. Plasma exchange and alkylating agents have been used, but have generally been found to be ineffective. Rituximab therapy has been reported to be effective in type II cryoglobulinemia.5 Because the lymphoplasmacytic cells that are the source of the monoclonal IgM protein strongly express CD20, this is a logical choice and its effectiveness has been demonstrated in corticosteroid failures. In this issue of Blood, Saadoun and coworkers studied 21 patients and demonstrated that, before therapy, there were dramatic reductions in CD19+ cells, increased numbers of memory B cells, and a high number of lymphoplasmacytic cells, and demonstrate the ability of rituximab to bring these abnormalities back to the normal range. This partially explains the mechanism of action of rituximab and the pretreatment immune dysregulation that exists in these patients.
Vasculitic ulcer in a patient with type II cryoglobulinemia. Reproduced from American Journal of Hematology, Vol. 77, No. 4, 2004, pages 329-330. Copyright 2004 Wiley-Liss, Inc. Reprinted with permission of Wiley-Liss, Inc, a subsidiary of John Wiley & Sons Inc.
Vasculitic ulcer in a patient with type II cryoglobulinemia. Reproduced from American Journal of Hematology, Vol. 77, No. 4, 2004, pages 329-330. Copyright 2004 Wiley-Liss, Inc. Reprinted with permission of Wiley-Liss, Inc, a subsidiary of John Wiley & Sons Inc.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal