Abstract
Regulatory T (Treg) cells contribute to immune evasion by malignancies. To investigate their importance in non-Hodgkin lymphoma (NHL), we enumerated Treg cells in peripheral blood mononuclear cells (PBMCs) and involved tissues from 30 patients. CD25+FoxP3+CD127lowCD4+ Treg cells were increased markedly in PBMCs (median = 20.4% CD4 T cells, n = 20) versus healthy controls (median = 3.2%, n = 13, P < .001) regardless of lymphoma subtype, and correlated with disease stage and serum lactate dehydrogenase (Rs = 0.79, P < .001). T-cell hyporesponsiveness was reversed by depleting CD25+ cells, or by adding anti–CTLA-4, supporting the view that Treg cells explain the systemic immunosuppression seen in NHL. A high proportion of Treg cells was also present in involved tissues (median = 38.8% CD4 T cells, n = 15) versus reactive nodes (median = 11.6%, n = 2, P = .02). When autologous CD25− PBMC fractions were incubated with tumor cells from patients (n = 6) in vitro, there was consistent strong induction and then expansion of cells with the CD4+CD25+FoxP3+ phenotype of classic “natural” Treg cells. This population was confirmed to be suppressive in function. Direct cell-cell interaction of tumor cells with CD25− PBMCs was important in Treg induction, although there was heterogeneity in the mechanisms responsible. We conclude that NHL cells are powerful inducers of Treg cells, which may represent a new therapeutic target.
Introduction
B-cell non-Hodgkin lymphoma (NHL) is a heterogeneous group of lymphoid malignancies, many of which remain incurable. Increasing evidence suggests that the tumor microenvironment plays an important role, in determining not only the severity of disease, but also response to therapy.1-4 Ansell et al4 demonstrated that higher numbers of activated CD4+ cells among the tumor-infiltrating lymphocytes of diffuse large B-cell NHL were associated with a better prognosis. More recently, analyses of gene expression using microchips in follicular and diffuse large B-cell lymphomas have indicated that the nature of nonmalignant lymphocytes is important prognostically.5,6
It is becoming clear that a critical component of the immune response to malignancies includes regulatory T (Treg) cells.7 There are at least 2 forms of Treg cells, “natural” and “induced.” The former suppress, at least in vitro, via undefined mechanisms dependent on cell-cell contact; the latter inhibit primarily through the secretion of immunosuppressive cytokines such as IL-10.8 Natural Treg cells constitutively express high levels of CD25 and are believed to arise as a suppressive population in Hassall corpuscles within the thymus,9 but there is evidence that they may also arise peripherally.10 There have been difficulties in identifying unique markers for the natural population.7 Expression of the forkhead/winged helix transcription factor (FoxP3) is important in the differentiation of natural Treg cells,11 and was therefore perceived to characterize this population,12 but Wang et al13 have recently demonstrated its up-regulation in other activated T cells. CD127 expression inversely correlates with FoxP3 levels in CD25+ Treg cells.14,15
CD25+ Treg cells have been shown to be overrepresented in several tumor types, including lung, breast, pancreatic, ovarian, and melanoma16-19 and able to suppress tumor-specific protective T-cell immunity.17-19 In hematologic malignancies, examples of up-regulation of CD25+ Treg cells include Hodgkin disease,20 tissues involved by NHL,21 chronic lymphocytic leukemia (CLL),22 acute myeloid leukemia (AML),23 myeloma,24 and high-grade myelodysplasia.25 Moreover, some subtypes of T-cell lymphoma cells exhibit a Treg phenotype.26-28 In NHL, Yang et al29 demonstrated that Treg cells attenuated CD8 T-cell function, thereby protecting lymphoma cells from cytotoxic activity. Likewise, Hilchey et al30 demonstrated that follicular lymphoma intratumoral Treg cells suppressed CD3/CD28-costimulated autologous and allogenic CD8+CD25− and CD4+CD25− effector T cells.
The origin of the increased proportion of Treg cells seen in malignancy needs to be determined. Possibilities include recruitment and/or expansion from the circulating Treg population. Tumor cell production of chemokine CCL22 recruits Treg cells in ovarian cancer and NHL.18,21 Treg expansion has been reported in Hodgkin lymphoma and myeloma.31,32 A third explanation is that the regulatory phenotype is induced from conventional T cells within the tumor microenvironment. Curti et al33 showed that AML cells induce CD25+ Treg cells from CD25− cells via modulation of tryptophan catabolism.
We therefore enumerated Treg cells in peripheral blood and involved tissue samples from NHL patients and determined whether the tumor cells induce their differentiation.
Methods
Patients
Between January 2006 and February 2007, 30 newly diagnosed patients with B-cell NHL (age range: 17 to 93 years, mean = 62, SD = 22) were recruited from Aberdeen Royal Infirmary, United Kingdom, which is the sole specialty hospital serving the Grampian area. The study received approval from The Grampian NHS local ethics committee, Aberdeen, and informed consent was obtained from all patients in accordance with the Declaration of Helsinki. An age-matched control group comprised 13 healthy individuals (age range: 20 to 90 years, mean = 58, SD = 17) admitted for minor surgical procedures and 2 individuals with benign lymphadenopathy. Patient characteristics are described in Table 1. All initial samples were obtained before any antilymphoma treatment was given.
Clinical characteristics of patients with B-cell NHL
| Patient no. . | Histologic diagnosis . | Sex, age, y . | Tissue used . |
|---|---|---|---|
| 1 | DLBCL | M, 61 | Node (new*) |
| 2 | High-grade transformation of CLL (DLBCL) | M, 54 | Node, PBMC |
| 3 | High-grade transformation of CLL (DLBCL) | F, 59 | Node, PBMC |
| 4 | MZL | F, 76 | Spleen, PBMC (new) |
| 5 | Follicular NHL, grade 1 | M, 52 | Node (new) |
| 6 | Relapsed Burkitt lymphoma | M, 17 | PBMC |
| 7 | Follicular NHL, grade II | M, 53 | Node, PBMC (new) |
| 8 | PTLD post-MUD transplantation for CML (DLBCL) | M, 27 | Node (new) |
| 9 | Mantle cell lymphoma | M, 76 | Node (new) |
| 10 | Follicular NHL | F, 93 | Node (new) |
| 11 | DLBCL | M, 69 | Node |
| 12 | Mantle cell lymphoma | M, 67 | Spleen, PBMC (new) |
| 13 | DLBCL | M, 56 | Node, PBMC (new) |
| 14 | MZL | F, 57 | Node (new) |
| 15 | DLBCL | F, 84 | Node, PBMC (new) |
| 16 | Small cell lymphocytic lymphoma | M, 67 | Node, PBMC (new) |
| 17 | Gastric DLBCL | M, 80 | PBMC (new) |
| 18 | MZL | M, 73 | Spleen, PBMC (new) |
| 19 | Pancreatic head DLBCL | M, 77 | PBMC (new) |
| 20 | Primary CNS DLBCL | M, 37 | PBMC (new) |
| 21 | DLBCL | M, 78 | PBMC (new) |
| 22 | Follicular NHL in leukemic phase | F, 83 | PBMC (new) |
| 23 | DLBCL | M, 46 | PBMC (new) |
| 24 | Small cell lymphocytic lymphoma | M, 48 | PBMC (new) |
| 25 | Follicular NHL | F, 56 | PBMC (new) |
| 26 | CNS DLBCL, previously treated nodal DLBCL | F, 55 | PBMC |
| 27 | DLBCL, previous low-grade NHL | F, 69 | PBMC |
| 28 | Follicular NHL | M, 66 | Node, PBMC (new) |
| 29 | Relapsed DLBCL, previous autologous transplantation | F, 61 | PBMC |
| 30 | DLBCL transformation from previous follicular NHL | M, 73 | PBMC |
| Patient no. . | Histologic diagnosis . | Sex, age, y . | Tissue used . |
|---|---|---|---|
| 1 | DLBCL | M, 61 | Node (new*) |
| 2 | High-grade transformation of CLL (DLBCL) | M, 54 | Node, PBMC |
| 3 | High-grade transformation of CLL (DLBCL) | F, 59 | Node, PBMC |
| 4 | MZL | F, 76 | Spleen, PBMC (new) |
| 5 | Follicular NHL, grade 1 | M, 52 | Node (new) |
| 6 | Relapsed Burkitt lymphoma | M, 17 | PBMC |
| 7 | Follicular NHL, grade II | M, 53 | Node, PBMC (new) |
| 8 | PTLD post-MUD transplantation for CML (DLBCL) | M, 27 | Node (new) |
| 9 | Mantle cell lymphoma | M, 76 | Node (new) |
| 10 | Follicular NHL | F, 93 | Node (new) |
| 11 | DLBCL | M, 69 | Node |
| 12 | Mantle cell lymphoma | M, 67 | Spleen, PBMC (new) |
| 13 | DLBCL | M, 56 | Node, PBMC (new) |
| 14 | MZL | F, 57 | Node (new) |
| 15 | DLBCL | F, 84 | Node, PBMC (new) |
| 16 | Small cell lymphocytic lymphoma | M, 67 | Node, PBMC (new) |
| 17 | Gastric DLBCL | M, 80 | PBMC (new) |
| 18 | MZL | M, 73 | Spleen, PBMC (new) |
| 19 | Pancreatic head DLBCL | M, 77 | PBMC (new) |
| 20 | Primary CNS DLBCL | M, 37 | PBMC (new) |
| 21 | DLBCL | M, 78 | PBMC (new) |
| 22 | Follicular NHL in leukemic phase | F, 83 | PBMC (new) |
| 23 | DLBCL | M, 46 | PBMC (new) |
| 24 | Small cell lymphocytic lymphoma | M, 48 | PBMC (new) |
| 25 | Follicular NHL | F, 56 | PBMC (new) |
| 26 | CNS DLBCL, previously treated nodal DLBCL | F, 55 | PBMC |
| 27 | DLBCL, previous low-grade NHL | F, 69 | PBMC |
| 28 | Follicular NHL | M, 66 | Node, PBMC (new) |
| 29 | Relapsed DLBCL, previous autologous transplantation | F, 61 | PBMC |
| 30 | DLBCL transformation from previous follicular NHL | M, 73 | PBMC |
DLBCL indicates diffuse large B-cell lymphoma; CLL, chronic lymphocytic leukemia; MZL, marginal zone lymphoma; NHL, non-Hodgkin lymphoma; PTLD, posttransplantation lymphoproliferative disease; MUD transplantation, matched unrelated donor transplantation; CML, chronic myeloid leukemia; and CNS, central nervous system.
New refers to a diagnosis of lymphoma with no previous history of lymphoproliferative disease.
Reagents
The control recall antigens, mycobacterial PPD (Statens Seruminstitut, Copenhagen, Denmark), diphtheria, poliomyelitis, and tetanus (DPT) vaccine (Aventis Pasteur, Strasbourg, France), T-cell mitogen concanavalin A (ConA; Sigma-Aldrich, Amersham, United Kingdom), and anti-CD3/28 Dynabeads (Dynal Biotec, Wirral, United Kingdom) were used to stimulate cultures. PPD and DPT readily provoke recall T-cell responses in vitro as most British citizens have been immunized with Bacille Calmette-Guérin and DPT vaccines during childhood. All antigens were dialyzed prior to use. Fluorochrome-conjugated antibodies for surface staining (anti-CD3, anti-CD4, anti-CD8, anti-CD25, anti-CD127, anti–annexin V, and anti-7AAD), intracellular staining antibody (anti–IL-10), and appropriate isotypes were purchased from BD Pharmingen (Oxford, United Kingdom). Carboxyfluorescein diacetate succinimidyl ester (CFSE) was obtained from Invitrogen (Paisley, United Kingdom). Phycoerythrin (PE) antihuman FoxP3 staining set, isotype control, and neutralizing antibodies antihuman B7-H1 (PDL1) and antihuman B7-DC (PDL2) were obtained from eBioscience (San Diego, CA). Monoclonal antihuman IL-9 receptor antibody, 1-methyl-d-tryptophan (1-MT), aspirin, sulindac, and anti–TGF-β blocking antibody were purchased from Sigma-Aldrich. A blocking anti–IL-10 antibody was obtained from BD Pharmingen; anti–CTLA-4 F(ab)2 from Ancell (Bayport, MN); and anti-CD25, anti-CD304 (anti-BDCA), anti-CD20 micromagnetic beads, and magnetic-activated cell sorting (MACS) columns (LS/MS), from Miltenyi Biotec (Bisley, United Kingdom). Detection and capture antihuman γ-IFN, anti–IL-4, and anti–IL-10 antibodies for enzyme-linked immunosorbent assay (ELISA) were obtained from BD Pharmingen. TGF-β1 ELISA kits were bought from eBioscience. Extravidin phosphatase, p-nitrophenyl phosphatase, and trypan blue were bought from Sigma-Aldrich. Transwell filters (0.4-μm polycarbonate membrane tissue-culture inserts) were obtained from Nunc (Roskilde, Denmark).
Mononuclear cell separation from peripheral blood and involved tissue samples
As described elsewhere,34 peripheral blood mononuclear cells (PBMCs) were separated from fresh blood samples by density gradient centrifugation. Single-cell suspensions of NHL-involved tissue cells or control nodes were prepared immediately after surgical removal, by gentle, repetitive scraping of immobilized nodal surface or spleen tissue with the use of a sharp scalpel with no enzymatic digestion. Red cells were removed from splenocytes using ammonium chloride lysis buffer.
Fractionation of PBMCs and involved tissue cells
CD25+ and CD25− fractions were isolated after incubating cells (PBMCs and involved tissue cell suspension) with 10 μL anti-CD25 microbeads per 107 cells for 15 minutes at 4°C and run through columns according to the manufacturer's instructions. For positive selection/depletion of lymphoplasmacytoid dendritic (CD304+) cells, involved tissue cell fractions were incubated with 100 μL FcR-blocking reagent and 100 μL anti-CD304 microbeads per 108 cells for 15 minutes at 6°C to 12°C, and different fractions were separated as before. Similarly, for positive selection of CD20+ tumor cells, we used 20 μL anti-CD20 magnetic beads per 107 cells. For higher purity, cells were run through the columns twice. Purity was checked by fluorescence-activated cell sorting (FACS) analysis and was typically greater than 95% for both positive and negative selections.
To assess the suppressive function, induced CD25+CD4+ and CD25−CD4+ fractions from CFSE-labeled CD25− PBMC samples were sorted (Becton Dickinson FACS DiVa; Mountain View, CA) on day 5, and both fractions were cocultured and stimulated with anti-CD3/28 beads for 3 days, or with PPD for 5 days, and proliferation of cells was assessed by incorporation of 3H-thymidine.
Cellular proliferation and (ELISA)
Cell fractions were cultured in 1-mL volumes at a cell concentration of 1.25 × 106/mL in complete medium (modification of Eagle medium [α-MEM; Gibco BRL, Carlsbad, CA] supplemented with 1% l-glutamine [Gibco BRL], 2% 1 M HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] buffer, pH range 7.2-7.4 [Sigma-Aldrich], 2% penicillin/streptomycin solution [Gibco BRL]) supplemented with 5% autologous serum at 37°C in a 5% CO2 humidified atmosphere for 5 days. ConA (10 μg/mL), PPD (10 μg/mL), DPT (10 μL/mL), and anti-CD3/28 Dynabeads (5 μL/mL; 1 bead/5 cells) were used as stimulants. Blocking anti–CTLA-4 F(ab)2 (0.5 ng/mL) and anti–IL-10 (1 ng/mL) antibodies were added to cultures in some experiments. Cellular proliferation was estimated from incorporation of 3H-thymidine into DNA in triplicate 100-μL aliquots taken from wells 5 days after stimulation, when recall T-cell responses are maximal.35 Incorporated 3H-thymidine was measured with a liquid scintillation counter (Microbeta; LKB Wallac, Turku, Finland). Proliferation results are presented as mean counts per minute plus or minus standard deviation (SD) of triplicate wells. The stimulation index (SI) is the ratio of mean counts obtained in the presence of antigen to mean counts obtained without antigen. A stimulation index of 3 or more indicates a positive proliferative response.34 Production of the Th cytokines γ-interferon (γ-IFN), IL-4, and IL-10 was assessed in duplicate 100-μL aliquots taken 5 days after stimulation of the cultures, with the use of a sensitive celELISA34 ; secretion of TGF-β1 was assessed according to the manufacturer's protocol. Positive responses are defined as 2 or more times background in unstimulated wells.
Flow cytometry and intracellular staining
Cells from peripheral blood or other involved tissues were stained with appropriate specific fluorochrome-conjugated surface antibodies after incubating for 30 minutes at 4°C in the dark. For intracellular FoxP3 and IL-10 staining, cells were first fixed and permeabilized and then incubated with an appropriate amount of antihuman FoxP3 and anti–IL-10 antibodies for 30 minutes at 4°C in darkness after blocking nonspecific binding using 2% rat serum (as per the eBioscience intracellular staining protocol). A minimum of 50 000 cells was acquired on a cytometer (LSR; Becton Dickinson). For induction experiments, where lower ratios of PBMCs were incubated with tumor cells, 200 000 events were acquired.
CFSE labeling and dilution assay
Unfractionated PBMC and CD25− fractions were washed, counted, and resuspended at a concentration of 107 cells/mL in PBS. A 5-mM stock solution of CFSE was prepared after dissolving CFSE lyophilized powder in dimethyl sulfoxide (DMSO) and used to stain different PBMC fractions, after further diluting with PBS to a final concentration of 0.25 mM per 107 cells. After 10-minute incubation at 37°C in darkness, cells were washed twice with sterile PBS containing 2% autologous serum. CFSE-labeled fractions from PBMCs were cocultured with different fractions from involved tissues, tumor cells (positively selected CD20+ or CD25+ and CD304+ cell–depleted cell fractions), tumor supernatant (prepared by incubating CD20+ tumor cells in complete medium with 5% autologous serum for 36-48 hours and by separating supernatant after centrifuging twice at 600g for 20 minutes), CD304+ cells, and unfractionated involved tissue cell suspension in different ratios in complete medium with 5% autologous serum. In some experiments, tumor cells were incubated with CFSE-labeled PBMC fractions separated with transwells. Cocultures were assessed for induction of CD25+FoxP3+ Treg cells from CFSE-labeled CD25− PBMC fractions or expansion of CD25+FoxP3+ cells in unfractionated PBMC samples at different time points (maximum 5 days). Purified blocking antihuman B7-H1 (5 μg/mL), antihuman B7-DC (5 μg/mL), antihuman IL-9 receptor antibody (2 ng/mL), 1-methyl-d-tryptophan (1 mM), aspirin (5 mM), sulindac (0.6 mM), and anti–TGF-β blocking antibody (10 μg/mL) were added in some of the cocultures.
Assessment of apoptosis in tumor cells
Apoptosis in CD20+ tumor cells from involved tissue samples with or without depletion of CD25+ T cells was assessed by determining annexin V–positive and 7AAD-negative cell percentages following 5 days of culture.
Results
Enumeration of Treg cells in patients' PBMCs and involved tissues
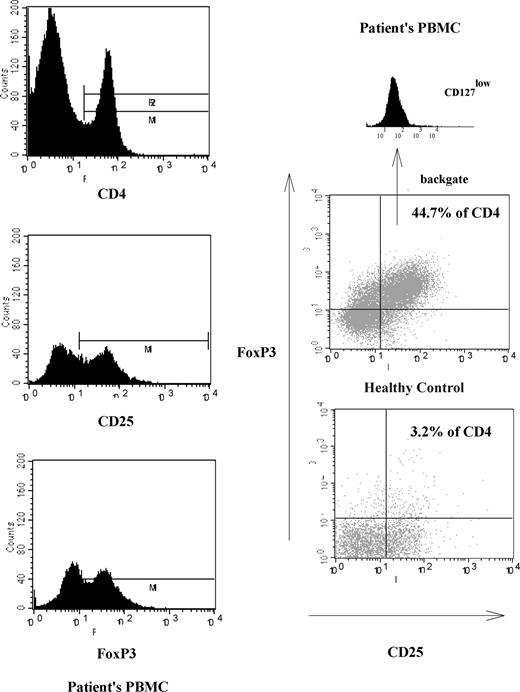
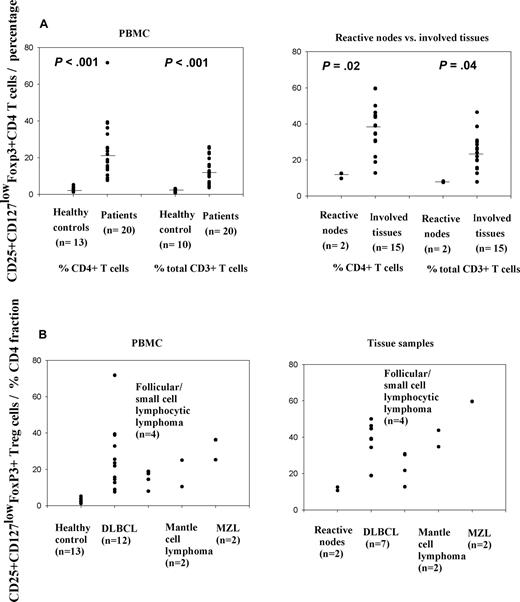
Natural Treg cells were counted as CD3+CD4+CD25+CD127lowFoxP3+ lymphocytes (representative flow cytometric data, Figure 1). The median proportion of these cells in peripheral blood samples from healthy controls (n = 13) was in the expected range at 3.2% of CD4+ cells (Figure 2A). In contrast, the median proportion of cells with this phenotype in PBMCs from patients (n = 20) was 20.4% of CD4 cells (P < .001, rank sum test; Figure 2A). These percentages were significantly higher both in aggressive and indolent subtypes compared with healthy controls (Figure 2B). In aggressive NHL (n = 14; DLBCL, n = 12; and mantle cell lymphoma, n = 2), the median percentage in PBMCs was 22.6% and in indolent NHL (n = 6; follicular lymphoma, n = 3; marginal zone lymphoma, n = 2; and small cell lymphocytic lymphoma, n = 1), it was 18.2%. There was no significant evidence for heterogeneity in Treg numbers between lymphoma subtypes, whether considered by all 5 different histologies (P = .37; Kruskal-Wallis test) or high grade versus low grade (P = .52).
Illustration of increased numbers of CD25+ Treg cells in one representative NHL patient. The 3 left-hand panels illustrate the gating strategy used to distinguish CD25+ Treg cells. The main panels on the right show representative FACS analyses demonstrating CD25+CD127lowFoxP3 Treg cell percentage in patient's PBMCs and comparison with a healthy control.
Illustration of increased numbers of CD25+ Treg cells in one representative NHL patient. The 3 left-hand panels illustrate the gating strategy used to distinguish CD25+ Treg cells. The main panels on the right show representative FACS analyses demonstrating CD25+CD127lowFoxP3 Treg cell percentage in patient's PBMCs and comparison with a healthy control.
Increased numbers of CD25+ Treg cells in patients with different specific subtypes of NHL. (A) Proportions of CD25+ Treg cells in patients' PBMCs and involved tissue samples compared with healthy control PBMCs and reactive nodes, respectively. (B) Proportions of CD25+ Treg cells in PBMCs and involved tissue samples from different specific subtypes of NHL and their comparison with control samples. Data are expressed as percentage of CD4+ T cells. DLBCL indicates diffuse large B-cell lymphoma; MZL, marginal zone lymphoma.
Increased numbers of CD25+ Treg cells in patients with different specific subtypes of NHL. (A) Proportions of CD25+ Treg cells in patients' PBMCs and involved tissue samples compared with healthy control PBMCs and reactive nodes, respectively. (B) Proportions of CD25+ Treg cells in PBMCs and involved tissue samples from different specific subtypes of NHL and their comparison with control samples. Data are expressed as percentage of CD4+ T cells. DLBCL indicates diffuse large B-cell lymphoma; MZL, marginal zone lymphoma.
Expressed as proportion of total CD3+ T cells, the median proportion of Treg cells was 11.2% in patients' PBMC samples (n = 20), compared with 1.9% in the healthy control group (n = 10; P < .001, rank sum test; Figure 2A).
In involved tissue samples from patients (n = 15), the median CD25+CD127lowFoxP3+ Treg was 38.2% of CD4 cells, whereas it was 11.6% in 2 reactive nodal tissue samples (P = .02, rank sum test; Figure 2A). In aggressive NHL (n = 9; DLBCL, n = 7; and mantle cell lymphoma, n = 2) involved tissue samples, the median was 39.1% and in indolent NHL (n = 6; follicular lymphoma n = 3; marginal zone lymphoma, n = 2; and small cell lymphocytic lymphoma, n = 1), it was 30.5% (Figure 2B).
Likewise, of total CD3+ T cells, the median percentage of Treg cells was 24% in patients' involved tissue samples (n = 15), whereas it was 7.9% in 2 reactive nodal tissue samples (P = .04, rank sum test) (Figure 2A).
Not only proportions, but also absolute numbers of Treg cells were elevated in patients' PBMCs (n = 10) compared with healthy control PBMC samples (n = 9; Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). Mean absolute Treg cell number in the patients' group was 0.22 × 109/L, whereas in the healthy control group, it was 0.026 × 109/L (P < .001; rank sum test).
In view of the previous demonstration that IL-10–expressing Tr1 CD4+ T cells were overexpressed in Hodgkin disease,20,36 we enumerated Tr1 cells both in peripheral blood and involved tissue samples and again compared with healthy controls. Median percentage of IL-10 plus Tr1 cells of CD4 T cells in patients' PBMCs (n = 20) versus healthy controls' PBMCs (n = 12), was 14.0% versus 7.1%, respectively (P = .144, rank sum test; Figure S2). Furthermore, there was no evidence for any histologic subtype having significantly raised numbers of Tr1 cells: in aggressive NHL (n = 14; DLBCL, n = 12; and mantle cell NHL, n = 2), the median percentage in PBMCs was 13.6% and in indolent NHL (n = 6; follicular lymphoma, n = 3; marginal zone lymphoma, n = 2; and small cell lymphocytic lymphoma, n = 1), it was 15.4%. Similarly, the median percentage of IL-10 plus Tr1 cells in patients' involved tissues (n = 15) versus healthy controls' PBMCs (n = 12) was 6.6% versus 7.1%, respectively (P = .449, rank sum test; Figure S2). Again, there was little evidence for heterogeneity in histologic subtypes: in aggressive NHL (n = 9; DLBCL, n = 7; and mantle cell NHL, n = 2) involved tissue samples, the median was 6.6% and in indolent NHL (n = 6; follicular NHL, n = 3; marginal zone lymphoma, n = 2; and small cell lymphocytic lymphoma, n = 1), it was 9%.
Proportion of CD25+CD127lowFoxP3+ Treg cells in peripheral blood correlates with tumor bulk
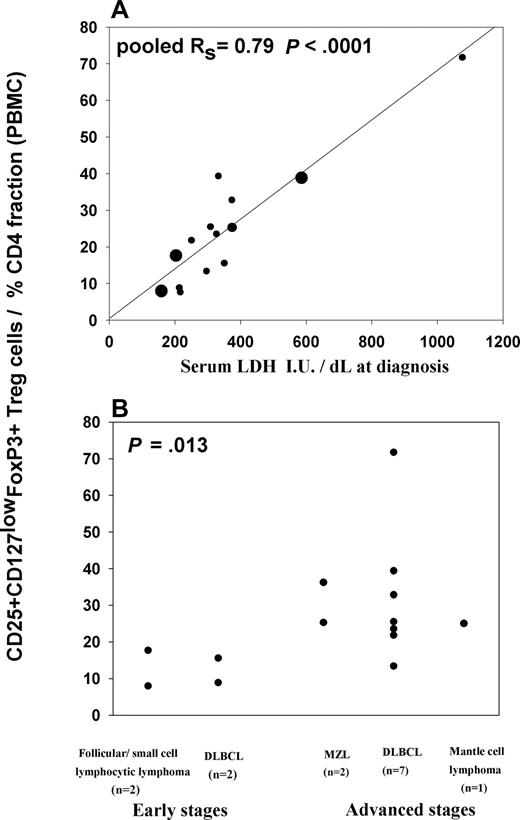
We next investigated whether CD25+ Treg cell numbers correlate with markers of tumor bulk. The proportion of CD25+ Treg cells is highly significantly correlated with serum lactate dehydrogenase (LDH) concentration, a tumor marker for DLBCL (n = 10, Rs = 0.8, P = .007). This correlation is also apparent for indolent (n = 4; follicular NHL, n = 2; small cell lymphocytic lymphoma, n = 1; and marginal zone lymphoma, n = 1; Rs = 1, P = .08) subtypes of NHL (pooled Rs = 0.79, P < .001; Figure 3A). Likewise, we found an increased proportion of CD25+ Treg cells in PBMCs in patients with more advanced clinical stages. We split the CD25+ Treg cell percentages of CD4 T cells in peripheral blood by early-stage disease (Ann Arbor stages I and II) and advanced disease (Ann Arbor stages III, IV, or bulky stage II, ≥ 5 cm). The median Treg percentage of CD4 T cells from early stages (n = 4; DLBCL, n = 2; follicular lymphoma, n = 1; and small cell lymphocytic lymphoma, n = 1) was 12.2%, whereas the median percentage in advanced disease (n = 10; DLBCL, n = 7; marginal zone lymphoma, n = 2; and mantle cell lymphoma, n = 1) was 25.4% (P = .013, rank sum test; Figure 3B).
Proportion of CD25+ Treg cells in patients with different specific subtypes of NHL correlates with disease bulk. Panels show relationships between proportions of CD25+ Treg cells within CD4 T cells in patients' PBMCs (n = 14) with (A) serum LDH at diagnosis and (B) clinical stages. P values were calculated using Spearman correlation test (A) and rank sum test (B). In panel A, large dots represent 3 follicular NHL/small cell lymphocytic lymphoma, an intermediate-sized dot represents a marginal zone lymphoma, and smaller dots (n = 10) represent DLBCL samples.
Proportion of CD25+ Treg cells in patients with different specific subtypes of NHL correlates with disease bulk. Panels show relationships between proportions of CD25+ Treg cells within CD4 T cells in patients' PBMCs (n = 14) with (A) serum LDH at diagnosis and (B) clinical stages. P values were calculated using Spearman correlation test (A) and rank sum test (B). In panel A, large dots represent 3 follicular NHL/small cell lymphocytic lymphoma, an intermediate-sized dot represents a marginal zone lymphoma, and smaller dots (n = 10) represent DLBCL samples.
CD25+ Treg cells and the immune suppression associated with NHL
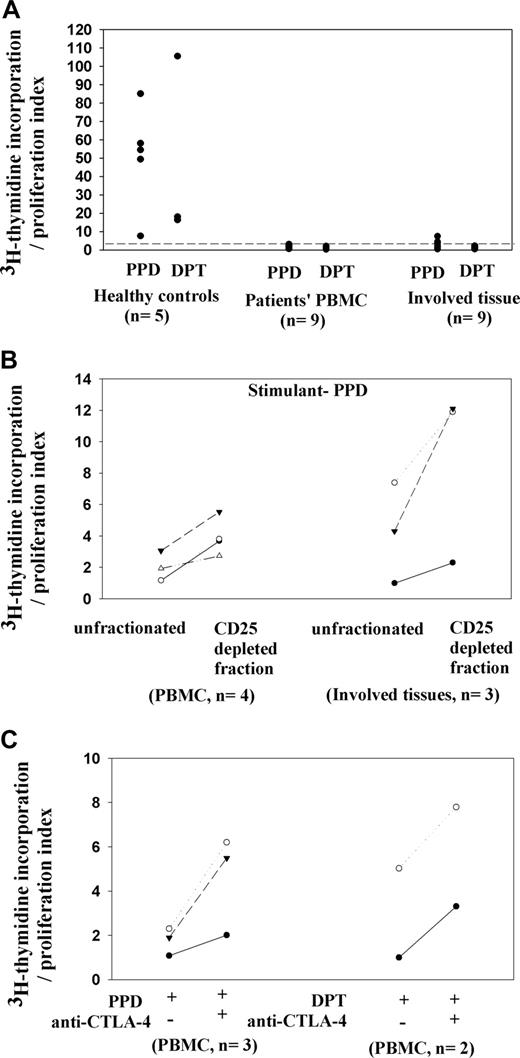
The question arises as to whether the increased numbers of CD25+ Treg cells contribute to immune suppression in NHL. We first corroborated there was poor effector T-cell activity in NHL. PBMCs and involved tissue mononuclear cells were stimulated with the recall antigens PPD and DPT and the mitogen ConA, which elicit strong T-cell responses in healthy controls (n = 5). As expected, we documented poor proliferation with ConA and almost none with recall antigens PPD and DPT in both PBMCs and involved tissue samples from patients with indolent and aggressive subtypes of NHL (patients 2, 4, 12, 15, 17, 18, 21, 23, and 24 for PBMCs, n = 9; and patients 2, 4, 5, 7, 10, 12, 15, 16, and 18 for involved tissue samples, n = 9; Figure 4A). Furthermore, there was little secretion of Th1 or Th2 cytokines (data not shown). However, PBMC samples from 2 patients with limited extranodal disease (patients 19 and 20) gave proliferation and cytokine responses similar to those of healthy controls (Figure S4), and their Treg cell percentages in peripheral blood were lower compared with those of other patients (12.7% and 8.8% of total CD4 T cells, respectively).
Contributions of CD25+ Treg cells to unresponsiveness of patients' T cells from peripheral blood and involved tissue samples. (A) Lack of T-cell proliferation in patients' samples, whereas control PBMC samples demonstrate significant proliferation with control stimuli PPD or DPT. (B) Increased proliferation to PPD by T cells from PBMCs and involved tissue samples after CD25+ cell depletion. (C) Increased T-cell proliferation on adding a blocking anti–CTLA-4 F(ab)2 fragment to patients' PBMC cultures stimulated by PPD or DPT.
Contributions of CD25+ Treg cells to unresponsiveness of patients' T cells from peripheral blood and involved tissue samples. (A) Lack of T-cell proliferation in patients' samples, whereas control PBMC samples demonstrate significant proliferation with control stimuli PPD or DPT. (B) Increased proliferation to PPD by T cells from PBMCs and involved tissue samples after CD25+ cell depletion. (C) Increased T-cell proliferation on adding a blocking anti–CTLA-4 F(ab)2 fragment to patients' PBMC cultures stimulated by PPD or DPT.
To determine whether the expanded CD25+ population in PBMCs and involved tissue samples of patients with NHL causes some of this unresponsiveness, we depleted CD25+ cells from patients' PBMCs (n = 4; patients 15, 17, 18, and 22) and involved tissue samples (n = 3; patients 5, 7, and 12) before stimulating T cells. In all cases, including both indolent and aggressive subtypes of lymphomas, greater T-cell proliferation and production of γ-IFN was noted in CD25-depleted samples compared with unfractionated PBMCs and involved tissue samples (Figure 4B). Furthermore, CD25+ T cells isolated from 2 involved tissue samples (patients 12 and 16) not only were hyporesponsive to stimulation with PPD, but also inhibited the response of the corresponding CD25− fraction to the antigen, confirming their suppressive nature (Figure S4).
The possibility that CD25+ cells in NHL may inhibit tumor killing, for example by cytotoxic T cells, was addressed by determining the levels of apoptosis of tumor cells, with or without depletion of CD25+ T cells, from involved tissue samples from 3 patients (patients 9, 10, and 18). Some increased apoptosis was noted when all 3 samples were depleted of CD25+ T cells, and in 2 cases the change was marked (Figure S5).
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is a costimulatory molecule expressed on the CD25+ Treg population. To assess whether CTLA-4 plays a role in increased Treg function in NHL, we added blocking antibody fragment anti–CTLA-4 F(ab)2 to stimulated PBMCs and involved tissue cultures. There were consistent increases in T-cell proliferation in treated cultures (Figure 4C; patients 17, 21, and 22). In contrast, neutralization of Tr1 cytokine IL-10 in stimulated cultures had little effect (results not shown).
Tumor cells induce and then expand functional CD25+ Treg cells from CD25− PBMCs in vitro
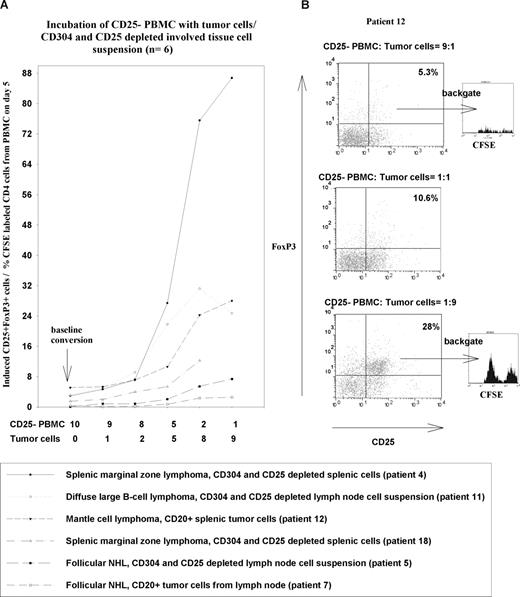
One hypothesis to explain the increased numbers of CD25+ Treg cells in NHL patients is that the tumor cells induce conventional CD25− T cells to adopt a regulatory phenotype. To test this possibility, we initially incubated PBMCs with crude involved tissue suspension from NHL patients (n = 2, patients 4 and 12) and demonstrated an increase in the numbers of CD25+ Treg cells in the cultures (results not shown). The next step was to identify the source of the additional CD25+ Treg cells, and to exclude cells from the involved tissues other than the tumor cells as the driver for Treg expansion. We therefore isolated CD25− PBMC fractions, labeled them with CFSE to follow their differentiation and division, and then incubated them with different fractions of involved tissue cell suspensions from NHL patients (n = 6, patients 4, 5, 7, 11, 12, and 18) enriched for tumor and depleted of CD25+ Treg cells (Figure 5).
NHL tumor cells induce CD25+ Treg cells. Panels show induction of CD25+FoxP3+ Treg cells from CFSE-labeled CD25− PBMC fractions with different ratios of tumor cells or CD304+ cell– and CD25+ cell–depleted involved tissue cell fractions. (A) The left-hand panel summarizes the percentages of CD25+FoxP3 Treg cells after 5 days of incubation with different ratios of tumor cells or CD304+- and CD25+-depleted involved tissue fractions (n = 6). (B) The plots shown on the right give examples of flow cytometric plots used to generate the graphs and typical examples of CFSE staining on cells gated on CD25 and FoxP3.
NHL tumor cells induce CD25+ Treg cells. Panels show induction of CD25+FoxP3+ Treg cells from CFSE-labeled CD25− PBMC fractions with different ratios of tumor cells or CD304+ cell– and CD25+ cell–depleted involved tissue cell fractions. (A) The left-hand panel summarizes the percentages of CD25+FoxP3 Treg cells after 5 days of incubation with different ratios of tumor cells or CD304+- and CD25+-depleted involved tissue fractions (n = 6). (B) The plots shown on the right give examples of flow cytometric plots used to generate the graphs and typical examples of CFSE staining on cells gated on CD25 and FoxP3.
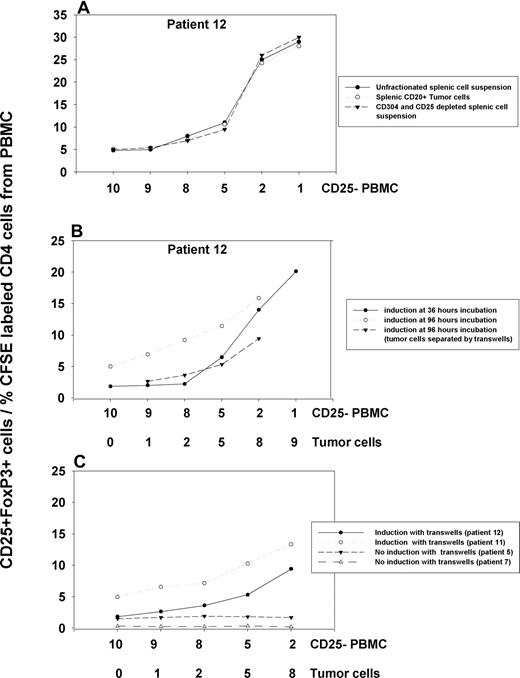
In all cases, we noted strong induction of CD25+ Treg cells from the CFSE-labeled CD25− PBMC population, and their proliferation. The induced CD25+ cells also stained for the Treg marker FoxP3. This induction was dependent on the dose of tumor cells, and was seen when we depleted lymphoid dendritic cells from the involved tissue cell suspension using anti-CD304, or enriched the tumor cells by positive selection of CD20+ cells. The degree of induction with unfractionated and CD304- and CD25-depleted involved tissue fractions was comparable (Figure 6A, patient 12), and no induction above background was noted when control CD304+ fractions from involved tissues were tested (data not shown). The induction in the presence in tumor cells continued over at least 96 hours of culture, with the numbers of CD25+ Treg cells steadily increasing (Figure 6B, patients 11 and 12). Analysis of CFSE dilution demonstrated that the induced Treg cells also proliferate to expand this population further. It is unlikely that this expanded population contains a significant contribution from any small numbers of preexisting CD25+ Treg cells that may have contaminated the CD25− fraction, since these would have had to proliferate many times more than the CFSE staining indicates. Furthermore, a substantial proportion of the induced CD25+ cells had not proliferated, indicating that they could not have arisen from proliferation of contaminating CD25+ cells (Figure 5).
Induction of CD25+ FoxP3+ Treg cells by NHL tumor cells under different conditions. (A) An example of comparable induction with unfractionated splenic cells, CD20+ tumor cells, and CD304+, CD25+–depleted splenic cell suspensions with CD25− PBMCs after 5 days incubation. (B) Increased induction at 96 hours of incubation compared with 36 hours. (C) The effects of separating tumor and PBMC fraction using transwells (n = 4 patients).
Induction of CD25+ FoxP3+ Treg cells by NHL tumor cells under different conditions. (A) An example of comparable induction with unfractionated splenic cells, CD20+ tumor cells, and CD304+, CD25+–depleted splenic cell suspensions with CD25− PBMCs after 5 days incubation. (B) Increased induction at 96 hours of incubation compared with 36 hours. (C) The effects of separating tumor and PBMC fraction using transwells (n = 4 patients).
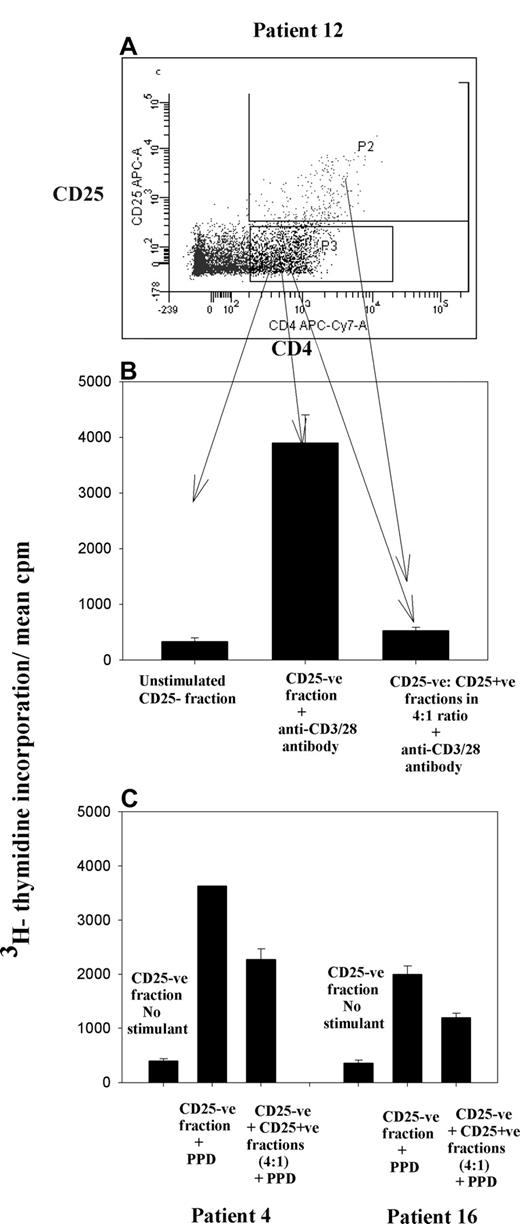
To confirm that these induced Treg cells do indeed have suppressive function, in 3 cases (patients 4, 12, and 16), we incubated CFSE-labeled CD25− PBMC fractions with CD20+ tumor cells and then flow-sorted the induced CD4+CD25+ and CD4+CD25− fractions. In each case, the CD25− fraction retained the ability to proliferate strongly when stimulated with anti-CD3/28 antibodies or PPD, but these responses were lost or diminished when the induced CD25+ fraction was added (Figure 7).
CD25+ Treg cells induced by NHL tumor cells are suppressive. CFSE-labeled CD25− fraction of PBMCs was incubated with tumor cells in the ratio of 1:4. On day 5, induced CD25+ and CD25−CD4+ fractions (n = 3 patients) were sorted by flow cytometry (A) and stimulated with either anti-CD3/28 or the recall antigen PPD. Histogram illustrates inhibition of proliferation of effector cells (CD25− fraction) on addition of CD25+ cells in a ratio of 4:1. (B) Patient 12. (C) Patients 4 and 16. Error bars represent SD.
CD25+ Treg cells induced by NHL tumor cells are suppressive. CFSE-labeled CD25− fraction of PBMCs was incubated with tumor cells in the ratio of 1:4. On day 5, induced CD25+ and CD25−CD4+ fractions (n = 3 patients) were sorted by flow cytometry (A) and stimulated with either anti-CD3/28 or the recall antigen PPD. Histogram illustrates inhibition of proliferation of effector cells (CD25− fraction) on addition of CD25+ cells in a ratio of 4:1. (B) Patient 12. (C) Patients 4 and 16. Error bars represent SD.
Mechanisms of Treg induction
Treg cells may be induced either by soluble factors or may be dependent on cell-cell contact. To determine the contribution of these mechanisms, we separated CD25− PBMC fractions from tumor cells with transwells and also tested the ability of tumor cell supernatant to induce CD25+ Treg cells. In 2 cases (patients 11 and 12) of 4, induction was reduced, but not abrogated, when the populations were separated (Figure 6C). Moreover, tumor cell–conditioned supernatant from these cases mediated an increase in CD25+ cell numbers (data not shown). Thus, both cell-cell contact and soluble factors appear important. However, in 2 other cases (patients 5 and 7), no induction was noted with transwell experiments, or with tumor cell–conditioned supernatant, indicating that cell-cell contact was an absolute requirement in those cases.
To identify molecules that may be important in inducing a Treg phenotype, we used several reagents to inhibit CD25+ Treg induction. Reports elsewhere suggest roles for prostaglandin E2,37 tryptophan catabolism,33 IL-9,38 PD-1 or PD-2 interactions,39,40 and TGF-β41 in inducing a Treg phenotype. In neither of the patients (5 and 7) that we identified where cell-cell contact was essential for Treg induction did blocking any of these potential soluble mediators inhibit the induction of Treg cells with NHL tumor cells. Thus, the cyclooxygenase inhibitors aspirin and sulindac; the indoleamine 2,3-dioxygenase (IDO) inhibitor 1-methyl tryptophan (1MT), anti–IL-9 receptor antibody, blocking PD-1 or PD-2 ligand interactions with anti–PDL-1 or anti–PDL-2, and anti–TGF-β blocking antibody had no effect in these cases. Anti–TGF-β blocking antibody was also used in one additional patient (patient 24) and again failed to inhibit Treg induction. Furthermore, no detectable TGF-β was noted in supernatant from CD25− PBMC fractions, CD20+ tumor cells, or cocultures containing CD25− PBMC/CD20+ tumor cells in a 1:1 ratio from all 3 patients (Figure S6).
In patient 12, blocking PD-1 ligand interactions with either anti–PDL-1 or anti–PDL-2 antibodies inhibited induction of the CD25+ phenotype (Figure S7), suggesting, in some cases of NHL, that these interactions may be important in inducing a Treg phenotype.
Discussion
The results demonstrate very high numbers of cells with a natural CD25+CD4+ Treg phenotype in the peripheral blood and intratumoral microenvironment of B-NHL patients, and we also show that suppressor cells of this type are potently induced by the lymphoma in vitro. This is the first direct evidence that NHL cells induce a recognized Treg population, and has implications for tumor immune evasion and therapy.
The proportion of Treg cells we recorded in patients with NHL was remarkably high, constituting up to 71% of CD4 T cells in PBMCs and 59% in involved tissues. The data support the findings of Yang et al,21 who reported up to 43% CD25+ Treg cells in the CD4+ population in involved tissues. Such high numbers of Treg cells have not been recorded in other hematologic malignancies with the exception of Hodgkin disease.20 Despite marked biologic differences between aggressive and indolent lymphomas, our data indicate overall consistency in Treg cell induction.
The relevance of the Treg population to tumor progression in NHL is suggested here by the close correlation between Treg numbers with both serum LDH levels and clinical staging. Our data add to the growing evidence that the degree of Treg expansion is associated with more advanced disease and poorer outcomes in different tumor types. Beyer et al22 found a significantly higher number of Treg cells in Binet stage C compared with Binet stage A disease in chronic lymphocytic leukemia patients. Curiel et al18 noted a poorer outcome with increased Treg cell number in patients with ovarian carcinoma. Similarly, Nadal et al42 demonstrated a higher probability of relapse in patients with chronic myeloid leukemia after allogenic transplantation with increased frequencies of CD25high Treg cells. However, in Hodgkin disease43 and follicular NHL,44 more FoxP3+ cells, assessed immunocytochemically, were associated with better survival, whereas in diffuse large B-cell lymphoma,45 they did not predict the outcome. More recently, Tzankov et al46 demonstrated a positive correlation of high numbers of intratumoral FoxP3+ Treg cells with improved survival in germinal center–like diffuse large B-cell lymphoma, follicular NHL, and classical Hodgkin disease. In contrast, they found a negative prognostic effect in non–germinal center diffuse large B-cell lymphoma. The reason for these discrepancies remains unclear, although the expression of FoxP3+ by activated Th1 cells may provide an explanation. We also demonstrated that depletion of CD25+ T cells increased apoptosis of tumor cells consistent with the mechanism described by Yang et al,29 who showed that CD25+ Treg cells suppressed degranulation and cytotoxic activity of infiltrating CD8+ T cells exposed to lymphoma cells.
Depressed cellular immunity has long been recognized in patients with NHL,47,48 making these patients prone to viral infections and transfusion-associated graft-versus-host disease.49 We confirmed a significant T-cell hyporesponsiveness to multiple control stimuli, both in peripheral blood and involved tissue samples in NHL patients. With depletion of the CD25+ fraction, T-cell proliferation and γ-IFN secretion increased, indicating that the Treg cells play an important role in mediating T-cell hyporesponsiveness in NHL. Blockade of CTLA-4 partially reversed hyporesponsiveness, suggesting that costimulation via this molecule expressed on Treg cells is necessary for their inhibitory effects, but we found no evidence that cytokines such as IL-10 were mediating suppression. Our demonstration of Treg-mediated suppression in low- and high-grade NHL confirms and extends a recent study that identified intratumoral T-cell hyporesponsiveness mediated by CD25+ cells in follicular NHL.30
We provide direct evidence that the NHL cells themselves are responsible for the increased numbers of Treg cells, since tumor cells potently induced a population with the same CD4+CD25+FoxP3+ phenotype from CD25− PBMCs in vitro. Depletion experiments demonstrated that the ability to induce Treg cells was a property of fractions containing tumor cells, rather than other populations such as lymphoplasmacytoid dendritic cells. The induced population also subsequently expanded, and was confirmed to have suppressive function. Yang et al21 reported Treg cell recruitment to the tumor by the chemokine CCL22 produced by lymphoma cells, but this mechanism cannot explain the expanded number of Treg cells in the peripheral circulation that we documented. Taken together, these studies demonstrate that changes to the Treg population associated with NHL can be attributed to several properties of the tumor cells, including the ability to induce, expand, and attract regulatory subsets. The observation that tumor cells from some individuals are more potent inducers than others may reflect the diversity and heterogeneity of the mechanisms proposed to influence overall Treg activity.
Our data are the first to show induction of a recognized natural CD25+ Treg population from PBMCs by NHL cells. Another group50 recently identified a novel population of Treg cells with an unconventional FoxP3+CD25− phenotype in NHL-involved tissues. A similar population was induced from CD25− T cells after nonspecific TCR stimulation, and this process was enhanced by NHL tumor cells. The relationships between the 2 CD25+ and CD25− populations in NHL, and their relative contribution to immune suppression, remain to be established.
In other examples of CD25+ Treg induction by tumor cells, a number of different mechanisms have been implicated. Our data indicate heterogeneity within NHL, since Treg induction was entirely dependent on direct contact between tumor and CD25− population in some cases, while there was a contribution by soluble factors in others. Possible mediators identified in other tumors include prostaglandin E2,37 tryptophan catabolism,33 IL-9,38 PD-1 ligands,39,40 and TGF-β.41 Of these, we demonstrated that blockade of PD-1 interaction prevented Treg induction in one NHL patient from our cohort. Clearly, the mechanisms that are the most important in NHL remain to be identified.
Our findings in NHL might be exploited clinically. For example, the strong positive correlation of Treg numbers with tumor bulk raises the possibility that the regulatory population may be a useful prognostic marker. Future treatments could also be targeted to the Treg cells associated with NHL, to reverse the immune suppression and possible immune evasion attributed to this population. This could be achieved not only by the development of selective immunotherapy, but also by the choice of current chemotherapy. Beyer et al22 in CLL noted a reduction of Treg cell number following a number of courses of chemotherapy especially fludarabine, and selective depletion of Treg cells by cyclophosphamide has been shown by several groups.51-53 It is, therefore, possible that that chemotherapy not only kills tumor cells but also preferentially targets Treg cells. The very high proportions of Treg cells, both locally and systemically, in NHL provide a model system in which to explore such possibilities.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr P. W. Johnston for assisting in acquiring involved tissue samples.
This work was supported by the Leukemia Research Fund and Friends of Anchor charitable fund.
Authorship
Contribution: S.M. recruited the patients and healthy controls, designed and performed experiments, analyzed data, and wrote the draft paper; L.D. provided technical support for flow cytometry; N.A.M. provided technical support, designed experiments, commented on data, and critically reviewed the paper; D.J.C. commented on data and critically reviewed the paper; R.N.B. conceived the study, commented on data, and critically reviewed the paper; M.A.V. designed and supervised the study, commented on data, and critically reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: R. N. Barker, Department of Medicine and Therapeutics, University of Aberdeen, Aberdeen AB25 2ZD, United Kingdom; e-mail: r.n.barker@abdn.ac.uk; or M. A. Vickers, Department of Medicine and Therapeutics, University of Aberdeen, Aberdeen AB25 2ZD, United Kingdom; e-mail: m.a.vickers@abdn.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal