Abstract
Extracellular adenosine has been implicated in vascular adaptation to hypoxia. Based on the observation that increases in intracellular adenosine can effectively elevate extracellular adenosine, we studied the contribution of adenosine kinase (AK, intracellular conversion of adenosine to adenosine monophosphate [AMP]) to vascular adenosine responses. Initial in vitro studies of ambient hypoxia revealed prominent repression of endothelial AK transcript (85% ± 2% reduction), protein, and function. Transcription factor binding assays and hypoxia inducible factor 1-α (HIF-1α) loss- and gain-of-function studies suggested a role for HIF-1α in transcriptional repression of AK. Moreover, repression of AK by ambient hypoxia was abolished in conditional HIF-1α mutant mice in vivo. Studies of endothelial barrier function revealed that inhibition or siRNA repression of AK is associated with enhanced adenosine-dependent barrier responses in vitro. Moreover, in vivo studies of vascular barrier function demonstrated that AK inhibition with 5′-iodotubericidin (1 mg/kg prior to hypoxia) significantly attenuated hypoxia-induced vascular leakage in multiple organs and reduced hypoxia-associated increases in lung water. Taken together, our data reveal a critical role of AK in modulating vascular adenosine responses and suggest pharmacologic inhibitors of AK in the treatment of conditions associated with hypoxia-induced vascular leakage (eg, sepsis or acute lung injury).
Introduction
Previous studies have shown that extracellular adenosine levels are increased during limited oxygen availability and play a critical role in vascular adaptation to hypoxia.1-3 Extracellular adenosine mainly stems from phosphohydrolysis of nucleotide precursor molecules (adenosine triphosphate [ATP], adenosine diphosphate [ADP], or adenosine monophosphate [AMP]) via the ecto-apyrase (CD39, conversion of ATP/ADP to AMP) and ecto-5′-nucleotidase (CD73, conversion of AMP to adenosine), which are both highly expressed in vascular endothelia.4 Once generated into the extracellular space, adenosine can signal through any of 4 G-protein–coupled adenosine receptors (A1AR, A2AAR, A2BAR, or A3AR). All of these receptors are expressed on vascular endothelia4 and have been implicated in tissue protection in different models of injury.1,5-9 Following receptor activation, vascular adenosine signaling is terminated by diffusion of adenosine away from the receptor and rapid transport from the extracellular toward the intracellular space mainly via equilibrative nucleoside transporters (ENTs).10 Having reached the intracellular space, adenosine can either undergo deamination by adenosine deaminase to inosine11 or conversion to AMP by adenosine kinase (AK, EC 2.7.1.20).12 Interestingly, despite its intracellular location, AK has been shown to regulate extracellular adenosine signaling.12-14 In fact, inhibition of AK efficiently elevates cytoplasmatic adenosine levels. For example, functional studies in the heart have shown that inhibition of AK can elevate extracellular adenosine levels12 and contributes to adenosine-dependent cardioprotection.13 Moveover, a very elegant study of rat hypocampal adenosine metabolism found that during conditions that provide adequate oxygen and glucose supply, adenosine kinase plays a much greater role than adenosine deaminase (metabolism of adenosine to inosine) in regulating the extracellular concentration of adenosine.15
As an important end point of vascular adenosine signaling, several studies have investigated the role of extracellular adenosine in vascular barrier function.4,16,17 In fact, changes in vascular barrier function closely coincide with tissue injuries of many etiologies, and result in fluid loss, edema, and organ dysfunction.18 As such, endothelial permeability is highly regulated and may increase markedly upon exposure to inflammatory stimuli or adverse conditions such as ischemia or hypoxia, and studies have shown a critical role of extracellular adenosine in attenuating hypoxia-associated vascular leakage.4,11,17,19-22 For example, gene-targeted mice with defects in extracellular generation of adenosine (cd39−/− or cd73−/− mice) show profound increases in vascular leakage and pulmonary edema when exposed to ambient hypoxia.4,19 Similarly, studies using inhibitors of extracellular adenosine deaminase (eg, deoxycoformycin) or adenosine uptake inhibitors (eg, dipyridamole) found attenuated vascular leakage during hypoxia.11,17 Along these lines, we pursued the hypothesis that inhibition of AK-dependent conversion of adenosine to AMP may increase vascular adenosine signaling, resulting in barrier protection from hypoxia-induced vascular leak.
Methods
Endothelial cell culture
Transcriptional analysis
The transcriptional profile of endothelial cells subjected to normobaric hypoxia (12 hours) was compared in RNA derived from control or hypoxic endothelia using quantitative genechip expression arrays (Affymetrix, Santa Clara, CA).11,17 mRNA was also quantified by real-time reverse transcriptase–polymerase chain reaction (RT-PCR; iCycler; Bio-Rad Laboratories, Hercules, CA).11,17 The following primer sets were used: human AK: sense 5′-TGC CCT AAT TGC TTC CTG AG-3′, antisense 5′-TTG GCA TTT AAG TGG CAC TAT C-3′; human Toll-like receptor 2 (TLR2): sense 5′-GGA GCT GGA GAA CTT CAA TC-3′, antisense 5′-TTG CAC CAC TCA CTT TTC AC-3′; human vascular endothelial growth factor (VEGF): sense 5′-CCC ACT GAG GAG TCC AAC AT-3′, antisense 5′- TTT CTT GCG CTT TCG TTT TT-3′; murine CD73: sense 5′-CAAATCCCACACAACCACTG-3′, antisense 5′-TGCTCACTTGGTCACAGGAC-3′; murine β-actin: sense 5′-ACATTGGCATGGCTTTGTTT-3′, antisense 5′-GTTTGCTCCAACCAACTGCT-3′; human β-actin: sense 5′-GGT GGC TTT TAG GAT GGC AAG-3′, antisense 5′-ACT GGA ACG GTG AAG GTG ACA-3′; β-actin was used in identical reactions to control for starting template. Real-time PCR was also performed from RNA isolations of human saphenous vein after ex vivo exposure to hypoxia as described previously.4
Protein analysis
HMEC-1, CaCo-2, or murine tissues were analyzed by Western blot using a rabbit polyclonal anti-AK (Santa Cruz Biotechnology, Santa Cruz, CA) or a rabbit monoclonal anti–β-actin antibody (Cell Signaling Technology, Danvers, MA), as described previously.17 Cardiac immunohistochemistry was performed as described previously.8,24
Evaluation of adenosine kinase activity
HMEC-1s were grown to full confluency and exposed as indicated to normoxia or different hours of hypoxia. In order to develop a model for kinetics of endothelial activity, we first measured etheno (E)–adenosine (E-Ado) conversion to E-AMP in lysates of HMEC-1 monolayers with different E-Ado concentrations (0.1 μM-20 μM), and found optimal resolution of E-Ado conversion to E-AMP with 5 μM E-Ado concentrations. These conditions were used throughout for further measurements. After washing in Hanks balanced salt solution (HBSS), HMEC-1s were lysed in cold H2O and E-Ado was added. E-AMP was detected via high-performance liquid chromatography (HPLC) as described previously.19 AK activity was expressed as percentage of conversion of E-Ado to E-AMP. In control experiments, the AK inhibitor 5′-iodotubericidin (ITU; 10 μM), was used to demonstrate specificity for AK.
Stable repression of AK or HIF-1α by siRNA
HMEC-1s or CaCo-2 cells stably expressing psiRNA against AK or HIF-1α (HMEC-AK, CaCo-HIF1α) were generated as previously described.22 The hairpin primer with the sequence 5′-ACC TCG CTG ACC AGT TAT GAT TGT GAT CAA GAG TCA CAA TCA TAA CTG GTC AGC TT-3′ and 5′-CAA AAA GCT GAC CAG TTA TGA TTG TGA CTC TTG ATC ACA ATC ATA ACT GGT CAG CG-3′ corresponds to position 2666-2685 of the HIF-1α gene. For AK, the hairpin sequence (position 401-422) was 5′-ACC TCG TGG CTC AGT GGA TGA TTC AAT CAA GAG TTG AAT CAT CCA CTG AGC CAC TT-3′ and 5′-CAA AAA GTG GCT CAG TGG ATG ATT CAA CTC TTG ATT GAA TCA TCC ACT GAG CCA CG-3′. The control cell lines (HMEC-Scr and CaCo-Scr) were transfected with psiRNA-hH1 neoscr plasmid.
Lentiviral vector design, production, and transduction in CaCo-2 cells
Expression of oxygen-stable HIF-1α in CaCo-2 (CaCo-ΔODD) was achieved as previously described.22 This cell line was a kind gift from Dr Colgan (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) and Dr Westerman (University of Colorado Health Sciences Center, Denver, CO).
Measurement of adenosine
Adenosine was measured in supernatants of endothelia or after endothelial lysis with cold H2O by HPLC as previously described.17
Macromolecule paracellular permeability assay
Endothelial barrier function was assessed as described previously.17
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was performed using a Chromatin Immunoprecipitation Assay Kit (Upstate Biotechnology, Charlottesville, VA). For this purpose, HMEC-1s were subjected to normoxia or hypoxia, and then processed as described by the manufacturer. The sequences of the AK promoter-specific primers spanning the putative hypoxia response elements (HREs) were as follows: sense, 5′-TGC GGA CTG GAA TTA GTA CG-3′ and antisense, 5′-TAG TAA CAG CGT CCC GTC AC-3′ (AK-ChIP-1, 309 bp), and sense, 5′-GTG ACG GGA CGC TGT TAC TA-3′ and antisense, 5′-CCG CTC GTT ACC TGA CTG AC-3′ (AK-ChIP-2, 360 bp). Chromatin incubated with only beads or IgG-control was used as control for nonspecific binding of DNA.25 As a positive control for a known HIF-1–dependent gene, a ChIP assay for TLR2 was performed.
In vivo hypoxia model
C57BL/6 mice were matched according to sex, age, and weight. In subsets of experiments, conditional colon mutants lacking hif1a allele, Fabpl4x at-132/Cre mice were bred and genotyped as described previously.26 Animals were exposed to normobaric hypoxia (8% O2, 92% N2) or room air for 4 hours (n = 6 animals per condition). Following hypoxia/normoxia exposure, AK transcript was determined. Primer sequences for murine AK were 5′-GCC CAC CAT GGT TCT CAT TA-3′ and 5′-CCT GCA TTG GAA GCA GAA AT-3′. Murine β-actin (sense primer, 5′-ACA TTG GCA TGG CTT TGT TT-3′ and antisense primer, 5′-GTT TGC TCC AAC CAA CTG CT-3′) in identical reactions was used to control for starting template. In other studies, total organ vascular permeability was quantified by intravascular administration of Evan blue as described previously.4,17,19 Pulmonary edema was assessed in additional experiments as described previously.17 This protocol was in accordance with National Institutes of Health (NIH) guidelines for use of live animals and was approved by the institutional animal care and use committee at the University of Colorado Health Science Center in Denver.
Data analysis
Data were compared by 1-factor analysis of variance (ANOVA), or by Student t test where appropriate. Values are expressed as the mean plus or minus the standard deviation (SD) from at least 3 separate experiments.
Results
AK transcript, protein, and function are repressed by hypoxia
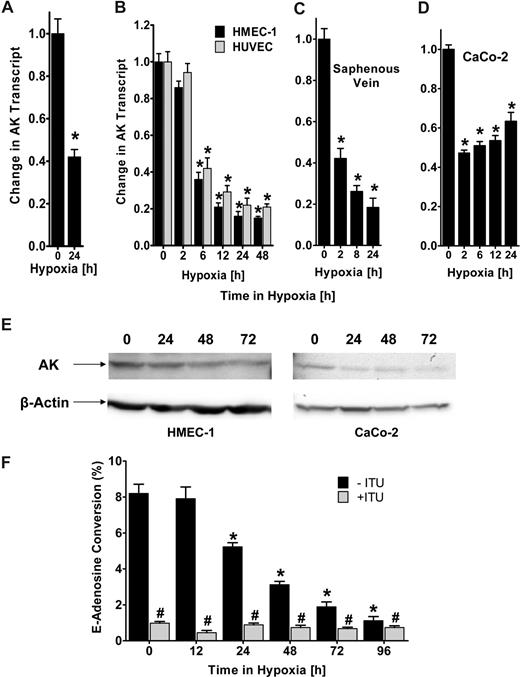
Recent studies suggest that hypoxia promotes a vascular phenotype that supports high capacity for rapid nucleotide phosphohydrolysis and enhanced adenosine signaling.4,20 Based on the notion that attenuation of AK-dependent intracellular metabolism of adenosine to AMP can effectively increase extracellular adenosine levels, we pursued transcriptional responses of AK during hypoxia. We gained initial insight from microarray analysis showing a 42% (± 0.06%) repression of AK mRNA expression in hypoxic endothelia (HMEC-1, pO2 20 torr, 24 hours; Figure 1A; P < .005). To confirm theses studies, we exposed confluent HMEC-1s or HUVECs to normoxia (ambient room air) or hypoxia (2% oxygen). As shown in Figure 1B, AK transcript levels are significantly repressed during hypoxia (P < .001). Similar results of AK repression were found when human saphenous vein tissues were subjected ex vivo to hypoxia, indicating that such findings are not limited to cultured endothelia (Figure 1C). Additionally, AK expression was also decreased in CaCo-2, an intestinal epithelial cell line (Figure 1D, P < .005), further suggesting that these findings are not limited to endothelial cells. We extended these mRNA findings to examine AK protein expression in cultured HMEC-1s. As shown in Figure 1E, Western blot analysis of lysates derived from HMEC-1s subjected to hypoxia (pO2 20 torr for 0 to 72 hours) revealed a significant loss of AK with increasing time in hypoxia. Similarly, AK protein was repressed in intestinal epithelia (CaCo-2, Figure 1E). In order to determine the functional consequences of hypoxia on AK activity, we measured the conversion of E-Ado into E-AMP by HPLC in lysates of HMEC-1 cells previously exposed to hypoxia (range 0-96 hours). The results show a time-dependent decrease in functional AK (Figure 1F), with an 8.6 (± 0.7-)-fold reduction after 72 hours of hypoxia exposure (P < .01). Selectivity for AK in this assay was demonstrated by parallel incubation with the adenosine kinase inhibitor ITU. Together, these results evidence transcriptional-dependent repression of AK by hypoxia.
Adenosine kinase (AK) expression and function during hypoxia. (A) Microarray analysis of AK in response to hypoxia. Confluent HMEC-1s were exposed to normoxia (21% oxygen) or hypoxia (2% oxygen, 24 hours) and relative AK expression was quantified from total RNA by microarray analysis. *Decreased fluorescence, P < .005. (B) Confluent HMEC-1/HUVEC monolayers were exposed to normoxia or hypoxia (2% oxygen) for the indicated time. Total RNA was isolated, and AK mRNA levels were determined by real-time RT-PCR. Data are expressed as fold change in transcript over normoxia plus or minus SD, and have been calculated relative to internal housekeeping gene (β-actin). Results are derived from 3 experiments in each condition (*P < .001). (C) Human saphenous vein was obtained from patients undergoing aorto-coronary bypass surgery and exposed ex vivo to normoxia (24 hours) or hypoxia. Real-time PCR was used to define AK mRNA levels. Results are derived from 3 experiments (*P < .001). (D) Confluent epithelial CaCo-2 cells were exposed to normoxia or hypoxia, and AK mRNA levels were determined (*P < .005). (E) Expression of AK protein during hypoxia in HMEC or CaCo-2 cells exposed to indicated periods of hypoxia. (F) Endothelial monolayers were exposed to indicated periods of hypoxia, and total AK activity was determined by HPLC (■, *P < .01). To determine specificity, cells were preincubated with 10 μM ITU ( , #P < .01). Data are derived from 5 monolayers in each condition, and results are expressed as mean percent E-adenosine conversion plus or minus SD.
, #P < .01). Data are derived from 5 monolayers in each condition, and results are expressed as mean percent E-adenosine conversion plus or minus SD.
Adenosine kinase (AK) expression and function during hypoxia. (A) Microarray analysis of AK in response to hypoxia. Confluent HMEC-1s were exposed to normoxia (21% oxygen) or hypoxia (2% oxygen, 24 hours) and relative AK expression was quantified from total RNA by microarray analysis. *Decreased fluorescence, P < .005. (B) Confluent HMEC-1/HUVEC monolayers were exposed to normoxia or hypoxia (2% oxygen) for the indicated time. Total RNA was isolated, and AK mRNA levels were determined by real-time RT-PCR. Data are expressed as fold change in transcript over normoxia plus or minus SD, and have been calculated relative to internal housekeeping gene (β-actin). Results are derived from 3 experiments in each condition (*P < .001). (C) Human saphenous vein was obtained from patients undergoing aorto-coronary bypass surgery and exposed ex vivo to normoxia (24 hours) or hypoxia. Real-time PCR was used to define AK mRNA levels. Results are derived from 3 experiments (*P < .001). (D) Confluent epithelial CaCo-2 cells were exposed to normoxia or hypoxia, and AK mRNA levels were determined (*P < .005). (E) Expression of AK protein during hypoxia in HMEC or CaCo-2 cells exposed to indicated periods of hypoxia. (F) Endothelial monolayers were exposed to indicated periods of hypoxia, and total AK activity was determined by HPLC (■, *P < .01). To determine specificity, cells were preincubated with 10 μM ITU ( , #P < .01). Data are derived from 5 monolayers in each condition, and results are expressed as mean percent E-adenosine conversion plus or minus SD.
, #P < .01). Data are derived from 5 monolayers in each condition, and results are expressed as mean percent E-adenosine conversion plus or minus SD.
AK is repressed in vascular organs during hypoxia in vivo
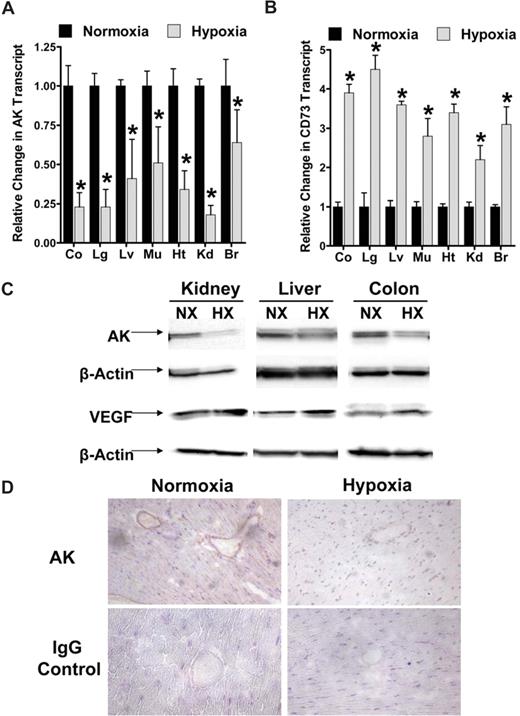
A model of murine hypoxia was employed to verify these results in vivo. To this aim, we exposed age-, sex-, and weight-matched mice to ambient hypoxia (4 hours, 8% oxygen) and examined AK transcript levels in vascular organs. As shown in Figure 2A, real-time PCR analysis revealed a significant decrease of AK mRNA in lung, kidney (P < .001), colon, liver, heart (P < .005), and muscle (P < .05) with hypoxia exposure. A known hypoxia-responsive gene (CD73)20 served as positive control. Western blot confirmed AK-protein repression as shown for kidney, liver, and colon (Figure 2B), whereas VEGF was induced. In addition, immunohistochemical staining of sections of cardiac tissue confirmed AK repression with hypoxia (Figure 2C). Taken together, these findings indicate that repression of AK also occurs during hypoxia in vivo.
Adenosine kinase (AK) expression during hypoxia in vivo. (A) C57BL/6 mice were exposed to ambient hypoxia (8% oxygen) or normoxia for 4 hours. The colon (Co), lungs (Lg), liver (Lv), muscle (Mu), heart (Ht), kidneys (Kd), and brain (Br) were harvested and AK or CD73 transcript was determined relative to β-actin by real-time RT-PCR (*P < .05). (B) Western-blot analysis of AK or VEGF protein from kidney, liver, or colon. (C) Normoxic or hypoxic cardiac tissue was harvested and stained with AK antibody. Note attenuated AK staining in hypoxic vasculature (magnification, ×400). IgG controls were used at identical concentrations and staining conditions as the target primary antibodies for AK. (D) The tissue was fixed in Tissue-Tek (Sakura) and cut in 3-μm slices, mounted on glass sildes, air dried, post-fixed in chilled methanol for 10 minutes, and permeabilized in chilled acetone for 1 minute. Immunohisto-chemical stainings were performed with polyclonal rabbit anti-AK antibody (dilution 1:100; Santa Cruz Biotechnology, Santa Cruz, CA) for AK and rabbit IgG serum (1:100; Santa Cruz Biotechnology) for isotype control. First endogneous peroxidase activity was quenched by incubating the specimens for 30 minutes with DAKO peroxidase blocking buffer (DAKO, Hamburg, Germany). Primary antibodies were applied for 30 minutes. Incubation with the DAKO evision secondary antibody followed for another 30 minutes. Staining was completed by 10-minute incubation with 3,3′-diaminobenzidine (DAB)+ substrate chromogen, which results in a brown-colored precipitate at the antigen side, and a hematoxylin counterstaining for 2 minutes. Evaluation of the immunohistochemical staining was performed using a LEITZ DMRBE light microscope (40×/0.75 lens), Leica DC300F camera, and Leica QWin software (Leica, Heidelberg, Germany).
Adenosine kinase (AK) expression during hypoxia in vivo. (A) C57BL/6 mice were exposed to ambient hypoxia (8% oxygen) or normoxia for 4 hours. The colon (Co), lungs (Lg), liver (Lv), muscle (Mu), heart (Ht), kidneys (Kd), and brain (Br) were harvested and AK or CD73 transcript was determined relative to β-actin by real-time RT-PCR (*P < .05). (B) Western-blot analysis of AK or VEGF protein from kidney, liver, or colon. (C) Normoxic or hypoxic cardiac tissue was harvested and stained with AK antibody. Note attenuated AK staining in hypoxic vasculature (magnification, ×400). IgG controls were used at identical concentrations and staining conditions as the target primary antibodies for AK. (D) The tissue was fixed in Tissue-Tek (Sakura) and cut in 3-μm slices, mounted on glass sildes, air dried, post-fixed in chilled methanol for 10 minutes, and permeabilized in chilled acetone for 1 minute. Immunohisto-chemical stainings were performed with polyclonal rabbit anti-AK antibody (dilution 1:100; Santa Cruz Biotechnology, Santa Cruz, CA) for AK and rabbit IgG serum (1:100; Santa Cruz Biotechnology) for isotype control. First endogneous peroxidase activity was quenched by incubating the specimens for 30 minutes with DAKO peroxidase blocking buffer (DAKO, Hamburg, Germany). Primary antibodies were applied for 30 minutes. Incubation with the DAKO evision secondary antibody followed for another 30 minutes. Staining was completed by 10-minute incubation with 3,3′-diaminobenzidine (DAB)+ substrate chromogen, which results in a brown-colored precipitate at the antigen side, and a hematoxylin counterstaining for 2 minutes. Evaluation of the immunohistochemical staining was performed using a LEITZ DMRBE light microscope (40×/0.75 lens), Leica DC300F camera, and Leica QWin software (Leica, Heidelberg, Germany).
HIF-1α in endothelial AK repression by hypoxia
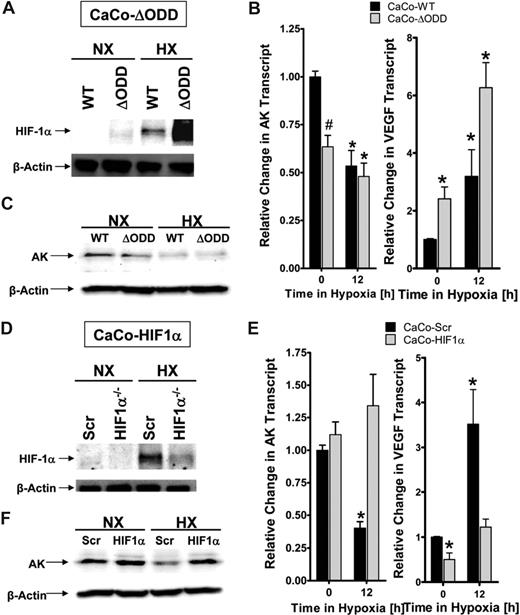
In an attempt to gain specific insight into the mechanisms of AK repression, we began examining repression pathways from hypoxia response genes. Consistent with previous studies showing HIF-1 as a critical regulator during inflammation or infection,27 we identified 4 previously unrecognized hypoxia response elements (HREs) in the AK promoter, including 2 HREs oriented on the sense strand (DNA consensus motif 5′-ACGTG-3′ and 5′-CCGTG-3′ located at positions −227 to −223 and +159 to +163, respectively, relative to the major transcription start site [TSS]), and 2 HREs located on the antisense strand (reversed HRE, rHRE, DNA consensus motif 5′-GTGCC-3′ and 5′-GTGCC-3′ located at positions −180 to −176 and −69 to −65, respectively, relative to the TSS, Figure 3A). To confirm that HIF-1α binds to the putative promoter, we employed chromatin immunoprecipitation (ChIP). For this purpose, 2 pairs of primer sets were designed (ADK-ChIP-1 or -2, Figure 3A). As shown in Figure 3B, ChIP analysis with the primers AK-ChIP-1 revealed a prominent band with hypoxia exposure. No bands were evident in the beads-only or IgG control, thereby indicating specific binding of HIF-1α to one of the HREs included in the AK-ChIP-1 primers. No significant signal was detected in the hypoxic fractions tested with the AK-ChIP-2 primer. As positive control, a ChIP assay with the known HIF-1–dependent gene TLR2 was used.28
Hypoxia inducible factor-1α (HIF-1α) in adenosine kinase (AK) repression. (A) Graphic representation of the putative AK promoter. Four putative hypoxia response elements (HREs) were identified (2 oriented forward [HRE], 2 on the antisense strand [rHRE], transcription start site [TSS]). The graph also displays the PCR product of the chromatin immunoprecipitation (ChIP) assays (AK-ChIP-1 or AK-ChIP-2). (B) ChIP assays were employed to examine HIF-1α binding to the human AK promoter in confluent HMEC-1 monolayers after hypoxia exposure. Controls for the ChIP assay included PCR performed with whole HMEC-1 cell genomic DNA (input), antibody control, samples precipitated with protein G sepharose beads alone (NX: normoxia, HX: hypoxia, 24 hours, 2% oxygen) and a known HIF-responsive gene (TLR2). (C) Confluent monolayers of control (HMEC-WT, ■) or oxygen-stable HIF-1α expressing (HMEC-ΔODD,  ) HMEC cell lines or (D) HMECs with psiRNA repression of HIF-1α (HMEC-HIF1α,
) HMEC cell lines or (D) HMECs with psiRNA repression of HIF-1α (HMEC-HIF1α,  ) or control transfected cells (HMEC-Scr, ■) were exposed over indicated time periods to hypoxia (2% oxygen). AK or TLR2 transcript or protein levels were determined by real-time RT-PCR or Western blot analysis, respectively. Results are derived from 3 experiments (*P < .005 indicates differences between normoxia and hypoxia; #P < .001 between different cell types).
) or control transfected cells (HMEC-Scr, ■) were exposed over indicated time periods to hypoxia (2% oxygen). AK or TLR2 transcript or protein levels were determined by real-time RT-PCR or Western blot analysis, respectively. Results are derived from 3 experiments (*P < .005 indicates differences between normoxia and hypoxia; #P < .001 between different cell types).
Hypoxia inducible factor-1α (HIF-1α) in adenosine kinase (AK) repression. (A) Graphic representation of the putative AK promoter. Four putative hypoxia response elements (HREs) were identified (2 oriented forward [HRE], 2 on the antisense strand [rHRE], transcription start site [TSS]). The graph also displays the PCR product of the chromatin immunoprecipitation (ChIP) assays (AK-ChIP-1 or AK-ChIP-2). (B) ChIP assays were employed to examine HIF-1α binding to the human AK promoter in confluent HMEC-1 monolayers after hypoxia exposure. Controls for the ChIP assay included PCR performed with whole HMEC-1 cell genomic DNA (input), antibody control, samples precipitated with protein G sepharose beads alone (NX: normoxia, HX: hypoxia, 24 hours, 2% oxygen) and a known HIF-responsive gene (TLR2). (C) Confluent monolayers of control (HMEC-WT, ■) or oxygen-stable HIF-1α expressing (HMEC-ΔODD,  ) HMEC cell lines or (D) HMECs with psiRNA repression of HIF-1α (HMEC-HIF1α,
) HMEC cell lines or (D) HMECs with psiRNA repression of HIF-1α (HMEC-HIF1α,  ) or control transfected cells (HMEC-Scr, ■) were exposed over indicated time periods to hypoxia (2% oxygen). AK or TLR2 transcript or protein levels were determined by real-time RT-PCR or Western blot analysis, respectively. Results are derived from 3 experiments (*P < .005 indicates differences between normoxia and hypoxia; #P < .001 between different cell types).
) or control transfected cells (HMEC-Scr, ■) were exposed over indicated time periods to hypoxia (2% oxygen). AK or TLR2 transcript or protein levels were determined by real-time RT-PCR or Western blot analysis, respectively. Results are derived from 3 experiments (*P < .005 indicates differences between normoxia and hypoxia; #P < .001 between different cell types).
Prompted by this finding, we next pursued HIF-1α loss- and gain-of-function studies in AK repression by hypoxia. As a first step, we used a previously described HMEC-1 cell line expressing oxygen-stable HIF-1α (HMEC-ΔODD, Figure 3C).22 AK transcript levels and protein were repressed in this cell line compared with controls (Figure 3C; TLR2 served as positive control). In contrast, hypoxia repression of AK transcript and protein were abolished using a previously characterized endothelial cell line (HMEC-1) with stable psiRNA repression of HIF-1a (Figure 3D, TLR2 served as positive control). To extend these findings to epithelia, we used a CaCo-2 cell line expressing oxygen-stable HIF-1α (CaCo-ΔODD) via lentiviral transduction (Figure 4A). Similar to our studies in HMEC-1, overexpression of HIF-1α in intestinal epithelia was associated with repression of AK transcript and protein under normoxic conditions (Figure 4B,C, VEGF served as positive control). In addition, we generated an intestinal epithelial cell line (CaCo-2) with psiRNA repression of HIF-1α. As shown in Figure 4D, Western blot analysis of HIF-1α confirmed repression of HIF-1α during normoxia or hypoxia. Using this cell line to study AK transcript and protein, we found abo-lished AK regulation by hypoxia (Figure 4E,F, VEGF served as positive control).
HIF-1α in epithelial adenosine kinase (AK) repression. (A) Characterization of CaCo-2 intestinal epithelia expressing oxygen-stable HIF-1α (CaCo-ΔODD) by Western blot analysis. (B) Confluent monolayers of control (CaCo-WT, ■) or oxygen-stable HIF-1α expressing (CaCo-ΔODD,  ) cell lines were exposed over indicated time periods to hypoxia (2% oxygen). Total RNA was isolated and AK or VEGF transcript was determined by real-time RT-PCR. Results are derived from 3 experiments (*P < .05 indicates differences between normoxia and hypoxia; #P < .05 between different cell types). (C) Western blot analysis of AK protein during similar conditions. (D) Characterization of CaCo-2 intestinal epithelia with psiRNA repression of HIF-1α (HIF1α−/−) or control transfected cells (Scr) by Western blot analysis. (E) Confluent monolayers of control (CaCo-Scr, ■) or HIF-1α–silenced CaCo-2 cells (CaCo-HIF1α) were exposed over indicated time periods to hypoxia (2% oxygen) and AK or VEGF transcript was determined by real-time RT-PCR (*P < .05 indicates differences between normoxia and hypoxia). (F) Western blot analysis of AK protein during similar conditions.
) cell lines were exposed over indicated time periods to hypoxia (2% oxygen). Total RNA was isolated and AK or VEGF transcript was determined by real-time RT-PCR. Results are derived from 3 experiments (*P < .05 indicates differences between normoxia and hypoxia; #P < .05 between different cell types). (C) Western blot analysis of AK protein during similar conditions. (D) Characterization of CaCo-2 intestinal epithelia with psiRNA repression of HIF-1α (HIF1α−/−) or control transfected cells (Scr) by Western blot analysis. (E) Confluent monolayers of control (CaCo-Scr, ■) or HIF-1α–silenced CaCo-2 cells (CaCo-HIF1α) were exposed over indicated time periods to hypoxia (2% oxygen) and AK or VEGF transcript was determined by real-time RT-PCR (*P < .05 indicates differences between normoxia and hypoxia). (F) Western blot analysis of AK protein during similar conditions.
HIF-1α in epithelial adenosine kinase (AK) repression. (A) Characterization of CaCo-2 intestinal epithelia expressing oxygen-stable HIF-1α (CaCo-ΔODD) by Western blot analysis. (B) Confluent monolayers of control (CaCo-WT, ■) or oxygen-stable HIF-1α expressing (CaCo-ΔODD,  ) cell lines were exposed over indicated time periods to hypoxia (2% oxygen). Total RNA was isolated and AK or VEGF transcript was determined by real-time RT-PCR. Results are derived from 3 experiments (*P < .05 indicates differences between normoxia and hypoxia; #P < .05 between different cell types). (C) Western blot analysis of AK protein during similar conditions. (D) Characterization of CaCo-2 intestinal epithelia with psiRNA repression of HIF-1α (HIF1α−/−) or control transfected cells (Scr) by Western blot analysis. (E) Confluent monolayers of control (CaCo-Scr, ■) or HIF-1α–silenced CaCo-2 cells (CaCo-HIF1α) were exposed over indicated time periods to hypoxia (2% oxygen) and AK or VEGF transcript was determined by real-time RT-PCR (*P < .05 indicates differences between normoxia and hypoxia). (F) Western blot analysis of AK protein during similar conditions.
) cell lines were exposed over indicated time periods to hypoxia (2% oxygen). Total RNA was isolated and AK or VEGF transcript was determined by real-time RT-PCR. Results are derived from 3 experiments (*P < .05 indicates differences between normoxia and hypoxia; #P < .05 between different cell types). (C) Western blot analysis of AK protein during similar conditions. (D) Characterization of CaCo-2 intestinal epithelia with psiRNA repression of HIF-1α (HIF1α−/−) or control transfected cells (Scr) by Western blot analysis. (E) Confluent monolayers of control (CaCo-Scr, ■) or HIF-1α–silenced CaCo-2 cells (CaCo-HIF1α) were exposed over indicated time periods to hypoxia (2% oxygen) and AK or VEGF transcript was determined by real-time RT-PCR (*P < .05 indicates differences between normoxia and hypoxia). (F) Western blot analysis of AK protein during similar conditions.
Role of HIF in AK expression in vivo
Next, we extended these findings of HIF-1–mediated repression of AK expression into a genetic in vivo model. Here, we examined baseline expression and the influence of hypoxia on AK mRNA levels in intestinal epithelia derived from conditionally gene-targeted Hif1α mice, in which intestinal epithelia lack detectable Hif1α expression in more than 70% of cells.26 As show in Figure 5A, AK levels in mice expressing wild-type Hif1α showed a normal pattern of hypoxia-associated AK repression (23% ± 9% decrease following hypoxia for 4 hours, P < .01). Consistent with our hypothesis that HIF-1 transcriptionally represses AK, real-time PCR analysis revealed a 8.2 (± 0.74)-fold increase in intestinal epithelial AK expression in Hif1α mutant animals (Figure 5A), relative to their littermate controls (P < .01). Exposure of HIF1α mutants to hypoxia decreased AK expression (P < .05), but to a far lesser extent than in wild-type animals. These latter findings likely reflect the remaining approximately 30% wild-type Hif1α expression in these mice.26 This difference was reflected only in the Fabpl4xat-132/Cre conditionally deleted tissue (colon; Figure 5A).26 In contrast, other organs demonstrated a wild-type phenotype with regard to AK repression in hypoxia (as shown for the lungs, Figure 5B). Taken together, such findings support our in vitro findings and indicate the likelihood that HIF-1 directly regulates murine AK expression.
HIF-1α in adenosine kinase (AK) repression in vivo. AK-mRNA levels were examined in mucosal scrapings from the colon (A) or lung tissue (B) derived from conditional Hif-1α–deficient mice targeted toward the intestinal epithelium (Hif-1α−/−,  , wild-type controls [WT], ■) subjected to either normoxia or normobaric hypoxia (8% oxygen) for 4 hours. *P < .01 indicates difference between normoxia and hypoxia; #P < .001 indicates difference between WT and Hif-1α−/−. Results are derived from 5 animals per group.
, wild-type controls [WT], ■) subjected to either normoxia or normobaric hypoxia (8% oxygen) for 4 hours. *P < .01 indicates difference between normoxia and hypoxia; #P < .001 indicates difference between WT and Hif-1α−/−. Results are derived from 5 animals per group.
HIF-1α in adenosine kinase (AK) repression in vivo. AK-mRNA levels were examined in mucosal scrapings from the colon (A) or lung tissue (B) derived from conditional Hif-1α–deficient mice targeted toward the intestinal epithelium (Hif-1α−/−,  , wild-type controls [WT], ■) subjected to either normoxia or normobaric hypoxia (8% oxygen) for 4 hours. *P < .01 indicates difference between normoxia and hypoxia; #P < .001 indicates difference between WT and Hif-1α−/−. Results are derived from 5 animals per group.
, wild-type controls [WT], ■) subjected to either normoxia or normobaric hypoxia (8% oxygen) for 4 hours. *P < .01 indicates difference between normoxia and hypoxia; #P < .001 indicates difference between WT and Hif-1α−/−. Results are derived from 5 animals per group.
AK in modulating endothelial adenosine concentration and uptake in vitro
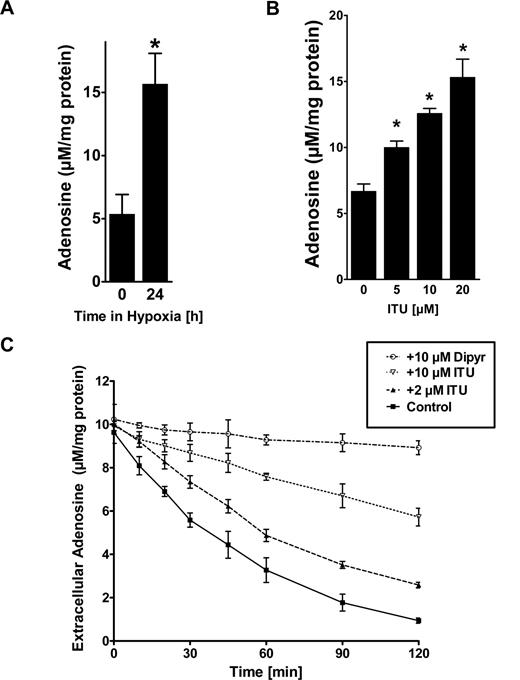
To study consequences of AK repression by hypoxia on endothelial adenosine, we first measured intracellular levels of adenosine during normoxia or hypoxia via HPLC. As shown in Figure 6A, hypoxia exposure (24 hours, 2% oxygen) was associated with an approximately 3-fold increase of intracellular adenosine as measured from lysates of HMEC-1s (Figure 6A, P < .05). The addition of ENT inhibitor dipyridamole to the experimental conditions was not associated with an additional change of intracellular adenosine concentrations, suggesting that flux through ENTs is minimal under these experimental conditions (data not shown). Similarly, inhibition of AK with ITU was associated with a dose-dependent increase in intracellular adenosine (Figure 6B, P < .01). As intracellular adenosine levels may affect endothelial adenosine uptake through diffusion limited ENTs, we next examined the capacity of HMEC-1s to transport extracellular adenosine by adding 50 μM adenosine to the supernatant. In fact, adenosine dissipation from the supernatant was significantly attenuated by inhibition of AK with ITU (Figure 6C, P < .05). Control experiments performed in the presence of the ENT-inhibitor dipyridamole (20 μM) revealed significant attenuation of Ado uptake (Figure 6C, P < .01). Taken together, these studies show endothelial AK in modulating intracellular adenosine concentrations and adenosine transport.
Adenosine kinase (AK) in modulating intracellular adenosine concentration and adenosine uptake. (A) Confluent HMEC-1s were exposed to normoxia or hypoxia (2% oxygen) for 24 hours, and intracellular levels of adenosine were determined by HPLC (*P < .05). (B) Confluent HMEC-1s were treated with indicated concentrations of the AK inhibitor 5′-iodotubericidin (ITU) over 30 minutes, and intracellular levels of adenosine were determined by HPLC (*P < .01). (D) HMEC-1 cells were treated with indicated concentrations of ITU and Ado transport was quantified. Control experiments were performed in the presence of dipyridamole (20 μM). Measurements were performed in triplicate (mean ± SD). Shown is 1 of 3 representative experiments.
Adenosine kinase (AK) in modulating intracellular adenosine concentration and adenosine uptake. (A) Confluent HMEC-1s were exposed to normoxia or hypoxia (2% oxygen) for 24 hours, and intracellular levels of adenosine were determined by HPLC (*P < .05). (B) Confluent HMEC-1s were treated with indicated concentrations of the AK inhibitor 5′-iodotubericidin (ITU) over 30 minutes, and intracellular levels of adenosine were determined by HPLC (*P < .01). (D) HMEC-1 cells were treated with indicated concentrations of ITU and Ado transport was quantified. Control experiments were performed in the presence of dipyridamole (20 μM). Measurements were performed in triplicate (mean ± SD). Shown is 1 of 3 representative experiments.
Role of AK in adenosine-dependent barrier function in vitro
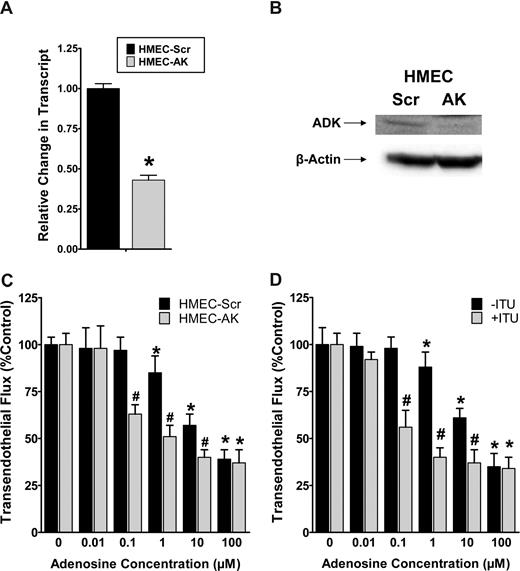
For functional studies of AK in endothelial adenosine responses, we first generated an HMEC-1 cell line stably expressing psiRNA directed against AK (HMEC-AK). In control studies, a nonspecific psiRNA was used (HMEC-Scr). These cells are characterized in Figure 7A,B by RT-PCR and Western blot analysis, confirming siRNA repression of AK. Next, we used these cell lines to study adenosine-dependent barrier responses using a previously described model of endothelial barrier function.4,17 In fact, adenosine-dependent increases in endothelial barrier function (decreased flux of 70-kDa FITC-dextran) were significantly enhanced following genetic repression of AK (Figure 7C). Similarly, inhibition of AK with 20 μM ITU was associated with significantly increased adenosine responses (Figure 7D, ANOVA P < .01). Taken together, these results suggest that adenosine-dependent barrier protection is enhanced during genetic repression or pharmacologic inhibition of AK.
Influence of adenosine kinase (AK) repression on endothelial barrier function in vitro. (A,B) Characterization of a HMEC-1 line with psiRNA repression of AK. PsiRNA-mediated silenced cells (HMEC-AK) or control transfected HMEC-1s (HMEC-Scr) were subjected to normoxia or hypoxia (12 hours). AK transcript was determined by real-time RT-PCR. Results are derived from 3 experiments. *Difference between normoxia and hypoxia (*P < .01). (B) Western blot analysis of AK protein. (C) Influence of extracellular adenosine on endothelial barrier function in HMEC-1 with psiRNA-repression of AK (HMEC-AK), or controls (HMEC-Scr). Indicated concentrations of adenosine were added to the apical surface and permeability to FITC-dextran was quantified. Data are expressed as mean plus or minus SD of percent control flux (*P < .01, significant differences between normoxia and hypo-xia; #P < .001, significant differences between HMEC-Scr and HMEC-AK). (D) Influence of AK inhibitor 5′-iodotubericidin (ITU, 20 μM) on adenosine-elicited barrier responses. (*P < .05, significant differences between normoxia and hypoxia; #P < .001, significant differences between treatment with ITU or control).
Influence of adenosine kinase (AK) repression on endothelial barrier function in vitro. (A,B) Characterization of a HMEC-1 line with psiRNA repression of AK. PsiRNA-mediated silenced cells (HMEC-AK) or control transfected HMEC-1s (HMEC-Scr) were subjected to normoxia or hypoxia (12 hours). AK transcript was determined by real-time RT-PCR. Results are derived from 3 experiments. *Difference between normoxia and hypoxia (*P < .01). (B) Western blot analysis of AK protein. (C) Influence of extracellular adenosine on endothelial barrier function in HMEC-1 with psiRNA-repression of AK (HMEC-AK), or controls (HMEC-Scr). Indicated concentrations of adenosine were added to the apical surface and permeability to FITC-dextran was quantified. Data are expressed as mean plus or minus SD of percent control flux (*P < .01, significant differences between normoxia and hypo-xia; #P < .001, significant differences between HMEC-Scr and HMEC-AK). (D) Influence of AK inhibitor 5′-iodotubericidin (ITU, 20 μM) on adenosine-elicited barrier responses. (*P < .05, significant differences between normoxia and hypoxia; #P < .001, significant differences between treatment with ITU or control).
Inhibition of AK during hypoxia attenuates vascular leak in vivo
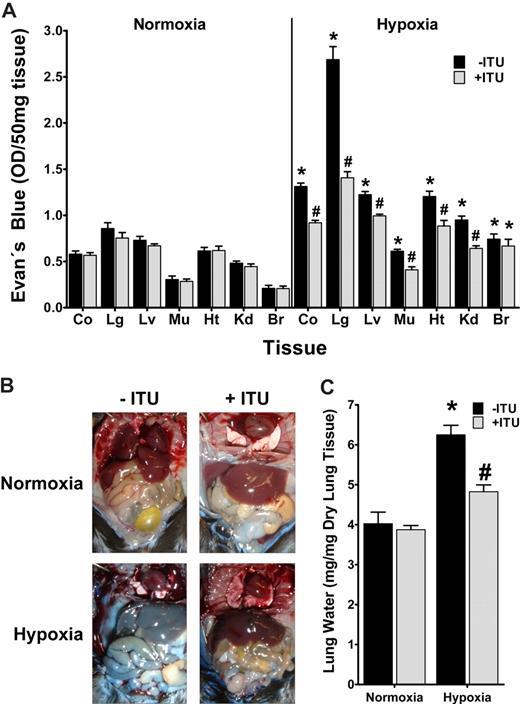
Prompted by these in vitro findings, we next pursued the role of AK in hypoxia-associated vascular leakage in vivo.19 For this purpose, mice were administered ITU intravenously (1 mg/kg) or vehicle (DMSO), a dose previously shown to effectively inhibit murine AK in vivo.13 To measure vascular barrier function, mice received the albumin marker Evan blue dye intravenously and were subjected to normoxia or nomobaric hypoxia (8% O2, 92% N2) for 4 hours. Comparison of vascular permeability in ITU or vehicle-treated mice subjected to normobaric hypoxia revealed significantly increased vascular leak in all animals, however to a smaller degree in ITU-treated animals (Figure 8A, P < .05). Such protective effect of ITU was observed in all organs except the brain, and was particularly evident in the lungs. Changes in overall vascular leakage (Evan blue extravasation) between ITU- or vehicle-treated mice were also evident in open abdominal images taken at necropsy (Figure 8B). In addition, we assessed pulmonary edema by determining lung water content (wet-dry ratio). As shown in Figure 8C, hypoxia-associated increases in lung water were attenuated following AK inhibition with ITU. These in vivo results suggest that inhibition of AK contributes to the in vivo resuscitation of vascular leak syndrome associated with hypoxia.
Influence of adenosine kinase (AK) inhibitor ITU on hypoxia elicited vascular leak in vivo. (A) C57BL/6 mice were injected with 5′-iodotubericidin (ITU, 1 mg/kg administered intravenously,  ) or vehicle control (DMSO, ■). Vascular leak was assessed by intravenous Evan blue. After exposure to normobaric hypoxia (8% oxygen) or room temperature air for 4 hours, animals were killed and Evan blue concentrations were determined in colon (Co), lung (Lg), liver (Lv), muscle (Mu), heart (Ht), kidney (Kd), and brain (Br). Data are expressed as mean plus or minus SD Evan blue OD/50 mg wet tissue and are pooled from 4 to 6 animals per condition (*P < .05, difference between normoxia and hypoxia; #P < .001, difference between ITU or vehicle). (B) Images of abdominal dissections taken at necropsy. Note the attenuated Evan blue leakage in hypoxic mice treated with ITU. The pictures were taken using a Canon digital IXUS 850 IS camera. (C) Measurement of lung water content in mice subjected to normoxia or hypoxia, following ITU treatment (
) or vehicle control (DMSO, ■). Vascular leak was assessed by intravenous Evan blue. After exposure to normobaric hypoxia (8% oxygen) or room temperature air for 4 hours, animals were killed and Evan blue concentrations were determined in colon (Co), lung (Lg), liver (Lv), muscle (Mu), heart (Ht), kidney (Kd), and brain (Br). Data are expressed as mean plus or minus SD Evan blue OD/50 mg wet tissue and are pooled from 4 to 6 animals per condition (*P < .05, difference between normoxia and hypoxia; #P < .001, difference between ITU or vehicle). (B) Images of abdominal dissections taken at necropsy. Note the attenuated Evan blue leakage in hypoxic mice treated with ITU. The pictures were taken using a Canon digital IXUS 850 IS camera. (C) Measurement of lung water content in mice subjected to normoxia or hypoxia, following ITU treatment ( ) or vehicle control (DMSO, ■). Data are expressed as mean plus or minus SD mgH2O/mg dry tissue and are pooled from 6 animals per condition (*P < .001 between normoxia and hypoxia, #P < .001 between treatment with ITU or control).
) or vehicle control (DMSO, ■). Data are expressed as mean plus or minus SD mgH2O/mg dry tissue and are pooled from 6 animals per condition (*P < .001 between normoxia and hypoxia, #P < .001 between treatment with ITU or control).
Influence of adenosine kinase (AK) inhibitor ITU on hypoxia elicited vascular leak in vivo. (A) C57BL/6 mice were injected with 5′-iodotubericidin (ITU, 1 mg/kg administered intravenously,  ) or vehicle control (DMSO, ■). Vascular leak was assessed by intravenous Evan blue. After exposure to normobaric hypoxia (8% oxygen) or room temperature air for 4 hours, animals were killed and Evan blue concentrations were determined in colon (Co), lung (Lg), liver (Lv), muscle (Mu), heart (Ht), kidney (Kd), and brain (Br). Data are expressed as mean plus or minus SD Evan blue OD/50 mg wet tissue and are pooled from 4 to 6 animals per condition (*P < .05, difference between normoxia and hypoxia; #P < .001, difference between ITU or vehicle). (B) Images of abdominal dissections taken at necropsy. Note the attenuated Evan blue leakage in hypoxic mice treated with ITU. The pictures were taken using a Canon digital IXUS 850 IS camera. (C) Measurement of lung water content in mice subjected to normoxia or hypoxia, following ITU treatment (
) or vehicle control (DMSO, ■). Vascular leak was assessed by intravenous Evan blue. After exposure to normobaric hypoxia (8% oxygen) or room temperature air for 4 hours, animals were killed and Evan blue concentrations were determined in colon (Co), lung (Lg), liver (Lv), muscle (Mu), heart (Ht), kidney (Kd), and brain (Br). Data are expressed as mean plus or minus SD Evan blue OD/50 mg wet tissue and are pooled from 4 to 6 animals per condition (*P < .05, difference between normoxia and hypoxia; #P < .001, difference between ITU or vehicle). (B) Images of abdominal dissections taken at necropsy. Note the attenuated Evan blue leakage in hypoxic mice treated with ITU. The pictures were taken using a Canon digital IXUS 850 IS camera. (C) Measurement of lung water content in mice subjected to normoxia or hypoxia, following ITU treatment ( ) or vehicle control (DMSO, ■). Data are expressed as mean plus or minus SD mgH2O/mg dry tissue and are pooled from 6 animals per condition (*P < .001 between normoxia and hypoxia, #P < .001 between treatment with ITU or control).
) or vehicle control (DMSO, ■). Data are expressed as mean plus or minus SD mgH2O/mg dry tissue and are pooled from 6 animals per condition (*P < .001 between normoxia and hypoxia, #P < .001 between treatment with ITU or control).
Discussion
In the present studies, we explored the contribution of AK to vascular adenosine signaling. As part of innate adaptation to hypoxia, we found transcriptional repression of AK by hypoxia. In addition, this response is mechanistically determined, at least in part, by HIF-1. Moreover, the present studies suggest inhibition of AK as a pharmacologic strategy to increase endothelial adenosine signaling and attenuate hypoxia-associated vascular leak.
These findings are consistent with previous work showing that hypoxia inhibits intracellular metabolism of adenosine to AMP. Decking et al12 showed, by measuring adenosine and AMP concentrations in the coronary sinus and coronary arteries of isolated guinea pig hearts, that hypoxia is associated with a functional inhibition of AK. The authors propose that hypoxia-associated inhibition of AK leads to site-specific increases of intracellular adenosine levels. As adenosine flux during ischemia or hypoxia is predominantly directed from the extracellular to the intracellular space,8,24,29,30 4 cellular strategies may contribute to increasing extracellular adenosine signaling during hypoxia. First, adenosine production from precursor nucleotides is increased during hypoxia (eg, by HIF-1–dependent induction of CD73).4,20 Second, hypoxia is associated with transcriptional induction of the A2BAR, thereby enhancing vascular adenosine signaling effects.4,22 Moreover, vascular adenosine uptake is attenuated due to transcriptional repression by ENTs.10,17 Finally, the present study reveals HIF-1–dependent repression of AK as additional mechanism to increase vascular adenosine signaling. Interestingly, all 4 mechanisms are transcriptionally regulated by HIF-1.17,20,22 In fact, this highlights the role of oxygen-sensing signaling pathways in transcriptional coordination of vascular responses during inflammation31 or hypoxia.32
Similar to the present studies showing AK in vascular adaptation, a previous study found AK inhibition in cardioprotection.13 In fact, this very elegant study used a rat model of myocardial infarction and found that infarct sizes were reduced from 58.0% to 37.5% of the area at risk following AK inhibition with ITU. Moreover, the protective effects of AK were abolished with inhibitors of extracellular adenosine signaling, suggesting that the reduction of infarct sizes with AK inhibition is mediated by extracellular adenosine signaling.13
It was recently appreciated that HIF can function as both a transcriptional activator and repressor.33 Therefore, a search of the putative AK gene promoter identified 4 potential HIF-1 binding DNA consensus motifs. However, the existence of a HIF-1α binding consensus is not evidence for HIF-1α–mediated response. Therefore, 2 strategies were used to define the role of HIF-1 in repression of AK. First, in vitro and in vivo studies of HIF-1 loss and gain of function suggested a functional role of HIF-1α in endothelial or epithelial AK repression by hypoxia. Second, ChIP analysis confirmed direct binding of HIF-1α to the putative AK promoter. At present, we do not know if this binding is direct or indirect, and we do not know the nature of HIF-mediated transcription. While previous work with peroxisome proliferators–activated receptor alpha (PPAR-α) or the ENT1 gene implicated potential repressor activity with HREs oriented on the antisense strand,17,34 implying some degree of transcriptional directionality, there is no direct evidence for such a mechanism. Moreover, while a recent study comparing transcriptional responses between hypoxia and constitutively active HIF1–1α identified a large cohort of transcriptionally repressed genes,33 no unique patterns of HRE expression were noted. Thus, more work will be necessary to define the nature of HIF-mediated repression.
In conclusion, the present studies indicate that as part of a vascular phenotype with increased extracellular nucleotide levels and enhanced adenosine signaling, hypoxia promotes transcriptional repression of AK. In fact, genetic studies, transcription factor binding assays, and HIF-1α loss- and gain-of-function studies point toward a role of HIF-1α in coordinating this response. In addition, in vivo inhibition of AK was associated with attenuated vascular leakage in mice exposed to ambient hypoxia. Extensions of these findings will determine whether AK regulation via HIF-1 might function as a pathway for development of therapies for disorders involving vascular leak syndromes or excessive inflammatory responses.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to thank Marion Faigle, Stephanie Zug, Edgar Hoffmann, and Stephanie Laucher for technical assistance, and Karen Westerman and Sean Colgan for kindly providing us with the CaCo-ΔODD cell line.
This work was supported by a Tübingen University Grant (IZKF Verbundprojekt) to J.C.M. and H.K.E., and grants from the German Research Foundation (DFG, no. EL274/2-2) and the Foundation for Anesthesia Research and Education (FAER) to H.K.E.
Authorship
Contribution: J.C.M.-G. designed research, analyzed data, and helped with manuscript writing; P.R. performed histologic studies and in vivo studies; J.K. designed research and helped with manuscript writing; and H.K.E. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no compet-ing financial interests.
Correspondence: Holger K. Eltzschig, Mucosal Inflammation Program, Department of Anesthesiology and Perioperative Medicine, Biochemistry Research Building (BRB), Room 852, 4200 E 9th Ave, Mailstop B112, Denver, CO 80262; e-mail: holger.eltzschig@uchsc.edu.

![Figure 3. Hypoxia inducible factor-1α (HIF-1α) in adenosine kinase (AK) repression. (A) Graphic representation of the putative AK promoter. Four putative hypoxia response elements (HREs) were identified (2 oriented forward [HRE], 2 on the antisense strand [rHRE], transcription start site [TSS]). The graph also displays the PCR product of the chromatin immunoprecipitation (ChIP) assays (AK-ChIP-1 or AK-ChIP-2). (B) ChIP assays were employed to examine HIF-1α binding to the human AK promoter in confluent HMEC-1 monolayers after hypoxia exposure. Controls for the ChIP assay included PCR performed with whole HMEC-1 cell genomic DNA (input), antibody control, samples precipitated with protein G sepharose beads alone (NX: normoxia, HX: hypoxia, 24 hours, 2% oxygen) and a known HIF-responsive gene (TLR2). (C) Confluent monolayers of control (HMEC-WT, ■) or oxygen-stable HIF-1α expressing (HMEC-ΔODD, ) HMEC cell lines or (D) HMECs with psiRNA repression of HIF-1α (HMEC-HIF1α, ) or control transfected cells (HMEC-Scr, ■) were exposed over indicated time periods to hypoxia (2% oxygen). AK or TLR2 transcript or protein levels were determined by real-time RT-PCR or Western blot analysis, respectively. Results are derived from 3 experiments (*P < .005 indicates differences between normoxia and hypoxia; #P < .001 between different cell types).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/12/10.1182_blood-2007-11-126763/6/m_zh80100819050003.jpeg?Expires=1767725235&Signature=uHfDf4VZdnz9XcDA3YQ21CVEpqxjes5Urr55CPyni-5vRVnjm1Eufv0VBo-hFH3NoY8f0nR-s4e3yHPmNSi0ofrqmu8JtSz07GWsRJVgz7FEF9E~l1VeazhZMJA3DKfULMO-QbKYIzKowpFDWuoTP~3jSLGcZyuS5Cm009kLl3XUyJECVEQYGTZ2DX6rEsUOW-H-IA~IQBugxWbJiOm20lR4jokI59sBtKYhayqLfwEXIcwQMM0XGcq6mhrot2hD5tmu22yGLup-a-30qhHWcSbam6ESGGZgY1tdG-9qhj9R2z3iIPxHEvk~17bTWmEt6un1oijCpxCQDtuwS7lCtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. HIF-1α in adenosine kinase (AK) repression in vivo. AK-mRNA levels were examined in mucosal scrapings from the colon (A) or lung tissue (B) derived from conditional Hif-1α–deficient mice targeted toward the intestinal epithelium (Hif-1α−/−, , wild-type controls [WT], ■) subjected to either normoxia or normobaric hypoxia (8% oxygen) for 4 hours. *P < .01 indicates difference between normoxia and hypoxia; #P < .001 indicates difference between WT and Hif-1α−/−. Results are derived from 5 animals per group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/12/10.1182_blood-2007-11-126763/6/m_zh80100819050005.jpeg?Expires=1767725235&Signature=UV5LxH4flGNxw7SpMXZfFqS1UdiVIFHDxFvf5jZmMfwf-ihZlxXm2w7YtA1bx1DCI-kcUkYEvaDRR3iDLorziQaAgbVmyWFwymF6tqi-NZbNEKJZ72ft-N-EFb0z6hRTSLjBDqUEG3erYBesuYUe8vSMAcQHOjmsFN0cRIR0CAvAWPwD3i1iNTe~k6PJesBzDXnb42F3317yS9U2VLR3ptpo43s6td31IaEC3Nq3vJ0gWai6x1MN-hpPJr3~o1PECnD9BQjDInTrjZAmaN1Vynrh--q3GwVtyrL6x616xZ80seoENR11rni8S~A~SvowHQ-SXdCRvPhn7wkaUKnehQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal