Abstract
The tyrosine kinase inhibitors sorafenib and sunitinib are approved for the treatment of patients with malignant diseases. To analyze the possible use of these compounds in combination with immunotherapeutic approaches, we analyzed the effects of both inhibitors on the immunostimulatory capacity of human dendritic cells (DCs) and the induction of primary immune responses in vivo. Sorafenib, but not sunitinib, inhibits function of DCs, characterized by reduced secretion of cytokines and expression of CD1a, major histocompatibility complex, and costimulatory molecules in response to TLR ligands as well as by their impaired ability to migrate and stimulate T-cell responses. These inhibitory effects are mediated by inhibition of PI3 and MAP kinases and NFκB signaling. In contrast, sorafenib had no influence on the phenotype and proliferation of T cells. To analyze the effects of both TKIs on cytotoxic T-cell induction in vivo, C57BL/6 mice were pretreated with sorafenib or sunitinib and immunized with OVA257-264 peptide. Sorafenib, but not sunitinib, application significantly reduced the induction of antigen-specific T cells. Numbers of regulatory T cells were reduced in peripheral blood mononuclear cells from mice treated with sunitinib. These results indicate that sunitinib, but not sorafenib, is suitable for combination with immunotherapeutic approaches for treatment of cancer patients.
Introduction
Metastatic renal cell cancer (RCC) has a very poor prognosis with a median survival of only 6 to 12 months from the time of diagnosis.1,2
Historically, there were no established effective treatment approaches for metastatic RCC because of its resistance to radiation and chemotherapy.3 Until recently, cytokine-based immunotherapy using interferon-α (IFN-α) and/or interleukin-2 (IL-2) was the only effective treatment resulting in response rates of 10% to 20%.4 The understanding of RCC pathogenesis and identification of molecular mechanisms responsible for the malignant transformation and metastatic spread led to the development of drugs that target cancer-specific pathways, such as the PI3K/AKT and Ras/Raf/MAPK pathways.5 RCC is often associated with up-regulated Raf1, EGFR, and VEGFR activity.5,6 Furthermore, in a high proportion of RCC, mutational aberrations of the von Hippel-Lindau (VHL) gene were identified. The loss-of-function of this tumor suppressor gene results in an accumulation of hypoxia inducible factor (HIF)-α subunits and stimulation of angiogenesis via VEGF- and PDGF-receptors.
Consequently, 2 novel tyrosine kinase inhibitors, sorafenib (Bayer HealthCare, Leverkusen, Germany)5 and sunitinib (Pfizer, New York, NY), were introduced in the treatment of RCC patients.
Sorafenib is a multikinase inhibitor initially developed to inhibit the Raf1-kinase pathway.2 However, besides the RAF/MEK/ERK pathway, sorafenib targets receptor tyrosine kinases (RTKs), such as VEGFR-2 and -3, PDGFR-β, Flt-3, and c-KIT.2,7 In several clinical and preclinical trials, sorafenib was revealed to be a promising anticancer therapeutic, which negatively regulates tumor growth, cell proliferation, and angiogenesis8,9 and additionally induces apoptosis in tumor cells.10 In December 2005, sorafenib was approved by the FDA for treatment of patients with advanced/metastatic RCC. In a randomized trial, sorafenib doubled the median duration of progression-free survival up to 24 weeks in patients refractory to or relapsed during cytokine treatment.2,11,12
Sunitinib inhibits multiple split kinase domain RTKs, including VEGFR-1 and -2, PDGFR-α and -β, c-KIT-receptor, and Flt-3.2,7,13 In 2 phase 2 studies, application of sunitinib resulted in response rates up to 40% and in a randomized phase 3 trial it showed an improved response rate and progression-free survival in comparison to IFN-α.12,14,15
However, until now the effects of sorafenib and sunitinib on development and function of normal nonmalignant hematopoetic cells have not been evaluated in detail. It is known that PBLs isolated from patients receiving clinically relevant doses of sorafenib show inhibition of ERK phosphorylation on ex vivo PMA stimulation.16 We therefore analyzed the immunomodulatory functions of these compounds using T cells and monocyte-derived dendritic cells (MDDCs), which were activated with ligands for TLR3 or 4. We found that sorafenib, but not sunitinib, has a detrimental effect on DC phenotype and inhibits cytokine secretion, migration ability, and T-cell stimulatory capacity, whereas the function and phenotype of T cells were not affected. In addition, vaccination of mice treated with sorafenib resulted in a severe, but reversible, inhibition of CD8+ T cell–mediated immune responses. In contrast, mice treated with subtoxic doses of sunitinib did not show an impaired CD8+ T-cell response, but a significant decrease in peripheral regulatory T cell (Treg) numbers was observed.
Methods
Approval was obtained from the institutional Ethics Committee of the University of Tübingen for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Media and reagents
Cells were cultured in RPMI 1640 with glutamax-I, supplemented with 10% inactivated fetal calf serum (RP10 medium) and the antibiotics penicillin/streptomycin (Invitrogen, Karlsruhe, Germany). Sorafenib tosylate and sunitinib malate were obtained from Eurasia Chemicals PVT (Mumbai, India). Identity and purity of these compounds were extensively analyzed by reverse phase high performance liquid chromatography, mass spectrometry, elemental analysis, and 1H-NMR. Granulocyte-macrophage colony-stimulating factor (GM-CSF, Leukine, Liquid Sargramostim) and human recombinant IL-4 both were purchased from R&D Systems (Wiesbaden, Germany). Escherichia coli lipopolysaccharide (LPS) was obtained from Sigma-Aldrich (St Louis, MO). Thioate-stabilized CpG-motive containing oligodeoxynucleotide 1668 (ODN-CpG; TCCATGACGTTCCTGATGCT) was purchased from TibMolbiol (Berlin, Germany). H2-Kb-restricted peptides SIINFEKL derived from chicken albumin (OVA257-264) and RGYVYQGL derived from vesicular stomatitis virus nucleoprotein (VSV NP52-59) for immunization experiments were synthesized and quality controlled by Immatics Biotechnologies' in-house peptide facility (Tübingen, Germany).
Generation of DCs
Dendritic cells were generated from peripheral blood adhering monocytes by plastic adherence as described previously.17-19 Adherent monocytes were cultured in RP10 medium supplemented with GM-CSF (100 ng/mL, Leucomax; Novartis, Basel, Switzerland) and IL-4 (20 ng/mL; R&D Systems). Cytokines were added to differentiate DCs every 2 to 3 days.
Sorafenib and sunitinib were dissolved in dimethyl sulfoxide and added to the culture media at day 5 in concentrations varying from 0.5 to 5 μg/mL for sorafenib and 50 to 200 ng/mL for sunitinib corresponding to serum levels achieved in treated patients. In each case, equal amounts of dimethyl sulfoxide were added as a control to exclude effects induced by the solvent. For most experiments, stimulation with TLR4L (LPS, 100 ng/mL; Sigma-Aldrich) was done at day 6 with the exception of Western blot analyses for which stimulation with LPS was performed at different time points before harvesting. In addition, TLR3L (polyriboinosinic:polyribocytidylic acid (poly I:C), 50 μg/mL; Sigma-Aldrich) was used to stimulate DCs. At day 7, cells were harvested for further experiments.
Immunostaining
Cells were stained using fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated mouse mAbs against CD14, CD80, CD86, HLA-DR (BD Biosciences, Heidelberg, Germany); CD1a (Dako Diagnostika, Hamburg, Germany); CD83 (Immunotech, Marseille, France); CCR7, DC-SIGN (R&D Systems) and mouse IgG isotype controls (BD Biosciences).
Mouse lymphocytes were stained with fluorescently labeled monoclonal antibodies against CD3ϵ, CD8a, CD4, CD25, CD45R/B220, CD19, and appropriate isotypic controls (all BD Biosciences). CD4+ CD25+ FoxP3+ mouse regulatory T cells were stained with the corresponding staining kit from eBiosciences (San Diego, CA) according to manufacturer's instructions. H2-Kb/peptide tetramers for analysis of specific CD8+ T cells were produced and used for staining as described previously.20,21
Flow cytometry was performed on a FACSCalibur or BD LSR II SORP cytometer (both BD Biosciences).
Migration assay
A total of 105 cells were seeded into a transwell chamber (8 μm; BD Falcon, Heidelberg, Germany) in a 24-well plate, and migration to CCL19/MIP-3β was analyzed after 4 hours by counting gated DCs for 1 minute in a FACSCalibur cytometer.22
Analysis of endocytotic capacity
Analysis of endocytotic activity was done as described previously.17,22 In brief, 105 cells were incubated with 5 μl FITC-dextran (Invitrogen) for 1 hour at 37°C or alternatively at 4°C. As a negative control, cells were incubated at room temperature without addition of FITC-dextran. FITC-dextran uptake was analyzed by flow cytometry.
Cytokine determination
After 7 days of DC culture supernatants were collected and stored at −80°C until use for cytokine determination. Cytokine concentrations were measured with commercially available 2-site sandwich ELISAs from Beckman Coulter (Hamburg, Germany; IL-12, IL-6, IL-10, and TNF-α) according to the manufacturer's instructions.
Mixed lymphocyte reactions
Various numbers of irradiated stimulator DCs were cultured in 96-well flat-bottom microtiter plates (Nunc, Roskilde, Denmark) with a total of 105 responding cells from allogenic peripheral blood mononuclear cells (PBMCs) or CD3+ T cells. Thymidine incorporation was measured on day 5 by a 16-hour pulse with [3H]-thymidine (18.5 kBq/well; GE Healthcare, Little Chalfont, United Kingdom).22 Different sets of experiments were performed. In one set of experiments, we used stimulator DCs treated with sorafenib or sunitinib and incubated them with untreated PBMCs for 5 days. For other experiments CD3+ T cells were purified from PBMCs making use of a MACS isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the instructions of the manufacturer. CD3+ T cells were treated with sorafenib for 24 hours and were then incubated with untreated DCs in the presence or absence of sorafenib.
Induction of Her-2/neu specific cytotoxic T-cell responses in vitro
The HLA-A*0201-binding peptides derived from Her-2/neu (E75; amino acids 369-377, KIFGSLAFL) and HIV (pol HIV-1 reverse transcriptase peptide; amino acids 476-484; ILKEPVHGV; used as an irrelevant control) were synthesized using standard F-moc chemistry on a peptide synthesizer (432A; Applied Biosystems, Weiterstadt, Germany) and analyzed by reverse-phase high-performance liquid chromatography and mass spectrometry. For cytotoxic T-cell (CTL) induction, 5 × 105 DCs were pulsed with 50 μg/mL of synthetic peptide for 2 hours, washed, and incubated with 3 × 106 autologous PBMCs in RP10 medium. After 7 days of culture, cells were restimulated with autologous peptide-pulsed PBMCs; 2 μg/mL of human recombinant IL-2 (R&D Systems) were added on days 1, 3, and 5. The cytolytic activity of induced CTLs was analyzed on day 5 after the last restimulation in a standard 51Cr release assay.
Standard 51Cr-release assay
The standard 51Cr release assay was performed as described previously.19 Target cells (autologous monocyte-derived DCs pulsed with 50 μg/mL of peptide for 2 hours, A-498, renal cell carcinoma cell line, HLA-A2 positive, Her-2/neu positive; SKOV-3, ovarian cancer cell line, HLA-A2 negative, Her-2/neu positive; K-562, chronic myelogenous leukemia cell line, HLA-A2 negative) were labeled with 51Cr sodium chromate in RP10 for 1 hour. In 96-well round-bottomed culture plates, 104 target cells were transferred to each well. Various numbers of CTLs were added to give a final volume of 200 μL. After 4 hours of incubation at 37°C, supernatants (50 μL/well) were harvested, transferred to scintillator-coated plates (LumaPlate-96; PerkinElmer, Waltham, MA), and counted in a beta plate counter. The percentage of specific lysis was calculated as 100 × (experimental release − spontaneous release/maximal release − spontaneous release). Spontaneous and maximal releases were determined in the presence of either medium or 2% Triton X-100, respectively.
PAGE and Western blotting
Nuclear extracts were prepared from DCs as described previously.23,24 Whole cell lysates were prepared using lysis buffer containing 1% Igepal CA-630, 0.5% sodium-deoxycholate, 0.1% SDS, 2 mM of EDTA, 1 mM of sodium-orthovanadate, 100 mM of sodium fluoride, and one Complete mini protease cocktail inhibitor tablet (Roche Diagnostics, Mannheim, Germany) per 8 mL buffer. Protein concentrations of cell lysates were determined using a bicinchoninic acid assay (Pierce, Perbio Science, Bonn, Germany).
For the detection of nuclear proteins, approximately 20 μg of nuclear extracts was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane (Whatman Schleicher & Schuell, Dassel, Germany). Ponceau S staining of the membrane was performed to confirm that equal amounts of protein were present in every lane. The blot was probed with Abs against c-Rel (B-6, mouse monoclonal), Rel-B (C-19, rat polyclonal), IRF-3 (C-20, goat polyclonal), IRF-8 (goat polyclonal), PU.1 (rabbit polyclonal), all from Santa Cruz Biotechnology (Santa Cruz, CA), or Rel-A (rabbit polyclonal; Upstate Biotechnology, Charlottesville, VA), followed by incubation with a horseradish peroxidase-conjugated secondary antibody. Bands were visualized by ECL staining (GE Healthcare).
To analyze MAPK cascades and TLR pathways, 20 μg of whole cell lysates was separated on a 10% SDS-PAGE and blotted onto nitrocellulose membranes. These were probed with phospho-specific Abs phospho-p38 (Thr180/Tyr182, rabbit polyclonal) and phospho-p44/42 (Thr202/Tyr204, mouse monoclonal), both from New England Biolabs (Frankfurt, Germany), TRAF-6 (mouse monoclonal) and MyD88 (N-19, goat polyclonal), both from Santa Cruz Biotechnology.
As a control, Abs against p38 MAP kinase (rabbit polyclonal; Cell Signaling Technology, Beverly, MA), ERK1 (C-16, rabbit polyclonal; Santa Cruz Biotechnology), and GAPDH (mouse monoclonal; HyTest, Turku, Finland) or actin (goat polyclonal; Santa Cruz Biotechnology) were used.
Detection of apoptosis
Apoptosis was detected as described by Nicoletti et al.25 In brief, DC nuclei were stained with propidium iodide in hypotonic buffer and the percentage of apoptotic nuclei was measured by flow cytometry.
Animal keeping
Female C57BL/6 mice (20-25 g; Charles-River WiGA, Sulzfeld, Germany) were used for all immunization experiments. Animals were kept in the animal facility of the Department of Immunology at the University of Tübingen. Animals were cared for by trained animal keepers, and the health status of animals was supervised by the veterinaries of the University of Tübingen. All animals were supplied with water and food ad libitum. The described animal experiments were approved by the Regierungspräsidium Tübingen (no. IM1/06).
Drug treatment
A liquid, viscous vehicle composed of 30% (w/v) Cremophor EL, 30% (w/v) PEG 400, 10% ethanol pro analysi, 10% glucose (all Sigma-Aldrich) was used. Sunitinib and sorafenib were suspended in vehicle in feasible concentrations. The deduced dosage was applied daily by gavage into the backward cavity of the mouth of the animal without anesthesia. Negative and positive control mice were fed with vehicle only. Drug delivery into blood plasma was controlled by reverse phase HPLC after acetonitril extraction.13
Analysis of specific T-cell responses and immune cell populations
Mice were immunized subcutaneously with a water-in-oil emulsion of 50 nmol ODN-CpG, 30 μg OVA257-264, and phosphate-buffered saline in Incomplete Freund Adjuvants/Titermax (4:1; both from Sigma-Aldrich). Negative control mice were immunized with VSV NP52-59 peptide. After 7 days, immune response was boosted with peptide emulsified in Incomplete Freund's Adjuvants. One week after the second immunization, mice were bled from the retrobulbar plexus under ether anesthesia and killed without awakening by CO2. Splenocytes were stained with PE-labeled H2-Kb/ OVA257-264 tetramer, antigen-presenting cell (APC)-labeled H2-Kb/ VSV NP52-59 tetramer, CD8-FITC, and CD3e-PerCP for analysis of induced peptide-specific CD8+ T-cell responses. In addition, splenocytes and blood cells were incubated with cell type–specific fluorescently labeled antibodies for population analysis. Stained cells were analyzed by flow cytometry.
Statistical analysis
In vitro DC experiments were performed at least 3 times; representative experiments are presented. To analyze statistical significance, Student t test was used. Immunization experiments and mixed lymphocyte reactions (MLRs) were performed at least 2 times. Unpaired, heteroscedastic, 2-tailed Student t test was used to assess statistical significant differences in tetramer-positive T-cell numbers between immunization groups.
Results
Exposure of immature DCs to sorafenib inhibits their activation via TLR signaling
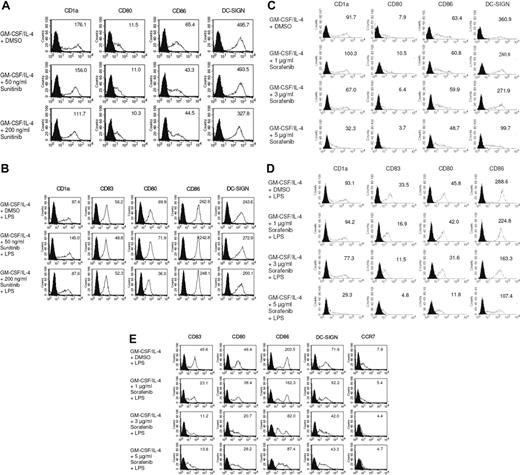
We first examined the effects of the multityrosine kinase inhibitors sorafenib and sunitinib on the phenotype of DCs generated from peripheral blood monocytes. We found that exposure to pharmacologic concentrations of sunitinib9,11,16,26 did not modify the phenotype of immature or mature MDDCs (Figure 1A,B). However, immature DCs exposed to sorafenib exhibited a pronounced down-regulation of CD1a, CD86, and the integrin DC-SIGN, a C-type lectin that mediates adhesion to T cells by stabilizing the DC/T cells' contact zone (Figure 1C).27 CD14 remained undetectable, thus ruling out dedifferentiation to monocytes (data not shown). In addition, treatment with sorafenib inhibited stimulation of DCs with LPS, a pathogen-derived signal that induces DC maturation.28,29 Namely, treatment with sorafenib prevented up-regulation of the costimulatory molecules CD80 and CD86 (Figure 1D). Levels of CD83, a surface marker expressed by mature DCs,28 and CD1a were markedly reduced (Figure 1D). In addition, the normal up-regulation of HLA class II molecules in response to LPS was reduced by sorafenib (data not shown). Even in immature DCs, sorafenib reduced levels of CD1a and CD86 (Figure 1C). Similar results were obtained when TLR3L poly I:C was used for maturation of DCs (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We next analyzed the effect of sorafenib on LPS matured DCs. To accomplish this, immature DCs were first incubated with LPS for 24 hours and treated afterward with increasing concentrations of sorafenib. As shown in Figure 1E, this compound also affected the phenotype of mature DCs.
Sorafenib lowers expression of cell surface molecules on DCs stimulated with LPS. DCs were generated by incubating adherent monocytes with GM-CSF and IL-4. On day 5 of culture, cells were exposed for 24 hours to sunitinib (A,B) or sorafenib at the indicated concentrations (C,D). LPS (100 ng/mL) was added as a stimulus to the culture media for the last 24 hours (B,D). In addition, to analyze the effects of sorafenib on the phenotype of mature DCs sorafenib was added to the culture medium after the activation of cells with LPS for 24 hours (E). The changes in phenotype of DCs by sorafenib treatment were analyzed by flow cytometry after staining with Abs against CD1a, CD80, CD86, CD83, DC-SIGN, or CCR7. Shaded histograms represent isotype control and open diagrams staining with the specific antibody. The numbers represent mean fluorescence intensity. One representative experiment of at least 3 is shown.
Sorafenib lowers expression of cell surface molecules on DCs stimulated with LPS. DCs were generated by incubating adherent monocytes with GM-CSF and IL-4. On day 5 of culture, cells were exposed for 24 hours to sunitinib (A,B) or sorafenib at the indicated concentrations (C,D). LPS (100 ng/mL) was added as a stimulus to the culture media for the last 24 hours (B,D). In addition, to analyze the effects of sorafenib on the phenotype of mature DCs sorafenib was added to the culture medium after the activation of cells with LPS for 24 hours (E). The changes in phenotype of DCs by sorafenib treatment were analyzed by flow cytometry after staining with Abs against CD1a, CD80, CD86, CD83, DC-SIGN, or CCR7. Shaded histograms represent isotype control and open diagrams staining with the specific antibody. The numbers represent mean fluorescence intensity. One representative experiment of at least 3 is shown.
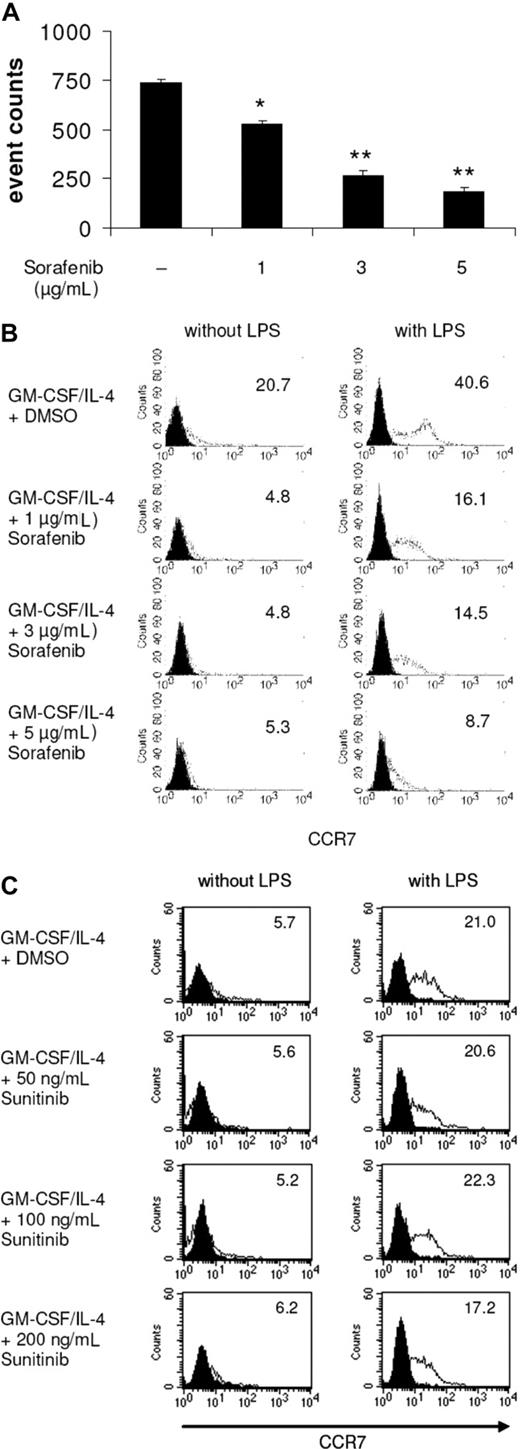
Sorafenib-treated DCs display impaired migratory capacity
Mature DCs express chemokine-CC motif receptor 7 (CCR7), the receptor for CCL19/MIP-3β, that guides DCs from peripheral tissues to the local lymph nodes across a CCL19/MIP-3β gradient.28,29 In our study, we found that sorafenib-treated and LPS-stimulated DCs showed reduced migration capacity toward MIP-3β (Figure 2A). In line with these results, FACS analyses revealed that sorafenib reduced the LPS-induced expression of CCR7 on DCs (Figure 2B). In contrast, incubation of cells with sunitinib did not affect the expression of CCR7 (Figure 2C). Comparable results for sorafenib-treated DCs were obtained using poly I:C for stimulation of DCs (Figure S2).
LPS-induced DC migration toward CCL19/MIP-3β is impaired by sorafenib. DCs were generated by incubating adherent monocytes with GM-CSF and IL-4. On day 5 of culture, cells were exposed for 24 hours to sorafenib (A,B) or sunitinib (C) at the indicated concentrations. LPS (100 ng/mL) was added as a stimulus to the culture media for the last 24 hours. (A) Migration toward CCL19 was analyzed using transwell chambers. DCs (105) were seeded in the upper chamber in triplicates, and the number of migrated DCs was analyzed after 4 hours by counting gated DCs for one minute by FACS analysis (*P < .05, **P < .001). (B,C) Surface expression of CCR7 was analyzed by staining of DCs with FITC conjugated antibodies against CCR7 and measuring by FACS analysis. Numbers represent mean fluorescence intensity.
LPS-induced DC migration toward CCL19/MIP-3β is impaired by sorafenib. DCs were generated by incubating adherent monocytes with GM-CSF and IL-4. On day 5 of culture, cells were exposed for 24 hours to sorafenib (A,B) or sunitinib (C) at the indicated concentrations. LPS (100 ng/mL) was added as a stimulus to the culture media for the last 24 hours. (A) Migration toward CCL19 was analyzed using transwell chambers. DCs (105) were seeded in the upper chamber in triplicates, and the number of migrated DCs was analyzed after 4 hours by counting gated DCs for one minute by FACS analysis (*P < .05, **P < .001). (B,C) Surface expression of CCR7 was analyzed by staining of DCs with FITC conjugated antibodies against CCR7 and measuring by FACS analysis. Numbers represent mean fluorescence intensity.
Sorafenib treatment enhances dextran uptake by DCs stimulated with TLR ligands
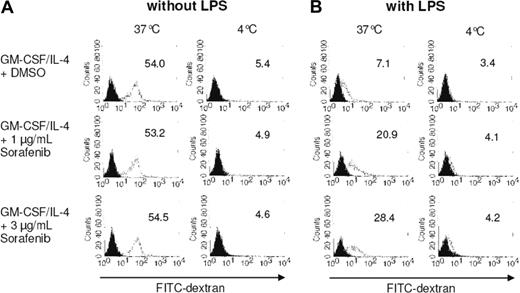
We further analyzed the effects of sorafenib on DC endocytotic capacity by monitoring FITC-dextran uptake. The internalization ability of immature DCs is higher than that of mature DCs.29 We found that immature DCs incubated at 37°C efficiently internalized FITC-dextran, which was inhibited by performing the experiments at 4°C (Figure 3A). Maturation of DCs with LPS reduced FITC-dextran uptake as expected (Figure 3B). Interestingly, although sorafenib had no significant effect on the uptake of FITC-dextran by immature DCs, it antagonized the TLR-induced inhibition of dextran endocytosis (Figure 3B). In line with the phenotype analysis, these results indicate that sorafenib is affecting maturation of DCs induced by LPS.
Treatment with sorafenib affects FITC-dextran uptake by mature DCs. Monocytes were cultured with GM-CSF and IL-4 for 7 days. On day 5 of culture, sorafenib was added at the indicated concentrations (A,B). LPS (100 ng/mL) was added as a maturation stimulus for the last 24 hours (B). A total of 105 cells were incubated with FITC-dextran for 1 hour at 37°C and 4°C. The cells were washed and analyzed by FACS analysis. Open histograms represent FITC-dextran treated cells; solid histograms cells incubated in the absence of FITC-dextran. Mean fluorescence intensity of DCs labeled with FITC-dextran is shown.
Treatment with sorafenib affects FITC-dextran uptake by mature DCs. Monocytes were cultured with GM-CSF and IL-4 for 7 days. On day 5 of culture, sorafenib was added at the indicated concentrations (A,B). LPS (100 ng/mL) was added as a maturation stimulus for the last 24 hours (B). A total of 105 cells were incubated with FITC-dextran for 1 hour at 37°C and 4°C. The cells were washed and analyzed by FACS analysis. Open histograms represent FITC-dextran treated cells; solid histograms cells incubated in the absence of FITC-dextran. Mean fluorescence intensity of DCs labeled with FITC-dextran is shown.
Pretreatment with sorafenib reduces the capacity of TLR4 ligand-activated APCs to stimulate lymphocyte proliferation and lowers DC cytokine secretion
The immunostimulatory capacity of the generated DCs was analyzed in a MLR. As shown in Figure 4A, pretreatment with sorafenib reduces the capacity of immature as well as LPS-primed DCs to stimulate allogenic lymphocyte proliferation. Interestingly, pretreatment of purified CD3+ T cells with sorafenib did not affect their proliferation (Figure 4B) or phenotype (data not shown) on stimulation with allogenic mature DCs, whereas the proliferation capacity was significantly reduced when sorafenib was added to the media during the incubation of pretreated CD3+ T cells with untreated mature DCs (Figure 4C). Pretreatment with sunitinib had no inhibitory effect on DCs to stimulate allogenic lymphocyte proliferation (Figure 4D). The finding that natural APCs are the target of inhibition by sorafenib was further confirmed by the observed lack of inhibition of either sorafenib or sunitinib on the expansion of human HLA-A*0201+ CD8+ T cells in a model system using HLA-A*0201/Melan-A(26-35, E27L peptide) and anti-CD28 loaded artificial APCs (data not shown). In these experiments addition of both TKIs to the culture medium had no effect on the generation of Melan-A specific CTLs. These results were further supported by the generation of primary CTL responses against a tumor-associated antigen using DCs pretreated with sunitinib or sorafenib. The incubation of DCs with sorafenib completely inhibited the induction of primary immune responses against Her-2/neu, whereas pulsing of sunitinib treated DCs with the HLA-A2 binding E75 peptide resulted in the induction of cytotoxic T cells, which recognized target cells pulsed with the cognate peptide or target cells constitutively expressing the antigen (Figure 4E).
Sorafenib reduces lymphocyte stimulation capacity of DCs. DCs were irradiated with 30 Gy and were incubated for 5 days with 105 allogenic PBMCs or CD3+ T cells. Thymidine incorporation was measured by a 16-hour pulse with [3H]thymidine. PBMCs or T cells without DCs were included as a control. (A) DCs were pretreated with sorafenib 48 hours before harvesting and immature as well as LPS-stimulated DCs (103 DCs per well) were incubated with untreated PBMCs. (B) Untreated mature DCs (103 DCs per well) were incubated with MACS isolated CD3+ T cells pretreated with different concentrations of sorafenib as indicated in the figure. (C) Untreated mature DCs (103 DCs per well) were incubated with MACS isolated CD3+ T cells pretreated with different concentrations of sorafenib as indicated in the figure. Sorafenib was added to the cell culture medium in the same concentrations during MLR. (D) DCs were pretreated with sunitinib, stimulated with LPS, and incubated with untreated PBMCs (*P < .05, ** P < .001). (E) Induction of Her-2/neu specific primary CTL responses was analyzed using LPS activated DCs pretreated with sorafenib or sunitinib and pulsed with an HLA-A2 binding Her-2/neu-derived E75 peptide. DCs pulsed with the E75 peptide as well as the HLA-A2-positive and Her-2/neu-expressing cell line A-498 were used as targets. The HLA-A2-negative and Her-2/neu-positive cell line SKOV-3, the HLA-A2-negative and Her-2/neu-negative cell line K-562, and DCs pulsed with an irrelevant HIV-derived peptide were included as negative controls.
Sorafenib reduces lymphocyte stimulation capacity of DCs. DCs were irradiated with 30 Gy and were incubated for 5 days with 105 allogenic PBMCs or CD3+ T cells. Thymidine incorporation was measured by a 16-hour pulse with [3H]thymidine. PBMCs or T cells without DCs were included as a control. (A) DCs were pretreated with sorafenib 48 hours before harvesting and immature as well as LPS-stimulated DCs (103 DCs per well) were incubated with untreated PBMCs. (B) Untreated mature DCs (103 DCs per well) were incubated with MACS isolated CD3+ T cells pretreated with different concentrations of sorafenib as indicated in the figure. (C) Untreated mature DCs (103 DCs per well) were incubated with MACS isolated CD3+ T cells pretreated with different concentrations of sorafenib as indicated in the figure. Sorafenib was added to the cell culture medium in the same concentrations during MLR. (D) DCs were pretreated with sunitinib, stimulated with LPS, and incubated with untreated PBMCs (*P < .05, ** P < .001). (E) Induction of Her-2/neu specific primary CTL responses was analyzed using LPS activated DCs pretreated with sorafenib or sunitinib and pulsed with an HLA-A2 binding Her-2/neu-derived E75 peptide. DCs pulsed with the E75 peptide as well as the HLA-A2-positive and Her-2/neu-expressing cell line A-498 were used as targets. The HLA-A2-negative and Her-2/neu-positive cell line SKOV-3, the HLA-A2-negative and Her-2/neu-negative cell line K-562, and DCs pulsed with an irrelevant HIV-derived peptide were included as negative controls.
In line with these results, we found that the secretion of cytokines known to be involved in T-lymphocyte activation by DCs, such as IL-6, TNF-α, IL-10, and IL-12, was inhibited by addition of sorafenib to DC cultures (Table 1).
Effect of sorafenib on cytokine secretion by DCs
| Cytokine . | No sorafenib . | Sorafenib concentration . | ||
|---|---|---|---|---|
| 1 μg/mL . | 3 μg/mL . | 5 μg/mL . | ||
| IL-12, pg/mL | 767 | 342 | 40 | 9 |
| IL-10, pg/mL | 325 | 425 | 88 | 82 |
| TNF-α, pg/mL | >10 000 | >10 000 | 4633 | 2824 |
| IL-6, pg/mL | 9758 | >10 000 | 7130 | 6546 |
| Cytokine . | No sorafenib . | Sorafenib concentration . | ||
|---|---|---|---|---|
| 1 μg/mL . | 3 μg/mL . | 5 μg/mL . | ||
| IL-12, pg/mL | 767 | 342 | 40 | 9 |
| IL-10, pg/mL | 325 | 425 | 88 | 82 |
| TNF-α, pg/mL | >10 000 | >10 000 | 4633 | 2824 |
| IL-6, pg/mL | 9758 | >10 000 | 7130 | 6546 |
Secretion of IL-12, IL-10, IL-6, and TNF-α was determined in the supernatant of cultured DCs treated with sorafenib and stimulated with LPS using commercially available ELISAs.
Effects of sorafenib on DC intracellular signaling cascades
In the next set of experiments, we determined the effect of the kinase inhibitor sorafenib on intracellular signaling in DCs after stimulation with LPS. LPS was added at different time points before harvesting.
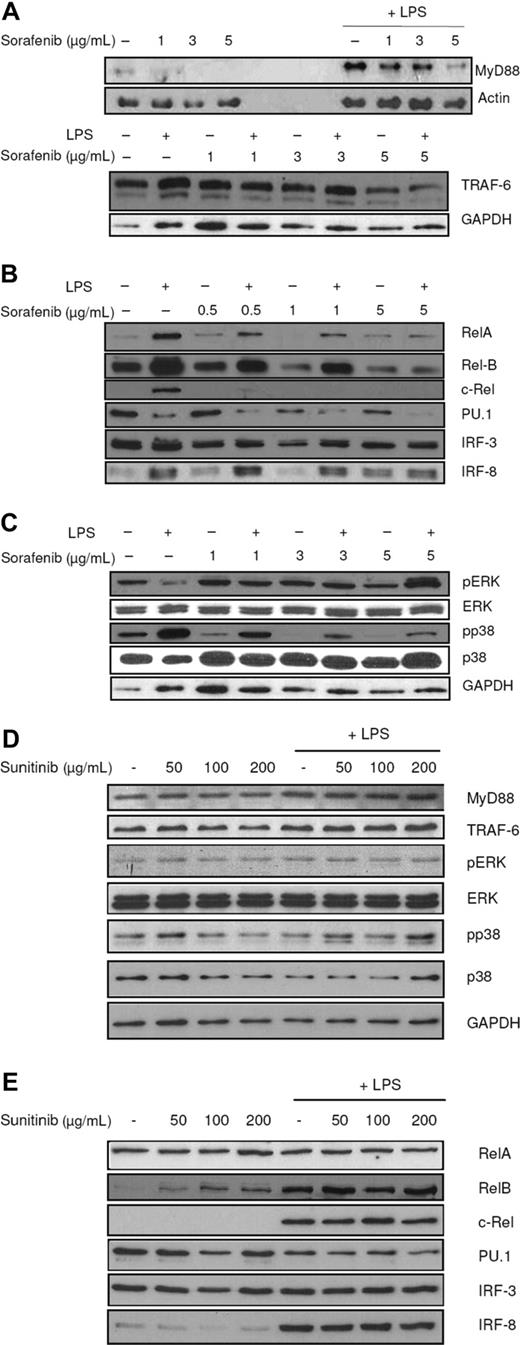
To analyze the MyD88-dependent pathway, protein levels of MyD88 and TRAF6 were analyzed in DCs that had been exposed to sorafenib and stimulated with LPS. The multikinase inhibitor reduced expression of MyD88 and TRAF6 (Figure 5A). Furthermore, nuclear localization of the transcription factors c-Rel, Rel-B, Rel-A, and PU.1 was reduced, whereas IRF-8 levels did not change after treatment with sorafenib, thus excluding an unselective unspecific effect (Figure 5B). These results were reproducibly obtained at all analyzed time points. The reduction of IRF-3 was not as efficient as observed for other transcription factors, indicating that sorafenib mainly affects the MyD88-dependent pathway.
Sorafenib affects intracellular signaling pathways. LPS (100 ng/mL) was added to immature DCs 15 minutes (A, top figure) or 24 hours (A, bottom figure, B-E) before harvesting. Protein levels were detected by SDS-PAGE and Western blot. Ponceau S staining was performed to ensure equal loading of the gel. (A) Sorafenib affects the TLR pathway. MyD88 and TRAF6 levels were reduced by sorafenib treatment. (B) Sorafenib reduces nuclear localization of the NF-κB family members c-Rel, Rel-B, Rel-A, and of PU.1 and IRF-3 in mature cells and nuclear translocation of Rel-B, IRF-3, and PU.1 also in immature cells. Protein levels of IRF-8 in nuclei were not affected. (C) The inhibitory effects of sorafenib partly are mediated by MAPK cascade and the PI3K/AKT pathway. Sorafenib reduces phosphorylation states of p38, whereas sorafenib induces phosphorylation of ERK. (D) Addition of sunitinib to the culture medium has no effect on the signaling pathways in DCs. (E) Addition of sunitinib to the culture medium has no effect on nuclear translocation of c-Rel, Rel-B, Rel-A, PU.1, IRF-3, and IRF-8. Representative experiments of at least 4 are presented.
Sorafenib affects intracellular signaling pathways. LPS (100 ng/mL) was added to immature DCs 15 minutes (A, top figure) or 24 hours (A, bottom figure, B-E) before harvesting. Protein levels were detected by SDS-PAGE and Western blot. Ponceau S staining was performed to ensure equal loading of the gel. (A) Sorafenib affects the TLR pathway. MyD88 and TRAF6 levels were reduced by sorafenib treatment. (B) Sorafenib reduces nuclear localization of the NF-κB family members c-Rel, Rel-B, Rel-A, and of PU.1 and IRF-3 in mature cells and nuclear translocation of Rel-B, IRF-3, and PU.1 also in immature cells. Protein levels of IRF-8 in nuclei were not affected. (C) The inhibitory effects of sorafenib partly are mediated by MAPK cascade and the PI3K/AKT pathway. Sorafenib reduces phosphorylation states of p38, whereas sorafenib induces phosphorylation of ERK. (D) Addition of sunitinib to the culture medium has no effect on the signaling pathways in DCs. (E) Addition of sunitinib to the culture medium has no effect on nuclear translocation of c-Rel, Rel-B, Rel-A, PU.1, IRF-3, and IRF-8. Representative experiments of at least 4 are presented.
Another important pathway regulating DC maturation and survival is the MAPK-pathway.30,31 In DCs stimulated with LPS, sorafenib inhibited phosphorylation of p38 (Figure 5C). Surprisingly, levels of phosphorylated extracellular signal-regulated kinase (ERK) 1/2 protein increased (Figure 5C). As shown in Figure 5D,E, we could not detect any effect of sunitinib on the expression of analyzed transcription factors or signaling pathways.
Sorafenib induces apoptosis of DCs
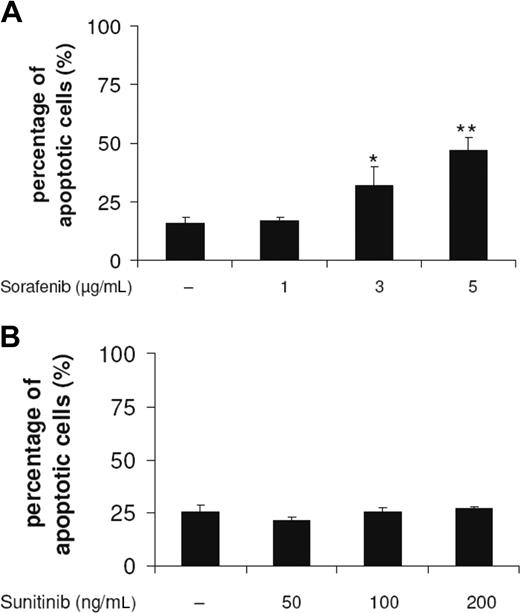
DC survival on exposure to sorafenib and sunitinib was monitored by analyzing the leakage of DNA from apoptotic nuclei as described by Nicoletti et al.25 Sorafenib concentrations that were able to modulate DC phenotype reduced the yield of viable immature DCs in a concentration-dependent manner (Figure 6A), whereas sunitinib did not induce apoptotic cell death (Figure 6B). These effects on the induction of cell death were detected after an incubation of DCs for 48 hours with the compound.
Sorafenib, but not sunitinib, induces apoptosis of DCs. DCs generated by culturing adherent monocytes in the presence of GM-CSF and IL-4 for 7 days were incubated for 48 hours with sorafenib (A) or sunitinib (B). Cells were harvested, washed, stained according to Nicoletti et al,25 and then analyzed by flow cytometry (*P < .05, **P < .001).
Sorafenib, but not sunitinib, induces apoptosis of DCs. DCs generated by culturing adherent monocytes in the presence of GM-CSF and IL-4 for 7 days were incubated for 48 hours with sorafenib (A) or sunitinib (B). Cells were harvested, washed, stained according to Nicoletti et al,25 and then analyzed by flow cytometry (*P < .05, **P < .001).
Sorafenib attenuates primary T-cell responses in vivo
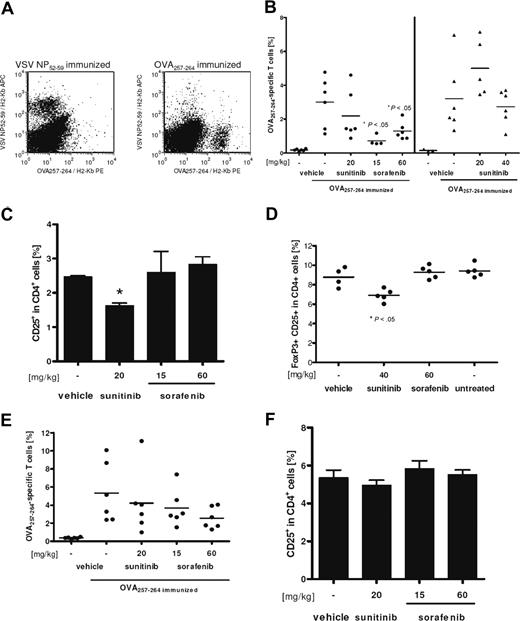
Finally, the influence of sorafenib and sunitinib on the induction of primary cytotoxic CD8+ T-cell responses was assessed in vivo. Mice were fed daily with doses of both compounds that have been shown to inhibit tumor growth in mice.32,33 Treatment of C57BL/6 mice started 2 weeks before first immunization and lasted until mice were killed. Measured blood plasma levels of both inhibitors in treated animals were comparable with published pharmacokinetics (data not shown). After 2 immunizations with OVA257-264 in a 1-week interval, ex vivo tetramer analysis of splenocytes revealed that the number of induced peptide-specific CD8+ T cells was significantly lower for sorafenib-treated mice compared with vehicle-treated controls (Figure 7A, left section of 7B). Animals treated with 20 mg/kg body weight of sunitinib did not show significantly reduced numbers of specific CD8+ T cells. Drug-induced toxicity was observed in a group treated with 80 mg/kg body weight of sunitinib for 4 weeks: animals showed weight loss and were hypoactive, 2 of 6 mice died during the experiments, and 2 of 4 survivors were not evaluable because of too low numbers of cells recovered from the shrunken spleens. The strongly reduced number of OVA257-264-specific CD8+ T cells in the remaining evaluable animals may be mainly caused by general toxic effects of the drug rather than by any distinct immune suppressive effect of sunitinib (data not shown). Supporting this hypothesis, 40 mg/kg of sunitinib applied in an independent experiment revealed no toxic effects, and the number of OVA257-264-specific CD8+ T cells was not altered compared with vehicle-treated controls (Figure 7B, right section).
Sorafenib reduces strongly, but reversible specific, CD8+ T-cell responses. C57BL/6 mice were pretreated for 2 weeks with the indicated dosage of tyrosine kinase inhibitors. Thereafter, animals were immunized twice in weekly interval with OVA257-264 and adjuvants under continued treatment, as described in “Methods.” Negative controls were immunized with VSV NP52-59 peptide. One week after the last immunization, mice were killed and spleen cells were analyzed ex vivo for peptide-specific CD8+ T cells by staining with anti-CD8-FITC, anti-CD3ϵ-PerCP, VSV NP52-59/H2-Kb-APC, and OVA257-264/H2-Kb-PE tetramers. (A) Staining for both tetramers gated on CD8+ CD3ϵ+ lymphocytes is shown for a representative negative (left) and positive (right) control sample. (B) Percentage of OVA257-264/H2-Kb tetramer-positive cells among CD8+ T cells for individual mice and means (lines) are shown. Treatment with 80 mg/kg body weight for 4 weeks resulted in severe toxicity. Therefore only 2 samples were evaluable, and this group was excluded from statistical analysis. (C) In addition, percentage of CD25+ cells among blood CD4+ cells was analyzed. Means of triplicates are shown and error bars indicate the standard deviations of means. Significance was tested by unpaired, heteroscedastic Student t test (*P < .05). (D) Reduced levels of CD25+ FoxP3+ T cells among CD4+ splenocytes of nonimmunized mice can also be observed after 14 days of daily sunitinib treatment. (E,F) Reversibility of the observed effects was assessed in an additional experiment with discontinued treatment 48 hours before first immunization. Mice had been fed daily for 2 weeks with sorafenib, sunitinib, or vehicle only in the indicated concentrations. Analysis was performed as described for panels B and C.
Sorafenib reduces strongly, but reversible specific, CD8+ T-cell responses. C57BL/6 mice were pretreated for 2 weeks with the indicated dosage of tyrosine kinase inhibitors. Thereafter, animals were immunized twice in weekly interval with OVA257-264 and adjuvants under continued treatment, as described in “Methods.” Negative controls were immunized with VSV NP52-59 peptide. One week after the last immunization, mice were killed and spleen cells were analyzed ex vivo for peptide-specific CD8+ T cells by staining with anti-CD8-FITC, anti-CD3ϵ-PerCP, VSV NP52-59/H2-Kb-APC, and OVA257-264/H2-Kb-PE tetramers. (A) Staining for both tetramers gated on CD8+ CD3ϵ+ lymphocytes is shown for a representative negative (left) and positive (right) control sample. (B) Percentage of OVA257-264/H2-Kb tetramer-positive cells among CD8+ T cells for individual mice and means (lines) are shown. Treatment with 80 mg/kg body weight for 4 weeks resulted in severe toxicity. Therefore only 2 samples were evaluable, and this group was excluded from statistical analysis. (C) In addition, percentage of CD25+ cells among blood CD4+ cells was analyzed. Means of triplicates are shown and error bars indicate the standard deviations of means. Significance was tested by unpaired, heteroscedastic Student t test (*P < .05). (D) Reduced levels of CD25+ FoxP3+ T cells among CD4+ splenocytes of nonimmunized mice can also be observed after 14 days of daily sunitinib treatment. (E,F) Reversibility of the observed effects was assessed in an additional experiment with discontinued treatment 48 hours before first immunization. Mice had been fed daily for 2 weeks with sorafenib, sunitinib, or vehicle only in the indicated concentrations. Analysis was performed as described for panels B and C.
Interestingly, the fraction of CD4+ CD25+ Tregs among blood CD4+ T cells was significantly reduced in mice treated with 20 mg/kg sunitinib but not with sorafenib (Figure 7C,D), whereas relative numbers of splenic CD8+, total CD4+, and B-cell populations as well as of blood lymphocytes were not altered in any drug-treated group compared with vehicle-treated animals (data not shown). The decrease of blood Tregs was even more pronounced in the 80 mg/kg sunitinib group, but we cannot exclude that this effect is caused or overlaid by the observed toxicity discussed above (data not shown). Reduction of Treg levels was independent of immunization with peptide and CpG, as splenic CD4+ CD25+ FoxP3+ percentages were also reduced in nonimmunized mice treated for 14 days with 40 mg/kg of sunitinib (Figure 7D).
Finally, the reversibility of the observed immunosuppressive effect of sorafenib in vivo was assessed. After 2 weeks of treatment with sorafenib, sunitinib, or vehicle alone, mice were immunized 48 hours after last drug application. At this time point, both tyrosine kinase inhibitors were cleared from blood, as plasma levels for both substances were below detection limit (data not shown). The immune response was boosted 7 days later and analyzed as described before. Levels of peptide-specific CD8+ T cells in drug-treated animals were not significantly different from the vehicle-treated controls, although still tending to the effects described for continuous treatment (Figure 7E). Therefore, the attenuation of specific CD8+ T-cell responses is rapidly reversed after discontinuation of sunitinib or sorafenib treatment. As expected, blood Treg levels were back to normal when mice were killed (Figure 7F).
Discussion
RCC is recognized as an immunogenic tumor. The discovery of tumor-associated antigens and development of efficient procedures to deliver these antigens into patients (eg, by peptide vaccination or using antigen [Ag]–pulsed DCs) led to clinical trials analyzing the clinical efficiency of these approaches in RCC that mounted promising results.34-39 Another important discovery was the detection of VHL gene alterations in RCC resulting in loss of function of this tumor-suppressor gene. VHL protein is mediating ubiquitination and proteasomal degradation of several cytosolic proteins, including HIFα-subunits.
Genetic mutations that disrupt the function of VHL protein result in an increased activity of HIF that leads to the up-regulation of genes involved in many aspects of cancer progression, such as resistance to apoptosis, metabolic adaptation, and angiogenesis. Thus, suppression of the HIF-induced VEGF pathway is an important and promising therapeutic strategy in RCC patients.40,41
Sorafenib and sunitinib represent 2 novel compounds that block VEGF- and PDGF-RTKs. In randomized trials, both agents demonstrated an improved progression-free survival and overall survival in patients with metastatic RCC that lead to their approval by FDA and European Medicines Agency.2,5,7
To evaluate the possible combination of drugs with Ag-specific immunotherapies, it is important to analyze the effects of these agents on the immune system and the induction of antigen-specific immune responses as several recently developed tyrosine kinase inhibitors were shown to have negative effects on the function and development of APCs and T lymphocytes.17,22,42
We found that sorafenib interferes with the function and maturation of MDDCs. Incubation of DCs with sorafenib inhibits the TLR3 or 4 ligand-induced up-regulation of costimulatory, adhesion and major histocompatibility complex molecules.
Furthermore, DCs pretreated with sorafenib showed a reduced capacity to secrete activating cytokines, such as IL-6, IL-12, and TNF-α, and to induce lymphocyte proliferation. Both effects, down-regulation of major histocompatibility complex and costimulatory molecules and decreased production of immunostimulatory cytokines, may cause the observed reduced proliferation of lymphocytes in allogenic settings and the impaired induction of Her-2/neu specific CTL responses in vitro.
In addition, DCs that were exposed to sorafenib showed impaired migratory capacity toward CCL19/MIP-3β, which was the result of a reduced expression of the corresponding receptor CCR7.
In the next set of experiments, we analyzed the pathways involved in the inhibitory effects mediated by sorafenib. LPS binding to TLR4 triggers the activation of 2 major signaling pathways43,44 : the MyD88-dependent and -independent pathways. The first event in the MyD88-dependent pathway is recruitment of Toll-IL-1 receptor (TIR) domain-containing adaptor protein and MyD88 to the receptor. Subsequently, MyD88 recruits members of IRAKs and TRAF6 and finally causes the activation of NF-κB and the MAPK pathways. The second signaling pathway uses adaptor proteins, such as TRIF-related adaptor molecule (TRAM) and TIR domain-containing adaptor-inducing IFN-γ (TRIF, also known as TICAM-1) to activate the IRFs (as well as NF-κB), thereby triggering production of type I IFNs. Members of the NF-κB family were shown to be relevant in controlling DC differentiation and function.28,29,43-50
Another important signaling cascade involved in DC function is the IFN pathway. Important members of this pathway are IRF-8, IRF-3, and PU.1. IRF-8 is a PU.1 interaction partner regulating lymphoid DC development and trafficking of Langerhans cells and dermal DCs.51,52 IRF-3 has an essential role in induction of IFN-α/β genes.53
Finally, the MAPK kinases p38 and ERK as well as the PI3K/AKT-pathway are also involved in modulating DC re-sponses to LPS.30,54,55
In our study, sorafenib inhibited nuclear translocation of the NF-κB family members Rel-B, Rel-A, and c-Rel on stimulation with LPS. In addition, nuclear translocation of PU.1 and to a lower extent of IRF-3 were reduced, whereas levels of IRF-8 in nuclear extracts did not change after treatment with sorafenib. Sorafenib down-regulated expression levels of MyD88 and TRAF6 proteins in response to LPS. The MAPK pathway in DCs stimulated with LPS was also affected as sorafenib reduced p38 phosphorylation. But in contrast to this, sorafenib stimulated ERK phosphorylation. A possible mechanism that explains this observation could be that sorafenib inhibits activation of AKT, which is known to be a Raf-1 inhibitor.56 AKT inhibits Raf-1 by phosphorylation of Ser259, which creates a binding site for the 14-3-3 protein, a negative regulator of Raf.56 Another explanation could be that LPS binding to TLR4 differentially triggers activation of ERK and p38. p38 directly gets activated through the MyD88-dependent pathway: TAK1 activates MAPK kinase 3/6 (MKK3/6), which in turn phosphorylates p38. ERK gets activated through a pathway involving Tlp2, which is possibly downstream of TRAF6.54,57 In addition, it was reported that ERK and p38 MAPK pathways differentially regulate maturation of MDDCs and that inhibition of p38 phosphorylation enhances phosphorylation of ERK.55,57-59
This severe impairment of DC function by sorafenib is reflected by the attenuated immune response observed in vivo. Both doses used (15 mg/kg and 60 mg/kg sorafenib) have proven antitumor efficacy in mouse tumor models.8 Because published and measured pharmacokinetics in our study were similar57 and because we did not see any signs of toxicity in sorafenib-treated animals, the immunosuppressive effect of this compound can be considered relevant for patients treated with this tyrosine kinase inhibitor. Nevertheless, pharmacokinetics are different for humans and mice60,61 with dramatically shorter serum half-life in mice (6 hours) compared with humans (> 1 day).
The second kinase inhibitor sunitinib did not show any inhibitory effects on maturation and function of DCs and peptide-induced CD8+ T-cell responses in vivo were not altered, at least at subtoxic doses. Interestingly, sunitinib reduced significantly the number of Tregs in blood. It has been shown convincingly in mice and humans that Tregs are major negative regulators of tumor immunity and that Treg levels in tumors are correlated with reduced survival.62-65 Similar reduction of Tregs has been reported for RCC patients under sunitinib therapy,66 and this might contribute to the efficacy of sunitinib, especially for immunogenic tumors.
In conclusion, the present study indicates the potential of sorafenib to interfere with DC function by down-modulating DC responsiveness to inflammatory signals, by reducing DC migration and their capacity for stimulation of lymphocytes. These data indicate that concomitant treatment with sorafenib during vaccination therapy is not recommendable. In contrast, sunitinib has no effects on DC maturation and function and did not impair the induction of primary T-cell responses in vivo. Furthermore, it reduced the number of Tregs that constitute a major immunosuppressive burden in cancer immunotherapy. Therefore, concomitant treatment of cancer patients with sunitinib could be even advantageous by reducing immunosuppression in cancer patients and increasing the efficacy of immunotherapeutic approaches.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Peter Lewandrowski for his quality control analyses for both TKIs and Silvia Roth, Sylvia Stephan, and Solveig Daecke for their excellent assistance.
This work was supported by the DFG SFB 685 “Immune therapy: From the molecular basics to clinical applications,” and the DFG Graduate Program GK 794 “Cellular mechanisms of immune-associated processes.”
Authorship
Contribution: M.M.H. performed research and prepared the manuscript; N.H., S.W., D.W., and M.P.R. performed research; K.M.B. analyzed data and prepared the manuscript; T.W., H.S.-J., and P.B. designed research and prepared the manuscript; all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Brossart, Department of Hematology and Oncology, University of Bonn, Wilhelmstrasse 35-37, D-53111 Bonn, Germany; e-mail: peter.brossart@ukb.uni-bonn.de.
References
Author notes
*M.M.H., N.H., and S.W. contributed equally to this study.

![Figure 4. Sorafenib reduces lymphocyte stimulation capacity of DCs. DCs were irradiated with 30 Gy and were incubated for 5 days with 105 allogenic PBMCs or CD3+ T cells. Thymidine incorporation was measured by a 16-hour pulse with [3H]thymidine. PBMCs or T cells without DCs were included as a control. (A) DCs were pretreated with sorafenib 48 hours before harvesting and immature as well as LPS-stimulated DCs (103 DCs per well) were incubated with untreated PBMCs. (B) Untreated mature DCs (103 DCs per well) were incubated with MACS isolated CD3+ T cells pretreated with different concentrations of sorafenib as indicated in the figure. (C) Untreated mature DCs (103 DCs per well) were incubated with MACS isolated CD3+ T cells pretreated with different concentrations of sorafenib as indicated in the figure. Sorafenib was added to the cell culture medium in the same concentrations during MLR. (D) DCs were pretreated with sunitinib, stimulated with LPS, and incubated with untreated PBMCs (*P < .05, ** P < .001). (E) Induction of Her-2/neu specific primary CTL responses was analyzed using LPS activated DCs pretreated with sorafenib or sunitinib and pulsed with an HLA-A2 binding Her-2/neu-derived E75 peptide. DCs pulsed with the E75 peptide as well as the HLA-A2-positive and Her-2/neu-expressing cell line A-498 were used as targets. The HLA-A2-negative and Her-2/neu-positive cell line SKOV-3, the HLA-A2-negative and Her-2/neu-negative cell line K-562, and DCs pulsed with an irrelevant HIV-derived peptide were included as negative controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/12/10.1182_blood-2007-02-075945/6/m_zh80100819110004.jpeg?Expires=1769088186&Signature=2Xu~Q7GRpfH9eZvUnAUVrjenDSgikTRzzb9czKliRMR6sZdcYBzm7wl1NQY9veSl68-EQ2B6AMJwdKZnVxAMk-Aw3ktLXwCSYgmtv0XYbwQj86jMJLJkR9lecNNzh~I845Kx9GdG6nef6nD-4dvFg~7SzkvhzmIQRFoVdBIn5TFKq1GTcOaQGXvPBTAg5oaH7OeUxwo43-3NC9VAxQT79R9En5NpSA~ke5bgxjArF2j8QybWHeqCFkcVLsn66DawgXMzlh3F7-2wDNz76nJkj-MaEbiWx4ngU56nXWii8v8KNpWEp6IEndBIro4waOfoW8JmncsP1Z9SweG3ABDYvA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)