Abstract
γδ T cells play an important role in innate immunity against infections; however, the regulation of these cells remains largely unknown. In the present study, we show that ESAT-6, an antigen of Mycobacterium tuberculosis, induces IFN-γ secretion by human γδ T cells. In addition, ESAT-6 also induces the activation and proliferation of γδ T cells. Phenotypic analysis indicates that IFN-γ–producing γδ T cells are mainly effector memory cells with the surface phenotype of CD45RA−CD62L−CCR7−. These results were further confirmed by the fact that naive γδ T cells from cord blood did not produce IFN-γ in response to ESAT-6. Further studies indicated that stimulation with ESAT-6 directly induced purified γδ T cells to produce IFN-γ, independent of both antigen-presenting cells and CD4+ T cells. Unexpectedly, depletion of CD4+ T cells markedly enhanced IFN-γ production by γδ T cells, indicating that CD4+ T cells regulate the response of γδ T cells. Importantly, CD4+CD25+ T regulatory (Treg) cells but not CD4+CD25− T cells significantly inhibited IFN-γ production by γδ T cells. Taken together, these data demonstrate for the first time that Treg cells can play an important role in the regulation of immune responses of antigen-specific human memory γδ T cells.
Introduction
γδ T cells represent a second subset of T cells, expressing T-cell antigen receptor (TCR), that have undergone γ and δ gene rearrangement. Normally, γδ T cells constitute 2% to 5% of the total T-cell population.1 However, during a variety of bacterial infections, γδ T cells have been found to expand to high levels, so that they represent the majority of circulating T cells in some individuals. Several lines of evidence suggest that γδ T cells contribute to the immune response to Mycobacterium tuberculosis.2-5 Intranasal infection of mice with M tuberculosis led to expansion of resident Vγ2 T cells and caused an influx of other γδ subsets in the lungs. These cells were capable of producing IFN-γ and were cytotoxic against infected macrophages in vitro. In humans, γδ T cells from Bacillus Calmette-Guerin (BCG)–vaccinated individuals expand upon restimulation with mycobac-terial antigen (Ag), displaying a memory-like phenotype.6
In contrast to recognition of antigens by αβ T cells, γδ T cells recognize antigens directly without any requirement for antigen processing and presentation on major histocompatibility complex (MHC) molecules.7-10 In humans, Vγ2Vδ2 T cells predominate in the peripheral blood and respond to microbial infections by recognizing small nonpeptide molecules.3,11 ESAT-6, a secreted Ag that is highly specific for the M tuberculosis complex, is a major target of IFN-γ–secreting CD4 T cells in the mouse model. Immunization of mice with ESAT-6 induces protective immunity against tuberculosis, which is mediated by ESAT-6–specific IFN-γ–secreting CD4 T cells. The recent observation in humans that ESAT-6 is recognized by a large portion of patients with active tuberculosis has generated much interest in this Ag as a diagnostic reagent.12,13 ESAT-6 and culture filtrate protein-10 (CFP-10) are cosecreted proteins of M tuberculosis that naturally form a tight 1:1 complex upon export from the bacterium and are potent IFN-γ–inducing antigens. It has been reported that γδ TCR+ and CD8+ cells, but not CD4+ cells, from M bovis–infected reindeer proliferated in response to the cosecreted protein stimulation.14
Much interest has recently arisen as to the role of naturally occurring CD4+CD25+ regulatory T (Treg) cells in immune homeostasis. These cells are characterized by expression of the fork-head transcription factor FOXP3 and increased levels of CD45RO, CTLA-4, and GITR.15-19 More recently, Treg cells with the CD4+CD25highFOXP3+ phenotype have been detected at elevated levels among peripheral blood mononuclear cells (PBMCs) or at disease site of patients with tuberculosis (TB) compared with uninfected control subjects or to patients with latent TB infection (LTBI).20-22 Furthermore, purified Treg cells have been shown to suppress antigen-specific T-cell responses in vitro. Several other studies have also shown that depletion of Treg cells from PBMCs of patients with TB leads to significantly higher production of IFN-γ, indicating that Treg cells play an important role in the suppressive function of effector CD4+ T cells.21,22 Although a regulatory role has been suggested for γδ T cells, the suppressive effect of human Treg cells on γδ T cells has not yet been reported.23-25 In the present study, we demonstrate that ESAT-6 induces IFN-γ production as well as activation and division of γδ T cells. These IFN-γ–producing γδ T cells were mostly effector memory cells with the phenotype of CD45RA−CD62L−CCR7−. Depletion of CD4+ T cells from PBMCs significantly enhanced IFN-γ production by γδ T cells. Addition of Treg cells but not CD4+CD25− T cells significantly inhibited IFN-γ production by γδ T cells. These data demonstrate for the first time that Treg cells can inhibit antigen-specific immune responses of memory γδ T cells, implying a new regulatory role for Treg cells.
Methods
Subjects
A total of 28 volunteers composed of 12 men and 16 women aged 18 to 52 years were enrolled in this study. Of these, 26 displayed positive reactions in a skin test for tuberculin. Umbilical cord blood from healthy, full-term newborn infants was collected from the First Affiliated Hospital of Sun Yat-Sen University. All of the blood samples were taken after adequate informed consent was obtained from all individuals involved in the study in accordance with the Declaration of Helsinki. The study was approved by the Medical School Review Board at Sun Yat-Sen University, China. Both adults and neonates that had been diagnosed with HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), or with a history of autoimmune diseases were excluded from the study.
mAbs
The following monoclonal antibodies (mAbs) were used for phenotypic and intracellular cytokine analysis: phycoerythrin (PE)–labeled anti-CD25, anti–IL-4, anti-CD62L, anti-CCR7, anti-γδ, anti–TNF-α, fluourescein isothiocyanate (FITC)–labeled anti-CD25, anti-CD45RA, anti-CD45RO, anti–IFN-γ, anti-CD8, peridin-chlorophyll protein (PerCP)–labeled anti-CD4, PE-cy7–conjugated anti-CD69, anti-CD56, allophycocyanin (APC)–labeled anti–IL-2 antibodies, anti–IFN-γ, anti-γδ, and isotype-matched control antibodies were purchased from BD Biosciences Pharmingen (San Jose, CA). APC-labeled anti-CD27 was obtained from Biolegend (San Diego, CA). Purified anti-CD28 mAb was purchased from BD Biosciences Pharmingen.
Cell preparation
For the isolation of umbilical cord blood mononuclear cells (CBMCs), heparinized cord blood was mixed sufficiently with an equal volume of Dextran 500 solution (6% in isotonic NaCl; GE Healthcare Bio-Sciences, Uppsala, Sweden) and incubated at 37°C in an atmosphere of 5% CO2 incubator to remove erythrocytes in cord blood. After incubation for 45 to 60 minutes, CBMCs were isolated by density centrifugation. PBMCs obtained from healthy donors were isolated from heparinized venous blood by Ficoll-Paque (Tianjin Hao Yang Biological Manufacture, Tianjin, China) gradient centrifugation within 24 hours of sampling. Cells were collected and washed twice in Hanks balanced salt solution. The cells were finally resuspended at a final concentration of 2 × 106/mL in complete RPMI 1640 medium (Invitrogen, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS; HyClone, Logan, UT), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol.
T-cell subset isolation
CD4+ T cells were positively purified from freshly isolated PBMCs using CD4 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. Briefly, after washing twice in buffer (phosphate-buffered saline [PBS] supplemented with 2 mM EDTA and 0.5% bovine serum albumin [BSA]), cells were resuspended in the buffer, mixed well with anti-CD4 microbeads, and incubated at 4°C for 15 minutes. The cells were washed and magnetically separated on a magnetic-activated cell sorter (MACS) magnet fitted with a MACS LS column. Unlabeled cells were collected and used as CD4-depleted cells. γδ T cells were isolated by anti-TCR γ/δ hapten antibody and antihapten microbeads (Miltenyi Biotec). In brief, cells were washed twice with buffer and resuspended in buffer, mixed well with anti-TCR γ/δ hapten antibody and incubated at 4°C. After incubation for 10 minutes, MACS antihapten microbeads labeled with FITC was added, mixed, and incubated at 4°C for 15 minutes. After washing, cells were resuspended in buffer and magnetically separated with an LS column. The purification of Treg cells was conducted as described previously.21 CD4+ T cells were first negatively isolated using a cocktail of anti–human biotin-conjugated monoclonal antibodies against CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCRγ/δ, glycophorin A, and antibiotin microbeads. Purified CD4+ T cells were then incubated with CD25 magnetic beads, CD4+CD25− cells were obtained by negative selection, and Treg cells were positively selected from the column (Miltenyi CD4+CD25+ regulatory isolation kit). The purity of the subpopulations was assessed by flow cytometry. We obtained more than 95% purity for Treg cells, CD4+, and γδ T cells, and more than 90% purity for CD4+CD25− and CD4− T-cell subsets.
Flow cytometric analysis of cell-surface markers and intracellular staining
Cells were washed twice with PBS buffer containing 0.1% BSA and 0.05% sodium azide. For surface staining, cells were incubated with the respective mAbs at 4°C in the dark for 30 minutes. The cells were thereafter washed twice and fixed in 0.5% paraformaldehyde before acquisition. For the detection of intracellular cytokines, cells were incubated with ESAT-6 plus anti-CD28 for 5 hours in the presence of brefeldin A (10 μg/mL; Sigma-Aldrich, St Louis, MO). After stimulation, cells were washed twice with PBS and fixed in 4% paraformaldehyde, followed by permeabilization and stained for the intracellular cytokines and molecules in PBS buffer containing 0.1% saponin. Lymphocytes were gated on forward- and side-scatter profiles. Flow cytometry was performed using a BD FACSCalibur (Becton Dickinson, San Jose, CA) and analyzed using FlowJo software (TreeStar, San Carlos, CA).
Antigen-specific suppressive function assay
Antigen-specific suppression assays were performed in 96-well flat-bottom plates. The ratio of Treg cells to γδ T responder cells was 1:1. In control cultures, responders were added instead of suppressor cells. All wells contained 5 μg/mL ESAT-6 (purchased from Shanghai H&G Biotechnology, Shanghai, China) and 1 μg/mL anti-CD28 mAb, and the optimal concentration of the ESAT-6 was chosen as a result of dose-dependent responses. The cells were cultured at a final volume of 200 μL in triplicate and incubated for 72 hours at 37°C with 5% CO2.
IFN-γ detection by ELISA and ELISPOT assay
PBMCs and CBMCs were resuspended in complete RPMI 1640 medium and stimulated with ESAT-6 plus anti-CD28 in a flat-bottom 96-well plate at a concentration of 4 × 105 cells/well in triplicate and incubated for 72 hours at 37°C with 5% CO2. The supernatants were harvested and assayed for IFN-γ production by enzyme-linked immunosorbent assay (ELISA; detection limit, 5 pg/mL) according to the manufacturer's protocol (BD Pharmingen, San Diego, CA). Human IFN-γ enzyme-linked immunospot (ELISPOT) assays were performed using a commercially available set (BD Pharmingen) according to the manufacturer's instructions. Briefly, 96-well nitrocellulose plates (Millipore, Billerica, MA) were coated overnight at 4°C with 5 μg/mL purified anti–human IFN-γ capture antibody. The wells were then washed and blocked for 2 hours at room temperature with 10% FCS-RPMI 1640 medium. A total of 2 × 105 PBMCs or CBMCs were added to microwells in triplicate and incubated for 20 hours with recombinant ESAT-6 and anti-CD28 mAbs. The plates were washed and subsequently incubated with 2 μg/mL biotinylated anti–human IFN-γ detection antibody for 2 hours at room temperature. After washing, wells were developed for 1 hour with streptavidin–horseradish peroxidase (HRP), followed by addition of AEC substrate reagent. Spot development was monitored and the reaction was quenched with water. Activated T cells were enumerated at the single-cell level by counting the number of spots per well using an ELISpot Reader (Champspot II; Sage Creation, Beijing, China). A spot represents an IFN-γ–producing cell. The average of spots in triplicate wells was calculated and considered as the number of specific spot-forming cells (SFCs)/2 × 105 PBMCs or CBMCs.
CFSE labeling
Purified γδ T cells were resuspended in complete RPMI 1640 medium at 107 cells/mL. Carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA) was added at a final concentration of 5 μM, and the cells were incubated for 10 minutes at 37°C in 5% CO2. The stain was quenched using 5 times the volume of ice-cold complete RPMI 1640 medium for 5 minutes. The cells were then washed 3 times and resuspended in complete RPMI 1640 medium and incubated with or without ESAT-6 plus anti-CD28 at a concentration of 106 cells/mL. After 6 days of culture, cells were acquired with FACSCalibur and analyzed using FlowJo software.
Statistical analysis
Comparison between 2 groups was performed by unpaired Student t tests. P values less than .05 were considered significant.
Results
ESAT-6 induces IFN-γ production by PBMCs
To determine whether ESAT-6 could induce IFN-γ production, PBMCs were cultured in the presence of various concentrations of ESAT-6 plus anti-CD28 (1 μg/mL) mAb. After stimulation for 72 hours, the level of IFN-γ in cell-free culture supernatants was assessed by ELISA. The results revealed very low levels of IFN-γ when PBMCs were cultured with medium alone (Figure 1A). Addition of ESAT-6 to cell cultures markedly enhanced the production of IFN-γ in a dose-dependent manner. The optimal concentration of ESAT-6 for the induction of IFN-γ was 5 μg/mL, which induced significantly higher levels of IFN-γ compared with medium alone (P < .05). The results of IFN-γ from 22 individuals showed that significantly higher levels of IFN-γ were detected when PBMCs were cultured with ESAT-6 compared with medium (P < .001; Figure 1B). To further analyze the frequency of IFN-γ producing cells, the ELISPOT assay was used to detect IFN-γ production after stimulation for 20 hours. Consistent with the results from ELISA, no IFN-γ+ spots were detected in 2 × 105 cells without stimulation. ESAT-6, however, elicited a strong antigen-specific T-cell response in normal donors with an average of 121 SFCs in 2 × 105 PBMCs (range, 17-300 SFCs) that were significantly higher than in medium alone (P < .001; Figure 1C,D). However, the range of ESAT-6–specific cell frequencies within healthy donors is broad and probably reflects the natural range of interindividual variability for the responses. Taken together, these results indicate that ESAT-6 can induce IFN-γ production by PBMCs.
ESAT-6–induced IFN-γ production by PBMCs. (A) Dose-dependent production of IFN-γ induced by ESAT-6 plus anti-CD28. Freshly isolated PBMCs were cultured with different concentrations of ESAT-6 in the presence of anti-CD28 (1μg/mL) for 72 hour. The production of IFN-γ in supernatants was detected by ELISA. (B) ESAT-6–specific IFN-γ secretion was measured by ELISA from PBMCs (n = 22). (C) Representation of ELISPOT results. (D) The frequency of IFN-γ–producing cells was enumerated by ELISPOT assay (n = 22). Cultures were set up in triplicate. Each symbol represents one data point from one donor. *P > .05; **P < .05; ***P < .001. Horizontal bars represent mean values.
ESAT-6–induced IFN-γ production by PBMCs. (A) Dose-dependent production of IFN-γ induced by ESAT-6 plus anti-CD28. Freshly isolated PBMCs were cultured with different concentrations of ESAT-6 in the presence of anti-CD28 (1μg/mL) for 72 hour. The production of IFN-γ in supernatants was detected by ELISA. (B) ESAT-6–specific IFN-γ secretion was measured by ELISA from PBMCs (n = 22). (C) Representation of ELISPOT results. (D) The frequency of IFN-γ–producing cells was enumerated by ELISPOT assay (n = 22). Cultures were set up in triplicate. Each symbol represents one data point from one donor. *P > .05; **P < .05; ***P < .001. Horizontal bars represent mean values.
ESAT-6–induced IFN-γ production by γδ T cells
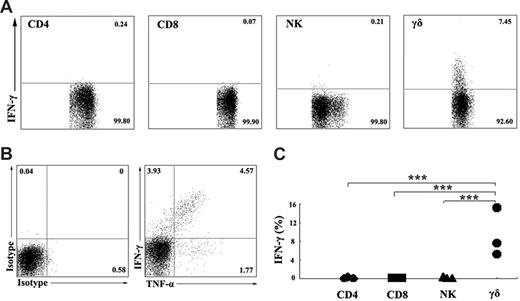
To characterize the subpopulation of IFN-γ–producing cells, PBMCs were stimulated in vitro with ESAT-6 plus anti-CD28 in the presence of 10 μg/mL brefeldin A. After stimulation for 5 hours, the cell-surface markers and intracellular cytokines were stained with anti-CD3, anti-CD4, anti-CD8, anti-CD56, anti-γδ, anti–IFN-γ, and isotype-matched controls. For flow cytometric analysis, the cells were first gated on CD3+CD4+, CD3+CD8+, CD3−CD56+, and γδ T cells, and the expression of IFN-γ was analyzed (Figure 2A). The results demonstrated that IFN-γ production in response to ESAT-6 was primarily due to γδ T cells but not CD3+CD4+, CD3+CD8+, and CD3−CD56+ cells. In addition, ESAT-6 induced γδ T cells to produce large amounts of TNF-α but less IL-2 (data not shown). Further analysis of TNF-α and IFN-γ expression demonstrated that γδ T cells could be divided into 3 distinct populations. Most cells expressed both TNF-α and IFN-γ, and small subsets of the cells expressed either TNF-α or IFN-γ alone (Figure 2B). Figure 2C shows the statistical results of IFN-γ expression from 3 independent experiments. Moreover, we also detected perforin and granzyme B expression by γδ T cells. The results indicate that γδ T cells express perforin and granzyme B constitutively. Stimulation of γδ T cells with ESAT-6 plus anti-CD28 resulted in induction of IFN-γ production but no significant change in the percentage of perforin and granzyme B (data not shown).
γδ T cells, but not CD4, CD8, or NK cells, express IFN-γ in ESAT-6–stimulated PBMCs. (A) Identification of IFN-γ–producing cells in PBMCs induced by ESAT-6. PBMCs were cultured in the presence of ESAT-6. After stimulation for 5 hours, cells were harvested and FACS stainings were conducted as described in “Flow cytometric analysis of cell-surface markers and intracellular staining.” Subpopulations of CD3+CD4+ T cells, CD3+CD8+ T cells, CD3−CD56+ NK cells, and γδ T cells were analyzed by FACS for IFN-γ expression. (B) One representative result from 3 independent experiments showing the expression of TNF-α and IFN-γ. The numbers in each quadrant represent the percentages of positive cells in gated γδ T cells. (C) Statistical results of IFN-γ expression by subpopulations of PBMCs from 3 independent experiments were shown. ***P < .001.
γδ T cells, but not CD4, CD8, or NK cells, express IFN-γ in ESAT-6–stimulated PBMCs. (A) Identification of IFN-γ–producing cells in PBMCs induced by ESAT-6. PBMCs were cultured in the presence of ESAT-6. After stimulation for 5 hours, cells were harvested and FACS stainings were conducted as described in “Flow cytometric analysis of cell-surface markers and intracellular staining.” Subpopulations of CD3+CD4+ T cells, CD3+CD8+ T cells, CD3−CD56+ NK cells, and γδ T cells were analyzed by FACS for IFN-γ expression. (B) One representative result from 3 independent experiments showing the expression of TNF-α and IFN-γ. The numbers in each quadrant represent the percentages of positive cells in gated γδ T cells. (C) Statistical results of IFN-γ expression by subpopulations of PBMCs from 3 independent experiments were shown. ***P < .001.
ESAT-6 induces activation and division of γδ T cells
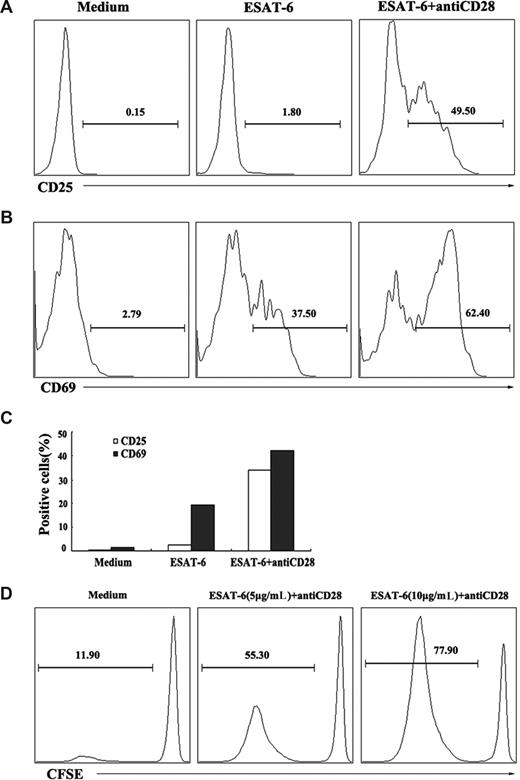
In addition to evaluation of the ability to induce the production of cytokines, we also assessed whether ESAT-6 could induce the activation and division of γδ T cells. PBMCs were cultured with ESAT-6 in the presence or absence of anti-CD28 for 72 hours. The expression of cell-surface activation markers CD25 and CD69 were analyzed on γδ T cells by flow cytometry. The results indicated that ESAT-6 plus anti-CD28, but not ESAT-6 alone induced the expression of CD25 on γδ T cells (Figure 3A). In contrast to CD25 expression, ESAT-6 alone was able to induce the expression of CD69 by γδ T cells (37.5%). Costimulation of γδ T cells with ESAT-6 and anti-CD28 further enhanced CD69 expression (62.4%; Figure 3B). The experiment was repeated several times and similar results were obtained. The mean value of the results is shown in Figure 3C. In addition, purified γδ T cells were labeled with CFSE and cultured with 5 μg/mL or 10 μg/mL ESAT-6 in the presence of anti-CD28 for 6 days. A small percentage (11.90%) of γδ T cells underwent division without ESAT-6 stimulation. The stimulation with ESAT-6 (5 μg/mL) resulted in the division of about half of γδ T cells (55.30%). Increase in the concentration of ESAT-6 to 10 μg/mL further enhanced the division of γδ T cells (77.90%; Figure 3D). These results demonstrate that ESAT-6 not only induces the activation but also the division of γδ T cells.
Activation and division of γδ T cells in response to ESAT-6. (A,B) Activation of γδ T cells induced by ESAT-6. PBMCs were cultured in the presence or absence of ESAT-6 and ESAT-6 plus anti-CD28. After stimulation for 72 hours, cells were harvested and stained for CD25 and CD69. γδ T cells were gated and analyzed for CD25 (A) and CD69 (B) expression. (C) Mean value of CD25 and CD69 expression on γδ T cells from 3 separate experiments was shown. (D) Division of γδ T cells induced by ESAT-6. Purified γδ T cells were labeled with CFSE (5μM) and cultured with or without ESAT-6 at the concentration of 5 and 10 μg/mL plus anti-CD28 for 6 days. CFSE-labeled cells were gated for FACS analysis. The percentages of divided cells in each condition were analyzed. Data are representative of 2 separate experiments with similar results.
Activation and division of γδ T cells in response to ESAT-6. (A,B) Activation of γδ T cells induced by ESAT-6. PBMCs were cultured in the presence or absence of ESAT-6 and ESAT-6 plus anti-CD28. After stimulation for 72 hours, cells were harvested and stained for CD25 and CD69. γδ T cells were gated and analyzed for CD25 (A) and CD69 (B) expression. (C) Mean value of CD25 and CD69 expression on γδ T cells from 3 separate experiments was shown. (D) Division of γδ T cells induced by ESAT-6. Purified γδ T cells were labeled with CFSE (5μM) and cultured with or without ESAT-6 at the concentration of 5 and 10 μg/mL plus anti-CD28 for 6 days. CFSE-labeled cells were gated for FACS analysis. The percentages of divided cells in each condition were analyzed. Data are representative of 2 separate experiments with similar results.
Memory γδ T cells produce IFN-γ in response to ESAT-6
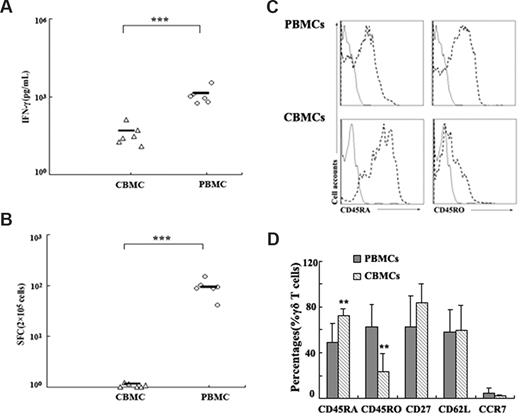
To characterize the phenotype of ESAT-6–specific γδ T cells on the basis of their ability to produce IFN-γ, PBMCs were stimulated with ESAT-6 plus anti-CD28. After 5 hours of incubation, cells were harvested, washed, and stained for cell-surface markers, including CD45RA as a marker for naive cells, CD62L and CCR7 as effector/memory subset markers, and CD25 as an activation marker. Cells were first gated on γδ T cells, and the phenotype of IFN-γ+ γδ T cells was analyzed. Consistent with previous reports, our results indicate that most IFN-γ–producing γδ T cells are CD45RA−. Moreover, most antigen-specific γδ T cells express low levels of CD62L and CCR7 (Figure 4A). The experiment was repeated 2 to 4 times, and the average percentages of each population are shown in the pie charts (Figure 4B). To further confirm whether ESAT-6 could only induce IFN-γ production by memory γδ T cells, we compared the reactivity of PBMCs with CBMCs in response to ESAT-6. Data in Figure 5A show that CBMCs did not respond to ESAT-6, whereas PBMCs reacted vigorously. The ELISPOT assay showed similar results to ELISA, as demonstrated by lack of SPCs from CBMCs (Figure 5B). In addition, we also compared the phenotype of γδ T cells in CBMCs and PBMCs. Most γδ T cells in CBMCs expressed CD45RA but lower levels of CD45RO compared with those in PBMCs (Figure 5C). In addition, other surface markers to distinguish naive/memory phenotype such as CD27, CD62L, and CCR7 were also compared. As illustrated in Figure 5D, γδ T cells in CBMCs expressed enhanced levels of CD45RA and diminished CD45RO with very low levels of cytokine production, suggesting that γδ cells in CBMCs are predominantly naive.
Memory γδ T cells produced IFN-γ in response to ESAT-6. (A) Several surface markers were included to define the phenotype of ESAT-6–specific γδ T cells. PBMCs were cultured in the presence of ESAT-6 plus anti-CD28 for 5 hours. IFN-γ–producing cells and cell-surface markers CD45RA, CD25, CD62L, and CCR7 were assessed by FACS. Numbers in quadrants indicate percentages of the cells in each population. Data are representative of 2 to 4 independent experiments. (B) The data were quantified and presented in a pie chart, in which each slice of the pie represents the fraction of the mean value of a given quadrant.
Memory γδ T cells produced IFN-γ in response to ESAT-6. (A) Several surface markers were included to define the phenotype of ESAT-6–specific γδ T cells. PBMCs were cultured in the presence of ESAT-6 plus anti-CD28 for 5 hours. IFN-γ–producing cells and cell-surface markers CD45RA, CD25, CD62L, and CCR7 were assessed by FACS. Numbers in quadrants indicate percentages of the cells in each population. Data are representative of 2 to 4 independent experiments. (B) The data were quantified and presented in a pie chart, in which each slice of the pie represents the fraction of the mean value of a given quadrant.
PBMCs but not CBMCs produced IFN-γ in response to ESAT-6. (A) Comparison of IFN-γ production by PBMCs and CBMCs in response to ESAT-6 measured by ELISA (n = 6). (B) The frequency of IFN-γ–producing cells were assessed by ELISPOT (n = 6). The cultures were set up in triplicate. Each symbol represents one datum from one donor. ***P < .001. Horizontal bars represent mean values. (C) The expression of CD45RA and CD45RO on γδ T cells in PBMCs and CBMCs were shown. Data are representative of 5 experiments yielding similar results. (D) γδ T cells from PBMCs and CBMCs were assessed by FACS for the expression of CD45RA, CD45RO, CD27, CD62L, and CCR7 in 5 independent experiments (mean ± SD); **P < .05 compared with PBMCs.
PBMCs but not CBMCs produced IFN-γ in response to ESAT-6. (A) Comparison of IFN-γ production by PBMCs and CBMCs in response to ESAT-6 measured by ELISA (n = 6). (B) The frequency of IFN-γ–producing cells were assessed by ELISPOT (n = 6). The cultures were set up in triplicate. Each symbol represents one datum from one donor. ***P < .001. Horizontal bars represent mean values. (C) The expression of CD45RA and CD45RO on γδ T cells in PBMCs and CBMCs were shown. Data are representative of 5 experiments yielding similar results. (D) γδ T cells from PBMCs and CBMCs were assessed by FACS for the expression of CD45RA, CD45RO, CD27, CD62L, and CCR7 in 5 independent experiments (mean ± SD); **P < .05 compared with PBMCs.
ESAT-6 directly induces IFN-γ production from purified γδ T cells and depletion of CD4+ T cells enhances IFN-γ production
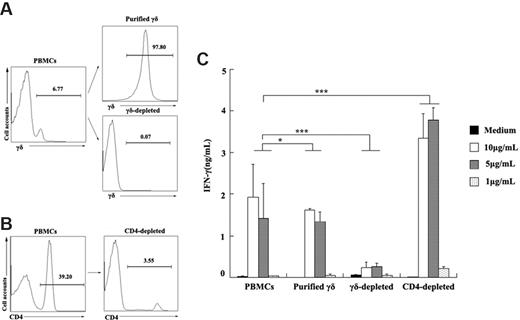
To determine whether ESAT-6 could directly act on γδ T cells to induce IFN-γ production, γδ T cells as well as γδ T cell–depleted cells were purified from PBMCs (Figure 6A). Both PBMCs and purified γδ T cells could induce IFN-γ production upon stimulation with ESAT-6 (Figure 6C). In addition, depletion of γδ T cells from PBMCs almost completely abrogated IFN-γ production. Moreover, to determine whether CD4+ T cells were required for the IFN-γ production by ESAT-6–induced γδ T cells, CD4+ T cells were depleted using MACS beads (Figure 6B) and stimulated with ESAT-6 at various concentrations. Results in Figure 6C demonstrate that depletion of CD4+ T cells from PBMCs markedly enhanced IFN-γ secretion, suggesting that subsets of CD4+ T cells might suppress the production of IFN-γ by γδ T cells when stimulated with ESAT-6. Taken together, these results show that purified γδ T cells can directly respond to ESAT-6.
Purified γδ T cells produced IFN-γ in response to ESAT-6. (A) Purified γδ T cells and γδ-depleted cells by magnetic beads from PBMCs are shown. The numbers represent the percentage of the purity of each population in one representative experiment. (B) CD4-depleted cells from PBMCs were prepared. (C) PBMCs, purified γδ T cells, γδ-depleted cells, and CD4-depleted cells were cultured with or without ESAT-6 at different concentrations. After stimulation, IFN-γ production was detected by ELISA (mean ± SD). *P > .05; ***P < .001.
Purified γδ T cells produced IFN-γ in response to ESAT-6. (A) Purified γδ T cells and γδ-depleted cells by magnetic beads from PBMCs are shown. The numbers represent the percentage of the purity of each population in one representative experiment. (B) CD4-depleted cells from PBMCs were prepared. (C) PBMCs, purified γδ T cells, γδ-depleted cells, and CD4-depleted cells were cultured with or without ESAT-6 at different concentrations. After stimulation, IFN-γ production was detected by ELISA (mean ± SD). *P > .05; ***P < .001.
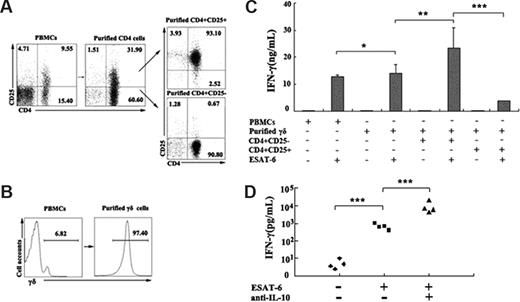
CD4+CD25+ Treg cells can inhibit IFN-γ production by γδ T cells in response to ESAT-6
As described, depletion of CD4+ T cells markedly enhanced IFN-γ production by ESAT-6–stimulated γδ T cells. Therefore, we wanted to determine whether the suppressive effect of CD4+ T cells on IFN-γ production was via the direct effect of Treg cells on ESAT-6–stimulated γδ T cells. CD4+CD25+ (Figure 7A), CD4+CD25− (Figure 7A), and γδ T cells (Figure 7B), respectively, were purified by magnetic beads. PBMCs or purified γδ T cells were cultured in the presence or absence of ESAT-6 plus anti-CD28. In the suppressive assay, purified γδ T cells were cocultured either with CD4+CD25− or CD4+CD25+ T cells at a ratio of 3:1 in the presence or absence of ESAT-6 plus anti-CD28. After 72 hours of incubation, supernatants were collected and the level of IFN-γ was detected by ELISA. The data shown in Figure 7C indicate that purified γδ T cells respond to ESAT-6 vigorously, which is consistent with previous experiments. Moreover, the addition of CD4+CD25− T cells had no significant effect on IFN-γ production. However, the addition of CD4+CD25+ T cells to purified γδ T cells significantly suppressed IFN-γ production (P < .001), indicating that Treg cells could inhibit the antigen-specific response of γδ T cells. The suppressive effects could be partially reversed by addition of neutralizing antibodies to IL-10 (Figure 7D). These results demonstrate that Treg cells can directly regulate the biologic functions of γδ T cells in addition to CD4+CD25− cells.
CD4+CD25+ Treg cells inhibited ESAT-6–specific IFN-γ production by γδ T cells. (A) Isolation of CD4+CD25+ and CD4+CD25− T cells from PBMCs. The numbers in each quadrant represent the percentages of positive cells in each cell population. (B) Purification of γδ T cells from PBMCs. (C) Suppressive effects of CD4+CD25+ T cells on IFN-γ production by γδ T cells. PBMCs or purified γδ T cells were cultured alone, with CD4+CD25− T cells, or with CD4+CD25+ T cells (at ratio of 3:1) in the presence or absence of ESAT-6 plus anti-CD28. The supernatants were collected for IFN-γ production. (D) Addition of neutralizing antibody to IL-10 promoted IFN-γ production. The results represent the average of triplicate wells (mean ± SD). *P > .05; **P < .05; ***P < .001.
CD4+CD25+ Treg cells inhibited ESAT-6–specific IFN-γ production by γδ T cells. (A) Isolation of CD4+CD25+ and CD4+CD25− T cells from PBMCs. The numbers in each quadrant represent the percentages of positive cells in each cell population. (B) Purification of γδ T cells from PBMCs. (C) Suppressive effects of CD4+CD25+ T cells on IFN-γ production by γδ T cells. PBMCs or purified γδ T cells were cultured alone, with CD4+CD25− T cells, or with CD4+CD25+ T cells (at ratio of 3:1) in the presence or absence of ESAT-6 plus anti-CD28. The supernatants were collected for IFN-γ production. (D) Addition of neutralizing antibody to IL-10 promoted IFN-γ production. The results represent the average of triplicate wells (mean ± SD). *P > .05; **P < .05; ***P < .001.
Discussion
γδ T cells represent less than 5% of total T cells in the blood, and their physiologic functions have been controversial for a long time. This is most likely because they have distinctive antigen-recognition properties and almost certainly recognize a different set of antigens.1,26,27 Similar to αβ T cells, γδ T cells are heterogeneous and comprise distinct populations which can be distinguished into naive, effector, central memory, and effector memory cells based on cell-surface phenotype and effector functions.28-34 Studies have reported the characterization and differentiation of γδ T cells in early life. Study on the lifespan of γδ T cells in adult thymectomized mice revealed that γδ T cells have a rapid turnover and display an activated/memory phenotype, suggesting a chronic response to environmental Ags.35 The antigen specificities of γδ T cells are not well understood; however, a major subset of γδ T cells in adult human PBMCs are known to respond to small molecular weight phosphoantigens after short-term stimulation.36 Although a variable percentage of γδ T cells in adults and children respond to this stimulation, few cord blood cells respond, which would indicate that γδ T cells with this specificity are not present in cord blood, or that they have not been previously activated.30
Antigen-specific γδ T cells may play a role in antimycobacterial immunity. Studies in humans and animal models have demonstrated complex patterns of γδ T-cell immune responses during early mycobacterial infections and chronic tuberculosis.37,38 Previous studies have also shown a clinical correlation between major recall expansion of antigen-specific γδ T cells and immunity against fetal early mycobacterial diseases. Multiple host and microbial factors can regulate diverse immune responses of phosphoantigen-specific γδ T cells during mycobacterial infections.11,39 A recent report indicated that natural killer T (NKT) cells were activated and up-regulated expression of CD25 in patients with TB.40
Although some phenotypic analyses of γδ T cells have been reported, few provide detailed phenotypic, functional, and developmental characterization of γδ T cells. Even fewer studies have been reported regarding the naive and memory phenotype of γδ T cells. In a prior report, consistent with our findings, most cord blood γδ T cells were CD45RO−.41 The author also noted an age-related increase in CD45RO expression on γδ T cells. In our previous study, comparison between γδ T cells from cord blood with the PBMCs indicated that the phenotype differentiated from naive to memory, suggesting exposure to Ag from birth onward. After short-term stimulation in vitro, memory γδ T cells secreted IFN-γ in response to specific Ag. This was further confirmed by the fact that γδ T cells from cord blood did not produce IFN-γ. In addition, we also observed a significantly lower proportion of γδ T cells in cord blood than that in PBMCs. Therefore, it cannot be excluded that the unresponsiveness of γδ T cells in cord blood may be due to the lower quantity. We also detected other marker characterization regarding naive/memory subsets in the γδ T-cell population. The results indicate that γδ T cells express low levels of CCR7 compared with αβ T cells, indicating that γδ T cells are mainly located in peripheral tissues. However, all of the results reported in this study are based on peripheral blood T cells, which is a limitation, especially when studying γδ T cells.
In our study, purified γδ T cells responded to ESAT-6 directly, independently of both antigen-presenting cells and CD4+ T cells, which is consistent with previous reports.8,9 However, there is also evidence that the γδ T-cell responses are dependent on the presence of CD4+ T cells for the cytokine IL-2.2 This may be due to the difference in antigens used for stimulation. Further investigation revealed that most of the responsive individuals were purified protein derivative (PPD) positive, suggesting latent infection with M tuberculosis. Moreover, we also found that addition of anti-CD28 could significantly enhance IFN-γ production and activation of γδ T cells, implicating a potential role for costimulatory signal in the magnitude of γδ T-cell responses similar to that of αβ T cells. Further phenotypic analysis indicated that most CD28+ cells among γδ T cells were CD45RO+ and CD45RA+ (data not shown), which might, in part, elucidate the greater and faster responsiveness by γδ T cells in the presence of anti-CD28.
Efforts have also been made in the past years to elucidate the mechanisms of suppression exerted by Treg cells. Among the variety of Treg cell populations, naturally occurring CD4+CD25+ Treg cells have been most intensively studied. Numerous putative mechanisms of Treg cell–mediated suppression have been proposed in the literature. The mechanisms include direct T-cell interaction involving TGF-β, Lag3, or CTLA-4; perforin- and/or granzyme B–dependent killing; IL-10–mediated suppression; modification of the function of dendritic cells; and IL-2 consumption by Treg.15,17 In addition to these in vitro analyses of Treg cell functions, attempts have been made to visualize their in vivo behavior. It now appears that more than one mechanism of suppression is operative in vivo, and that one Treg cell may exert suppression by more than one mechanism depending on in vivo and in vitro situations.17
In the present study, we found that addition of anti–IL-10 reversed IFN-γ production induced by ESAT-6 significantly, suggesting that IL-10 played an important role in the suppression of immune responses of γδ T cells. In our previous study, we demonstrated that the suppressive effect of Treg cells on BCG-specific IFN-γ production by CD4+CD25− T cells was mainly mediated via the release of IL-10 but not TGF-β, implicating a similar suppressive mechanism of Treg cells.21
A regulatory role has been implicated for γδ T cells; however, the influence of naturally occurring Treg cells on γδ T cells has not yet been studied.23,24,42 We observe that Treg cells can inhibit antigen-specific memory γδ T cells from producing IFN-γ; however, the mechanism remains unclear. Our preliminary data indicates that a soluble factor, IL-10, might play an important role in the suppressive function of Treg cells. It cannot be excluded that other mechanisms, including direct cell-surface contact, play an important role as well. Nevertheless, our data provide a novel insight into the immune responses mediated by γδ T cells and enhance our understanding of the regulation of this unconventional T-cell lineage.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Daniel Bailey, scientific writer and editor, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, for his kind critical reading and linguistic revision of the manuscript.
This study was supported by a grant from the National Key Basic Research Program of China (973; no. 2007CB512404).
Authorship
Contributions: L.L. performed the experiments, analyzed results, and prepared the manuscript; C.-Y.W. designed the experiments, analyzed data, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Changyou Wu, Department of Immunology, Zhongshan School of Medicine, Sun Yat-Sen University, 74 Zhongshan 2nd Road, Guangzhou 510080, PR China; e-mail: changyou_wu@yahoo.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal