Abstract
The accumulation of eosinophils in inflammatory foci is a hallmark characteristic of Th2 inflammation. Nevertheless, the expression of inhibitory receptors such as paired immunoglobulin-like receptor B (PIR-B) and their function regulating eosinophil accumulation have received limited attention. We now report that Pirb was up-regulated in an eosinophil-dependent manner in the lungs of allergen-challenged and interleukin (IL)-13–overexpressing mice. Eosinophils expressed high levels of PIR-B, and Pirb−/− mice displayed increased gastrointestinal eosinophils. Consistent with these findings, PIR-B negatively regulated eotaxin-dependent eosinophil chemotaxis in vivo and in vitro. Surprisingly, Pirb−/− eosinophils and neutrophils had decreased leukotriene B4 (LTB4)–dependent chemotactic responses in vitro. Furthermore, eosinophil accumulation was decreased in a chitin-induced model, partially dependent on LTB4. Mechanistic analysis using a miniphosphoproteomic approach revealed that PIR-B recruits activating kinases after LTB4 but not eotaxin stimulation. Consequently, eotaxin-activated Pirb−/− eosinophils displayed markedly increased extracellular signal-related kinase 1 and 2 (ERK1/2) phosphorylation, whereas LTB4-activated eosinophils had reduced ERK1/2 phosphorylation. We provide multiple lines of evidence supporting a model in which PIR-B displays opposing but potent regulatory functions in granulocyte activation. These data change the conventional wisdom that inhibitory receptors are restricted to inhibitory signals; we therefore propose that a single receptor can have dual functionality in distinct cell types after unique cellular signals.
Introduction
Eosinophils are proinflammatory cells implicated in the pathogenesis of numerous inflammatory processes. Under normal conditions, eosinophils mainly reside in mucosal sites, such as the gastrointestinal tract. However, after stimulation, they are recruited into the inflamed tissue, where they can modulate responses by releasing an array of molecules, including cytotoxic proteins, cytokines, and lipid mediators.1,2
Multiple data show that eotaxins are significant regulators of eosinophil recruitment.3,4 Support for this concept is the demonstration that eotaxins are critical for regulating baseline levels of eosinophils in the gastrointestinal tract and for the recruitment of eosinophils in the allergen-challenged lung.5-10 In addition, nonspecific mediators such as leukotrienes and especially leukotriene B4 (LTB4) also promote eosinophil recruitment in various settings as well.11-13
Although the activation pathways regulating eosinophil recruitment have been studied extensively, the role of inhibitory signaling pathways that could restrain eosinophil activation has been scarcely examined. However, recently, inhibitory pathways (ie, Siglec-8, Mig, IRp60) have been shown to regulate interleukin-5 (IL-5) and eotaxin-mediated eosinophil functions.14-16 Notably, the importance of identifying the processes regulating eosinophil trafficking has been reinforced by the demonstration of a critical role for eosinophils in regulating mucus production and airway remodeling in allergic airway inflammation in 2 different eosinophil-deficient mouse models.17,18
Paired immunoglobulin-like receptors A and B (PIR-A and PIR-B) were first identified as homologues to the human Fc receptor for immunoglobulin A (IgA).19 PIR-A requires a homodimeric Fc common γ chain, which harbors an immunoreceptor tyrosine-based activation motif, for its efficient cell surface expression and for the delivery of activation signals. In contrast, PIR-B contains immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in its cytoplasmic portion and can inhibit receptor-mediated activation signaling upon cellular engagement with other activating-type receptors through the binding of intracellular phosphatases such as SH2-homology–containing protein-tyrosine phosphatase-1 (SHP-1) and SHP-2. Based on their similarities in structure, ligand binding, expression patterns, and genomic localization, PIR-A and PIR-B are probably orthologs of the human leukocyte immunoglobulin-like receptor (LIR) family of receptors.20,21 Importantly, several of the LIRs, including LIR-1, LIR-2, LIR-3, and LIR-7, are expressed and functional in human eosinophils.22 Thus, identifying the role of PIR-B in regulating murine eosinophil responses is probably relevant to human eosinophils as well.
PIR-B has been shown to negatively regulate the activation of multiple cell types, including mast cells, dendritic cells, neutrophils, and macrophages.20 Pirb deficiency impairs dendritic cell maturation by perturbation of intracellular signaling pathways that have yet to be defined.23 Nevertheless, its role in regulating eosinophil functions, has not been assessed.
In this study, we demonstrate that PIR-B inhibits eotaxin-mediated eosinophil responses. Notably, we also provide evidence demonstrating an activating role for PIR-B in the regulation of LTB4-mediated eosinophil (and neutrophil) responses. To the best of our knowledge, this is the first line of evidence suggesting a role for PIR-B as an activation molecule.
Methods
Mice
Male and female 8- to 12-week-old Pirb−/− mice (backcrossed to c57BL/6 background at least 9 generations) were kindly provided by Dr Hiromi Kubagawa (University of Alabama, Birmingham).24 For all experiments, 4- to 5-week-old wild-type mice were obtained from Taconic Laboratories (Hudson, NY) and environmentally matched with the Pirb−/− for 2 to 3 weeks. Stat6−/− mice (BALB/c background) from The Jackson Laboratory (Bar Harbor, ME). Pirb−/− mice were mated with CD2–IL-5 transgenic (IL-5Tg) mice (BALB/c).25 Pirb−/−/IL-5Tg, Pirb+/+/IL-5Tg controls, or CD2–IL-5Tg mice were used as a source of eosinophils. Bitransgenic mice (CC10–IL-13) were generated as described previously.26 Δdbl-GATA mice were generously provided by Drs Alison Humbles and Craig Gerard (Children's Hospital, Boston, MA).27 All mice were housed under specific pathogen-free conditions and treated according to institutional guidelines.
Microarray analysis
Microarray hybridization was performed by the Affymetrix Gene Chip Core facility at Cincinnati Children's Hospital Medical Center as described.28
Flow cytometry
Analysis of PIR-B expression on the various cells and of CCR3 on wild-type and Pirb−/− eosinophils was achieved using the following antibodies: anti–PIR-A/B (6C1; BD PharMingen, San Diego, CA), anti–PIR-B (p91; Santa Cruz Biotechnology, Santa Cruz, CA), anti-CCR3–fluorescein isothiocyanate (FITC; R&D Systems, Minneapolis, MN), anti-GR-1–FITC, anti-B220–phycoerythrin (PE), anti-CD3–FITC, FITC–rat IgG2b isotype control, FITC–rat IgG2a isotype control, and PE–rat IgG2a isotype control (BD PharMingen), and anti–MAC-3 (eBioscience, San Diego, CA). Cy-5–anti-rabbit secondary antibody was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Splenocytes or enzymatically digested jejunum cells (105) of wild-type or Pirb−/− mice were incubated with the aforementioned antibodies in a final volume of 100 μL Hanks balanced salt solution (HBSS) supplemented with 0.1% bovine serum albumin (BSA) and 0.02% sodium azide for 30 minutes on ice. Thereafter, differential cell populations or eosinophils were electronically gated and assessed for expression of PIR-B or CCR3, respectively. After staining, cells were analyzed on a FACSCalibur System (BD Biosciences, San Jose, CA). For each staining, a minimum of 10 000 events was collected and data analysis performed using CellQuest (BD Biosciences) or FlowJo 8.3.3 (TreeStar, Ashland, OR).
Animal models
IL-13 was administered via intratracheal delivery (10 μg/mouse) every other day for 6 days as described previously.6 Chitin (New England Biolabs, Ipswich, MA) was administered intranasally according to the methods of Reese et al.29 After IL-13 or chitin instillation, mice were held upright for 20 to 30 seconds. Forty-eight hours after the final IL-13 or chitin challenge, the mice were killed, a midline neck incision was made, and the trachea was cannulated. Subsequently, bronchoalveolar lavage (BALF) was performed and the lungs excised for further histologic measurements.
BALF fluid collection and analysis
Mice were killed as described previously.26 The lungs were lavaged 3 times with 1 mL phosphate-buffered saline (PBS) containing 1% fetal calf serum (FBS-1%). The retained BALF was centrifuged at 400g for 5 minutes at 4°C. The recovered supernatants were collected and stored at −70°C until assessed for cytokine concentration, and the cell pellets were resuspended in 200 μL PBS-1%. Total cell numbers were counted using a hemacytometer, and cytospins were prepared (105 cells/slide), stained with the Hema 3 Staining System (Fisher Diagnostics, Middletown, VA), and differential cell counts were determined.
Histology
Mice were killed as described above, and the tissues were excised and fixed with 10% formalin, embedded in paraffin, and stained with either hematoxylin and eosin or antieosinophil major basic protein (MBP). Quantification of eosinophil numbers in the tissue was performed as described previously using a computerized morphometric analysis.26
Enzyme-linked immunosorbent assay
BALF and jejunum eotaxin-1 and eotaxin-2 levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to manufacturer instructions (R&D Systems). Lower detection limits for eotaxin-1 and eotaxin-2 were 15.6 pg/mL. LTB4 was determined using an enzyme immunoassay kit according to manufacturer instructions (Cayman Chemical, Ann Arbor, MI). The lower detection limit for LTB4 was 31.25 pg/mL.
Eosinophil and neutrophil isolation
Eosinophils were purified from the spleen of Pirb−/−/IL-5Tg or Pirb+/+/IL-5Tg mice by immunomagnetic negative selection as described previously.15 Eosinophil purity was evaluated using Diff-Quick stain and was generally 91% to 95%. Thioglycolate-elicited neutrophils were obtained from Pirb−/− and wild-type mice after thioglycolate injection as described.30 Briefly, 4 hours after thioglycolate injection, peritoneal lavage cells were adhered in a 12-well plate (Nalge Nunc International, Rochester, NY; 1 hour; 37°C; 5% CO2). One hour later, the nonadherent fraction was removed and contained 99% purified neutrophils.
Chemotaxis assays
Chemotaxis was assessed using a transwell system (6.5-mm inserts, 3.0-μm pore size; Corning Life Sciences, Lowell, MA). Isolated eosinophils or neutrophils (1.5 × 106) in HBSS plus 0.5% BSA (low endotoxin; Sigma-Aldrich, St Louis, MO) were placed in the upper chamber, and the chemoattractant (eotaxins or LTB4) was placed in the lower chamber. After 3 hours, total cells in the lower chamber were assessed using a hemacytometer.
Immunoprecipitation
Eosinophils obtained from CD2–IL-5Tg mice were activated with either 10 nM LTB4 or 10 ng/mL eotaxin-1 or eotaxin-2 in 96-well plates (37°C; 5% CO2). The reaction was stopped at the indicated time points by adding M-PER lysis buffer (Pierce Endogen, Rockford, IL) supplemented with protease inhibitor cocktail (Sigma-Aldrich) on ice for 30 minutes. The cell lysate was transferred to an eppendorf tube, and preclearing was performed using protein A/G beads (Pierce Endogen). Importantly, all immunoprecipitation steps were conducted with the p91 anti–PIR-B antibody (unless indicated otherwise) that specifically recognizes the C terminus of PIR-B. The p91 antibody was added to the precleared lysate (8 μg/mL; 1 hour; 4°C; rotation) followed by protein A/G (1 hour; 4°C; rotation). The immunoprecipitated complex was eluted from the protein A/G beads using Ig-elution buffer (Pierce Endogen).
Protein phosphorylation array
Custom-designed membranes coated with various antibodies were purchased from Hypromatrix (Worcester, MA) and performed according to manufacturer instructions. Briefly, the immunoprecipitated PIR-B complex was diluted in 2 mL of PBS and incubated with the anti-kinase–specific precoated membrane. After washing, the membrane was incubated with anti-mouse phosphotyrosine conjugated to horseradish peroxidase (pY99; Santa Cruz Biotechnology). The membrane was developed using enhanced chemiluminescence (ECL)-plus (GE Healthcare, Little Chalfont, United Kingdom) and analyzed using ImageJ version 1.37 (National Institutes of Health [NIH], Bethesda, MD). Briefly, autoradiography films were scanned (CanonScan Lide 30), images inverted, and the bands were gated using ImageJ version 1.37 (NIH). Numeric parameters reflecting band intensity were normalized to untreated cells (time 0), and the percent change was calculated relative to the baseline expression.
Western blot
In assays determining phosphorylated forms of extracellular signal-related kinase (ERK), eotaxin-2– or LTB4-activated eosinophils were lysed as described above. Cell lysates were loaded to 4% to 12% Bis-Tris Gels and transferred to a nitrocellulose membrane (Invitrogen, Carlsbad, CA). Detection of phosho–ERK1/2 was achieved by blotting the membranes with anti–phospho-specific antibodies (Cell Signaling Technology, Danvers, MA) followed by ECL-plus detection (GE Healthcare). Numeric parameters reflecting band intensities were normalized to untreated cells (time 0) using ImageJ version 1.37 (NIH), and fold change was calculated relative to the baseline expression.
Statistical analysis
Data were analyzed by analysis of variance followed by Tukey post hoc test using GraphPad Prism 4 (GraphPad Software, San Diego, CA). Data are presented as means plus or minus SD; values of P less than .05 were considered statistically significant.
Results
PIR-B is an allergen and IL-13–dependent gene correlating with eosinophil infiltration
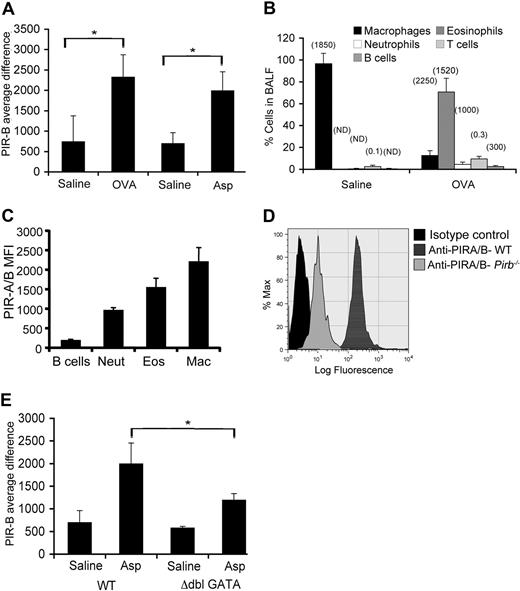
Quantitative microarray analysis revealed that PIR-B mRNA expression was significantly increased in both the lungs of ovalbumin (OVA)– and Aspergillus-induced allergic eosinophilic airway inflammation models (Figure 1A). Because IL-13 has been shown to be a major effector molecule in allergic settings,31 we examined the expression of Pirb in the lungs of doxycycline (dox)–inducible IL-13 transgenic mice. Lung Pirb was found to be up-regulated by dox exposure of these mice as well (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article; Figure S1). Interestingly, Pirb was also up-regulated in the esophagus after IL-13 gene induction (Figure S1).
Assessment of PIR-B expression in the allergic lung. (A) Expression of PIR-B was assessed by gene chip analysis in saline-challenged, OVA-challenged, and Aspergillus fumigatus (Asp)–challenged mice; *P < .05 when comparing OVA- and Asp-treated mice with saline groups. (B) The cellular source for PIR-B expression was assessed in the BALF of OVA-challenged mice. Data are represented as percentage of cell population from total BALF cells plus or minus SD and was defined by flow cytometric analysis as follows: macrophages (Mac, FSChigh, Mac-3+, CCR3−), (Eos, SSChigh, CCR3+), neutrophils (Neut, SSCintermediate, CCR3−, Gr-1+), T cells (FSClow, SSClow, B220−, CD3+), and B cells (FSClow, SSClow, B220+, CD3−). Values in parentheses indicate the mean fluorescent intensity (MFI) of PIR-A/B expression on the various cell population. (C) Analysis of the expression of PIR-A/B on various cell populations in the BALF of OVA-challenged mice. (D) The relative expression of PIR-B and PIR-A on eosinophils was assessed by FACS analysis by staining for PIR-A/B on wild-type (WT) or Pirb−/− eosinophils. (E) Data shown are a representative histogram plot of n = 4. The lungs of Asp-challenged wild-type and Δdbl-GATA were assessed for PIR-B expression; *P < .05 when comparing Asp-treated wild-type and Asp-treated Δdbl-GATA mice. The average difference for the hybridization signal after saline and allergen challenge is depicted (n = 3 mice for saline groups, n = 2-4 mice for OVA and Aspergillus experimental groups). PIR-B expression was assessed on gated eosinophils. Error bars represent SD.
Assessment of PIR-B expression in the allergic lung. (A) Expression of PIR-B was assessed by gene chip analysis in saline-challenged, OVA-challenged, and Aspergillus fumigatus (Asp)–challenged mice; *P < .05 when comparing OVA- and Asp-treated mice with saline groups. (B) The cellular source for PIR-B expression was assessed in the BALF of OVA-challenged mice. Data are represented as percentage of cell population from total BALF cells plus or minus SD and was defined by flow cytometric analysis as follows: macrophages (Mac, FSChigh, Mac-3+, CCR3−), (Eos, SSChigh, CCR3+), neutrophils (Neut, SSCintermediate, CCR3−, Gr-1+), T cells (FSClow, SSClow, B220−, CD3+), and B cells (FSClow, SSClow, B220+, CD3−). Values in parentheses indicate the mean fluorescent intensity (MFI) of PIR-A/B expression on the various cell population. (C) Analysis of the expression of PIR-A/B on various cell populations in the BALF of OVA-challenged mice. (D) The relative expression of PIR-B and PIR-A on eosinophils was assessed by FACS analysis by staining for PIR-A/B on wild-type (WT) or Pirb−/− eosinophils. (E) Data shown are a representative histogram plot of n = 4. The lungs of Asp-challenged wild-type and Δdbl-GATA were assessed for PIR-B expression; *P < .05 when comparing Asp-treated wild-type and Asp-treated Δdbl-GATA mice. The average difference for the hybridization signal after saline and allergen challenge is depicted (n = 3 mice for saline groups, n = 2-4 mice for OVA and Aspergillus experimental groups). PIR-B expression was assessed on gated eosinophils. Error bars represent SD.
To determine the cellular source accounting for the expression of PIR-B in the allergic lung, flow cytometry was conducted on BALF cells obtained from OVA-challenged mice. Eosinophils, the major constituent cells of the BALF in allergen-challenged mice (Figure 1B),1 were found to express PIR-A/B (Figure 1C). In addition to eosinophils, BALF B cells, macrophages, and neutrophils but not T cells were found to express PIR-A/B (Figure 1C; data not shown). Quantification of the flow cytometric analysis revealed that eosinophils express high levels of PIR-B comparable to other myeloid cells (Figure 1B parentheses; Figure 1C). The expression of PIR-A and PIR-B in eosinophils was confirmed by immunoprecipitation (using the 6C1 antibody clone) followed by Western blot analysis. Eosinophils were found to express both PIR-A (∼80 kDa) and PIR-B (∼120 kDa; data not shown). Flow cytometric analysis of Pirb−/− eosinophils (using the 6C1 antibody) confirmed that eosinophils express PIR-B and PIR-A (Figure 1D). Together, these data suggest that eosinophils are a major source for PIR-B in the allergen-challenged lung. In support of this finding, microarray analysis of allergen-challenged Δdbl-GATA mice, which lack eosinophils,17 revealed reduced expression of Pirb (Figure 1E). Together, these data suggest that the increased expression in PIR-B is attributable to tissue infiltration of inflammatory cells, including eosinophils.
Pirb−/− mice display increased eosinophil homing to the gastrointestinal tract
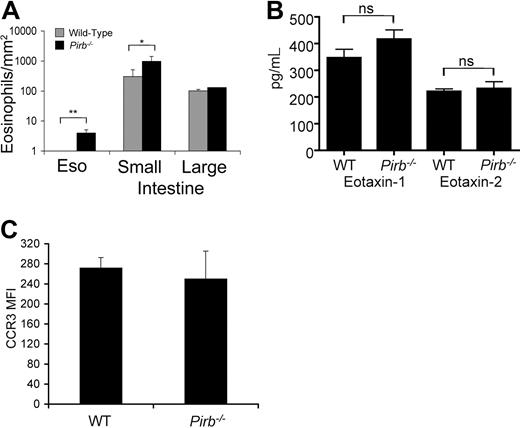
Subsequently, we analyzed the distribution of eosinophils in Pirb−/− mice. No significant alteration in eosinophil numbers or percentages was observed in the bone marrow, spleen, blood, or lungs of Pirb−/− mice (data not shown). However, assessment of the gastrointestinal tract revealed increased eosinophils in the lamina propria of the esophagus (0.5±0.1 and 5±2 eosinophils/mm2 in wild-type and Pirb−/−, respectively) and small intestine (516±92 and 1420±129 eosinophils/mm2 in wild-type and Pirb−/− mice, respectively; Figure 2A; Figure S2). Eosinophil homing to the gastrointestinal tract has been shown previously to be dependent on the eotaxin chemokines.5 Therefore, we examined the expression of eotaxin-1 and eotaxin-2 in the small intestine of Pirb−/− mice. As shown in Figure 2B, the expression of eotaxin-1 and eotaxin-2 was similar in wild-type and Pirb−/− mice. Thus, the increase in eosinophil levels in the gastrointestinal tract is not attributable to overproduction of eotaxins. Furthermore, CCR3 expression was comparable on splenic (data not shown) and intestinal eosinophils in wild-type and Pirb−/− mice (Figure 2C).
Assessment of eosinophil distribution, eotaxin, and CCR3 expression in Pirb−/− mice. (A) Quantification of eosinophil distribution in the gastrointestinal tract of naive wild-type (WT) and Pirb−/− mice. Eosinophils were identified by MBP-stained sections and quantified using digital morphometric analysis. Data represent means plus or minus SD of 4 or 5 random sections per mouse for 6 or 8 animals per group; *P < .05; **P < .01 when comparing Pirb−/− and wild-type mice. Eso indicates esophagus. (B) The levels of eotaxin-1 and eotaxin-2 were assessed in small intestinal homogenates of WT and Pirb−/− mice using a commercial ELISA. Data represent mean plus or minus SD of homogenates from 8 mice. ns indicates nonsignificant. (C) The expression of CCR3 as assessed by FACS on intestinal eosinophils from WT and Pirb−/− mice. MFI indicates mean fluorescent intensity. Data represent n = 4 experiments. Error bars represent SD.
Assessment of eosinophil distribution, eotaxin, and CCR3 expression in Pirb−/− mice. (A) Quantification of eosinophil distribution in the gastrointestinal tract of naive wild-type (WT) and Pirb−/− mice. Eosinophils were identified by MBP-stained sections and quantified using digital morphometric analysis. Data represent means plus or minus SD of 4 or 5 random sections per mouse for 6 or 8 animals per group; *P < .05; **P < .01 when comparing Pirb−/− and wild-type mice. Eso indicates esophagus. (B) The levels of eotaxin-1 and eotaxin-2 were assessed in small intestinal homogenates of WT and Pirb−/− mice using a commercial ELISA. Data represent mean plus or minus SD of homogenates from 8 mice. ns indicates nonsignificant. (C) The expression of CCR3 as assessed by FACS on intestinal eosinophils from WT and Pirb−/− mice. MFI indicates mean fluorescent intensity. Data represent n = 4 experiments. Error bars represent SD.
Increased eosinophil recruitment to the lung and esophagus of Pirb−/− mice after IL-13 challenge
To examine the possibility that Pirb−/− eosinophils are hyper-responding to eotaxin(s)-dependent signaling, we used a model of IL-13–induced airway inflammation. IL-13 is a potent inducer of CC-chemokines and especially those belonging to the eotaxin family of chemokines. Furthermore, in this model, eosinophil recruitment is critically dependent on eotaxin-1 and eotaxin-2.6,32 Analysis of BALF from IL-13–treated Pirb−/− mice showed a marked increase in BALF total cells (0.5 ± 0.1 × 106 cells and 2.6 ± 0.1 × 106 cells in wild-type and Pirb−/− mice, respectively) and a significant increase in eosinophil (1.2 ± 0.5 × 105 cells and 9.9 ± 0.4 × 105 cells, wild-type and Pirb−/− mice, respectively) and neutrophil (7.1 ± 4.2 × 104 cells and 6.6 ± 2.3 × 105 cells in wild-type and Pirb−/− mice, respectively) cell populations (Figure 3A,B). Assessment of tissue eosinophils in the lung and esophagus revealed a statistically significant increase in baseline eosinophilia (Figure 3C,D) in the esophagus (0.2 ± 0.03 cells/HPF and 2.2 ± 1.2 cells/HPF in wild-type and Pirb−/− mice, respectively) but not in the lung. Nevertheless, after IL-13 challenge a marked increase in eosinophil numbers was observed both in the lungs and esophagus of IL-13–challenged Pirb−/− mice (Figure 3C,D). Importantly, analysis of eotaxin-1 and eotaxin-2 levels in the BALF showed no difference between wild-type and Pirb−/− mice (Figure 3E,F). Thus, these data support an inhibitory role for PIR-B in the regulation of eosinophil recruitment in response to eotaxin.
Assessment of IL-13–induced eosinophil recruitment in Pirb−/− mice. Mice received intranasal treatment of 10 μg/day every other day of either IL-13 or control saline solution. The total cell count (A) and differential cell counts (B) in BALF 48 hours after the final saline or IL-13 challenge are shown. Data are expressed as means plus or minus SD; *P < .05; **P < .01; ns indicates nonsignificant; n = 3 (4-6 mice/experimental group). Quantification of eosinophil numbers in the lung (C) and esophagus (D) was assessed by digital morphometric analysis. Data are expressed as means plus or minus SD; *P < .05; **P < .01 when comparing IL-13–treated WT and Pirb−/− mice; n = 3 experiments (4-6 mice/experimental group). The levels of eotaxin-1 and eotaxin-2 (E,F) were assessed in the BALF of WT and Pirb−/− mice using a commercial ELISA. Data represent means plus or minus SD of n = 3 experiments (4-6 mice/experimental group).
Assessment of IL-13–induced eosinophil recruitment in Pirb−/− mice. Mice received intranasal treatment of 10 μg/day every other day of either IL-13 or control saline solution. The total cell count (A) and differential cell counts (B) in BALF 48 hours after the final saline or IL-13 challenge are shown. Data are expressed as means plus or minus SD; *P < .05; **P < .01; ns indicates nonsignificant; n = 3 (4-6 mice/experimental group). Quantification of eosinophil numbers in the lung (C) and esophagus (D) was assessed by digital morphometric analysis. Data are expressed as means plus or minus SD; *P < .05; **P < .01 when comparing IL-13–treated WT and Pirb−/− mice; n = 3 experiments (4-6 mice/experimental group). The levels of eotaxin-1 and eotaxin-2 (E,F) were assessed in the BALF of WT and Pirb−/− mice using a commercial ELISA. Data represent means plus or minus SD of n = 3 experiments (4-6 mice/experimental group).
PIR-B negatively regulates eotaxin-induced eosinophil chemotaxis
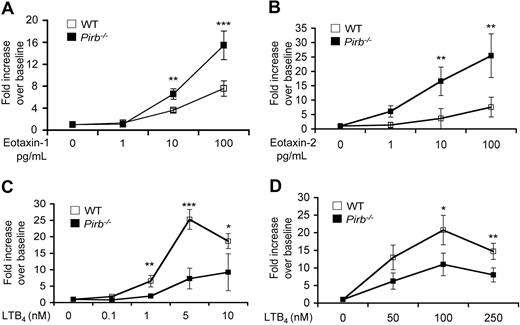
To examine the direct effect of eotaxin on PIR-B–deficient eosinophils, in vitro chemotaxis assays were performed on eosinophils obtained from the Pirb+/+/IL-5Tg or Pirb−/−/IL-5Tg mice. Notably, under baseline conditions (ie, unstimulated cells), no difference was found between wild-type and Pirb−/− eosinophils. However, after eotaxin-1 and eotaxin-2 stimulus, Pirb−/− eosinophils displayed significantly increased chemotaxis in a dose-dependent fashion (Figure 4A,B).
Chemotactic responses of Pirb−/−-granulocytes to chemokine stimulation. Chemotaxis was assessed in eosinophils (A-C) isolated from CD2–IL-5Tg/Pirb−/− (WT), CD2–IL-5Tg/Pirb+/+ (Pirb+/+), or thioglycolate-elicited neutrophils (D) obtained from wild-type (WT) or Pirb−/− mice using a transwell-chamber system in response to recombinant mouse eotaxin-1 (1-100 ng/mL; A), eotaxin-2 (1-100 ng/mL; B), LTB4 (0.1-250 nM; C,D) for 180 minutes. Data are expressed as means plus or minus SD fold increase over baseline chemotaxis of n = 4; *P < .05; **P < .01; ***P < .001 when comparing WT and Pirb−/− cells.
Chemotactic responses of Pirb−/−-granulocytes to chemokine stimulation. Chemotaxis was assessed in eosinophils (A-C) isolated from CD2–IL-5Tg/Pirb−/− (WT), CD2–IL-5Tg/Pirb+/+ (Pirb+/+), or thioglycolate-elicited neutrophils (D) obtained from wild-type (WT) or Pirb−/− mice using a transwell-chamber system in response to recombinant mouse eotaxin-1 (1-100 ng/mL; A), eotaxin-2 (1-100 ng/mL; B), LTB4 (0.1-250 nM; C,D) for 180 minutes. Data are expressed as means plus or minus SD fold increase over baseline chemotaxis of n = 4; *P < .05; **P < .01; ***P < .001 when comparing WT and Pirb−/− cells.
PIR-B positively regulates LTB4-induced eosinophil chemotaxis
Intriguingly, and contrary to the hypothesis that PIR-B negatively regulates eosinophil chemotaxis, Pirb−/− eosinophils displayed decreased chemotactic responses toward LTB4 activation (Figure 4C). To identify whether this effect was specific to eosinophils or a shared phenomenon between granulocytes, chemotaxis assays were performed on Pirb−/− and wild-type neutrophils. No difference was observed in the chemotactic activity of unstimulated cells. However, Pirb−/− neutrophils displayed decreased chemotactic responses toward LTB4 activation (Figure 4D). Importantly, and as already described by Zhang et al, Pirb−/− neutrophils displayed increased chemotaxis toward macrophage inflammatory protein-1α activation (Figure S3).33
PIR-B positively regulates eosinophil responses to chitin
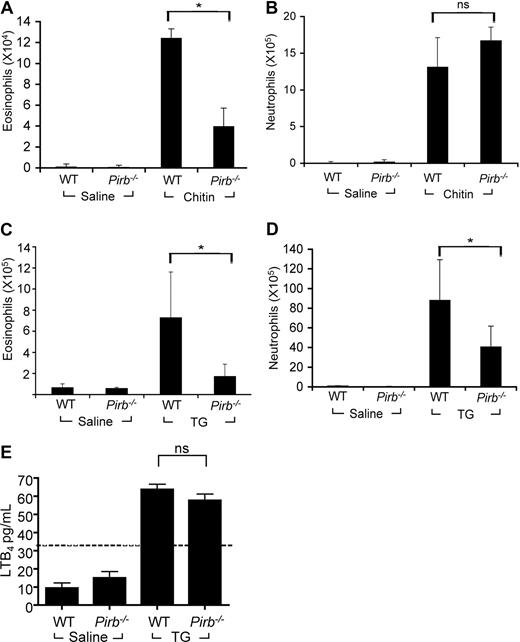
Accordingly, we assessed whether PIR-B positively regulates eosinophils in an in vivo model known to be partially dependent on LTB4, a chitin-induced airway inflammation model.29 Pirb−/− mice displayed a significant reduction in BALF eosinophils after chitin exposure compared with wild-type mice (Figure 5A). Interestingly, this effect was specific for eosinophils because the recruitment of neutrophils in response to chitin was similar in wild-type and Pirb−/− mice (Figure 5B). Moreover, intraperitoneal thioglycolate injection was shown recently to recruit eosinophils to the peritoneum in a partial LTB4-dependent fashion as well.30 Indeed, thioglycolate-challenged Pirb−/− mice displayed a marked decrease in peritoneal eosinophils (Figure 5C). Interestingly, although the recruitment of neutrophils in this model was not reported to be dependent of LTB4,30 Pirb−/− mice displayed a significant decrease in neutrophil accumulation in the peritoneum as well (Figure 5D). To determine whether decreased eosinophil and neutrophil chemotaxis was attributable to decreased LTB4 production in the Pirb−/− mice, LTB4 levels were assessed. Importantly, LTB4 levels were comparable between wild-type and Pirb−/− thioglycolate-challenged mice (Figure 5E). Although we were unable to detect LTB4 in the BALF of chitin-challenged mice, the thioglycolate-challenged data are consistent with an activating role for PIR-B in the regulation of LTB4-induced responses.
Assessment of granulocyte recruitment in response to chitin and thioglycolate. The accumulation of eosinophils (A,C) and neutrophils (B,D) in the BALF of chitin-challenged (A) and the peritoneum of thioglycolate (TG)–challenged Pirb−/− or wild-type (WT) mice was assessed by differential cell counts. Data are expressed as means plus or minus SD of n = 3 (6-8 mice per experimental group); *P < .05; ns indicates nonsignificant when comparing chitin-challenged or TG-challenged WT and Pirb−/− mice. (E) LTB4 was assessed in the peritoneal lavage fluid of thioglycolate-challenged WT and Pirb−/− mice. Data are expressed as means plus or minus SD of n = 3 (6-8 mice per experimental group). The dashed line represents the detection limit of the assay.
Assessment of granulocyte recruitment in response to chitin and thioglycolate. The accumulation of eosinophils (A,C) and neutrophils (B,D) in the BALF of chitin-challenged (A) and the peritoneum of thioglycolate (TG)–challenged Pirb−/− or wild-type (WT) mice was assessed by differential cell counts. Data are expressed as means plus or minus SD of n = 3 (6-8 mice per experimental group); *P < .05; ns indicates nonsignificant when comparing chitin-challenged or TG-challenged WT and Pirb−/− mice. (E) LTB4 was assessed in the peritoneal lavage fluid of thioglycolate-challenged WT and Pirb−/− mice. Data are expressed as means plus or minus SD of n = 3 (6-8 mice per experimental group). The dashed line represents the detection limit of the assay.
Identification of the PIR-B–kinase complex in murine eosinophils
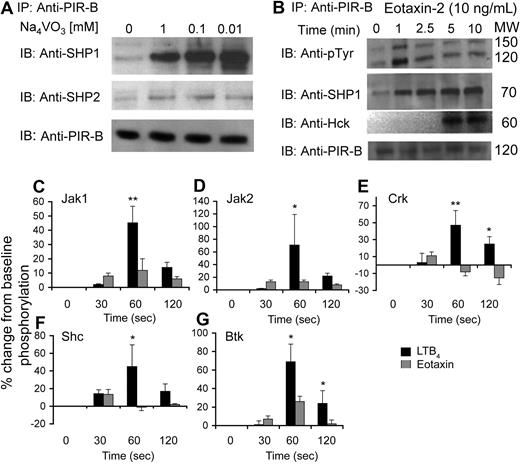
The opposing functions of PIR-B prompted us to investigate the underlying mechanism of PIR-B signal transduction in eosinophils. First, we examined the capability of PIR-B to undergo tyrosine phosphorylation and recruit intracellular phosphatases. For this set of experiments, PIR-B was first immunoprecipitated and then blotted with various antiphosphatase or antikinase antibodies. Although PIR-B displayed tyrosine phosphorylation at baseline, it underwent substantial tyrosine phosphorylation and recruited the phosphatases SHP-1 (a 232% increase over baseline phosphorylation at 0.1 mM of NaVO3), and to a lesser extent SHP-2 (a 37% increase over baseline phosphorylation at 0.1 mM of NaVO3), after orthovanadate treatment but did not recruit SHIP-1 (Figure 6A; data not shown). After eotaxin stimulation (10 ng/mL), PIR-B displayed increased tyrosine phosphorylation. Interestingly, blotting the PIR-B precipitate with anti–phospho-tyrosine revealed a high molecular band (Figure 6B top panel, upper band) of approximately 150 kDa that undergoes similar tyrosine phosphorylation as PIR-B (Figure 6B top panel, lower band). Furthermore, eotaxin increased the recruitment of SHP-1 and Hck but not Fgr to PIR-B (Figure 6B; data not shown). These changes were not observed in response to LTB4 stimulation (data not shown).
Assessment of PIR-B with various signaling molecules at baseline and after eotaxin and LTB4 stimulation. Eosinophils were obtained from the spleens of CD2–IL-5Tg mice. Freshly isolated eosinophils were incubated with various concentrations of sodium orthovanadate (A). Thereafter, the cells were lysed, precleared, PIR-B immunoprecipitated (IP), analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a membrane, and blotted (IB) with antibodies to SHP-1, SHP-2, and PIR-B as a loading control. After eotaxin-2 stimulation (B), the eosinophils were lysed, precleared, IP, analyzed by SDS-PAGE, transferred to a membrane, and IB with antibodies to phospho-tyrosine (pTyr), SHP-1, and Hck. As a loading control, samples were also IB with anti–PIR-B. PIR-B–kinase complexes were assessed using an antibody-coated membrane. Densitometric analysis of phosphorylation patterns after eotaxin or LTB4 stimulation (C-G) is shown. Data are normalized to baseline phosphorylation status and are shown as the percentage change (increase or decrease) from unstimulated eosinophils. Data represent means plus or minus SD of n = 4; *P < .05; **P < .01; when comparing LTB4- and eotaxin-treated cells.
Assessment of PIR-B with various signaling molecules at baseline and after eotaxin and LTB4 stimulation. Eosinophils were obtained from the spleens of CD2–IL-5Tg mice. Freshly isolated eosinophils were incubated with various concentrations of sodium orthovanadate (A). Thereafter, the cells were lysed, precleared, PIR-B immunoprecipitated (IP), analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a membrane, and blotted (IB) with antibodies to SHP-1, SHP-2, and PIR-B as a loading control. After eotaxin-2 stimulation (B), the eosinophils were lysed, precleared, IP, analyzed by SDS-PAGE, transferred to a membrane, and IB with antibodies to phospho-tyrosine (pTyr), SHP-1, and Hck. As a loading control, samples were also IB with anti–PIR-B. PIR-B–kinase complexes were assessed using an antibody-coated membrane. Densitometric analysis of phosphorylation patterns after eotaxin or LTB4 stimulation (C-G) is shown. Data are normalized to baseline phosphorylation status and are shown as the percentage change (increase or decrease) from unstimulated eosinophils. Data represent means plus or minus SD of n = 4; *P < .05; **P < .01; when comparing LTB4- and eotaxin-treated cells.
PIR-B has been shown to interact with various Src-family kinases.34,35 Thus, we aimed to determine which kinases interacted with PIR-B in eosinophils. For this set of experiments, eosinophils were activated with eotaxin-1 or LTB4 using similar concentrations that were used for the chemotaxis assays (ie, 10 ng/mL eotaxin and 10 nM LTB4) and PIR-B was immunoprecipitated using the anti–PIR-B–specific p91 antibody.21,36 Thereafter, the PIR-B precipitate was incubated with a membrane that was precoated with antibodies against various signaling molecules (Figure S4A,B). After incubation, the membrane was blotted with anti–phospho-tyrosine. Because PIR-B is constitutively activated, we hypothesized that the kinases interacting with PIR-B will display tyrosine phosphorylation as well.37 Under baseline conditions, we found that PIR-B was strongly associated with Btk, Src, Syk, and Yes, and to a lesser extent with Csk, JAK1, JAK2, Lyn, Nck, and Vav. PIR-B was not associated with Fgr, Crk, GRB14, GRB2, JAK3, PTEN, or SHIP (Figure S4C). Next, we assessed the possibility that the kinases complexed with PIR-B will undergo differential tyrosine phosphorylation in response to eotaxin or LTB4 activation. Interestingly, after LTB4 but not eotaxin stimulation, JAK1, JAK2, Shc, and Crk underwent rapid tyrosine phosphorylation (45.2 ± 11.6%, 71 ± 48.3%, 45.1 ± 24.7%, and 47.3 ± 17.3% increase over baseline phosphorylation at 60 seconds, respectively; Figure 6C-F). Furthermore, Btk was phosphorylated in response to both eotaxin and LTB4 stimulation, although LTB4 had a greater and long-lived impact on Btk phosphorylation (69.1 ± 19.2 vs 26.2 ± 5.6% increase from baseline at 60 seconds and 24 ± 13.7 vs 2 ± 4.3% increase from baseline at 120 seconds, LTB4 and eotaxin, respectively; Figure 6G). Because changes in PIR-A expression may also modify the activation status of PIR-B, PIR-A expression was analyzed on eosinophils after activation with eotaxin and LTB4. Indeed, staining of Pirb−/−-eosinophils using the 6C1 antibody revealed no change in the expression of PIR-A (data not shown). Thus, the differential responses in the formation of kinase complexes with PIR-B are independent of PIR-A expression.
The effects of PIR-B on ERK1/2 phosphorylation
ERK1/2 has been proposed to be an essential downstream kinase involved in eosinophil migration.14,38-40 We thus hypothesized that the upstream phosphorylation events in the kinase–PIR-B complex after LTB4 or eotaxin stimulation will accordingly affect ERK1/2 phosphorylation. Indeed, as assessed by Western blot, we observed an increase in ERK1/2 phosphorylation after eotaxin stimulation in Pirb+/+ eosinophils. At baseline conditions, Pirb−/− eosinophils displayed significantly increased ERK1/2 phosphorylation (Figure 7A,B). This baseline effect was specific to ERK1/2 because p38 was not changed between Pirb−/− and Pirb+/+ eosinophils (data not shown). Furthermore, this phosphorylation pattern was significantly increased in Pirb−/− eosinophils (Figure 7A,C). For example, 3 minutes after eotaxin stimulation, wild-type mice displayed an increase in ERK phosphorylation, whereas Pirb−/− eosinophils showed a far greater increase (as observed by band intensity). These changes were even more intensified in later time points (ie, 3-5 minutes), whereas ERK1/2 phosphorylation was normalized to baseline in Pirb+/+ eosinophils but still phosphorylated in Pirb−/− eosinophils. Moreover, Pirb+/+ eosinophils displayed enhanced ERK1/2 phosphorylation in response to LTB4 stimulation peaking after 1 minute. However, Pirb−/− eosinophils displayed a significant decrease in ERK1/2 phosphorylation (ie, starting after 1 minute and the maximum decrease observed at 3 minutes), supporting an activation role for PIR-B (Figure 7B,D).
Assessment of ERK1/2 phosphorylation after eotaxin and LTB4 stimulation. Pirb−/− or wild-type (WT) eosinophils were stimulated with eotaxin-2 (10 ng/mL; A,C) or LTB4 (10 nM; B,D) at the indicated time points. Thereafter, the cells were lysed, analyzed by SDS-PAGE, transferred to a membrane, and blotted with antibodies to phospho-specific ERK1/2 (pERK1/2) or total ERK1/2 as loading control. Quantification of band intensity (C,D) of eotaxin and LTB4 stimulated eosinophils. Each time point was normalized to the baseline phosphorylation state of WT mice and presented as fold increase plus or minus SD. Data represent n = 3 experiments; *P < .05; **P < .01; ***P < .001 when comparing WT and Pirb−/− cells.
Assessment of ERK1/2 phosphorylation after eotaxin and LTB4 stimulation. Pirb−/− or wild-type (WT) eosinophils were stimulated with eotaxin-2 (10 ng/mL; A,C) or LTB4 (10 nM; B,D) at the indicated time points. Thereafter, the cells were lysed, analyzed by SDS-PAGE, transferred to a membrane, and blotted with antibodies to phospho-specific ERK1/2 (pERK1/2) or total ERK1/2 as loading control. Quantification of band intensity (C,D) of eotaxin and LTB4 stimulated eosinophils. Each time point was normalized to the baseline phosphorylation state of WT mice and presented as fold increase plus or minus SD. Data represent n = 3 experiments; *P < .05; **P < .01; ***P < .001 when comparing WT and Pirb−/− cells.
Discussion
Understanding the properties of inhibitory receptors has considerable implications for disease therapy.41 However, there is a surprising paucity of studies concerning inhibitory mechanisms regarding eosinophils. In this study, we aimed to dissect the activity of the inhibitory receptor PIR-B in murine eosinophils. Our results demonstrate several findings regarding the functions of PIR-B in the regulation of eosinophil chemotaxis as well as several novel findings that could be extended to other cell types. First, we demonstrate that PIR-B is highly expressed on eosinophils, and that tissue expression of PIR-B in response to allergen or IL-13 challenge is partially eosinophil dependent. Second, we demonstrate that PIR-B initiates an inhibitory signaling pathway that results in reduced eotaxin-induced eosinophil chemotaxis and tissue recruitment. Unexpectedly, PIR-B initiates an activating signaling pathway resulting in increased eosinophil chemotaxis in response to innate stimuli such as chitin- and thioglycollate-induced inflammation, both involving LTB4-dependent eosinophil migration. Third, we characterize the ability of PIR-B to interact with various kinases and to differentially affect the activation of downstream kinases, such as ERK1/2, in a fashion that supports a role for PIR-B as a receptor capable to induce both inhibitory and activation signals. Moreover, it is important to note that although signaling events in human eosinophils have been described, downstream pathways in murine eosinophils have received relatively limited attention. Therefore, the findings and methodology described herein contribute significantly to further elucidating signaling pathways in murine eosinophils. Because most of the in vitro studies described herein were conducted on eosinophils from IL-5Tg mice, we cannot exclude an interaction between IL-5 and PIR-B that may affect our results. Nevertheless, inflammatory conditions promoting tissue eosinophilia are associated with increased IL-5. Thus, our results may actually reflect how inhibitory receptors regulate eosinophils under disease conditions.
Recent research on Pirb−/− mice has provided insight into the physiologic significance of the major histocompatibility complex (MHC) H-2 recognition by PIR-B in the immune response, especially in antigen presentation, humoral immunity, and transplantation.20 Thus, the finding that Pirb−/− mice under baseline conditions display enhanced eosinophil recruitment to the gastrointestinal tract suggests an active regulation of eosinophil homing by MHC class I molecules. Indeed, recent studies in human eosinophils revealed that approximately 25% of human peripheral blood eosinophils express p140, a member of the killer immunoglobulin-like receptor family that is known to inhibit natural killer cell functions in response to human leukocyte antigen-A (HLA-A) alleles such as HLA-A3 and HLA-A11.14 This finding provides another set of evidence for negative regulation of eosinophils by MHC class I molecules. Our finding that the eosinophils are mobilized to the esophagus of Pirb−/− mice indicates that PIR-B has an important role in normally suppressing eosinophil recruitment to the esophagus. We demonstrated previously that the esophagus is unique compared with all other gastrointestinal segments because it is devoid of resident eosinophils at baseline despite baseline eotaxin-1 expression comparable to the rest of the gastrointestinal tract.5 Our current findings provide the first molecular explanation for this paradox by demonstrating that PIR-B is a key inhibitory checkpoint for esophageal eosinophil trafficking. This suggests the importance of assessment of PIR-B ligand expression (class I molecules) in the esophagus at baseline and in eosinophilic esophagitis.
Several inhibitory receptors such as Siglec-8 and IRp60/CD300a have been shown to inhibit human eosinophil functions in vitro.14,16 However, direct roles for inhibitory receptors in the regulation of eosinophil functions in vivo have been scantly characterized. Recent data support a model in which PIR-B negatively regulates neutrophil and dendritic cell chemokine signaling.33 We therefore hypothesized that PIR-B will negatively regulate eosinophil responses to eotaxins, the cardinal and eosinophil-specific chemokines involved in eosinophil recruitment.3-9 Indeed, in response to IL-13, a potent regulator of lung chemokine expression, Pirb−/− mice display augmented eosinophil recruitment to the lungs and the esophagus. These data were corroborated in vitro because Pirb−/− eosinophils displayed increased chemotaxis toward eotaxin-1 and eotaxin-2. Surprisingly, Pirb−/− eosinophils and neutrophils displayed decreased chemotaxis in response to LTB4. LTB4 has been shown to be a potent chemotactic factor for eosinophils and neutrophils and involved in several diseases with an eosinophilic component, such as asthma and inflammatory bowel disease.12,13,39,42 In fact, BLT1, the LTB4 receptor, has been shown to regulate eosinophil chemotaxis in 2 independent models of eosinophilic inflammation (ie, chitin-induced and thioglycolate-induced eosinophilic inflammation).29,30 Thus, we investigated whether PIR-B can positively regulate LTB4-dependent eosinophil recruitment in vivo and determined that LTB4-dependent responses were significantly attenuated in the absence of PIR-B. Importantly, in the thioglycolate-induced inflammatory model, a dramatic decrease was also observed in neutrophil recruitment to the peritoneum. Because the accumulation of neutrophils in this experimental regime has not been reported to be dependent on LTB4,29 these data suggest that PIR-B can positively regulate other (yet to be defined) pathways. Importantly, this is the first report of an activation function for PIR-B.
PIR-B has been shown to interact with various kinases that may induce its tyrosine phosphorylation.20,33-35,37 The Src family kinases Hck and Fgr function as negative regulators of myeloid cell chemokine signaling by maintaining phosphorylation of PIR-B on neutrophils.33 Because Hck and Fgr have been suggested as downstream kinases of CCR3 signaling,43 it is likely that these kinases would interact with PIR-B in a similar fashion among eosinophils and neutrophils. We show that upon activation with eotaxin but not LTB4, PIR-B undergoes further tyrosine phosphorylation and recruitment of Hck but not Fgr. In fact, our findings concur with previous reports indicating that LTB4 does not stimulate Crk, Hck, or Fgr in human eosinophils.44 This observation is quite different from neutrophils, in which chemokine activation decreases tyrosine phosphorylation and decreases recruitment of Hck.33 Interestingly, Hck is recruited to PIR-B at a relatively late time point after eotaxin stimulation. This may suggest that other kinases rather than Hck phosphorylate PIR-B in eosinophils and that PIR-B recruits Hck to regulate other pathways other than inhibitory ones. Another difference between the kinases governing chemotactic responses in eosinophils and neutrophils can be observed from the function of Fgr. Fgr−/− neutrophils display increased chemotactic responses, whereas Fgr−/− mice display decreased recruitment of eosinophils to the allergen-challenged lung.33,45 Furthermore, Lyn kinase has been shown to play an essential role in the phosphorylation of PIR-B in neutrophils.34,37 In fact, in lyn−/− mice, PIR-B tyrosine phosphorylation was greatly reduced, and biochemical analysis of macrophages from lyn−/− mice revealed that Lyn has an essential role in the adhesion-dependent phosphorylation of the ITIM of the inhibitory receptors signal regulatory protein-1α (SIRP1α) and PIR-B.34,37 We report that Lyn kinase is probably not associated with PIR-B phosphorylation in eosinophils.
To assess whether the differential role of PIR-B in regulating eotaxin- or LTB4-induced responses can be determined by the formation of a different PIR-B–kinase complex, we characterized the ability of PIR-B to interact with various kinases after eotaxin and LTB4 activation. Our data support a model in which PIR-B after LTB4 activation can interact with several activating kinases, such as JAK1, JAK2, Shc, and Crk, that, in turn, execute a positive signaling cascade. For example, IL-5 recruits JAK2 and Shc in a similar kinetic fashion to activate eosinophils.46,47 Furthermore, the inhibitory receptor SIRPα, a close relative of PIR-B, was reported recently to stimulate nitric oxide production in macrophages via the Jak/STAT and PI3K/Rac1 pathways,48 thereby suggesting that an activating role for SIRPα uses a similar molecular mechanism to that of PIR-B. In fact, ITIM-bearing receptors such as TREM-like transcript-1 (TLT-1) and the G protein–coupled cholecystokinin (CCK) receptor have been shown to enhance cellular activation. For example, TLT-1 amplifies FcεRI-mediated calcium signaling, and CCK (via recruitment of SHP-2 to its ITIM) activates the AKT pathway.49,50 Although PIR-B contains 4 ITIMs in its cytoplasmic tail (SVYATL, VTYAQL, ETYAQV, and SLYASV), PIR-B contains a fifth tyrosine residue within the sequence TEYEQA. This sequence is a noncanonical immunoreceptor tyrosine-bases switch motif (ITSM) and resembles several switch motifs in the signaling lymphocytic activation molecule (SLAM) and Siglec family proteins.51 The coexistence of an ITSM-like domain within the PIR-B ITIMs raises the possibility that PIR-B may interact with adaptor molecules such as SLAM-associated protein and Ewing's sarcoma-related transcript and may explain the dual function of PIR-B similar to those found for other SLAM family receptors such as 2B4 (CD244).51,52 Further experiments will need to determine whether PIR-B uses a functional ITSM motif.
Recently, PIR-B has been shown to regulate dendritic cell maturation. Nevertheless, the precise intracellular mechanism responsible for this phenomenon is still unclear.23 Based on our findings, it is tempting to hypothesize that PIR-B may actually induce an activation signal rendering dendritic cells toward the myeloid phenotype.
In summary, we provide novel findings for the regulation of eosinophil chemotaxis by PIR-B. Our results substantially contribute to our understanding of inhibitory signaling in eosinophils and, perhaps, other myeloid cells. This insight into negative and positive regulation of eosinophils by the same cell surface receptor may help use inhibitory receptor–based tools for future therapeutic intervention in inflammatory diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Ido Bachelet, Simon P. Hogan, Nives Zimmermann, Bruce Bochner, Yi Zheng, and Hiromi Kubagawa for critical review of this manuscript and helpful discussions, Drs James and Nancy Lee (Mayo Clinic, Scottsdale, AZ) for the generous supply of anti-MBP, and Dr Joshua Boyce for helpful discussions about LTB4 measurements. We also thank Danielle Kroetz for technical assistance and Linda Keller for editorial assistance.
This work was supported by NIH grants P01 HL-076383 (M.E.R.) and R01 AI057803 (M.E.R.), the CURED and Buckeye Foundations, the Food Allergy Project, and a fellowship award (A.M.) from the Machiah Foundation, a supporting foundation of the Jewish Community Endowment Fund, the generous support of the Alexander M. and June L. Maisin Foundation, and Maurice Kanbar.
National Institutes of Health
Authorship
Contribution: A.M. designed research, performed research, analyzed and interpreted data, performed statistical analysis, and drafted the manuscript; M.L.M. performed research; J.S.B. performed research and analyzed and interpreted data; and M.E.R. designed research, drafted the manuscript, and supervised experiments.
Conflict-of-interest disclosure: M.E.R. is a consultant for Merck and Ception Therapeutics. A.M., M.L.M., and J.S.B. declare no competing financial interests.
Correspondence: Marc Rothenberg, MD, PhD, Division of Allergy and Immunology, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: Rothenberg@cchmc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal