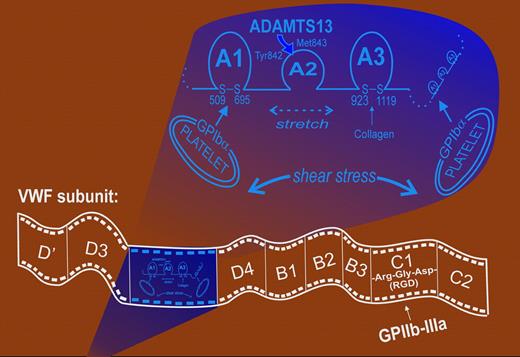
VWF is made up of subunits (consisting of domains D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2) forming disulfide-linked mutltimers of 20 million daltons or more. Multimer size is critical to the prothrombotic function of VWF, and regulation of multimer size by the ADAM (A Disintegrin And a Metalloproteinase)–family metalloproteinase ADAMTS13 controls a clinically important class of VWF-based pathologies, including thrombotic thrombocytopenic purpura (TTP). The more high molecular weight the VWF multimers (including those arising from ADAMTS13 dysfunction), the greater the potential for thrombosis.2 Establishing the mechanisms for regulating ADAMTS13-mediated VWF cleavage is the focus of much recent research because of the pathologic importance of understanding how this cleavage is regulated in vivo.1,–3
One of VWF multimers' primary roles is to support formation of platelet adhesion and aggregation. VWF contains 2 major platelet-binding domains: the A1 domain, which binds GPIbα of the GPIb-IX-V complex, and an Arg-Gly-Asp (RGD) sequence in the C1 domain that binds the platelet integrin αIIbβ3 (GPIIb-IIIa). ADAMTS13 cleaves at Tyr842/Met843 within the A2 domain, between A1 (which binds GPIbα) and A3 (which binds collagen). This cleavage, however, does not occur efficiently in native solution-phase VWF, and conformational changes of A2 are required to enable cleavage.2 Unlike A1 and A3, the A2 domain lacks an intra-domain disulfide bond, presumably enabling mechanical distortion and exposure of the cryptic Tyr842/Met843 cleavage site.
Projecting a dual role for platelet GPIbα in regulating ADAMTS13-mediated proteolysis of VWF. First, GPIbα binding to VWF A1 removes an inhibitory constraint of ADAMTS13-dependent A2 proteolysis at Tyr842/Met843. Second, binding of 2 or more platelets to an individual VWF multimer through GPIbα stretches the flexible A2 domain under shear to facilitate ADAMTS13 cleavage of A2, thereby decreasing the prothrombotic activity of high-molecular-weight multimers.
Projecting a dual role for platelet GPIbα in regulating ADAMTS13-mediated proteolysis of VWF. First, GPIbα binding to VWF A1 removes an inhibitory constraint of ADAMTS13-dependent A2 proteolysis at Tyr842/Met843. Second, binding of 2 or more platelets to an individual VWF multimer through GPIbα stretches the flexible A2 domain under shear to facilitate ADAMTS13 cleavage of A2, thereby decreasing the prothrombotic activity of high-molecular-weight multimers.
Previous studies suggest tensile stress applied across A1-A2-A3 facilitates ADAMTS13-dependent cleavage of A2.3 In this article, Shim et al use purified components in a reconstituted system under uniform shear conditions in a cone-and-plate viscometer to show shear-dependent platelet- and GPIbα-dependent ADAMTS13-mediated cleavage of VWF (inhibitable by anti-GPIbα antibodies or soluble GPIbα fragments).Thus, platelet GPIbα acts in 2 ways to favor VWF cleavage: by binding A1 (to remove an inhibitory effect on A2 cleavage)1 and by anchoring different A1 domains on an individual multimer to 2 or more platelets, interactions that under sufficient shear stress stretch the A2 domain and promote ADAMTS13-dependent proteolysis (see figure). Dong and colleagues have previously reported that ultralarge VWF strings expressed on activated endothelial cells (via P-selectin) can be decorated by multiple platelets under flow, and that in this form the VWF is susceptible to proteolysis by added plasma (ADAMTS13 dependent).2 These results suggest that a developing thrombus under arterial flow involving platelet attachment to VWF would be self-limiting in size, since shear applied to platelet-VWF-platelet complexes would promote ADAMTS13-mediated cleavage of VWF.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal