The production of hematopoietic cells is under the tight control of a group of hematopoietic cytokines. Each cytokine has multiple actions mediated by receptors whose cytoplasmic domains contain specialized regions initiating the various responses—survival, proliferation, differentiation commitment, maturation, and functional activation. Individual cytokines can be lineage specific or can regulate cells in multiple lineages, and for some cell types, such as stem cells or megakaryocyte progenitors, the simultaneous action of multiple cytokines is required for proliferative responses. The same cytokines control basal and emergency hematopoietic cell proliferation. Three cytokines, erythropoietin, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor, have now been in routine clinical use to stimulate cell production and in total have been used in the management of many millions of patients. In this little review, discussion will be restricted to those cytokines well established as influencing the production of hematopoietic cells and will exclude newer candidate regulators and those active on lymphoid cells. As requested, this account will describe the cytokines in a historical manner, using a sequential format of discovery, understanding, validation, and puzzlement, a sequence that reflects the evolving views on these cytokines over the past 50 years.
Discovery
Erythropoietin has had a long history, dating back to 1906, when an unfortunate publication1 described the discovery of hemopoietine (erythropoietin), based on impossible data. This unpromising start to the history of a distinguished hematopoietic regulator was followed in the 1940s and 1950s by increasing data from experimental animals showing that a humoral regulator of red cell formation, by then termed erythropoietin (EPO), did exist and was elevated in anemic plasma and urine. The original in vivo bioassays became based on the uptake of radiolabeled iron2 and were sophisticated for their time but, in fact, were cumbersome when used as a bioassay to monitor the purification of erythropoietin. It was therefore not until 1977 that EPO purification was achieved, having used urine from anemic patients as the source material.3 Based on amino acid sequence data from purified EPO, cDNAs for EPO were successfully cloned in 1985.4,5
The example set by erythropoietin led to the expectation that comparable regulators might exist that controlled cell production in other hematopoietic lineages. For the granulocyte-macrophage lineages, no progress was possible until the accidental discovery6,7 that solid-state culture systems could allow specific precursors of granulocytes and macrophages to proliferate and form colonies of maturing progeny. Cell proliferation was not autonomous, and the unknown cell product needing to be added to stimulate colony formation was given the operational name colony-stimulating factor (CSF). The expectation was that a single CSF might regulate granulocyte and macrophage colony formation in this culture system. CSF was a promising candidate regulator because levels fluctuated during infections and in other situations where perturbations of granulocyte-macrophage populations occurred. The CSF characterization project became progressively more complex with the final recognition that there were 4 different CSFs, all active on granulocyte-macrophage populations.
Initial attempts to purify CSF, using in vitro cultures of mouse bone marrow as the bioassay system, were commenced in 1968, again using human urine as the source material. By 1975, reasonable purity had been achieved of this material,8 but it proved disappointing in bioassay because it merely stimulated small macrophage colonies to develop. It was consequently termed M-CSF in this laboratory. Later, Richard Stanley turned to mouse L-cell–conditioned medium as a better starting material and achieved purification of this more active murine M-CSF (which he termed CSF-1) in 1977.9
Meantime, mouse lung–conditioned medium had been found to be a rich source of CSF that stimulated the formation of a more diverse set of granulocyte-macrophage colonies.10 Purification of this CSF, termed GM-CSF, also was published in 197711 —a golden year for hematopoietic cytokines. Obtaining enough GM-CSF for sequencing and then cloning a GM-CSF cDNA were more difficult tasks and were achieved only in 1984.12
Overlapping this work with GM-CSF was work on another type of CSF in mouse lung–conditioned medium. This CSF, when purified, was exceptionally hydrophobic and stimulated the formation only of tiny granulocytic colonies, earning it both the name G-CSF and the expectation that this CSF might not be of much importance in the biology of granulocytic populations.13,14 How misleading in vitro assays were to prove!
Confusing much of the early work on GM-CSF was the apparent presence in some materials, like medium conditioned by WEHI-3 leukemic cells or by mitogen-stimulated lymphocytes, of one or more factors stimulating not only granulocyte and macrophage colony formation, but also eosinophil, megakaryocyte, and erythroid colony formation. Events showed that these multiple actions were in fact the properties of a single cytokine. Using a simpler assay based on the measurement of 20 α-steroid dehydrogenase activity, Ihle's group succeeded in purifying this cytokine under the name interleukin-3 (IL-3).15 After purification was achieved, Ihle's group then had the luxury of watching others scramble to obtain IL-3 and establish its broad properties. At issue was the possibility that this might be a master CSF (termed by some pluripoietin) that might regulate the complete range of blood cells.
In general, the discovery and purification of the murine CSFs occurred before the corresponding human CSFs were characterized. Purified human GM-CSF was achieved in 198416 and cloned in 1985.17,18 The group at Sloan Kettering were convinced they had purified a human pluripoietin.19 By a twist of fate, their “pluripoietin” was in fact G-CSF, and this molecule was purified then sequenced and cloned simultaneously in the United States20 and Japan.21 The successful cloning and clinical development of G-CSF and erythropoietin were 2 major reasons for the astonishing emergence of Amgen as a force in molecular medicine.
The cloning of human M-CSF was achieved in 1985.22 Purification of native human IL-3 was only achieved in 1991.23 The isolation of the cDNA for human IL-3 proved a difficult technical task because of insufficient homology with murine IL-3. However, this was finally achieved and recombinant human IL-3 generated in 1986.24
Thus, between 1983 and 1986, cDNAs for EPO and all the CSFs were isolated and expressed in one short creative period in the history of hematopoietic cytokines.
In the period after CSF, 5 more hematopoietic cytokines of major interest were discovered.
Interleukin-5 (IL-5) was purified and cloned as a B-cell differentiation factor,25 but it soon became apparent that IL-5 was in fact the major regulator of eosinophil production.26 This was intriguing because the signaling β-chain of the IL-5 receptor is shared with the 2 other factors able to stimulate eosinophil proliferation: GM-CSF and IL-3.27,28
A factor was purified and cloned on the basis of its capacity to induce differentiation and suppress clonogenicity in a murine myeloid leukemia cell line.29 It was termed leukemia inhibitory factor (LIF) and found to be a cofactor in stimulating megakaryocyte colony formation.30
A factor was purified from rat liver–conditioned medium31 that had a prominent action on mast cell proliferation but, when in combination with other hematopoietic growth factors, this factor proved to be a powerful stimulus for the proliferation of stem cells and of early hematopoietic progenitor cells. Most commonly, it is now referred to as stem cell factor (SCF), and a cDNA for SCF was cloned in 1990.32,33 SCF was recognized to be the ligand for the membrane receptor c-kit, and deficiencies in SCF or c-kit were recognized to be the basis for the anemia in various mutant mouse strains.33,34
Simultaneously, with the discovery of SCF, a factor was cloned from a stromal cell line with the capacity to stimulate the proliferation of a murine plasmacytoma cell line.35 It was named interleukin-11 (IL-11) and proved to have actions on multiple hematopoietic lineages, including the stimulation of megakaryocytopoiesis.36
Flt3 is a tyrosine kinase receptor that was noted on stem cells and committed lymphoid precursors.37 The ligand for this receptor was cloned (FL)38 and shown to be an active proliferative stimulus for stem and developing dendritic cells, particularly when acting in synergy with other growth factors.
It had long been postulated that a humoral agent existed that regulated platelet production in an analogous action to that of EPO. The agent was termed thrombopoietin (TPO), and many attempts were subsequently made to purify and characterize this agent. Success came in an unexpected manner with the discovery of a murine leukemia virus whose active genome included Mpl, whose structure resembled that of a cytokine receptor,39 and cMpl appeared to be selectively expressed in megakaryocytes.40 With these clues, TPO was recognized as the ligand of Mpl and purified and cloned by purification from plasma, affinity purification, or expression cloning.41,,–44 TPO is a strong stimulus for megakaryocyte colony formation in vitro, particularly in combination with IL-3 and SCF.
At this stage, a somewhat simple view was developed by some that there may be growth factors active on early or stem cells (SCF, FL) that are coupled with factors acting on more mature precursors (eg, the CSFs) with finally several purely lineage-specific factors—EPO, TPO, and IL-5. Events were to shown that this model was far too naive and was incorrect in many details (Figure 1).
Three types of action of hematopoietic cytokines. (A) Lineage restricted. (B) Action on multiple lineages; broken line shows actions only at high concentrations. (C) Sequential actions; SCF acts on stem and early erythroid progenitors, while EPO acts on more mature precursors. The notion of sequential actions was later found to be incorrect.
Three types of action of hematopoietic cytokines. (A) Lineage restricted. (B) Action on multiple lineages; broken line shows actions only at high concentrations. (C) Sequential actions; SCF acts on stem and early erythroid progenitors, while EPO acts on more mature precursors. The notion of sequential actions was later found to be incorrect.
In the 1970s, the emerging work on hematopoietic regulators was largely dismissed by cellular immunologists as not being of relevance for lymphoid cells. Their data persuaded them that antigens were what controlled the proliferation of B and peripheral T cells. What resulted was a delay of almost a decade before the beginning of work on lymphoid-active cytokines. The first agent to be characterized was IL-2, a T cell–derived agent stimulating the proliferation of T cells, which was purified in 1982 then cloned in 1983.45,46 Thereafter, in this initial phase, additional agents active on T or B cells were uncovered (IL-4 mainly acting as a cofactor47 ; IL-6 active on B-cell differentiation,48 and IL-7 cloned49 as a B-cell stimulus that later emerged as a key factor in T-lymphocyte formation).
The present regulatory situation for lymphoid and dendritic cells is highly complex and not appropriate for the present discussion. With one or 2 minor exceptions, it is quite intriguing that there is little overlap in biologic actions between hematopoietic and lymphoid regulatory factors. Lymphoid cells do differ importantly in their possession of major cell-cell membrane triggering systems with exquisite specificity based on immunoglobulin and T-cell receptor heterogeneity. Possibly this results in a more sophisticated regulatory system, but some of the features to be discussed in the following section remain common to both hematopoietic and lymphoid-active regulators.
As a side comment on lymphoid cytokines, it is curious that none have yet entered routine clinical usage. Interest in clinical applications for IL-2 was high initially but led to disappointment. More recent agents seem scarcely to have entered clinical trials, and this remains a curious aspect of what should be a closely allied field to hematopoietic cytokines.
Understanding
The hematopoietic cytokines exhibited features that were initially met with puzzlement and skepticism but have since become accepted as features of these cytokines. The first novel feature concerned the unusual tissue sources of the cytokines (Figure 2). The initial expectation was that cytokines would be similar to hormones and have a limited, or single, organ source. For EPO this seemed to be the case, with the kidney being the outstanding source of EPO and with renal disease leading to low EPO levels and anemia.50 Similarly, a major source of TPO appears to be the liver.51
Varying tissue origin of hematopoietic cytokines. (A) EPO is mainly a product of kidney tissue. (B) GM-CSF is a product of multiple tissues and cell types. (C) M-CSF, CSF-1 can be a humoral factor and the product of many tissues or a membrane-displayed factor on local stromal cells.
Varying tissue origin of hematopoietic cytokines. (A) EPO is mainly a product of kidney tissue. (B) GM-CSF is a product of multiple tissues and cell types. (C) M-CSF, CSF-1 can be a humoral factor and the product of many tissues or a membrane-displayed factor on local stromal cells.
The CSFs proved to be radically different. Multiple organs were found to be capable of producing one or other CSF52 and, indeed, individual organs were able to produce multiple forms of CSFs simultaneously. This situation proved also to be the case for IL-6, LIF, and IL-5.53,54 The exact cellular sources of each cytokine have yet to be established, but clearly, for the CSFs, multiple cell types have synthetic activity.
Further complicating the biology was the recognition that these cytokines could be humoral or paracrine in nature and that for some, such as M-CSF or SCF, active membrane-displayed molecules were documented in addition to the secreted forms.32,55 Typically, serum concentrations of cytokines were low, but these could be rapidly elevated up to 1000-fold by inducing agents such as blood loss or endotoxin injection.56
All of these features continue to present difficulties in precisely measuring concentrations of cytokines impinging on hematopoietic cells and in establishing the precise source of a cytokine in any particular situation and its ultimate fate. For some, such as M-CSF,57 TPO,58 or G-CSF,59 a strong case has been made that target cell receptor-mediated endocytosis, then destruction, are the major fates of the cytokines. For other CSFs, the situation is less clear, and degradation in the liver and kidney also appears to be a feature of their biology.56
Cytokines proved to be highly active agents with maximal responses, often able to be induced with concentrations in the picogram-to-nanogram per milliliter range. There are specific membrane receptors for each cytokine that transmit signals to the cell following interaction with the cytokine. The numbers of such receptors per cell are surprisingly low—typically a few hundred per cell. Commonly, receptors share subunits with other receptors, suggesting close ancestral relationships between these systems despite the differing amino acid sequences of the cytokines.27,56
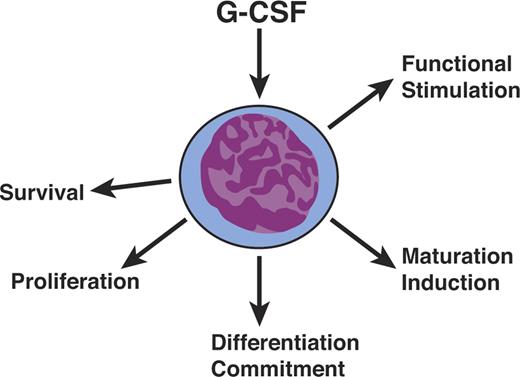
The most unexpected feature of cytokines, as best illustrated by the CSFs, was their polyfunctionality (Figure 3). They were not simply proliferative stimuli but also had actions affecting survival, differentiation commitment, induction of maturation, and functional activation of mature cells. These diverse actions were initially widely disputed but have become accepted as characteristics of cytokine action. Acceptance was greatly enhanced when the unique receptors for cytokines were examined in detail and their cytoplasmic domains found to have specialized regions, providing the physical basis for the ability of a single receptor type to signal disparate effects within a cell.60,61
Hematopoietic cytokines are polyfunctional. Hematopoietic cytokines such as G-CSF are not simply mandatory proliferative stimuli but also act on cell survival, differentiation commitment, maturation induction, and the functional stimulation of mature cells.
Hematopoietic cytokines are polyfunctional. Hematopoietic cytokines such as G-CSF are not simply mandatory proliferative stimuli but also act on cell survival, differentiation commitment, maturation induction, and the functional stimulation of mature cells.
A survival action of cytokines, now known to be mediated by suppression of apoptosis, on both progenitor and mature cells was readily documented and was uncontroversial.62,63 However, this action led some to propose that cytokines had no proliferative actions, merely survival actions that then allowed hematopoietic cells to proliferate spontaneously. This notion was difficult to refute experimentally, and it required characterization of the specific signaling pathways activated by cytokine action that were necessary for progression through the cell cycle.64 Demonstration that the survival-inducing domains were in a different location on the receptor from proliferation-inducing domains finally settled this dispute.61
The action of cytokines in inducing differentiation commitment also was disputed because of what appeared to be firm evidence that insertion of receptors for EPO or M-CSF into inappropriate cell types did not appear to influence their subsequent ligand-driven lineage commitment or maturation.65,66 It was equally clear, however, that cytokines such as G-CSF, IL-6, or LIF could induce differentiation commitment and suppress self-generation in various leukemic cell lines.67 With certain leukemias, cytokines had an even more dramatic action in inducing the leukemic cells to behave as appropriate normal progenitor cells.68 Definitive evidence that inserted receptors could induce differentiation commitment was obtained by inserting IL-2Rβ receptors into common lymphoid progenitor cells, which up-regulated GM-CSF receptors, allowing stimulation of granulocyte and macrophage formation by GM-CSF.69
Maturation initiation was reported in unstimulated factor-dependent continuous cell lines, where viability was maintained by overexpression of bcl2.70 On this basis, maturation was proposed to not be a function needing to be induced by cytokines. However, maturation-inducing domains have since been documented in cytokine receptors that are mandatory for full cellular maturation to occur in cells.61
Activation by cytokines of various functional properties of mature cells was easy enough to document and was noncontroversial but further added to the conceptual difficulty in explaining how a single receptor system could initiate responses that had to be located in quite different regions of the responding cell.
As the discovery of additional cytokines continued, the situation became more confusing because cells in any one lineage were clearly responsive to more than one cytokine. For example, G-CSF, GM-CSF, IL-3, M-CSF, SCF, and IL-6 all were found to have proliferative effects on granulocyte colony formation.56 Were different subsets of the precursor cells responsive to these different cytokines? Was there massive redundancy between cytokines? Was there a necessity for complex consortia of cytokines to act, or act most efficiently, to achieve the proliferation of these populations?
An important facet of the action of cytokines is, indeed, that there are situations where a combination of cytokines is essential to stimulate the proliferation of cells. For example, stem cells,71,–73 immature cells surviving 5FU treatment,74 and a majority of megakaryocytic precursors75 all require simultaneous stimulation by a combination of cytokines before proliferation occurs. In addition, some combinations of cytokines were found to be capable of stimulating more proliferation than was able to be stimulated by either cytokine alone, regardless of the concentration used.76 Interestingly, the cytokine combinations able to exhibit synergy of this type usually involve one cytokine that uses a tyrosine kinase receptor and a second cytokine that uses one of the various nonkinase-containing cytokine receptors.
The slow and labor-intensive work involved in purifying the early cytokines such as EPO and the CSFs later paid a dividend in that there were relatively few surprises in the biologic actions of these agents when tested in pure form, either in vitro or in vivo. This is in sharp contrast to many of the newer candidate cytokines that have been cloned with minimal knowledge of their possible functions. For these, a long period of characterization lies in the future that will possibly end up taking as much time as was spent with the earlier cytokines.
Validation
The low concentrations of cytokines in the blood, but their elevation in emergencies and the possible redundancy of cytokines, suggested to some that these molecules might simply be emergency molecules and that basal hematopoiesis might be regulated in some other manner.
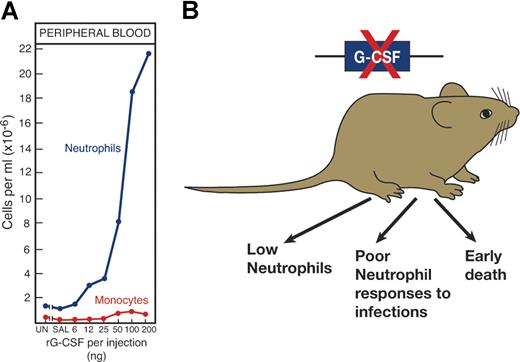
Validation that cytokines are major regulators of hematopoiesis came from several directions (Figure 4). First, in vivo injection of recombinant cytokines produced effects on relevant hematopoietic lineages that were clearly evident in the case of EPO,3 G-CSF,77 GM-CSF, and IL-3.78,79 For reasons yet to be established, the injection of some other cytokines did not produce clear increases in cell production.
The importance of a hematopoietic cytokine such as G-CSF can be validated in several ways. (A) By injecting G-CSF to elevate neutrophil levels and (B) by deleting the gene, a procedure resulting in low neutrophil levels and poor neutrophil responses to challenge infections.
The importance of a hematopoietic cytokine such as G-CSF can be validated in several ways. (A) By injecting G-CSF to elevate neutrophil levels and (B) by deleting the gene, a procedure resulting in low neutrophil levels and poor neutrophil responses to challenge infections.
The multiorgan origin of many cytokines prevented simple organ removal as a means of documenting the role of the cytokine concerned. Fortunately, molecular biology presented an exquisitely selective method for suppressing or promoting production of the cytokine by gene inactivation or overexpression. For some, such as EPO,80 G-CSF,81 TPO,43 and M-CSF,82 deletion of the gene or the gene for the relevant receptor produced dramatic reductions in cell populations that were, in fact, lethal in the case of EPO.80 In other instances, such as with GM-CSF, cell numbers appeared unaltered, but mature cell functional activity was unequivocally reduced.83,84
All deletions, either naturally occurring, as in the case of M-CSF (op/op mice)85 or SCF (Wv and Sl mutants) or artificially induced, have resulted in phenotypic changes in virtually all cases.
Further documentation was obtained by creating transgenic mice or mice engrafted with marrow cells engineered to overproduce the cytokine in question. This approach documented dramatic effects of overproduction of EPO, G-CSF, GM-CSF, IL-3, and LIF.56
Above all, the validation of the cytokines EPO, G-CSF, GM-CSF, SCF, and IL-11 came from their trial in humans. In particular, clinical trials showed EPO to be highly effective in correcting the anemia of renal disease,86 and G-CSF and GM-CSF were effective in correcting low neutrophil levels most commonly encountered as a complication of chemotherapy.87,,–90 The agents were relatively nontoxic, had no serious side effects due to actions on other cell types, and were not antigenic. These 3 agents have entered routine clinical use, and in total have been used in millions of patients.
Other cytokines have not become successful clinical agents because of side effects mediated by activated mature cells (IL-3, M-CSF) or antigenicity of the form used (TPO).
Of special importance was the observed ability of injected G-CSF and GM-CSF to elevate the levels of stem and progenitor cells in the peripheral blood.91,,–94 This has resulted in the use of peripheral blood stem cells as a more effective and simpler substitute for bone marrow cells95 in the now-expanded clinical transplantation applications.
Perplexity
Until the mid-1980s, the world of hematopoietic regulators appeared logical enough despite the unexpected multilineage actions, multipotential actions, and multiorgan origins. There was little suggestion that hematopoietic regulators were concerned with anything else but hematopoiesis.
This orderly world came tumbling down almost simultaneously with 2 regulators, IL-6 and LIF. Both had been purified and cloned as apparently unexceptional hematopoietic-active agents—IL-6 as a B-lymphocyte differentiating factor48 and LIF as an agent able to induce maturation and suppression of self-replication in a myeloid leukemic cell line.29,96 Both received validation by in vivo overexpression systems, and LIF also emerged as a reasonably active stimulus for platelet formation. Thereafter, IL-6 increasingly proved to have actions on hepatocytes, neuronal precursor cells, mesangial cells, osteoclasts, and many other cell types.97 LIF had an equally astonishing set of actions ranging from being necessary to maintain murine embryonic stem (ES) cells in an undifferentiated state (the opposite action to the one used to purify LIF) to being necessary for blastocyst implantation and successful pregnancy with, in addition, effects on autonomic nerve signaling, adipocyte function, liver production of acute phase proteins, pituitary production of adrenocorticotrophic hormone (ACTH), muscle regeneration, osteoblast proliferation, and still other actions (Figure 5).30 What possible logic could allow such potentially dangerous disparate functional activities to be mediated by a single molecule? For LIF, the presence of blocking soluble receptors98 could serve to restrict LIF action to local sites of production, thus removing some of the potential hazards of polyfunctionality. However, for IL-6, soluble receptors accentuated the problem because complexes of IL-6 soluble receptor with IL-6 could bind to any cell expressing the gp130 β-chain, allowing the IL-6 receptor complex then to deliver a positive stimulus.97 Most relevant to the present discussion, should IL-6 and LIF ever have been regarded as bona fide hematopoietic regulators? Why focus on their hematopoietic actions and ignore their other powerful actions?
The confusing nature of some hematopoietic cytokines as illustrated by LIF. LIF was isolated as a factor active in suppressing a myeloid leukemic cell line. However, it has obvious additional actions on multiple tissues and is essential for blastocyst implantation and successful pregnancy.
The confusing nature of some hematopoietic cytokines as illustrated by LIF. LIF was isolated as a factor active in suppressing a myeloid leukemic cell line. However, it has obvious additional actions on multiple tissues and is essential for blastocyst implantation and successful pregnancy.
Almost predictably, the situation became worse. IL-11, until then a reasonable stimulus for platelet formation, was shown to have many of the polyfunctional actions of LIF and IL-6,99 as also was oncostatin M.100
Meantime, even the gold standard regulators are now recognized to have some nonhematopoietic actions. M-CSF is necessary for placental development and pregnancy and male fertility.82,101 Most puzzling, EPO has been claimed to have protective actions in tissue, and particularly neuronal, injury.102 It has yet to be established how many of the newer candidate regulators will also prove to have unexpected polyfunctional properties.
The future
Considering the results of gene deletion and the interbreeding of such manipulated animals, we still have missing regulators for the formation of macrophages, megakaryocytes, platelets, granulocytes, eosinophils, and possibly even factors active on erythropoietic cells. We still remain unable to direct effectively the proliferation and self-generation of hematopoietic stem cells. Even including the possible actions of stromal factors, there clearly are additional agents at work in vivo to control stem cells in a manner that cannot yet be mimicked in vitro.
In the early days of the past 50-year saga of hematopoietic regulators, the future appeared uncomplicated. Candidate factors emerged, assays were developed, and it remained only for hard work to purify, clone, and mass produce a limited number of factors for cells of each lineage.
For some companies and clinicians, this remains the mental approach. Simply find a good agent for each lineage and the job is finished—EPO for erythropoiesis, G-CSF for neutrophil production, et cetera. For those who actually work in the field, the enormous complexity of new information and unanticipated numbers of candidate regulators are very much at odds with the simple view held 50 years ago.
Given the now-formidable numbers of established regulators and the even larger number of candidate regulators that all potentially interact with one another in inhibitory or synergistic actions, is there any possibility of understanding and intervening intelligently in hematopoiesis—particularly if many of these “hematopoietic” regulators are in fact polyfunctional agents with hematopoietic effects that may be peripheral in importance?
The present situation is confusing and may encourage a certain level of frustration. However, certain facts stand out clearly. The administration of single agents can have predictable and dramatic effects on hematopoiesis despite all the potentially interacting and buffering networks. EPO does increase red cell production, and G-CSF does stimulate neutrophil production, regardless of potential interactions with other agents. We need to keep these simple facts in mind. Every member of this network of regulators is likely to prove to have a unique role in a particular situation, and our future task is now to establish precisely what roles are played by each of these candidate regulators and in what clinical situations the application of these regulators may prove to be of value.
Acknowledgments
This work was supported by grants from the Cancer Council Victoria and the National Health and Medical Research Council, Canberra, and grant CA22556 from the National Institutes of Health, Bethesda, MD.
The Walter and Eliza Hall Institute shares the patents rights for GM-CSF.
National Institutes of Health
Authorship
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Donald Metcalf, The Walter and Eliza Hall Institute of Medical Research; 1G Royal Parade, Parkville Victoria 3050, Australia; e-mail: metcalf@wehi.edu.au.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal