Computed tomography (CT) is currently the most commonly used means for staging malignant lymphoma. 18F-fluoro-2-deoxyglucose positron emission tomography (FDG-PET), FDG-PET/CT fusion, and whole-body magnetic resonance imaging (WB-MRI) are potential alternatives. The purpose of this study was to systematically review published data on the diagnostic performance of CT, FDG-PET, FDG-PET/CT fusion, and WB-MRI in staging of malignant lymphoma. In addition, technical aspects, procedures, advantages, and drawbacks of each imaging modality are outlined. Three CT studies, 17 FDG-PET studies, and 4 FDG-PET/CT fusion studies were included in this systematic review. The studies were of moderate methodological quality and used different scoring systems to stage malignant lymphoma. CT remains the standard imaging modality for initial staging of malignant lymphoma, while FDG-PET has an essential role in restaging after treatment. Early results suggest that FDG-PET/CT fusion outperforms both CT alone and FDG-PET alone. Data on the diagnostic performance of WB-MRI are lacking. Future well-designed studies, expressing their results according to the Ann Arbor staging system, are needed to determine which imaging modality is most accurate and cost-effective in staging malignant lymphoma.
Introduction
The malignant lymphomas, Hodgkin disease (HD) and non-Hodgkin lymphoma (NHL), comprise approximately 5% to 6% of all malignancies and are the fifth most frequently occurring type of cancer in the United States. In 2007, an estimated 9260 new cases of HD and 81 850 new cases of NHL will be diagnosed in the United States.1 Once the diagnosis HD or NHL has been established by biopsy of a particular site, determination of disease extent (staging) is important for appropriate treatment planning and determining prognosis. In addition, knowing the sites of involvement at time of diagnosis makes it possible to accurately restage at the end of therapy and document a complete remission.2,3 Staging of HD and NHL is based on the Ann Arbor classification with the addition of a definition of bulky disease often referred to as the Cotswold modification (Table 1). This staging system encompasses the number of sites of disease involved, the type of involvement (nodal or extranodal), and the distribution of disease.4
Computed tomography (CT) is currently the most commonly used means for staging patients with malignant lymphoma.2,3 However, CT lacks functional information, which impedes identification of disease in normal-sized organs. 18F-fluoro-2-deoxyglucose positron emission tomography (FDG-PET) may be an alternative to CT.5,6 Other promising alternatives to CT are FDG-PET/CT fusion7,8 and whole-body magnetic resonance imaging (WB-MRI).9,10 The purpose of this study is to provide an up-to-date overview of the diagnostic performance of CT, FDG-PET, FDG-PET/CT fusion, and WB-MRI in staging of malignant lymphoma.
Cotswold-modified Ann Arbor classification
| . | Involvement/features . |
|---|---|
| Stage | |
| I | Single lymph node region (I) or one extralymphatic site (IE) |
| II | Two or more lymph node regions, same side of the diaphragm (II) or local extralymphatic extension plus one or more lymph node regions same side of the diaphragm (IIE) |
| III | Lymph node regions on both sides of the diaphragm (III), which may be accompanied by local extralymphatic extension (IIIE) |
| IV | Diffuse involvement of one or more extralymphatic organs or sites |
| Suffix | |
| A | No B symptoms |
| B | Presence of at least one of the following: unexplained weight loss >10% baseline during 6 months before staging; recurrent unexplained fever >38°C; recurrent night sweats |
| X | Bulky tumor is defined as either a single mass of tumor tissue exceeding 10 cm in largest diameter or a mediastinal mass exceeding one-third of the maximum transverse transthoracic diameter measured on a standard posterior-anterior chest radiograph |
| . | Involvement/features . |
|---|---|
| Stage | |
| I | Single lymph node region (I) or one extralymphatic site (IE) |
| II | Two or more lymph node regions, same side of the diaphragm (II) or local extralymphatic extension plus one or more lymph node regions same side of the diaphragm (IIE) |
| III | Lymph node regions on both sides of the diaphragm (III), which may be accompanied by local extralymphatic extension (IIIE) |
| IV | Diffuse involvement of one or more extralymphatic organs or sites |
| Suffix | |
| A | No B symptoms |
| B | Presence of at least one of the following: unexplained weight loss >10% baseline during 6 months before staging; recurrent unexplained fever >38°C; recurrent night sweats |
| X | Bulky tumor is defined as either a single mass of tumor tissue exceeding 10 cm in largest diameter or a mediastinal mass exceeding one-third of the maximum transverse transthoracic diameter measured on a standard posterior-anterior chest radiograph |
In the first part of this article, technical aspects and procedures of each imaging modality are summarized. Subsequently, we describe our literature search strategy, study selection methods, and study analysis. Next, literature search results and results of analyzed articles are demonstrated. Finally, results are discussed, and advantages, drawbacks, and limitations of each imaging technique are outlined.
Technical aspects and procedures
CT
Before the CT era, patients with a diagnosis of malignant lymphoma were subjected to a battery of radiologic studies that included chest radiography, intravenous pyelography, lymphangiography, skeletal surveys, and isotope scans. On top of this, most patients with HD underwent staging laparotomy with its attendant risks.11 The introduction of CT in the early 1970s was a tremendous breakthrough in noninvasive imaging, and its potential for staging malignant lymphoma was soon recognized and investigated.12 Since then, CT has gradually become the imaging modality of choice for staging malignant lymphoma. CT technology has continuously been developed and refined; major milestones include the introduction of spiral CT in the early 1990s and the advent of multidetector-row CT in 1998. The concept of multidetector-row CT deserves special attention; multidetector-row CT scanners have multiple (X) data acquisition systems connected to multidetector arrays to provide a multiple (X)-section scan, increasing the speed of data collection by a factor X over single detector-row CT scanners. In addition, current multidetector-row CT scanners have a faster gantry rotation. These 2 properties enable acquisition of thinner slices in a shorter time, compared with single detector-row CT scanners, which minimizes or eliminates breathing artifacts.13,14 As a result, lymph nodes of 5 mm or less in diameter can be detected throughout the whole body. In combination with powered injectors for rapid bolus administration of intravenous contrast medium, focal extranodal lesions on the order of a few millimeters can be identified.15,16 State-of-the-art CT for staging malignant lymphoma is currently performed on at least 4-section multidetector-row CT scanners. Patients receive an intravenous injection of iodinated contrast medium and generally are given oral contrast agent prior to scanning.
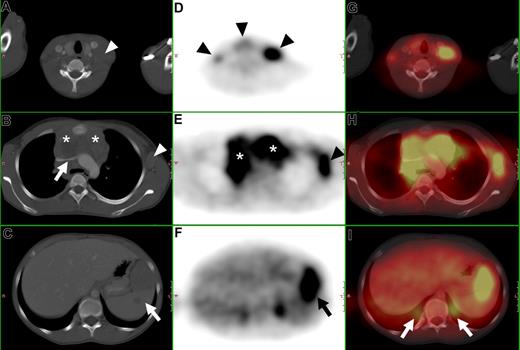
Determination of nodal involvement is based on size criteria. Lymph nodes with a short-axis diameter greater than 10 mm are generally considered positive. Furthermore, clustering of normal-sized but prominent lymph nodes in the anterior mediastinum and the mesentery is suspicious for disease. The use of intravenous contrast medium is not helpful in differentiating normal from malignant lymph nodes. General criteria for extranodal involvement are organomegaly, abnormal mass or structural changes in a normal-sized organ, and abnormal contrast enhancement (Figures 1A-C).16
CT, FDG-PET, and FDG-PET/CT fusion of a 13-year-old female with Hodgkin disease. (A-C) Axial CT images show (A) a cervical lymph node mass ( ), (B) a large mediastinal mass (*) compressing the left brachiocephalic vein (
), (B) a large mediastinal mass (*) compressing the left brachiocephalic vein ( ), an enlarged axillary lymph node (
), an enlarged axillary lymph node ( ), and (C) an enlarged inhomogeneous spleen (419). (D-F) Axial PET images show pathologic FDG uptake in (D) cervical lymph nodes (
), and (C) an enlarged inhomogeneous spleen (419). (D-F) Axial PET images show pathologic FDG uptake in (D) cervical lymph nodes ( ), (E) in the mediastinum (*), in a left axillary lymph node mass (
), (E) in the mediastinum (*), in a left axillary lymph node mass ( ), and (F) in the spleen (
), and (F) in the spleen ( ). (G-I) Fused PET/CT images. (I) Note the misregistration of normal renal FDG excretion (
). (G-I) Fused PET/CT images. (I) Note the misregistration of normal renal FDG excretion ( ).
).
CT, FDG-PET, and FDG-PET/CT fusion of a 13-year-old female with Hodgkin disease. (A-C) Axial CT images show (A) a cervical lymph node mass ( ), (B) a large mediastinal mass (*) compressing the left brachiocephalic vein (
), (B) a large mediastinal mass (*) compressing the left brachiocephalic vein ( ), an enlarged axillary lymph node (
), an enlarged axillary lymph node ( ), and (C) an enlarged inhomogeneous spleen (419). (D-F) Axial PET images show pathologic FDG uptake in (D) cervical lymph nodes (
), and (C) an enlarged inhomogeneous spleen (419). (D-F) Axial PET images show pathologic FDG uptake in (D) cervical lymph nodes ( ), (E) in the mediastinum (*), in a left axillary lymph node mass (
), (E) in the mediastinum (*), in a left axillary lymph node mass ( ), and (F) in the spleen (
), and (F) in the spleen ( ). (G-I) Fused PET/CT images. (I) Note the misregistration of normal renal FDG excretion (
). (G-I) Fused PET/CT images. (I) Note the misregistration of normal renal FDG excretion ( ).
).
FDG-PET
Positron emission tomography (PET) was developed in the early 1970s soon after CT.17 PET is based on the use of positron-emitting radiopharmaceuticals and the detection in coincidence of the 2 nearly collinear 511-keV photons emitted following positron annihilation with an electron. The increased glycolytic rate of malignant cells is the rationale behind the common use of 18F-fluoro-2-deoxyglucose (FDG) as a radiotracer in oncological PET studies.6 Imaging of malignant lymphoma with FDG was first described in 1987,18 and the first reports on FDG-PET as a whole-body staging method in malignant lymphoma appeared in the 1990s.19,,–22 PET technology has improved dramatically since its development. Initial patient imaging units had a system resolution greater than 15 mm, whereas current units have a 4 to 5 mm resolution.6 FDG-PET examinations for staging malignant lymphoma should be performed on dedicated (full ring) PET scanners, because dual-head gamma cameras in coincidence mode are unreliable in detecting lesions less than 15 to 20 mm in diameter.23,,–26 Raw data should be reconstructed by means of iterative expectation maximization algorithms, which provide superior signal-to-noise ratio compared with filtered back-projection images.6 Attenuation effects (scatter or absorption of emitted photons in the body) produce regional nonuniformities, distortions of intense structures, and edge effects. To improve anatomic delineation, additional transmission scanning for attenuation correction using an external radiation source is required. Attenuation correction also allows for semiquantitative evaluation, which offers a more objective way to assess FDG uptake. Nonattenuation corrected images should, however, also be evaluated, because the attenuation correction itself may also introduce image artifacts.6,27 Patients are required to fast for at least 4 to 6 hours prior to scanning, which starts approximately 60 minutes after the injection of a typical FDG dose of 370 MBq. Serum glucose levels of less than 150 mg/dL are desirable. Patients are also instructed to avoid any kind of strenuous activity prior to the examination and following injection of the radioisotope to avoid physiologic muscle uptake of FDG.
Any focus of visually elevated FDG uptake relative to the background, not located in areas of physiologically increased uptake or where the clinical data do not suggest the presence of a nonmalignant hypermetabolic lesion, is regarded as positive for malignant lymphoma. In organs with physiologic FDG uptake (eg, spleen and liver), focal or inhomogeneous uptake patterns are considered to be indicative of malignant lymphoma (Figure 1D-F). Cut-off values for semiquantitative evaluation (measurement of standardized uptake values) of suspected foci have not been reported yet in the literature, to our knowledge.
Although beyond the scope of this review, FDG-PET also has high potential as a biomarker of response to chemotherapy in malignant lymphoma.28,29 Early interim FDG-PET has already proven to overshadow the prognostic value of the International Prognostic Score and appears to be the single most important tool for planning risk-adapted treatment in advanced HD.30
FDG-PET/CT fusion
FDG-PET and CT provide functional and anatomic information, respectively. Integration of both modalities may outperform both FDG-PET alone and CT alone in staging of malignant lymphoma. In traditional visual image fusion, FDG-PET and CT images are viewed and compared next to each other, with the fusion taking place in the interpreter's mind. Integration of separate FDG-PET and CT image sets into a single study can be achieved with software fusion. However, differences in scanner bed profiles, external patient positioning, and internal organ movement present a challenge to the software approaches (Figure 1I). These challenges have recently been addressed by the introduction of the combined PET/CT scanner, a hardware-oriented approach to image fusion. With this type of scanner, accurately registered anatomic and functional images can be acquired in a single examination, which has been shown to increase both the accuracy of the interpretation and the confidence level of the readers. Several manufacturers are now offering integrated FDG-PET/CT systems combining different models of dedicated PET scanners and multidetector-row CT scanners in line with a common imaging bed.7,8
Patient preparation is similar to that of FDG-PET alone. It is important to apply an appropriate respiration protocol to minimize the mismatch between CT and FDG-PET. On completion of the CT portion of the examination, the patient couch is advanced into the PET field-of-view, and a multibed PET study is acquired over the same range as the CT scan. CT images are used for attenuation correction of the FDG-PET emission data. Again, both images with and without attenuation correction should be evaluated. There is no consensus yet whether intravenous and oral contrast materials are needed for a combined FDG-PET/CT examination.7,8
Fused FDG-PET/CT images are interpreted using a combination of the criteria as described for CT and FDG-PET alone (Figure 1G-I).
WB-MRI
The concept that MRI might become the ultimate whole-body imaging tool was initially proposed by the MRI pioneers Damadian and Lauterbur in 1980.31,32 The high spatial resolution and excellent soft-tissue contrast make MRI an ideal tool for the detection of parenchymal and osseous lesions. However, because of long imaging time, limited availability, and extensive costs, MRI was previously used only as a tool to image limited anatomical areas of the body. Recent improvements in MRI technology have resulted in the availability of sufficiently fast and diagnostic sequences for WB-MRI. Additionally, the introduction of a rolling bed patient platform, enabling data acquisition of different anatomical regions in rapid succession, has overcome the time-consuming problem of stepwise manual repositioning of the patient. As a result, WB-MRI has become feasible for staging malignancies, including malignant lymphoma.9,10
MRI systems operating at 1.5 T are widely available and provide high image quality of all body regions in reasonable measuring times. WB-MRI at higher field strength (3.0 T) has high potential, but has not yet proven to be equal or superior to WB-MRI at 1.5 T.33,34 The use of a phased-array surface coil is preferred because it provides an increased signal-to-noise ratio and spatial resolution compared with an integrated body coil. There is no standard WB-MRI protocol for staging malignant lymphoma yet; data regarding preferred sequence and imaging plane are lacking. A commonly recommended approach for tumor staging in general is the application of fat-suppressed, T1-weighted gradient echo sequences, before and after the administration of intravenous gadolinium. The fluid-sensitive, fat-suppressed, T2-weighted short-tau-inversion-recovery sequence is useful for the assessment of the bone marrow (Ann Arbor stage IV) and the pelvis.9,10
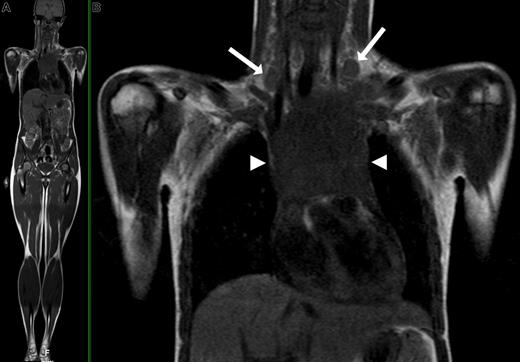
Although MRI inherently provides superior soft-tissue contrast to CT and has the potential to characterize lesions on the basis of signal characteristics, assessment of nodal involvement is still based on size criteria, where lymph nodes with a short-axis diameter greater than 10 mm are generally considered positive (similar to CT). General criteria for extranodal involvement are any signal abnormalities or mass lesions involving soft tissues, bones, parenchymal organs, and serosal cavities (Figure 2).
WB-MRI of a 16-year-old female with Hodgkin disease. (A) Coronal T1-weighted WB-MRI. (B) Close-up image shows bilateral enlarged cervical lymph nodes (arrows) and a large mediastinal mass (arrowheads) consistent with confluent lymphadenopathy.
WB-MRI of a 16-year-old female with Hodgkin disease. (A) Coronal T1-weighted WB-MRI. (B) Close-up image shows bilateral enlarged cervical lymph nodes (arrows) and a large mediastinal mass (arrowheads) consistent with confluent lymphadenopathy.
Methods
Data sources
A computer-aided search of the PubMed/Medline and Embase databases was conducted to find relevant publications on the diagnostic performance of CT, FDG-PET, FDG-PET/CT fusion, and WB-MRI in the staging of malignant lymphoma. The search strategy is presented in Table 2. No beginning date limit was used. The search was updated until July 25, 2007. Only English-, German-, or French-language studies were considered because the investigators were familiar with these languages. To expand our search, bibliographies of articles that finally remained after the selection process were screened for potentially suitable references.
Search strategy and results as on 25 July 2007
| Search number . | Search string . | PubMed/Medline citations, no. . | Embase citations, no. . |
|---|---|---|---|
| 1 | Computed tomography OR Computerized tomography OR Computed tomographic OR CT OR CAT | 414 143 | 258 730 |
| 2 | Fluorodeoxyglucose OR 2-fluoro-2-deoxy-D-glucose OR FDG OR Positron emission tomography OR Positron-emission tomography OR PET | 36 616 | 41 132 |
| 3 | Magnetic resonance OR MR imaging OR MRI OR Magnetic resonance tomography OR Nuclear magnetic resonance OR NMR | 349 864 | 342 766 |
| 4 | Hodgkin OR Lymphoma | 170 373 | 114 531 |
| 5 | Staging OR Stage OR Follow-up OR Remission OR Relapse OR Recurrence OR Progression OR Progressive | 1 323 267 | 1 002 278 |
| 6 | (Search 1 OR Search 2 OR Search 3) AND Search 4 AND Search 5 | 4003 | 2937 |
| Search number . | Search string . | PubMed/Medline citations, no. . | Embase citations, no. . |
|---|---|---|---|
| 1 | Computed tomography OR Computerized tomography OR Computed tomographic OR CT OR CAT | 414 143 | 258 730 |
| 2 | Fluorodeoxyglucose OR 2-fluoro-2-deoxy-D-glucose OR FDG OR Positron emission tomography OR Positron-emission tomography OR PET | 36 616 | 41 132 |
| 3 | Magnetic resonance OR MR imaging OR MRI OR Magnetic resonance tomography OR Nuclear magnetic resonance OR NMR | 349 864 | 342 766 |
| 4 | Hodgkin OR Lymphoma | 170 373 | 114 531 |
| 5 | Staging OR Stage OR Follow-up OR Remission OR Relapse OR Recurrence OR Progression OR Progressive | 1 323 267 | 1 002 278 |
| 6 | (Search 1 OR Search 2 OR Search 3) AND Search 4 AND Search 5 | 4003 | 2937 |
Study selection
At the first stage, 2 researchers (T.C.K., R.M.K.) independently reviewed the titles and abstracts of the retrieved articles. Studies investigating the diagnostic performance of CT, FDG-PET, FDG-PET/CT fusion, or WB-MRI in staging or restaging of patients with histologically proven malignant lymphoma were included. Only studies in which at least the area from the neck to the pelvis was imaged were included. In oncology, histological proof of presence or absence of viable tumor is the most accurate reference test. However, in an often diffuse disease such as malignant lymphoma, surgical exploration of all possibly involved sites and subsequent histological examination is not possible for practical and ethical reasons. As there is no other gold standard, follow-up is required to validate or invalidate index test findings that could not be histologically verified. Therefore, we included only those studies that applied a clinico-radiological follow-up period of at least 6 months as the standard of reference in all patients. Studies performed in animals, review articles, meta-analyses, abstracts, editorials or letters, case reports, studies investigating 10 or fewer patients, tutorials, and guidelines for management were excluded. Studies that did not make a separate analysis for either initial staging or restaging of malignant lymphoma and studies only dealing with the detection of bone marrow metastases in patients with malignant lymphoma were excluded. Studies investigating older generations of CT scanners (incremental, single-section, or dual-section CT), studies that examined FDG with a gamma camera in coincidence mode, and studies using low-field scanners (< 1.0 T) for WB-MRI also were excluded. Articles were rejected if they were clearly ineligible.
At the second stage, the same researchers (T.C.K., R.M.K.) independently evaluated the full-text version of all articles that were found to be potentially eligible for inclusion, using the same inclusion and exclusion criteria as mentioned above. If it was unclear from the information provided in a study as to whether all inclusion criteria were fulfilled and all exclusion criteria were absent, the study was excluded. Studies were included only if absolute numbers could be extracted to calculate confidence intervals for the reported estimates of diagnostic performance. When data or subsets of data were presented in more than one article, the article with the most details or the most recent article was chosen.
At both stages, disagreements between the 2 researchers (T.C.K., R.M.K.) were discussed and resolved in a consensus meeting.
Study analysis
The methodological quality of the included studies was assessed in terms of the potential for bias (internal validity) and lack of generalizibility (external validity). For this purpose, a checklist adapted from Kelly et al35 and Whiting et al36,37 was used. The complete criteria list is presented in Table 3. Internal validity criteria and external validity scores were scored as positive (adequate methods) or negative (inadequate methods, potential bias). If insufficient information was provided on a specific item, a negative score was given. Two reviewers (T.C.K., R.M.K.) independently assigned the scores. Disagreements were discussed and resolved by consensus. Subtotals were calculated for internal (maximum 7) and external (maximum 5) validity separately. Total quality scores were expressed as a percentage of the maximum score.
Criteria list used to assess the methodologic quality of the studies
| Criteria of validity . | Positive score . |
|---|---|
| Internal validity | |
| 1. Prospective study | Mentioned in publication |
| 2. Avoidance of incorporation bias | The index test did not form part of the reference test |
| 3. Avoidance of diagnostic review bias | Blind interpretation of index test without knowledge of reference test |
| 4. Avoidance of test review bias | Blind interpretation of reference test without knowledge of index test |
| 5. Avoidance of comparator review bias | Blinding index test to the other imaging modality, if more than one imaging modality was applied |
| 6. Minimum of withdrawal bias | < 10% of patients withdrew after the index test |
| 7. Avoidance of study examination bias | < 10% of indeterminate or uninterpretable results |
| External validity | |
| 1. Avoidance of patient filtering bias | Selection criteria described, age and sex of patients reported |
| 2. Avoidance of spectrum bias | All stages of disease |
| 3. Avoidance of selection bias | Consecutive series of patients |
| 4. Standard execution of index test | Application of the same hardware and imaging protocol in all patients |
| 5. Avoidance of observer variability bias | Interpreter(s) of index test described |
| Criteria of validity . | Positive score . |
|---|---|
| Internal validity | |
| 1. Prospective study | Mentioned in publication |
| 2. Avoidance of incorporation bias | The index test did not form part of the reference test |
| 3. Avoidance of diagnostic review bias | Blind interpretation of index test without knowledge of reference test |
| 4. Avoidance of test review bias | Blind interpretation of reference test without knowledge of index test |
| 5. Avoidance of comparator review bias | Blinding index test to the other imaging modality, if more than one imaging modality was applied |
| 6. Minimum of withdrawal bias | < 10% of patients withdrew after the index test |
| 7. Avoidance of study examination bias | < 10% of indeterminate or uninterpretable results |
| External validity | |
| 1. Avoidance of patient filtering bias | Selection criteria described, age and sex of patients reported |
| 2. Avoidance of spectrum bias | All stages of disease |
| 3. Avoidance of selection bias | Consecutive series of patients |
| 4. Standard execution of index test | Application of the same hardware and imaging protocol in all patients |
| 5. Avoidance of observer variability bias | Interpreter(s) of index test described |
Separate analyses were made for studies or subsets in studies investigating patients for initial staging (ie, staging prior to any treatment) and studies or subsets in studies investigating patients for restaging (ie, staging after treatment). Separate analyses also were made for HD and NHL, where possible. Reported estimates of diagnostic performance, with corresponding 95% CIs, were calculated from the original numbers given in the included studies. Statistical analyses were executed using Statistical Package for the Social Sciences version 12.0 software (SPSS, Chicago, IL).
Results
Literature search
The computer-aided search revealed 4003 articles from PubMed/Medline and 2973 articles from Embase (Table 2). Reviewing titles and abstracts from PubMed/Medline revealed 99 articles potentially eligible for inclusion. Reviewing titles and abstracts from Embase revealed 89 articles potentially eligible for inclusion, of which 88 were already identified by the PubMed/Medline search. Thus, 100 studies remained for possible inclusion and were retrieved in full-text version. After reviewing the full article, 81 articles were excluded, the majority (54%) because of the lack of an adequate standard of reference (Table 4). Eventually, 19 studies38,,,,,,,,,,,,,,,,,–56 met all inclusion and exclusion criteria, and they were included in this review. Screening references of these articles did not result in other potentially relevant articles. Of the 19 included studies, 3 studies investigated CT, 17 studies investigated FDG-PET, and 3 studies investigated FDG-PET/CT fusion for staging malignant lymphoma. No eligible studies on WB-MRI were identified for inclusion. The characteristics of the included CT, FDG-PET, and FDG-PET/CT fusion studies are presented in Tables 5 through 7.
Reasons for exclusion of full text articles
| Reason for exclusion . | No. of studies . |
|---|---|
| Not all patients received an adequate reference test (clinico-radiologic follow-up period of at least 6 months), or this was unclear | 44 |
| Only discrepant findings between the index test and conventional imaging procedure(s) were verified using a standard of reference | 11 |
| Absolute numbers to calculate confidence intervals for the reported estimates of diagnostic performance could not be extracted | 7 |
| The area from the neck to the pelvis was not imaged in all patients or it was unclear whether this was done | 5 |
| The prognostic value of interim FDG-PET in predicting treatment outcome was investigated | 4 |
| Ten or less patients with malignant lymphoma were included | 3 |
| Patients were not examined using a quad-section (or higher) multidetector-row CT scanner, or it was unclear whether this was done | 3 |
| Only patients with negative FDG-PET scans were included | 1 |
| Patients were examined using a dual-head gamma cameras in coincidence mode for FDG imaging | 1 |
| Prior FDG-PET studies were included in the interpretation of findings | 1 |
| No separate analysis was made of patients undergoing initial staging and patients undergoing restaging | 1 |
| Total | 81 |
| Reason for exclusion . | No. of studies . |
|---|---|
| Not all patients received an adequate reference test (clinico-radiologic follow-up period of at least 6 months), or this was unclear | 44 |
| Only discrepant findings between the index test and conventional imaging procedure(s) were verified using a standard of reference | 11 |
| Absolute numbers to calculate confidence intervals for the reported estimates of diagnostic performance could not be extracted | 7 |
| The area from the neck to the pelvis was not imaged in all patients or it was unclear whether this was done | 5 |
| The prognostic value of interim FDG-PET in predicting treatment outcome was investigated | 4 |
| Ten or less patients with malignant lymphoma were included | 3 |
| Patients were not examined using a quad-section (or higher) multidetector-row CT scanner, or it was unclear whether this was done | 3 |
| Only patients with negative FDG-PET scans were included | 1 |
| Patients were examined using a dual-head gamma cameras in coincidence mode for FDG imaging | 1 |
| Prior FDG-PET studies were included in the interpretation of findings | 1 |
| No separate analysis was made of patients undergoing initial staging and patients undergoing restaging | 1 |
| Total | 81 |
Characteristics of the 3 included studies investigating CT
| HD/NHL . | Study, year . | Patients/scans, no. . | Median age, y (range) . | Sex, M/F . | Criteria for positivity . | Interpreter(s) . |
|---|---|---|---|---|---|---|
| HD | Rigacci et al,38 2005 | 28/28 | 30.6 (16-73) | 14/14 | Any mass above the limit of 1.5 cm in the longest diameter or any abnormalities that were not consistent with previous therapy | NR |
| Mixed | La Fougere et al,39 2006 | 50/50 | NR (19-70) | 24/26 | Anatomical abnormalities; abnormal contrast enhancement patterns of solid organs; lymph nodes with a short-axis diameter >1.0 cm in the transverse plane, combined with abnormal contrast uptake or absence of hilar fatty degeneration | Two board-certified radiologists and nuclear medicine specialists with more than 10 years of experience in CT and PET |
| Mixed | Hernandez-Pampaloni et al,40 2006 | 16/21 | NR | NR | Suspected tumorous lesion; lymph nodes with a short-axis diameter in the axial direction >15 mm in the axillary and iliac/inguinal regions and >10 mm for the rest of the regions | An experienced pediatric radiologist |
| HD/NHL . | Study, year . | Patients/scans, no. . | Median age, y (range) . | Sex, M/F . | Criteria for positivity . | Interpreter(s) . |
|---|---|---|---|---|---|---|
| HD | Rigacci et al,38 2005 | 28/28 | 30.6 (16-73) | 14/14 | Any mass above the limit of 1.5 cm in the longest diameter or any abnormalities that were not consistent with previous therapy | NR |
| Mixed | La Fougere et al,39 2006 | 50/50 | NR (19-70) | 24/26 | Anatomical abnormalities; abnormal contrast enhancement patterns of solid organs; lymph nodes with a short-axis diameter >1.0 cm in the transverse plane, combined with abnormal contrast uptake or absence of hilar fatty degeneration | Two board-certified radiologists and nuclear medicine specialists with more than 10 years of experience in CT and PET |
| Mixed | Hernandez-Pampaloni et al,40 2006 | 16/21 | NR | NR | Suspected tumorous lesion; lymph nodes with a short-axis diameter in the axial direction >15 mm in the axillary and iliac/inguinal regions and >10 mm for the rest of the regions | An experienced pediatric radiologist |
NR indicates not reported.
Characteristics of the 17 included studies investigating FDG-PET
| HD/NHL, study and year . | Patients/scans, no. . | Median age, y (range) . | Sex, M/F . | Criteria for positivity . | Interpreter(s) . |
|---|---|---|---|---|---|
| HD | |||||
| Meany et al,41 2007 | 23/23 | 15 (5-19) | 10/13 | NR | NR |
| Bjurberg et al,42 2006 | 26/34 | NR (8-70) | NR | Any focus of elevated FDG metabolism not located in areas of normal FDG uptake or where the clinical data did not suggest the presence of nonmalignant hypermetabolic lesions. | Two experienced investigators |
| Zinzani et al,43 2006 | 40/40 | 32 (14-48) | 19/21 | Areas of focal uptake, unless they were at the sites of known accumulation, including the kidney and bladder, gastrointestinal tract, skeletal areas showing symmetric uptake (especially in the shoulder) were considered as due to arthritis | Three experienced readers |
| Rigacci et al,38 2005 | 28/28 | 30.6 (16-73) | 14/14 | Foci of hyperactivity outside areas of known physiologic uptake, in comparison with liver and mediastinum | NR |
| Filmont et al,44 2004 | 32/32 | 30 (6-65) | 15/17 | NR | An experienced reader |
| Dittmann et al,45 2001 | 47/47 | NR (18-63) | 27/20 | Focally increased uptake, exceeding that of the surrounding tissue and/or contralateral body regions | Two experienced nuclear medicine physicians |
| NHL | |||||
| Filmont et al,46 2003 | 78/78 | 57 (21-84) | 46/32 | NR | An experienced reader |
| Mikhaeel et al,47 2000 | 45/45 | NR | NR | Residual increased FDG uptake in previously diagnosed disease sites or the appearance of new uptake indicative of progressive disease | Two nuclear medicine physicians |
| Mixed | |||||
| La Fougere et al,39 2006 | 100/100 | NR (19-70) | 24/26 | Regions of focally increased tracer uptake; well-circumscribed areas of tracer uptake in the liver; focal lung lesions identifiable on both attenuation-corrected and noncorrected images; in doubtful cases an SUVmax ≥2.0 | Two board-certified radiologists and nuclear medicine specialists with more than 10 years of experience in CT and PET |
| Hernandez-Pampaloni et al,40 2006 | 16/21 | NR | NR | A focus of increased activity, not corresponding to the known physiologic distribution of FDG | Two experienced nuclear medicine physicians |
| Reinhardt et al,48 2005 | 101/101 | NR | 72/29 | All foci of elevated FDG uptake | NR |
| Freudenberg et al,49 2004 | 27/27 | 46†(19-70) | 16/11 | A SUVmax ≥2.5 in an area of focal tracer uptake | Two experienced nuclear medicine physicians |
| Mikosch et al,50 2003* | 93/121 | NR | NR | NR | NR |
| Mikhaeel et al,51 2000* | 32/32 | NR | 22/10 | Residual tracer uptake | Two independent nuclear medicine physicians |
| Bangerter et al,52 1999 | 36/36 | 31 (17-74) | 16/20 | Any foci of increased FDG uptake over background value that were not located in an area of physiologically increased uptake | Two independent investigators |
| Bangerter et al,53 1999 | 58/58 | NR | NR | Any clearly delineated uptake in the hilar and mediastinal regions | Two nuclear medicine physicians |
| Stumpe et al,54 1998* | 50/71 | NR (17-88) | 31/19 | A focus of increased FDG uptake above the intensity of the background as long as it was outside the renal pelvis, urinary bladder and myocardium | At least 2 board-certified nuclear medicine physicians |
| HD/NHL, study and year . | Patients/scans, no. . | Median age, y (range) . | Sex, M/F . | Criteria for positivity . | Interpreter(s) . |
|---|---|---|---|---|---|
| HD | |||||
| Meany et al,41 2007 | 23/23 | 15 (5-19) | 10/13 | NR | NR |
| Bjurberg et al,42 2006 | 26/34 | NR (8-70) | NR | Any focus of elevated FDG metabolism not located in areas of normal FDG uptake or where the clinical data did not suggest the presence of nonmalignant hypermetabolic lesions. | Two experienced investigators |
| Zinzani et al,43 2006 | 40/40 | 32 (14-48) | 19/21 | Areas of focal uptake, unless they were at the sites of known accumulation, including the kidney and bladder, gastrointestinal tract, skeletal areas showing symmetric uptake (especially in the shoulder) were considered as due to arthritis | Three experienced readers |
| Rigacci et al,38 2005 | 28/28 | 30.6 (16-73) | 14/14 | Foci of hyperactivity outside areas of known physiologic uptake, in comparison with liver and mediastinum | NR |
| Filmont et al,44 2004 | 32/32 | 30 (6-65) | 15/17 | NR | An experienced reader |
| Dittmann et al,45 2001 | 47/47 | NR (18-63) | 27/20 | Focally increased uptake, exceeding that of the surrounding tissue and/or contralateral body regions | Two experienced nuclear medicine physicians |
| NHL | |||||
| Filmont et al,46 2003 | 78/78 | 57 (21-84) | 46/32 | NR | An experienced reader |
| Mikhaeel et al,47 2000 | 45/45 | NR | NR | Residual increased FDG uptake in previously diagnosed disease sites or the appearance of new uptake indicative of progressive disease | Two nuclear medicine physicians |
| Mixed | |||||
| La Fougere et al,39 2006 | 100/100 | NR (19-70) | 24/26 | Regions of focally increased tracer uptake; well-circumscribed areas of tracer uptake in the liver; focal lung lesions identifiable on both attenuation-corrected and noncorrected images; in doubtful cases an SUVmax ≥2.0 | Two board-certified radiologists and nuclear medicine specialists with more than 10 years of experience in CT and PET |
| Hernandez-Pampaloni et al,40 2006 | 16/21 | NR | NR | A focus of increased activity, not corresponding to the known physiologic distribution of FDG | Two experienced nuclear medicine physicians |
| Reinhardt et al,48 2005 | 101/101 | NR | 72/29 | All foci of elevated FDG uptake | NR |
| Freudenberg et al,49 2004 | 27/27 | 46†(19-70) | 16/11 | A SUVmax ≥2.5 in an area of focal tracer uptake | Two experienced nuclear medicine physicians |
| Mikosch et al,50 2003* | 93/121 | NR | NR | NR | NR |
| Mikhaeel et al,51 2000* | 32/32 | NR | 22/10 | Residual tracer uptake | Two independent nuclear medicine physicians |
| Bangerter et al,52 1999 | 36/36 | 31 (17-74) | 16/20 | Any foci of increased FDG uptake over background value that were not located in an area of physiologically increased uptake | Two independent investigators |
| Bangerter et al,53 1999 | 58/58 | NR | NR | Any clearly delineated uptake in the hilar and mediastinal regions | Two nuclear medicine physicians |
| Stumpe et al,54 1998* | 50/71 | NR (17-88) | 31/19 | A focus of increased FDG uptake above the intensity of the background as long as it was outside the renal pelvis, urinary bladder and myocardium | At least 2 board-certified nuclear medicine physicians |
NR indicates not reported; and SUVmax, maximum standardized uptake value.
*This study allowed separate analysis of HD and NHL patients.
†Indicates a mean age.
Characteristics of the 4 included studies investigating FDG-PET/CT fusion
| HD/NHL . | Study, year . | Patients/scans, no. . | Median age, (range) . | Sex, M/F . | Criteria for positivity . | Interpreter(s) . |
|---|---|---|---|---|---|---|
| HD | Schaefer et al,55 2007 | 66/66 | 35.2 (11-76) | 46/20 | NR | Two board-certified nuclear medicine physicians (6 and 3 years of PET experience) and 2 board-certified radiologists (10 and 6 years of CT experience) |
| Mixed | La Fougere et al,39 2006 | 100/100 | NR (29-70) | 40/10 | A combination of the criteria mentioned in Tables 5 and 6 for this study | Two board-certified radiologists and nuclear medicine specialists with more than 10 years of experience in CT and PET |
| Mixed | Rhodes et al,56 2006 | 41/247 | 13 (3-18) | 23/18 | Nonphysiologic focal FDG uptake; FDG uptake equal to or greater than that seen in the liver corresponding to a lymph node or abnormality on simultaneously performed diagnostic CT scan | NR |
| Mixed | Freudenberg et al,49 2004 | 27/27 | 46* (19-70) | 16/11 | A SUVmax ≥2.5 in an area of focal tracer uptake and lymph nodes with a diameter >15 mm in the groin or >10 mm in all other regions | Two experienced nuclear medicine physicians and radiologists |
| HD/NHL . | Study, year . | Patients/scans, no. . | Median age, (range) . | Sex, M/F . | Criteria for positivity . | Interpreter(s) . |
|---|---|---|---|---|---|---|
| HD | Schaefer et al,55 2007 | 66/66 | 35.2 (11-76) | 46/20 | NR | Two board-certified nuclear medicine physicians (6 and 3 years of PET experience) and 2 board-certified radiologists (10 and 6 years of CT experience) |
| Mixed | La Fougere et al,39 2006 | 100/100 | NR (29-70) | 40/10 | A combination of the criteria mentioned in Tables 5 and 6 for this study | Two board-certified radiologists and nuclear medicine specialists with more than 10 years of experience in CT and PET |
| Mixed | Rhodes et al,56 2006 | 41/247 | 13 (3-18) | 23/18 | Nonphysiologic focal FDG uptake; FDG uptake equal to or greater than that seen in the liver corresponding to a lymph node or abnormality on simultaneously performed diagnostic CT scan | NR |
| Mixed | Freudenberg et al,49 2004 | 27/27 | 46* (19-70) | 16/11 | A SUVmax ≥2.5 in an area of focal tracer uptake and lymph nodes with a diameter >15 mm in the groin or >10 mm in all other regions | Two experienced nuclear medicine physicians and radiologists |
*Indicates a mean age.
HD indicates Hodgkin disease; NHL, non-Hodgkin lymphoma; and NR, not reported.
Methodological quality assessment
Methodological quality was assessed by 12 items. The scores for internal and external validity are presented in Tables 8 (CT), 9 (FDG-PET), and 10 (FDG-PET/CT fusion). For the CT studies, the FDG-PET studies, and the FDG-PET/CT fusion studies, the total scores for combined internal and external validity, expressed as a fraction of the maximum score, ranged from 50% to 58% (median, 50%), from 42% to 58% (median, 50%), and from 50% to 67% (median, 54%), respectively.
Quality assessment of the 3 included studies investigating CT
| Study and year . | Criteria . | Total scores . | % of maximum score (IV and EV) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IV . | EV . | ||||||||||||||
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 1 . | 2 . | 3 . | 4 . | 5 . | IV . | EV . | ||
| Rigacci et al,38 2005 | + | − | − | − | − | + | + | − | + | + | + | − | 3 | 3 | 50 |
| La Fougere et al,39 2006 | − | − | − | − | + | + | + | − | + | + | − | + | 3 | 3 | 50 |
| Hernandez-Pampaloni et al,40 2006 | − | − | + | − | + | + | + | − | + | − | + | + | 4 | 3 | 58 |
| Study and year . | Criteria . | Total scores . | % of maximum score (IV and EV) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IV . | EV . | ||||||||||||||
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 1 . | 2 . | 3 . | 4 . | 5 . | IV . | EV . | ||
| Rigacci et al,38 2005 | + | − | − | − | − | + | + | − | + | + | + | − | 3 | 3 | 50 |
| La Fougere et al,39 2006 | − | − | − | − | + | + | + | − | + | + | − | + | 3 | 3 | 50 |
| Hernandez-Pampaloni et al,40 2006 | − | − | + | − | + | + | + | − | + | − | + | + | 4 | 3 | 58 |
IV indicates internal validity; EV, external validity; +, quality item fulfilled; and −, quality item not fulfilled or unclear.
Quality assessment of the 17 included studies investigating FDG-PET
| Study and year . | Criteria . | Total scores . | % of maximumscore(IV and EV) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IV . | EV . | ||||||||||||||
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 1 . | 2 . | 3 . | 4 . | 5 . | IV . | EV . | ||
| Meany et al,41 2007 | − | − | − | − | − | + | + | - | + | + | + | − | 2 | 3 | 42 |
| Bjurberg et al,42 2006 | − | − | − | − | − | + | + | − | + | + | + | − | 2 | 3 | 42 |
| Zinzani et al,43 2006 | + | − | − | − | − | + | + | − | − | + | + | − | 3 | 2 | 42 |
| Rigacci et al,38 2005 | + | − | − | − | − | + | + | − | + | + | + | − | 3 | 3 | 50 |
| Filmont et al,44 2004 | − | − | + | − | + | + | + | + | + | + | − | − | 4 | 3 | 58 |
| Dittmann et al,45 2001 | − | − | − | − | + | + | + | + | + | − | + | + | 3 | 4 | 58 |
| Filmont et al,46 2003 | − | − | + | − | + | + | + | + | + | + | − | − | 4 | 3 | 58 |
| Mikhaeel et al,47 2000 | − | − | − | − | + | + | + | − | + | − | + | + | 3 | 3 | 50 |
| La Fougere et al,39 2006 | − | − | − | − | + | + | + | − | + | + | − | + | 3 | 3 | 50 |
| Hernandez-Pampaloni et al,40 2006 | − | − | + | − | + | + | + | − | + | − | − | + | 4 | 2 | 50 |
| Reinhardt et al,48 2005 | − | − | − | − | − | + | + | − | + | + | + | − | 2 | 3 | 42 |
| Freudenberg et al,49 2004 | − | − | − | − | + | + | + | − | + | − | + | + | 3 | 3 | 50 |
| Mikosch et al,50 2003 | − | + | + | − | − | + | + | − | + | − | + | − | 4 | 2 | 50 |
| Mikhaeel et al,51 2000 | − | − | − | − | − | + | + | − | + | − | + | + | 2 | 3 | 42 |
| Bangerter et al,52 1999 | + | − | + | − | − | + | + | + | + | − | + | − | 4 | 3 | 58 |
| Bangerter et al,53 1999 | − | − | − | − | − | + | + | − | + | + | − | + | 2 | 3 | 42 |
| Stumpe et al,54 1998 | − | − | − | − | − | + | + | − | + | − | + | + | 2 | 3 | 42 |
| Study and year . | Criteria . | Total scores . | % of maximumscore(IV and EV) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IV . | EV . | ||||||||||||||
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 1 . | 2 . | 3 . | 4 . | 5 . | IV . | EV . | ||
| Meany et al,41 2007 | − | − | − | − | − | + | + | - | + | + | + | − | 2 | 3 | 42 |
| Bjurberg et al,42 2006 | − | − | − | − | − | + | + | − | + | + | + | − | 2 | 3 | 42 |
| Zinzani et al,43 2006 | + | − | − | − | − | + | + | − | − | + | + | − | 3 | 2 | 42 |
| Rigacci et al,38 2005 | + | − | − | − | − | + | + | − | + | + | + | − | 3 | 3 | 50 |
| Filmont et al,44 2004 | − | − | + | − | + | + | + | + | + | + | − | − | 4 | 3 | 58 |
| Dittmann et al,45 2001 | − | − | − | − | + | + | + | + | + | − | + | + | 3 | 4 | 58 |
| Filmont et al,46 2003 | − | − | + | − | + | + | + | + | + | + | − | − | 4 | 3 | 58 |
| Mikhaeel et al,47 2000 | − | − | − | − | + | + | + | − | + | − | + | + | 3 | 3 | 50 |
| La Fougere et al,39 2006 | − | − | − | − | + | + | + | − | + | + | − | + | 3 | 3 | 50 |
| Hernandez-Pampaloni et al,40 2006 | − | − | + | − | + | + | + | − | + | − | − | + | 4 | 2 | 50 |
| Reinhardt et al,48 2005 | − | − | − | − | − | + | + | − | + | + | + | − | 2 | 3 | 42 |
| Freudenberg et al,49 2004 | − | − | − | − | + | + | + | − | + | − | + | + | 3 | 3 | 50 |
| Mikosch et al,50 2003 | − | + | + | − | − | + | + | − | + | − | + | − | 4 | 2 | 50 |
| Mikhaeel et al,51 2000 | − | − | − | − | − | + | + | − | + | − | + | + | 2 | 3 | 42 |
| Bangerter et al,52 1999 | + | − | + | − | − | + | + | + | + | − | + | − | 4 | 3 | 58 |
| Bangerter et al,53 1999 | − | − | − | − | − | + | + | − | + | + | − | + | 2 | 3 | 42 |
| Stumpe et al,54 1998 | − | − | − | − | − | + | + | − | + | − | + | + | 2 | 3 | 42 |
IV indicates internal validity; EV, external validity; +, quality item fulfilled; and −, quality item not fulfilled or unclear.
Quality assessment of the 4 included studies investigating FDG-PET/CT fusion
| Study and year . | Criteria . | Total scores . | % of maximum score (IV and EV) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IV . | EV . | ||||||||||||||
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 1 . | 2 . | 3 . | 4 . | 5 . | IV . | EV . | ||
| Schaefer et al,55 2007 | − | − | − | − | + | + | + | + | + | + | + | + | 3 | 5 | 67 |
| La Fougere et al,39 2006 | − | − | − | − | + | + | + | − | + | + | − | + | 3 | 3 | 50 |
| Rhodes et al,56 2006 | − | − | − | − | + | + | − | + | + | + | + | + | 2 | 5 | 58 |
| Freudenberg et al,49 2004 | − | − | − | − | + | + | + | − | + | − | + | + | 3 | 3 | 50 |
| Study and year . | Criteria . | Total scores . | % of maximum score (IV and EV) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IV . | EV . | ||||||||||||||
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 1 . | 2 . | 3 . | 4 . | 5 . | IV . | EV . | ||
| Schaefer et al,55 2007 | − | − | − | − | + | + | + | + | + | + | + | + | 3 | 5 | 67 |
| La Fougere et al,39 2006 | − | − | − | − | + | + | + | − | + | + | − | + | 3 | 3 | 50 |
| Rhodes et al,56 2006 | − | − | − | − | + | + | − | + | + | + | + | + | 2 | 5 | 58 |
| Freudenberg et al,49 2004 | − | − | − | − | + | + | + | − | + | − | + | + | 3 | 3 | 50 |
IV indicates internal validity; EV, external validity; +, quality item fulfilled; and −, quality item not fulfilled or unclear.
Diagnostic performance
CT.
Results of the 3 studies investigating CT38,–40 are displayed in Table 11. Only one study (separately) investigated patients with HD, with sensitivity and specificity of 87.5% and 85.6% (region-based) for initial staging, and sensitivity and specificity of 85.7% and 75.6% (region-based) for restaging, respectively.39 The other 2 studies were performed in a mixture of patients with HD and NHL, with sensitivities of 25% and 100% (patient-based) and specificities of 41.7% and 58.8% (patient-based) for restaging, respectively.38,40
Results of the 3 included studies investigating CT
| HD/NHL . | Study, year . | Scoring system . | Initial staging . | Restaging . | ||
|---|---|---|---|---|---|---|
| Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | Sensitivity,% (95% CI) . | Specificity, % (95% CI) . | |||
| HD | La Fougere et al,39 2006 | Region-based analysis (7 predefined regions) | 87.5 (75.3-94.1) | 85.6 (78.0-91.2) | 85.7 (68.5-94.3) | 75.6 (68.6-81.5) |
| Mixed | Hernandez-Pampaloni et al,40 2006 | Patient-based analysis | — | — | 100 (51.0-100) | 58.8 (36.0-78.4) |
| Mixed | Rigacci et al,38 2005 | Patient-based analysis | — | — | 25 (4.6-70.0) | 41.7 (24.5-61.2) |
| HD/NHL . | Study, year . | Scoring system . | Initial staging . | Restaging . | ||
|---|---|---|---|---|---|---|
| Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | Sensitivity,% (95% CI) . | Specificity, % (95% CI) . | |||
| HD | La Fougere et al,39 2006 | Region-based analysis (7 predefined regions) | 87.5 (75.3-94.1) | 85.6 (78.0-91.2) | 85.7 (68.5-94.3) | 75.6 (68.6-81.5) |
| Mixed | Hernandez-Pampaloni et al,40 2006 | Patient-based analysis | — | — | 100 (51.0-100) | 58.8 (36.0-78.4) |
| Mixed | Rigacci et al,38 2005 | Patient-based analysis | — | — | 25 (4.6-70.0) | 41.7 (24.5-61.2) |
— indicates no entry.
FDG-PET.
Results of the 17 studies investigating FDG-PET38,,,,,,,,,,,,,,,–54 are displayed in Table 12. Nine studies (separately or exclusively) investigated patients with HD.38,41,,,–45,50,51,54 Only one study investigated FDG-PET for initial staging of HD, with sensitivity and specificity of 87.5% and 100% (lesion-based), respectively.54 Sensitivities and specificities for restaging HD ranged between 85% (lesion-based)54 and 100% (patient-based),38,41,44,50,51 and between 57.1% (patient-based)41 and 100% (patient-based),43 respectively. Five studies (separately or exclusively) investigated patients with NHL.46,47,50,51,54 One study did not describe the histologic subtype(s) of NHL investigated,50 2 studies investigated patients with both high-grade and low-grade NHL,46,54 and 2 studies investigated patients with aggressive NHL47,51 (Table 12). Only one study investigated FDG-PET for initial staging of NHL (high-grade and low-grade), with sensitivity and specificity of 83.3% and 100% (lesion-based), respectively.54 Sensitivities and specificities for restaging NHL ranged between 60% (patient-based)47 and 100% (lesion-based),54 and between 80% (patient-based)50 and 100% (patient-based and lesion-based),47,51,54 respectively.
Results of the 17 included studies investigating FDG-PET
| HD/NHL; Study, year . | Scoring system . | Initial staging . | Restaging . | ||
|---|---|---|---|---|---|
| Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | ||
| HD | |||||
| Meany et al,41 2007 | Patient-based analysis | — | — | 100 (34.2-100) | 57.1 (36.6-75.5) |
| Bjurberg et al,42 2006 | Patient-based analysis | — | — | 91.7 (64.6-98.5) | 95.2 (77.3-99.2) |
| Zinzani et al,43 2006 | Patient-based analysis | — | — | 87.5 (52.9-97.8) | 100 (89.0-100) |
| Rigacci et al,38 2005 | Patient-based analysis | — | — | 100 (51.0-100) | 83.3 (64.2-93.3) |
| Filmont et al,44 2004 | Patient-based analysis | — | — | 100 (74.1-100) | 85.7 (65.4-95.0) |
| Mikosch et al,50 2003 | Patient-based analysis | — | — | 100 (87.1-100) | 81.8 (65.6-91.4) |
| Dittmann et al,45 2001 | Patient-based analysis | — | — | 86.2 (69.4-94.5) | 94.4 (74.2-99.0) |
| Mikhaeel et al,51 2000 | Patient-based analysis | — | — | 100 (43.9-100) | 91.7 (64.6-98.5) |
| Stumpe et al,54 1998 | Lesion-based analysis | 87.5 (52.9-97.8) | 100 (34.2-100) | 85.0 (64.0-94.8) | 95.7 (79.0-99.2) |
| NHL | |||||
| Mikosch et al,50 2003* | Patient-based analysis | — | — | 81.5 (63.3-91.8) | 80.0 (64.1-90.0) |
| Filmont et al,46 2003† | Patient-based analysis | — | — | 87.0 (74.3-93.9) | 93.8 (79.9-98.3) |
| Mikhaeel et al,47 2000‡ | Patient-based analysis | — | — | 60.0 (35.8-80.2) | 100 (88.7-100) |
| Mikhaeel et al,51 2000§ | Patient-based analysis | — | — | 71.4 (35.9-91.8) | 100 (72.3-100) |
| Stumpe et al,54 1998¶ | Lesion-based analysis | 83.3 (43.7-97.0) | 100 (20.7-100) | 100 (43.9-100) | 100 (67.6-100) |
| Mixed | |||||
| La Fougere et al,39 2006 | Region-based analysis (7 predefined regions) | 97.9 (89.1-99.6) ‖; 100 (87.5-100)† | 98.1 (93.4-99.5) ‖; 100 (93.7-100) † | 96.4 (82.3-99.4) ‖; 97.3 (90.8-99.3) † | 99.4 (96.7-99.9) ‖; 98.4 (95.5-99.5) † |
| Hernandez-Pampaloni et al,40 2006 | Patient-based analysis | — | — | 100 (51.0-100) | 88.2 (65.7-96.7) |
| Reinhardt et al,48 2005 | Patient-based analysis | — | — | 71.4 (52.9-84.8) | 94.5 (86.7-97.9) |
| Freudenberg et al,48 2004 | Region-based analysis (5 predefined regions); | — | — | 78.3 (58.1-90.3) | 98.2 (93.7-99.5) |
| Freudenberg et al,48 2004 | Patient-based analysis | — | — | 85.7 (60.1-96.0) | 100 (77.2-100) |
| Bangerter et al,52 1999 | Patient-based analysis | — | — | 71.4 (35.9-91.8) | 86.2 (69.4-94.5) |
| Bangerter et al,53 1999 | Patient-based analysis | — | — | 85.7 (48.7-97.4) | 96.1 (86.8-98.9) |
| HD/NHL; Study, year . | Scoring system . | Initial staging . | Restaging . | ||
|---|---|---|---|---|---|
| Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | ||
| HD | |||||
| Meany et al,41 2007 | Patient-based analysis | — | — | 100 (34.2-100) | 57.1 (36.6-75.5) |
| Bjurberg et al,42 2006 | Patient-based analysis | — | — | 91.7 (64.6-98.5) | 95.2 (77.3-99.2) |
| Zinzani et al,43 2006 | Patient-based analysis | — | — | 87.5 (52.9-97.8) | 100 (89.0-100) |
| Rigacci et al,38 2005 | Patient-based analysis | — | — | 100 (51.0-100) | 83.3 (64.2-93.3) |
| Filmont et al,44 2004 | Patient-based analysis | — | — | 100 (74.1-100) | 85.7 (65.4-95.0) |
| Mikosch et al,50 2003 | Patient-based analysis | — | — | 100 (87.1-100) | 81.8 (65.6-91.4) |
| Dittmann et al,45 2001 | Patient-based analysis | — | — | 86.2 (69.4-94.5) | 94.4 (74.2-99.0) |
| Mikhaeel et al,51 2000 | Patient-based analysis | — | — | 100 (43.9-100) | 91.7 (64.6-98.5) |
| Stumpe et al,54 1998 | Lesion-based analysis | 87.5 (52.9-97.8) | 100 (34.2-100) | 85.0 (64.0-94.8) | 95.7 (79.0-99.2) |
| NHL | |||||
| Mikosch et al,50 2003* | Patient-based analysis | — | — | 81.5 (63.3-91.8) | 80.0 (64.1-90.0) |
| Filmont et al,46 2003† | Patient-based analysis | — | — | 87.0 (74.3-93.9) | 93.8 (79.9-98.3) |
| Mikhaeel et al,47 2000‡ | Patient-based analysis | — | — | 60.0 (35.8-80.2) | 100 (88.7-100) |
| Mikhaeel et al,51 2000§ | Patient-based analysis | — | — | 71.4 (35.9-91.8) | 100 (72.3-100) |
| Stumpe et al,54 1998¶ | Lesion-based analysis | 83.3 (43.7-97.0) | 100 (20.7-100) | 100 (43.9-100) | 100 (67.6-100) |
| Mixed | |||||
| La Fougere et al,39 2006 | Region-based analysis (7 predefined regions) | 97.9 (89.1-99.6) ‖; 100 (87.5-100)† | 98.1 (93.4-99.5) ‖; 100 (93.7-100) † | 96.4 (82.3-99.4) ‖; 97.3 (90.8-99.3) † | 99.4 (96.7-99.9) ‖; 98.4 (95.5-99.5) † |
| Hernandez-Pampaloni et al,40 2006 | Patient-based analysis | — | — | 100 (51.0-100) | 88.2 (65.7-96.7) |
| Reinhardt et al,48 2005 | Patient-based analysis | — | — | 71.4 (52.9-84.8) | 94.5 (86.7-97.9) |
| Freudenberg et al,48 2004 | Region-based analysis (5 predefined regions); | — | — | 78.3 (58.1-90.3) | 98.2 (93.7-99.5) |
| Freudenberg et al,48 2004 | Patient-based analysis | — | — | 85.7 (60.1-96.0) | 100 (77.2-100) |
| Bangerter et al,52 1999 | Patient-based analysis | — | — | 71.4 (35.9-91.8) | 86.2 (69.4-94.5) |
| Bangerter et al,53 1999 | Patient-based analysis | — | — | 85.7 (48.7-97.4) | 96.1 (86.8-98.9) |
— indicates no entry.
*Histologic subtype(s) of NHL not described.
† High-grade NHL in 51 patients, low-grade NHL in 11 patients, and unknown histologic grade in 16 patients.
‡All patients had aggressive NHL (diffuse mixed, diffuse large-cell, or large-cell immunoblastic lymphoma).
§Aggressive histology NHL (diffuse mixed, diffuse large-cell, and large-cell immunoblastic lymphoma).
¶Nine with high-grade and 6 with low-grade NHL.
‖ Dedicated PET-scanner.
†FDG-PET portion of a combined FDG-PET/CT examination.
The remaining 6 studies were performed in a mixture of patients with HD and NHL. Sensitivity and specificity for initial staging approached 100% in the study of La Fougere et al.39 Sensitivities and specificities for restaging ranged between 71.4% (patient-based)48,52 and 100% (patient-based),40 and between 86.2% (patient-based)52 and 100% (patient-based),49 respectively.
FDG-PET/CT fusion.
Results of the 4 studies investigating FDG-PET/CT fusion39,49,55,56 are displayed in Table 13. Only one study (exclusively) investigated patients with HD, with sensitivity and specificity of 100% and 90.7% (patient-based) for restaging.55 The other 3 studies were performed in a mixture of patients with HD and NHL. Sensitivity and specificity for initial staging approached 100% in the study of La Fougere et al.39 Sensitivities and specificities for restaging exceeded 90% (region-based and patient-based).39,49,56
Results of the 4 included studies investigating FDG-PET/CT fusion
| HD/NHL . | Study, year . | Scoring system . | Initial staging . | Restaging . | ||
|---|---|---|---|---|---|---|
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | |||
| HD | Schaefer et al,55 2007 | Patient-based analysis | — | — | 100 (85.7-100) † | 90.7 (78.4-96.3) † |
| Mixed | La Fougere et al,39 2006 | Region-based analysis; (7 predefined regions) | 97.9 (89.1-99.6) *; 100 (87.5-100) † | 100 (97.7-100) *; 100 (93.7-100) † | 96.4 (82.3-99.4) *; 97.3 (90.8-99.3) † | 100 (97.8-100) *; 99.5% (97.1-99.9) † |
| Mixed | Rhodes et al,56 2006 | Patient-based analysis | — | — | 94.7 (75.4-99.1) ‡ | 90.6 (85.3-94.1) ‡ |
| Mixed | Freudenberg et al,49 2004 | Region-based analysis; (5 predefined regions) | — | — | 91.3 (73.2-97.6) *; 95.7 (79.0-99.2) † | 99.1 (95.1-99.8) *; 99.1 (95.1-99.8) † |
| Mixed | Freudenberg et al,49 2004 | Patient-based analysis | — | — | 92.9 (68.5-98.7) *; 92.9 (68.5-98.7) † | 10 (77.2-100) *,100 (77.2-100) † |
| HD/NHL . | Study, year . | Scoring system . | Initial staging . | Restaging . | ||
|---|---|---|---|---|---|---|
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | |||
| HD | Schaefer et al,55 2007 | Patient-based analysis | — | — | 100 (85.7-100) † | 90.7 (78.4-96.3) † |
| Mixed | La Fougere et al,39 2006 | Region-based analysis; (7 predefined regions) | 97.9 (89.1-99.6) *; 100 (87.5-100) † | 100 (97.7-100) *; 100 (93.7-100) † | 96.4 (82.3-99.4) *; 97.3 (90.8-99.3) † | 100 (97.8-100) *; 99.5% (97.1-99.9) † |
| Mixed | Rhodes et al,56 2006 | Patient-based analysis | — | — | 94.7 (75.4-99.1) ‡ | 90.6 (85.3-94.1) ‡ |
| Mixed | Freudenberg et al,49 2004 | Region-based analysis; (5 predefined regions) | — | — | 91.3 (73.2-97.6) *; 95.7 (79.0-99.2) † | 99.1 (95.1-99.8) *; 99.1 (95.1-99.8) † |
| Mixed | Freudenberg et al,49 2004 | Patient-based analysis | — | — | 92.9 (68.5-98.7) *; 92.9 (68.5-98.7) † | 10 (77.2-100) *,100 (77.2-100) † |
— indicates no entry.
*Side-by-side assessment of separately obtained CT and FDG-PET images.
†Hardware-oriented approach to image fusion; combined PET/CT.
‡Both * and † were available.
Discussion
This systematic review included 19 studies, of which 3 studies investigated CT, 17 studies investigated FDG-PET, and 4 studies investigated FDG-PET/CT fusion for initial staging and/or restaging of malignant lymphoma. No WB-MRI studies were found eligible for inclusion. The number of included studies investigating CT, FDG-PET, or FDG-PET/CT fusion for initial staging of malignant lymphoma was scarce. Many of the included studies did not separately analyze HD and NHL patients and/or mixed different histologic subtypes of NHL. Results of individual studies could not be meta-analyzed, because different scoring systems were used. Furthermore, none of the studies expressed their results according to the Ann Arbor staging system, which limits estimation of the therapeutic impact of each imaging modality.
The included studies had moderate methodological quality. Only one CT study was performed in a prospective fashion, and only one study explicitly mentioned that CT images were interpreted without knowledge of the reference test. None of the CT studies adequately described selection criteria, age, and sex of patients. Only 18% of the included FDG-PET studies were conducted prospectively, and only 29% of the studies explicitly mentioned that FDG-PET was interpreted without knowledge of the reference test. Up to 59% of the FDG-PET studies were potentially threatened by comparator review bias. Selection criteria, age, and sex of patients were adequately described in only 24% of the FDG-PET studies. None of the FDG-PET/CT fusion studies was prospectively executed, and none of the studies explicitly mentioned that FDG-PET/CT fusion was interpreted without knowledge of the reference test. Selection criteria, age, and sex of patients were adequately described in only 2 FDG-PET/CT fusion studies. In addition, all CT, FDG-PET, and FDG-PET/CT fusion studies used follow-up as the standard of reference. As a consequence, incorporation bias and/or test review bias could have been present in almost all studies.
CT is the most commonly used imaging modality for staging malignant lymphoma because of its widespread availability and relatively low cost. The exact values of FDG-PET, FDG-PET/CT fusion, and WB-MRI in comparison to CT for initial staging of HD and NHL have not been determined yet. Therefore, CT remains the standard imaging modality for initial staging of malignant lymphoma. An important drawback of CT is its failure to detect pathologic changes in normal-sized structures and to detect lesions that have poor contrast with surrounding tissue. Another weakness of CT is that it is not reliable in the detection of bone marrow disease, which, if present, by definition indicates stage IV disease.16 Furthermore, CT may not be able to differentiate residual viable tumor tissue from therapy-induced fibrosis; the 3 CT studies included in this review indeed reported a low-to-moderate specificity in restaging malignant lymphoma (Table 11).39,40 Comparing current with previous CT scans may improve diagnostic reliability. Nevertheless, the use of CT alone in restaging malignant lymphoma can be limited. Another disadvantage of CT is exposure of the patient to ionizing radiation, which may induce second cancers. Each CT scan, covering the neck, thorax, abdomen, and pelvis, is associated with an effective dose of approximately 20 to 25 mSv.57,58 The Food and Drug Administration estimates that a CT examination with an effective dose of 10 mSv may be associated with an increased risk of developing fatal cancer for approximately one in 2000 patients, and the Biological Effects of Ionizing Radiation VII lifetime risk model predicts that with the same effective dose of 10 mSv, approximately one individual in 1000 will develop cancer.57,58 In patients with malignant lymphoma, imaging will repetitively be performed during follow-up, thereby increasing the risk.59 This health risk caused by ionizing radiation is especially of concern in children, because they have a higher radiosensitivity than adults and they have more years ahead in which cancerous changes might occur.57 In the present era, where HD can be cured in at least 80% of patients3 and similar proceedings have been achieved in the treatment of certain types of NHLs,60,61 prevention of second malignancies due to CT radiation is an important issue. Another disadvantage of CT is the administration of iodinated contrast agents, which may cause adverse reactions, including rarely occurring but life-threatening contrast-induced nephrotoxicity and anaphylactic shock.62
The main advantage of FDG-PET over anatomical imaging techniques, such as CT, is its ability to detect metabolic changes in areas involved with malignant lymphoma before structural changes become visible. A pretreatment FDG-PET scan may identify additional cases of focal bone marrow involvement that would be missed by bone marrow biopsy or CT. However, it is not clear yet whether this complementary information affects patient prognosis and is cost-effective.63 It also is likely that FDG-PET surpasses CT in differentiating residual viable tumor tissue from therapy-induced fibrosis. Indeed, accuracy of FDG-PET in restaging of malignant lymphoma seems to be higher than that of CT (Tables 11,12). Routinely, FDG avid malignant lymphomas (HD, diffuse large B-cell lymphoma, follicular lymphoma, and mantle cell lymphoma) are well visualized, both in initial staging and restaging.64,65 However, some subtypes of NHL, predominantly low-grade lymphomas, may have low or even no uptake of FDG. Nodal and extranodal marginal zone lymphomas,64,,,,,,–71 small lymphocytic lymphomas,64,68,72,73 primary duodenal follicular lymphoma,64,74 cutaneous T-cell lymphomas,64,75 and peripheral T-cell lymphomas65 all have been reported to be possibly FDG negative. Caution is warranted in these histologic subtypes of NHL because a negative FDG-PET scan does not necessarily rule out disease; complimentary anatomical imaging (CT or MRI) is mandatory to increase detection rate of lesions. It also is considered mandatory to perform a pretreatment FDG-PET scan in these variably FDG avid NHLs; comparison of a post-treatment FDG-PET scan to a pretreatment FDG-PET scan will lead to more accurate restaging.76 Gastric marginal zone mucosa-associated lymphoid tissue (MALT) lymphoma constitutes a special group and may not be visualized with either CT or FDG-PET.77 In this instance, endoscopy and/or endoscopic ultrasonography may be of value. The included studies in this systematic review mixed patients with HD and NHL and/or mixed different histologic subtypes of NHL. Therefore, more studies are needed to determine the diagnostic performance of FDG-PET in staging the different histologic subtypes of NHL. A major drawback of FDG-PET is its lack of detailed anatomic information, which impedes precise localization of sites with FDG uptake and identification of other clinical problems such as spinal cord compression and ureteral, biliary, or central venous obstruction. Another disadvantage of FDG-PET is the possibility of FDG uptake in benign conditions with increased glycolysis such as infection, inflammation, and granulomatous disease. Additionally, high physiological uptake within the brain, myocardium, gastrointestinal tract, urinary tract, muscle, brown adipose tissue, salivary glands, lymphoid tissue, axillary skinfolds, apocrine sweat glands, and thymus may obscure or mimic the presence of tumor deposits. Caution also is warranted in patients receiving chemotherapy in conjunction with cytokines, such as granulocyte (-macrophage) colony stimulating factor, because these patients may have increased bone marrow FDG uptake up to 3 weeks after the last dose of cytokines. Another pitfall is that any process that stimulates the bone marrow, including bone marrow hyperplasia in HD or bone marrow hyperplasia as a consequence of recovery from a chemotherapeutic insult, also may result in increased FDG uptake. Similar effects can be seen in the spleen.78,–80 A careful evaluation of FDG-PET findings, along with a patient's accurate history and clinical examination, is necessary to minimize the number of false-positive interpretations. Another disadvantage of FDG-PET is exposure of the patient to ionizing radiation; the effective dose is approximately 3.3 to 7.6 mSv per examination.58
FDG-PET/CT fusion, using a combined PET/CT scanner, allows more accurate localization of foci with increased FDG uptake than stand-alone PET, and this may reduce the problems of physiological FDG uptake being misinterpreted as pathological and false localization of disease. An additional advantage of combined PET/CT is the use of the CT images for attenuation correction of the PET emission data, which reduces whole-body scanning times by 25% to 40% to 30 minutes or less. This approach also provides low-noise attenuation correction factors, compared with those from standard PET transmission measurements using an external radiation source, and eliminates bias from emission contamination of postinjection transmission scans. A pitfall of CT-based attenuation correction, however, is that the use of concentrated CT contrast agents, CT beam-hardening artifacts due to metallic implants, and physiologic motion can result in alterations of standardized uptake values of lesions or in the appearance of artifactual lesions. Images without attenuation correction also should be evaluated to avoid misinterpretations.7,8 The limited evidence suggests FDG-PET/CT fusion to be superior to CT alone and FDG-PET alone in initial staging and restaging of malignant lymphoma. However, more well-designed studies are needed to establish the additional value of FDG-PET/CT fusion compared with CT alone and FDG-PET alone in initial staging and restaging of malignant lymphoma. Future studies also should investigate whether the accuracy of FDG-PET/CT fusion is reproducible among the different histologic subtypes of malignant lymphoma. Radiation dose is a point of concern in FDG-PET/CT fusion. Although the CT portion of a PET/CT scan is usually performed at different settings than a standard diagnostic CT to decrease the radiation burden, the effective dose is still on the order of 25 mSv per examination.57,81
WB-MRI is a feasible technique for staging malignant lymphoma.82,83 However, no eligible WB-MRI studies were identified for inclusion in this systematic review. Large prospective trials are needed to determine the value of WB-MRI. WB-MRI may be of particular value for the assessment of bone marrow involvement.84 In contrast to CT, FDG-PET, and FDG-PET/CT fusion, WB-MRI has the advantage of not exposing the patient to ionizing radiation, which is especially important in children. In addition, the safety profile of magnetic resonance contrast agents is favorable when compared with that of iodinated contrast with CT.85 However, WB-MRI cannot be performed in patients with pacemakers, defibrillators, or other implanted electronic devices, and in case of claustrophobia. Other disadvantages of conventional (anatomical) WB-MRI are the lack of functional information, which may result in failure to detect pathologic changes in normal-sized structures, and the large amounts of image data obtained, which may result in the possibility of overlooking subtle pathological findings. Last-mentioned drawbacks, however, may be overcome with recently developed functional WB-MRI techniques, such as diffusion-weighted imaging. Diffusion-weighted imaging highlights areas with restricted diffusion, such as occurs in many malignant tumors, including malignant lymphoma.86,–88 Additionally, superparamagnetic iron oxide nanoparticles, which are MRI-specific lymphographic agents, are currently under investigation and can potentially play a role in staging of malignant lymphoma by identifying involved lymph nodes independently of lymph node size.89
Data on the cost-effectiveness of CT, FDG-PET, FDG-PET/CT fusion, and WB-MRI in staging malignant lymphoma are lacking. Only Klose et al90 attempted to address this issue and found an incremental cost-effectiveness ratio of FDG-PET versus CT of 3133 euros per correctly staged patient, in initial staging of malignant lymphoma.90 However, this study included only a small number of patients, mixed patients with HD and NHL, did not apply an appropriate standard of reference in all patients, and did not take into account long-term patient outcomes.90
In conclusion, the studies included in this systematic review were of moderate methodological quality and used different scoring systems to stage malignant lymphoma. CT remains the standard imaging modality for initial staging of malignant lymphoma, while FDG-PET has an essential role in restaging. Early results suggest that FDG-PET/CT fusion outperforms both CT alone and FDG-PET alone. Data on the diagnostic performance of WB-MRI are lacking. Future well-designed studies, expressing their results according to the Ann Arbor staging system, are needed to determine which imaging modality is most accurate and cost-effective in staging malignant lymphoma.
Authorship
Contribution: T.C.K., R.M.K., and R.A.J.N. designed the research, analyzed results, and wrote the paper. T.C.K. created the figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas C. Kwee, University Medical Center Utrecht, Department of Radiology, Heidelberglaan 100, 3584 CX Utrecht, the Netherlands; e-mail: thomaskwee@gmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal