We demonstrate mechanisms by which HIV-1 appears to facilitate its own infection in ex vivo–infected human lymphoid tissue. In this system, HIV-1 readily infects various CD4+ T cells, but productive viral infection was supported predominantly by activated T cells expressing either CD25 or HLA-DR or both (CD25/HLA-DR) but not other activation markers: There was a strong positive correlation (r = 0.64, P = .001) between virus production and the number of CD25+/HLA-DR+ T cells. HIV-1 infection of lymphoid tissue was associated with activation of both HIV-1–infected and uninfected (bystanders) T cells. In these tissues, apoptosis was selectively increased in T cells expressing CD25/HLA-DR and p24gag but not in cells expressing either of these markers alone. In the course of HIV-1 infection, there was a significant increase in the number of activated (CD25+/HLA-DR+) T cells both infected and uninfected (bystander). By inducing T cells to express particular markers of activation that create new targets for infection, HIV-1 generates in ex vivo lymphoid tissues a vicious destructive circle of activation and infection. In vivo, such self-perpetuating cycle could contribute to HIV-1 disease.
Introduction
CD4+ T lymphocytes are the major target for HIV-1 infection,1 and their loss is the hallmark of HIV-1 disease.2,–4 It is well established that the critical event of HIV-1 infection occurs in lymphoid tissue where T lymphocytes expressing CD4 constitute a highly heterogeneous population different in many parameters, in particular, their activation status.5,,–8 Unlike single cell cultures in vitro, the tissue microenvironment provides conditions for both activated and nonactivated cells to be productively infected.9,10 Nevertheless, CD4+ T-cell activation is thought to be a major factor in facilitating HIV-1 infection of these cells.11,12 This, and several other observations, have led to the widely accepted hypothesis that tissue activation is a major force, driving HIV-1 disease progression (for review, see Grossman et al13,14 ). The fraction of activated T lymphocytes and other cell types is increased in HIV-1–infected patients, and we have recently documented distorted activation pattern of lymphocytes in lymph nodes and tonsils from HIV-1–infected patients.15 However, the patterns of activation determining cell susceptibility to productive HIV-1 infection, the contribution of nonactivated cells to the viral load, and the relationship between activation status and cell loss in lymphoid tissues remain largely unknown, in part because of the lack of an adequate experimental model to address these problems
Here, to reveal mechanisms connecting cell activation and HIV-1 infection, we used ex vivo–infected human lymphoid tissues. These tissues support productive HIV-1 infection ex vivo without exogenous activation16,17 that is needed to efficiently infect peripheral blood mononuclear cells (PBMCs); such conditions are unlikely to reflect the conditions of cell activation in vivo even remotely. Moreover, in ex vivo tissues, similar to in vivo,10,18 both activated and nonactivated cells become productively infected,19 providing an experimental system to address some aspects of cell activation in HIV-1–infected human lymphoid tissue.
By comparing matched infected and noninfected lymphoid tissues from individual donors, we demonstrated here that viral load in this system depends on the number of activated target cells, but only of a particular pattern, CD25+/HLA-DR+. Furthermore, we found that viral infection mobilizes new HIV-1 cell targets by activating uninfected cells to express the very same pattern of markers that is associated with the efficient HIV-1 replication. HIV-1 infection of these cells drives them into apoptosis. Thus, HIV-1 creates in lymphoid tissue ex vivo a cycle of activation and infection that is also directly associated with tissue destruction. A similar pathogenic cycle operating in vivo might partly explain the role of cell activation in disease progression.
Methods
The use of anonymous surgical waste was approved by the National Institute of Child Health and Human Development and Children's National Medical Center Institutional Review Boards.
Lymphoid tissue histocultures and HIV-1 infection
Tonsillar tissue obtained at the Children's National Medical Center (Washington, DC) during routine therapeutic tonsillectomy according to an IRB-approved protocol were dissected into approximately 3-mm blocks and placed on top of collagen sponge gels. Tissue blocks were infected with the HIV-1 X4 variants LAI.04 (X4LAI.04) and NL4.3 (X4NL4.3) or with the R5 variants SF162 (R5SF162) and AD8 (R5AD8; National Institutes of Health AIDS Research Program), as described earlier.16,17 In a typical experiment, 3 to 5 μL of clarified virus-containing medium (approximately 300 TCID50 per block) were applied to the top of each tissue block. In each experiment, we compared with matched tissues, ie, tissue blocks obtained from the same donor. Tissue blocks (both HIV-1–infected and matched uninfected) were cultured for 12 days in RPMI 1640 (GibcoBRL; Invitrogen, Carlsbad, CA) containing 15% heat-inactivated fetal calf serum (Summit Biotechnology, Fort Collins, CO), nonessential amino acids (1 mM), sodium pyruvate (1 mM), l-glutamine (292 μg/mL), amphotericin B (2.5 μg/mL; GibcoBRL; Invitrogen), and gentamicin (50 μg/mL; Quality Biological, Rockville, MD). HIV-1 replication was assessed as described in “Evaluation of HIV-1 replication.”
Evaluation of HIV-1 replication
We assessed productive infection by measuring HIV-1 antigen p24gag accumulated in the culture medium during the 3 days between the successive medium changes, using p24gag antigen enzyme-linked immunosorbent assay (ELISA) detection kits (Perkin Elmer, Wellesley, MA; and Beckman Coulter, Miami, FL).
Flow cytometry
Single-cell suspensions were prepared from tissue blocks by digestion with Collagenase IV (GibcoBRL) at 5 mg/mL in RPMI 5% fetal calf serum for 30 minutes, followed by a wash in staining buffer (phosphate-buffered saline supplemented with 2% normal mouse serum, Gemini Bioproducts, West Sacramento, CA). Lymphocytes were identified according to their light-scattering properties and then analyzed for the expression of activation markers. To determine the proportion of infected cells, we washed the cells 3 times and stained them with different combinations of the following monoclonal antibodies: anti-CD3, anti-CD4, anti-CD25, anti-CD69, anti-CD38, anti-HLA-DR, and anti-CD95 coupled to a combination of fluorochroms: fluorescein isothiocyanate (FITC), phycoerythrin (PE)–Alexa610, PE-Cy7, PE-Cy5, PerCP-Cy5.5, or APC (Caltag Laboratories, Burlingame, CA; Becton Dickinson, San Jose, CA). After surface staining, the cells were permeabilized with Fix&Perm reagent (Caltag) and stained with anti-HIV-1-p24gag monoclonal antibody (KC57-RD1(PE), Beckman Coulter) and with APO 2.7 (PE-Cy5; Beckman Coulter) a monoclonal antibody specific for an early apoptosis mitochondrial antigen. Cells washed and fixed in phosphate-buffered saline, containing 4% formaldehyde, were acquired on an LSRII flow cytometer equipped with 355, 488, 532, 407, and 638 nm LASER lines using DIVA 4.1.2 software. Data were analyzed with FlowJo version 8.3 software (Tree Star, Ashland, OR). The percentage of infected T cells is reported for events gated on CD3+ cells with anti-HIV-1-p24gag-PE for intracellular staining.
Statistical analysis
Data obtained with tissue (27 or 54 tissue blocks for each experimental condition) from one donor were considered as one experiment (n). Because both the levels of viral replication and the proportions of cells in various leukocyte subsets varied from donor to donor,17,20 the results of different experiments were normalized per number of blocks (either uninfected or infected), averaged, and analyzed statistically. Statistical analysis performed on the normalized results data included the calculation of mean, SEM, and P values by use of a multiple comparison test (2-way analysis of variance test). The significance level was set as P at 0.5 or less, and the actual P values are indicated for each series of experiments. Statistical analysis of p24gag ELISA data was performed with Deltasoft version 3.0 software (BioMetallics, Princeton, NJ), by combining data from 3 dilutions and calculating a weighted interpolated p24gag concentration and SEM using a 4-parameter fitting algorithm.
Results
Activated T cells in human lymphoid tissue ex vivo
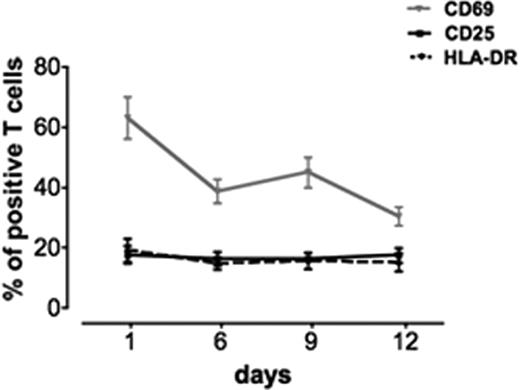
We evaluated the number of activated T cells in cultured noninfected blocks of human tonsils. In this study, we followed activation by expression of CD69 (early activation marker21,22 ) and of CD25 and HLA-DR (late activation markers23,24 ; Figure 1). Flow cytometric analysis on day 1 in culture revealed that, on average, 63 plus or minus 6.9% of T cells expressed CD69 (n = 13). This fraction is not different from that observed in uncultured lymphoid tissue after surgery (data not shown). The other activation markers were expressed to a lower extent: HLA-DR was expressed on 19 plus or minus 3.8% (n = 12) and CD25 on 17.5 plus or minus 3% (n = 12) of T cells (Figure 1).
Kinetics of expression of different activation markers in human lymphoid tissue ex vivo. Tissues from 8 to 22 different donors were immunostained for different activation markers (CD69, CD25, and HLA-DR). The graphs represent the means (± SEM) of T cells expressing these markers at days 1, 6, 9, and 12 after the beginning of culture. Shown are the fractions of cells positive for CD25, CD69, and HLA-DR expression.
Kinetics of expression of different activation markers in human lymphoid tissue ex vivo. Tissues from 8 to 22 different donors were immunostained for different activation markers (CD69, CD25, and HLA-DR). The graphs represent the means (± SEM) of T cells expressing these markers at days 1, 6, 9, and 12 after the beginning of culture. Shown are the fractions of cells positive for CD25, CD69, and HLA-DR expression.
With time, the number of cells expressing CD69 dramatically decreased: on day 6 in culture these cells constituted 38.7 plus or minus 3.9% (n = 22) of the T cells and on day 12 this number dropped to 30 plus or minus 3% (n = 22; P = .002). In contrast, the numbers of T cells that expressed CD25 and HLA-DR remained at approximately the same levels as on day 1, and on day 12 these cells constituted 15 plus or minus 3% (n = 22) and 17 plus or minus 2.5% (n = 22) of the total number of T cells, respectively. In addition, expression of other activation markers (CD38 and CD95) was also stable over the 12 days of culture (not shown). CD38 was expressed in 26.1 plus or minus 4.7% (n = 5) of T cells at day 1 and 16 plus or minus 4.9% (n = 5) at day 12 (P = .15). CD95 was expressed in 28.6 plus or minus 4% of T cells at day 1 and in 28 plus or minus 7% at day 12 (P = .1). Thus, in uninfected human lymphoid tissue ex vivo, the pattern of T-cell activation changes in time with the decrease in CD69 expression, whereas the levels of expression of CD25, CD38, CD95, and HLA-DR remain stable.
HIV-1 infection in activated and nonactivated tissue CD4+ T cells
Next, we investigated the relative contributions of differently activated CD4+ T cells to viral production. We stained cells for activation markers (CD25/HLA-DR) and for the intracellular viral antigen p24gag. Flow cytometric analysis of these cells revealed that both CD25+/HLA-DR+ CD4+ T cells and CD25−/HLA-DR− CD4+ T cells were productively infected (Figure 2).
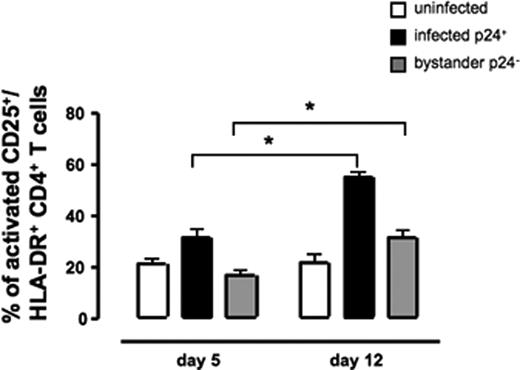
Activation in infected and uninfected CD4+ T cells in HIV-1–infected tissues. Tissue blocks from 3 different donors (54 blocks for each data point) were infected with X4LAI.04. CD4+ T cells from infected and matched uninfected tissues were stained for activation markers (HLA-DR and CD25) and for p24gag and analyzed by flow cytometry. The graphs represent the averages (± SEM) of CD25+/HLA-DR+ CD4+ T cells at days 5 and 12 after infection. CD25+/HLA-DR+ CD4+ T cells in uninfected control tissue. CD25+/HLA-DR+ CD4+ T cells were divided in X4LAI.04 infected tissue in productively infected cells (p24gag+) and bystander cells (p24gag−). * Represents significant differences for a nonparametric paired T test.
Activation in infected and uninfected CD4+ T cells in HIV-1–infected tissues. Tissue blocks from 3 different donors (54 blocks for each data point) were infected with X4LAI.04. CD4+ T cells from infected and matched uninfected tissues were stained for activation markers (HLA-DR and CD25) and for p24gag and analyzed by flow cytometry. The graphs represent the averages (± SEM) of CD25+/HLA-DR+ CD4+ T cells at days 5 and 12 after infection. CD25+/HLA-DR+ CD4+ T cells in uninfected control tissue. CD25+/HLA-DR+ CD4+ T cells were divided in X4LAI.04 infected tissue in productively infected cells (p24gag+) and bystander cells (p24gag−). * Represents significant differences for a nonparametric paired T test.
We investigated whether activated and nonactivated cells are infected with differential efficiency. The ratios of infected (p24gag+) to uninfected (p24gag−) CD4+ T cells among activated (CD25+/HLA-DR+) and nonactivated (CD25-/HLA-DR−) subsets were 1.9 plus or minus 0.18 and 0.97 plus or minus 0.16, respectively (n = 5, P = .005). Thus, in ex vivo–infected human lymphoid tissue, an activated CD4+ T cell is twice as likely as a nonactivated CD4+ T cell to be productively infected with HIV-1.
Tissue T-cell activation and HIV-1 production
Here, we investigated whether there is a correlation between the efficiency of viral production in tissue as evaluated from p24gag accumulation (“viral load”) and the number of activated T cells (Figure 3). We evaluated the proportion of T cells expressing either CD69 or CD25/HLA-DR at day 6 when the infection is readily detectable in the culture medium bathing blocks of tissues. In matched tissues infected with either X4LAI.04 (Figure 3A,C) or R5SF162 (Figure 3B,D), we evaluated the cumulative production of p24gag over the duration of the experiment.
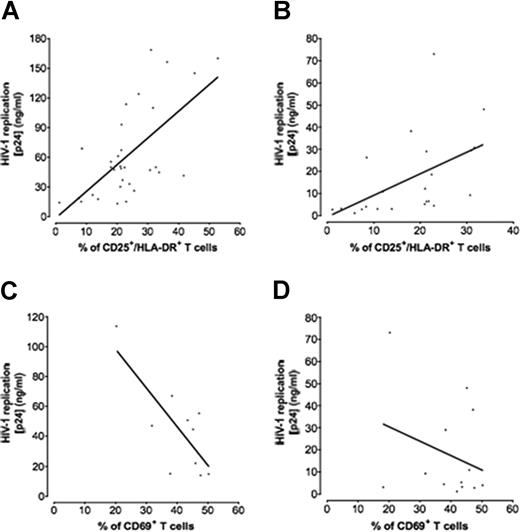
Correlation between p24gag production and T-cell activation. Tissues from 8 to 32 different donors were infected with X4LAI.04 or R5SF162, and for each data point the amount of p24gag accumulated in culture medium bathing 54 infected tissue blocks was measured by p24gag ELISA. Matched uninfected tissue blocks were used as controls. T cells of either CD69+ or CD25+/HLA-DR+ phenotype were considered as activated. The graphs represent the linear regression between the fraction of activated T cells in noninfected tissues and the maximal p24gag production in the matched infected tissue. (A) X4LAI.04 production correlated with CD25+/HLA-DR+ T cells. (B) R5SF162 production correlated with CD25+/HLA-DR+ T cells. (C) X4LAI.04 production correlated with CD69+ T cells. (D) R5SF162 production correlated with CD69+ T cells.
Correlation between p24gag production and T-cell activation. Tissues from 8 to 32 different donors were infected with X4LAI.04 or R5SF162, and for each data point the amount of p24gag accumulated in culture medium bathing 54 infected tissue blocks was measured by p24gag ELISA. Matched uninfected tissue blocks were used as controls. T cells of either CD69+ or CD25+/HLA-DR+ phenotype were considered as activated. The graphs represent the linear regression between the fraction of activated T cells in noninfected tissues and the maximal p24gag production in the matched infected tissue. (A) X4LAI.04 production correlated with CD25+/HLA-DR+ T cells. (B) R5SF162 production correlated with CD25+/HLA-DR+ T cells. (C) X4LAI.04 production correlated with CD69+ T cells. (D) R5SF162 production correlated with CD69+ T cells.
There was a strong positive linear correlation between both X4LAI.04 and R5SF162 viral loads and the fraction of T cells that expressed CD25/HLA-DR (r = 0.64, P < .001, n = 34, for X4LAI.04, and r = 0.5, P = .01, n = 22, for R5SF162; Figure 3A,B). In contrast, there was no correlation between these viral loads and the fraction of T cells expressing either CD38, CD95 (data not shown) or CD69 at day 6 (r = −0.2 for X4LAI.04 and r = −0.1 for R5SF162; n = 10; P = .57 and P = .71, respectively). Because of the fast down-regulation of CD69 in culture (Figure 1), we correlated the expression of CD69 in T cells at day 1 with viral loads and found a negative correlation for X4LAI.04 (r = −0.75, P = .01, n = 10; Figure 3C) and no correlation with R5 HIV-1-viral load (r = −0.28, P = .32, n = 13; Figure 3D).
We performed the same experiments with 2 other viral strains, X4NL4-3 andR5AD8. Akin to the correlation between the fraction of activated T cells and X4LAI.04 replication, we observed a negative trend between X4NL4-3 replication and the fraction of T cells that expressed CD69 at day 1 (r = −0.54, n = 4, P = .45), whereas there was no correlation between R5AD8 replication and expression of this marker (r = −0.1, n = 4, P = .89). There were positive trends between replication of these viruses and the fractions of T cells that expressed CD25/HLA-DR (r = 0.59 for X4NL4-3 and r = 0.63 for R5AD8; n = 6; P = .21 and P = .12, respectively).
To investigate whether there is a correlation between the viral load and the number of activated CD4+ T cells, we evaluated the proportion of CD4+ T cells expressing CD25/HLA-DR. As in the case of the correlation described above for activated T cells taken together, there was a positive linear correlation between the X4LAI.04 viral load and the fraction of CD4 T cells that expressed CD25/HLA-DR (r = 0.70, n = 7, P = .004). Thus, in human lymphoid tissue ex vivo, the number of CD4+ T cells expressing CD25/HLA-DR but not CD69 is a major positive correlate of the efficiency of HIV-1 replication.
HIV-1–driven activation of CD4+ T cells
We studied whether infection of lymphoid tissue by HIV-1 modulates their activation status. On day 5 after infection, on average 90 plus or minus 2% of CD4+ T cells were uninfected (n = 5). As shown in Figure 2, on day 5 after infection, the presence of infected CD4+ T cells did not significantly change the fraction of CD25+/HLA-DR+ cells among uninfected CD4+ T cells residing in the same tissue (bystander cells): they constituted 20.8 plus or minus 1.9% and 16.6 plus or minus 1.8% in uninfected and infected tissues, respectively (n = 5, P = .14). Thus, on day 5 after infection, the frequency of activated bystander CD4+ T cells remained similar to that in matched uninfected control tissues (P = .14).
In contrast, on day 12 after infection, the fractions of activated cells among bystander and among productively infected CD4+ T cells increased to 31.3 plus or minus 2.6% (n = 5, P = .001) and 54.6 plus or minus 2% (n = 5, P < .001), respectively (Figure 2). In uninfected control tissue, however, the fraction of activated cells did not change and remained 21.5 plus or minus 2.9% (n = 5, P = .85) at day 12.
We also calculated the ratio of activated to nonactivated CD4+ T cells for the 3 populations: p24gag− in uninfected, p24gag− and p24gag+ in infected tissues. We found that there was no change in uninfected tissue. This ratio (activated to nonactivated CD4+ T cells) was 1 plus or minus .01 (n = 5) in uninfected tissue, whereas for infected tissue, these ratios were on average 1.9 plus or minus 0.2 for p24gag− CD4+ T cells (n = 5, P < .001) and 1.8 plus or minus 0.2 for p24gag+ CD4+ T cells (n = 5, P = .002). Thus, on day 12 there was a higher proportion of both bystander and HIV-1 productively infected activated T CD4+ cells suggesting that HIV-1 drives activation of CD4+ T cells.
Preferential apoptosis of activated CD4+ T cells
As in the case of infection in vivo, productive HIV-1 infection in ex vivo tissue results in the depletion of CD4+ T cells. Here, we studied the relation between the activation status of an infected cell and its fate. We evaluated the fractions of CD4+ T cells expressing an early apoptotic marker: the mitochondrial antigen Apo2.7, and the activation markers CD25+/HLA-DR+ in HIV-1–infected and in matched control tissues (Table 1). In uninfected tissues, the frequencies of apoptosis in CD4+ T cells of the CD25+/HLA-DR+ subset were not significantly different from that in CD4+ T cells of the CD25−/HLA-DR− subset (8 ± 2% vs 6 ± 1% and 10.5 ± 2% vs 5 ± 2% for days 5 and 12, respectively; n = 3; P = .3 and P = .054). In infected tissues on day 5 after infection, the Apo2.7+ cells constituted 13 plus or minus 0.5% of the CD25+/HLA-DR+ CD4+ T cells, and this fraction increased significantly on day 12, on average to 31 plus or minus 3% (n = 3, P = .003). In contrast, apoptosis was not significantly increased among CD25−/HLA-DR− CD4+ infected T cells: on day 5, Apo2.7+ cells constituted 6 plus or minus 0.6%, and on day 12, 10 plus or minus 4%, of these cells (n = 3, P = .27). Thus, infection with HIV-1 selectively increased apoptosis among activated CD4+ T cells.
Preferential depletion of activated CD4+ T cells
| Activation status by day . | Frequencies of apoptotic cells, % . | |
|---|---|---|
| Uninfected . | Infected . | |
| Activated | ||
| Day 5 | 8 ± 2 | 13 ± 0.5 |
| Day 12 | 10.5 ± 2 | 31 ± 3 |
| P*, day 12 | .3 | .003 |
| Nonactivated | ||
| Day 5 | 6 ± 1 | 6 ± 0.6 |
| Day 12 | 5 ± 2 | 10 ± 4 |
| P*, day 12 | .054 | .27 |
| Activation status by day . | Frequencies of apoptotic cells, % . | |
|---|---|---|
| Uninfected . | Infected . | |
| Activated | ||
| Day 5 | 8 ± 2 | 13 ± 0.5 |
| Day 12 | 10.5 ± 2 | 31 ± 3 |
| P*, day 12 | .3 | .003 |
| Nonactivated | ||
| Day 5 | 6 ± 1 | 6 ± 0.6 |
| Day 12 | 5 ± 2 | 10 ± 4 |
| P*, day 12 | .054 | .27 |
P values are calculated for a paired t test measured between day 5 and day 12.
Discussion
In this work, we investigated in an ex vivo model of human lymphoid tissue the role of cell activation in HIV-1 replication because this activation was hypothesized to be the driving force of HIV-1 disease in general13,14,25,,,,–30 and T-cell turnover in particular.31,–33 The system of ex vivo–infected human lymphoid tissue used in our experiments is more adequate to address this question than conventional cell culture systems in which blasting PBMCs with phytohemagglutinin/interleukin-2 changes their activation status. As reported earlier and confirmed here, ex vivo HIV-1 inoculation of tissue blocks results in efficient productive infection34,,–37 without exogenous activation or stimulation, and therefore both activation and infection occur in our system under conditions that resemble in many ways those prevailing in lymphoid tissue in vivo. However, ex vivo HIV-1–infected tissues do not reflect some important aspects of HIV-1 pathogenesis, eg, changes in lymphocyte circulation, aberrant lymphocyte proliferation, and various systemic factors. These limitations should be kept in mind when extrapolating our results to an in vivo situation. The kinetics of HIV-1 replication in ex vivo lymphoid tissue evaluated by the release of p24gag into the medium is highly reproducible.34,38 However, the absolute level of viral infection significantly varied from donor to donor (see also Glushakova et al,34 Penn et al,38 and Grivel et al39 ). Here we found that the efficiency of viral infection is determined by the tissue T-cell expression of the particular activation markers. For this study, we monitored expression of CD69, CD25/HLA-DR, CD38, and CD95. In the course of a 12-day experiment, the expression of CD25 and HLA-DR, as well as of CD95 and CD38 was stable, whereas that of CD69 was downregulated. The rapid decrease of CD69 expression may reflect the physiology of T-cell circulation in vivo. Indeed, in secondary lymphoid tissues (ie, tonsils, lymph nodes), T cells express CD69 for 18 to 24 hours40 while they are retained in lymph nodes.41,42 Later, T cells lose CD69 and move out of the lymphoid tissue. In isolated tissue blocks in which the normal cell trafficking is disrupted, these cells nevertheless down-regulate CD69 at the same time they would do it in vivo.
We studied whether the expression of these markers that reflect various aspects of tissue activation status determines the viral load on tissue HIV-1 infection. Our analysis revealed in this system a strong linear correlation between HIV-1 production (both X4 and R5 variants) and the number of CD25+/HLA-DR+ T cells at the time of viral spread (day 6 after infection).34 In contrast, there was no correlation between HIV-1 production and expression of either CD69 or CD38, or CD95 at that time. However, because CD69 expression is transient and is lost within 72 hours of its initial appearance, CD69 measurement after 6 days of culture ex vivo does not reflect the activation status of the tissue at the time of HIV-1 infection (day 1). Therefore, we measured the expression of CD69 at the day of infection and found no correlation (for R5 variants) and a negative correlation (in case of X4 variants) with HIV-1 production.
It seems that in human lymphoid tissue, expression of the early activation marker CD69 is the attribute of tissue less susceptible to productive HIV-1 infection, whereas expression of late activation markers (CD25/HLA-DR) is the attribute of tissue more susceptible to productive HIV-1 infection. Unfortunately, the physiologic role of most of the activation markers is not known. Likewise, the expression of a particular marker CD69 or CD25/HLA-DR can be either a cause or consequence of the susceptibility to HIV-1 replication, whose underlying molecular mechanisms need to be addressed in future studies. Nevertheless, our results clearly establish that in lymphoid tissue a particular pattern, rather than general activation, is required for efficient HIV-1 replication. This implies that the use of the term “activation” in the context of HIV-1 infection requires qualification of which markers have been used to define activation. The role of the molecules that are now used as activation markers for most cases remains unclear. Future studies may reveal that the molecular mechanisms that lead to expression of particular set of markers are different and thus would define the diverse processes that are now collectively and vaguely referred to as “activation.”
Unlike in various in vitro systems based on isolated cell cultures, even nonactivated T cells in human lymphoid tissue (both in vivo and ex vivo) support productive HIV-1 infection.19,35,37,43 However, we found here that the frequency of infection, as evaluated from intracellular p24gag staining, was twice higher among activated HLA-DR+/CD25+ T cells than among nonactivated HLA-DR−/CD25− ones. Moreover, in contrast to the correlation of the number of activated T cells with viral production, there was no such correlation between HIV-1 replication and the number of nonactivated T cells. In the future, it would be interesting to directly compare the individual productivity of an activated and a nonactivated infected cell. Nevertheless, our data indicate that infected, nonactivated T cells produce much less virus than activated cells, as was previously reported in mucosal tissue and in PBMCs.9,18
Remarkably, HIV-1 infection seems to facilitate the very same pattern of lymphoid tissue activation that is associated with enhanced HIV-1 replication. The number of T cells of this phenotype increased with the duration of tissue infection not only among HIV-1–infected cells but also among uninfected bystander cells. A possible mechanism of T-cell activation in HIV-1–infected cells would occur via production of the Nef and Tat proteins, which activate both NF-AT and NF-κB, was reported earlier,18,44 whereas the mechanism of activation of bystander cells remains to be fully understood. Whichever this mechanism, such HIV-1–induced activation of bystander cells should promote viral spreading by creating a pool of new viral targets that on infection produce virus at a higher rate. Both in in vivo and in ex vivo tissues, HIV-1 infection of T cells leads to their death through apoptosis.45,,,,–50 The resultant kinetics of viral replication depends on how quickly apoptosis occurs in infected and activated cells, at which stage of apoptosis HIV-1 production is shut down, and how HIV-1 propagates through the tissue in the course of experiment.
Do activated and nonactivated T cells survive differentially in productively infected tissues? In the present work, we monitored the coexpression of an early apoptotic marker, the mitochondrial antigen Apo2.7,51,52 and the activation markers HLA-DR and CD25. Our results show that productively infected activated cells tend to enter apoptosis, whereas productively infected but nonactivated T cells do not express this apoptotic marker. Thus, infection of T cells does not seem to be sufficient to efficiently draw them into apoptosis. This may be simply related to the low amount of the virus produced by nonactivated cells but may also depend on yet unknown processes that are triggered by HIV-1 exclusively in CD25+/HLA-DR+ CD4 T cells. Neither does activation alone seem to be sufficient to induce apoptosis because, in our experiments in uninfected tissues, apoptotic frequencies among activated T cells were not different from that among nonactivated T cells. It seems that a combination of productive infection and activation is necessary for efficient T-cell apoptosis. Therefore, the frequency of activated infected T cells in tissues reported in our results may be underestimated because of their death before the time point of our analysis. If so, their contribution to HIV-1 production and T-cell depletion is even more significant.
Extrapolation of this observation to the in vivo situation suggests that nonactivated T cells are producing HIV-1 with low efficiency but survive longer than activated infected cells and therefore may contribute to the long-lived viral reservoir. In general, immunoactivation may be one of the critical factors for T-cell depletion in HIV-1–infected individuals.13,53
In summary, the amount of T cells of a particular activation pattern determines the efficiency of viral production in human lymphoid tissue. HIV-1 infection induces tissue cells to express this particular pattern of activation markers, both on infected and on the uninfected bystander T cells, thus creating new HIV-1 cell targets. Although activated and nonactivated cells support productive HIV-1 infection, the size of the latter pool is smaller than that of the former and the number of activated T cells productively infected with HIV-1 is the major correlate for viral load. A combination of HIV-1 infection and activation, but neither of these factors alone, is sufficient to drive cells into apoptosis.
In conclusion, our results are in general agreement with the in vivo observations on the central role of cell activation in HIV-1 disease but go beyond confirmation of this phenomenon in ex vivo tissues by demonstrating the existence of an activation–infection cycle that locally enhances the replication of HIV-1 in lymphoid tissues and drives cells into apoptosis. Such a cycle operating in vivo would facilitate viral pathogenesis in infected tissues leading to AIDS. The ex vivo tissue system can be used to further explore the details of that cycle; targeting individual elements of this cycle may become part of an anti-HIV-1 strategy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Dr Michael Lederman for helpful suggestions and constructive criticism and to all members of the Bad Boys of Cleveland Consortium of HIV immunologists and virologists for helping to set a general framework for our approach to HIV pathogenesis. We thank Dr M. Santi and the entire staff of the Department of Pathology of Children's National Medical Center for their generous assistance in obtaining human tonsillar tissues.
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Child Health and Human Development.
Authorship
Contribution: A.B., I.H., L.B.M., and J.-C.G. wrote the paper and designed the research; and A.B., S.J.H., C.V., C.E.C., A.L., and E.R. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angélique Biancotto, Laboratory of Cellular and Molecular Biology, Bldg 10, Rm 9D51, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892; e-mail: biancoa@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal