We hypothesized that increased monoclonal free kappa or lambda immunoglobulin light chains in smoldering multiple myeloma (SMM), as detected by the serum free light chain (FLC) assay, indicates an increased risk of progression to active myeloma. Baseline serum samples obtained within 30 days of diagnosis were available in 273 patients with SMM seen from 1970 to 1995. At a median follow-up of surviving patients of 12.4 years, transformation to active disease has occurred in 59%. The best breakpoint for predicting risk of progression was an FLC ratio of 0.125 or less, or 8 or more (hazard ratio, 2.3; 95% CI, 1.6-3.2). The extent of abnormality of FLC ratio was independent of SMM risk categories defined by number of bone marrow plasma cells (BMPCs) and size of serum M proteins (BMPC ≥ 10% and serum M protein ≥ 3 g/dL; BMPC ≥ 10% but serum M protein < 3 g/dL; and serum M protein≥ 3 g/dL but BMPC < 10%). Incorporating the FLC ratio into the risk model, the 5-year progression rates in high-, intermediate-, and low-risk groups were 76%, 51%, and 25%, respectively. The serum immunoglobulin FLC ratio is an important additional determinant of clinical outcome in patients with SMM.
Introduction
Smoldering multiple myeloma (SMM), first reported in 1980,1 is an asymptomatic plasma cell disorder with a high risk of progression to symptomatic multiple myeloma. Through the years, different definitions have been used, making reported outcomes difficult to interpret.2,,,,–7 In 2003, however, progress was made when the International Myeloma Working Group agreed upon the following definition: a serum M protein level greater than or equal to 3 g/dL and/or bone marrow plasma cells (BMPCs) greater than or equal to 10%, plus no anemia, hypercalcemia, renal failure, or lytic bone lesions (ie, no end organ damage), or symptoms of myeloma.8
It is crucial that SMM be distinguished from the clinically aggressive symptomatic MM as well as from the more benign monoclonal gammopathy of undetermined significance (MGUS), which is associated with a much lower risk of progression.9 Moreover, identification of risk factors that predict progression of SMM to symptomatic MM could identify higher-risk patients who might benefit from new active agents that can be tested as chemoprevention.
Recently, we reported our experience of a large cohort of patients with strictly defined SMM to determine its clinical course and outcome.10 We determined that the risk of progression is significantly affected by whether patients with SMM meet both the M protein and the bone marrow criteria, or just 1 of the 2 criteria. At 10 years, progression occurred in 77% if both plasma cells were greater than or equal to 10% and M spike was greater than or equal to 3 g/dL; in 64% of those with plasma cells greater than or equal to 10% but M spike less than 3 g/dL; and in 33% of patients if plasma cells less than 10% but M spike was greater than or equal to 3 g/dL.10
An abnormal serum immunoglobulin free light chain (FLC) ratio at baseline could help to further refine prognosis in SMM based on the predictive power demonstrated for the FLC ratio in patients with other plasma cell disorders.11,,–14 For those patients with clonal lambda (λ) disorders, ratios of serum levels of kappa (κ) to λ FLCs lower than the normal range have been associated with poorer outcomes; correspondingly, for patients with clonal κ disorders, ratios of serum levels of κ to λ FLCs higher than the normal range have been associated with poorer outcomes.11,–13 The presence of a monoclonal FLC excess in SMM could prove to be a marker of clonal evolution in the neoplastic plasma cell, since it likely indicates a loss of control over the proportion of heavy and light chains synthesized. Previously, Weber et al demonstrated that 50 mg/day of a monoclonal light chain in the urine was a risk factor for SMM progression.6 We undertook this study to test the hypothesis that an abnormal FLC ratio at baseline is a risk factor for the progression of SMM to malignancy.
Methods
Institutional Review Board approval was obtained from the Mayo Clinic for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Patients
We searched a computerized database and reviewed medical records of all patients seen at Mayo Clinic within 30 days of recognition of an IgG or IgA monoclonal protein of 3 g/dL or more or a bone marrow containing 10% or more plasma cells between 1970 through 1995, which allowed for a minimum potential follow-up of 10 years. Patients found to have end organ damage at diagnosis as a result of the plasma cell proliferative disorder (including symptomatic MM or light chain amyloidosis) were excluded. Patients who received chemotherapy at diagnosis were also excluded. A total of 276 patients fulfilled clinical criteria for a diagnosis of SMM and had a bone marrow available for central review during this 26-year period. Of these patients who have recently been reported,10 273 had serum available within 30 days of diagnosis for evaluation by the FLC assay.
Samples
The FLC assay (Freelite; the Binding Site, Birmingham, United Kingdom) was performed on a Dade-Behring Nephelometer (Dade-Behring, Marburg, Germany).15,16 The assay consists of 2 separate measurements, 1 to detect free κ (normal range, 0.33-1.94 mg/dL) and the other to detect free λ (normal range, 0.57-2.63 mg/dL) light chains.16 In addition to measuring the absolute levels of FLC, the test also allows an assessment of clonality based on the ratio of κ/λ light chain levels (normal reference range, 0.26-1.65).16 Patients with a κ/λ FLC ratio lower than 0.26 are typically defined as having a monoclonal λ FLC, and those with ratios greater than 1.65 are defined as having a monoclonal κ FLC.
Study endpoints and statistical considerations
Follow-up included review of each patient's medical record at the Mayo Clinic and review of death certificates for those who died. Patients were sent letters of inquiry if they had not visited Mayo Clinic in the preceding year. During 2097 cumulative years of follow-up (range, 0-29 years; median, 6.0 years), 85% of the patients with SMM died (median follow-up of those still living, 12.4 years). A total of 3 patients had no follow-up for progression, 2 of whom also had no survival follow-up. During this period of observation, MM developed in 156 (57%) persons, while 5 (2%) developed immunoglobulin light chain amyloidosis (AL), leaving only 8.8% of the population without an outcome event—that is, without progression or death.
The prognostic effect of abnormal κ to λ FLC ratio on progression of SMM was studied. To estimate the continuous risk effect of the FLC ratio, a smoothing spline17 was used in univariate and multivariate Cox proportional hazards models.18 The risk of progression depending on the extent to which the FLC ratio was abnormal was also estimated after adjusting for the prognostic system for SMM that we have recently described (high-risk: BMPCs greater than or equal to 10% and serum M protein greater than or equal to 3 g/dL; intermediate-risk: BMPCs greater than or equal to 10% but serum M protein less than 3 g/dL; and low-risk: and serum M protein greater than or equal to 3 g/dL but BMPCs less than 10%).10
The primary study endpoint was progression to symptomatic MM or light chain amyloidosis requiring therapy. The endpoint with respect to progression was calculated in terms of both the cumulative probability and the cumulative incidence of progression. The cumulative probability was calculated with a Kaplan-Meier estimate19 in which patients who died were censored; curves were compared by the log-rank test.20 The cumulative incidence curve, which explicitly accounted for death as a competing risk, was computed by the method of Gooley et al21 The effects of potential risk factors on progression rates were examined in a Cox proportional-hazards model.18
Results
Patient characteristics and clinical features
The characteristics of the 273 patients are shown in Table 1. Their median age at diagnosis was 64 years (range, 26-90 years), and only 13% were younger than 50 years. Of these, 169 (62%) were men. The light-chain type was κ in 68% and λ in 32%. A total of 3% were biclonal. The serum M protein at diagnosis of SMM ranged from 0.5 g/dL to 5.4 g/dL (median, 2.9 g/dL). An abnormal FLC ratio (κ to λ ratio < 0.26 or > 1.65) was detected in 245 (90%) patients. The median level of the involved immunoglobulin FLC was 7.4 mg/dL (range, 0.1-1, 110 mg/dL), and the median ratio of involved to uninvolved was 11.2 (range, 0.3-11 186).
Clinical characteristics of 273 patients with SMM
| Characteristic . | Quantity . |
|---|---|
| Median age, y (range) | 64 (26-90) |
| Cutoff age, % patients | |
| Younger than 40 y | 3 |
| Younger than 50 y | 13 |
| 50 to 59 y | 23 |
| 60 to 69 y | 33 |
| Older than 70 y | 30 |
| Male sex, % patients | 62 |
| Hemoglobin, median g/L (range) | 130 (100-168) |
| Creatinine median (range): greater than or equal to 176.8 μM, % | 97.24 (53.04-167.96); <1 |
| Calcium median (range): greater than or equal to 2.75 μM, % | 9.3 (8.2-10.8); 0 |
| Serum M protein, median g/L (range) | 29 (5-54) |
| Heavy chain type, IgG/IgA/IgD/biclonal, % | 74/23/<1, and 3 |
| Reduced uninvolved immunoglobulins, % | 81 |
| Kappa FLC for kappa patients, median mg/L (range) | 62 (1-11 100) |
| Lambda FLC for lambda patients, median mg/L (range) | 105 (11-3450) |
| Ratio of involved-uninvolved, median (range) | 11.2 (0.3-11 186) |
| Urine M protein present, % | 54 |
| Urine M protein g/24 hours, median (range) | 0.001 (0.0-1.7) |
| BMPC, median % | 17 |
| Cutoff level, % | |
| Less than 10 | 10 |
| 10 to 19 | 50 |
| 20 to 29 | 13 |
| 30 to 39 | 9 |
| 40 to 49 | 8 |
| 50 or more | 10 |
| Characteristic . | Quantity . |
|---|---|
| Median age, y (range) | 64 (26-90) |
| Cutoff age, % patients | |
| Younger than 40 y | 3 |
| Younger than 50 y | 13 |
| 50 to 59 y | 23 |
| 60 to 69 y | 33 |
| Older than 70 y | 30 |
| Male sex, % patients | 62 |
| Hemoglobin, median g/L (range) | 130 (100-168) |
| Creatinine median (range): greater than or equal to 176.8 μM, % | 97.24 (53.04-167.96); <1 |
| Calcium median (range): greater than or equal to 2.75 μM, % | 9.3 (8.2-10.8); 0 |
| Serum M protein, median g/L (range) | 29 (5-54) |
| Heavy chain type, IgG/IgA/IgD/biclonal, % | 74/23/<1, and 3 |
| Reduced uninvolved immunoglobulins, % | 81 |
| Kappa FLC for kappa patients, median mg/L (range) | 62 (1-11 100) |
| Lambda FLC for lambda patients, median mg/L (range) | 105 (11-3450) |
| Ratio of involved-uninvolved, median (range) | 11.2 (0.3-11 186) |
| Urine M protein present, % | 54 |
| Urine M protein g/24 hours, median (range) | 0.001 (0.0-1.7) |
| BMPC, median % | 17 |
| Cutoff level, % | |
| Less than 10 | 10 |
| 10 to 19 | 50 |
| 20 to 29 | 13 |
| 30 to 39 | 9 |
| 40 to 49 | 8 |
| 50 or more | 10 |
Outcomes
As shown in Table 2 and Figure 1, an increasingly abnormal FLC ratio was associated with a higher risk for progression to active MM. Patients with a normal (0.26 to 1.65) or near normal (0.25 to 4) ratio had a rate of progression of 5% per year, while patients with increasingly abnormal ratios had a progressive increase in the risk of progression. Patients with markedly abnormal ratios either less than 0.0312 (1 to 32) or more than 32 had a rate of progression of 8.1% per year. This increase persisted after adjusting for the competing risk of death (Table 2).
Absolute risk of progression of SMM to MM or related disorders based on the serum FLC ratio
| . | FLC Ratio . | |||
|---|---|---|---|---|
| 0.25 to 4 . | 0.125 to 0.25, or 4 to 8 . | 0.0312 to 0.125 or 8 to 32 . | Less than 0.0312 or more than 32 . | |
| Patients, no. | 63 | 46 | 93 | 71 |
| Years of follow-up, % (95% CI) | ||||
| 1 | 6.7 (0.1-12.8) | 2.2 (0.0-6.4) | 20.3 (11.5-28.2) | 22.1 (11.5-31.3) |
| 2 | 11.9(3.2-19.8) | 19.3 (6.3-30.5) | 38.4 (27.1-47.9) | 42.7 (29.2-53.5) |
| 5 | 28.1 (14.8-39.4) | 35.3 (18.4-48.7) | 58.0 (45.3-67.8) | 70.9 (56.9-80.3) |
| 10 | 50 (30.9-63.8) | 55.2 (34.5-69.3) | 70.4 (57.0-79.6) | 81.1 (67.3-89.1) |
| Cumulative annual rate of progression, % per y | 5.0 | 5.5 | 7.0 | 8.1 |
| Cumulative annual rate of progression, adjusted for competing risk of death, % per y | 3.6 | 4.5 | 6.0 | 7.1 |
| . | FLC Ratio . | |||
|---|---|---|---|---|
| 0.25 to 4 . | 0.125 to 0.25, or 4 to 8 . | 0.0312 to 0.125 or 8 to 32 . | Less than 0.0312 or more than 32 . | |
| Patients, no. | 63 | 46 | 93 | 71 |
| Years of follow-up, % (95% CI) | ||||
| 1 | 6.7 (0.1-12.8) | 2.2 (0.0-6.4) | 20.3 (11.5-28.2) | 22.1 (11.5-31.3) |
| 2 | 11.9(3.2-19.8) | 19.3 (6.3-30.5) | 38.4 (27.1-47.9) | 42.7 (29.2-53.5) |
| 5 | 28.1 (14.8-39.4) | 35.3 (18.4-48.7) | 58.0 (45.3-67.8) | 70.9 (56.9-80.3) |
| 10 | 50 (30.9-63.8) | 55.2 (34.5-69.3) | 70.4 (57.0-79.6) | 81.1 (67.3-89.1) |
| Cumulative annual rate of progression, % per y | 5.0 | 5.5 | 7.0 | 8.1 |
| Cumulative annual rate of progression, adjusted for competing risk of death, % per y | 3.6 | 4.5 | 6.0 | 7.1 |
Effect of increasingly abnormal FLC ratio on the relative risk of progression of SMM to MM or related disorder. As the serum kappa/lambda FLC ratio becomes increasingly abnormal, the risk of progression increases. The middle curve in represents relative risk; upper and lower curves represent 95% confidence intervals. Vertical bars represent the normal range for kappa to lambda ratio. (A) Unadjusted. (B) Adjusted for BMPC count and M protein.
Effect of increasingly abnormal FLC ratio on the relative risk of progression of SMM to MM or related disorder. As the serum kappa/lambda FLC ratio becomes increasingly abnormal, the risk of progression increases. The middle curve in represents relative risk; upper and lower curves represent 95% confidence intervals. Vertical bars represent the normal range for kappa to lambda ratio. (A) Unadjusted. (B) Adjusted for BMPC count and M protein.
The best cut-point for progression was a FLC ratio less than 0.125 (can be alternatively expressed as a ratio less than 1:8) or greater than 8. A total of 164 patients (60% of the cohort) had this degree of abnormality, and their hazard ratio for progression to active multiple myeloma was 2.3 (95% CI, 1.6-3.2) compared with patients with FLC ratios of 0.125 to 8 (Figure 2).
Risk of progression to myeloma or related disorder in 273 patients with SMM. Risk of progression of SMM to active myeloma using serum κ to λ FLC ratio of less than 0.125 (< 1:8) or more than 8 (top curve) versus 0.125 to 8 (bottom curve).
Risk of progression to myeloma or related disorder in 273 patients with SMM. Risk of progression of SMM to active myeloma using serum κ to λ FLC ratio of less than 0.125 (< 1:8) or more than 8 (top curve) versus 0.125 to 8 (bottom curve).
We conducted a multivariate analysis incorporating the FLC ratio into the recently described risk categories based on bone marrow plasmacytosis (cutoff ≥ 10%) and/or serum M spike (≥ 3 g/dL).10 An FLC ratio of either less htan 0.125 or more than 8 retained prognostic value (Table 3). In fact, the adjusted hazard ratio of 1.9 for the abnormal FLC ratio was comparable to—and independent of—serum M protein of 3 g/dL and higher. An FLC ratio of less than 0.125 or more than 8 divided the intermediate- and high-risk groups into subgroups with significantly different likelihoods of progression (Table 4). Immunoglobulin A isotype was a risk factor independent of the other prognostic variables, but less powerful than the other 3 variables. A 4-variable model including it was possible, but for simplicity it was not used in our model system.
Multivariate analysis of prognostic factors for progression of SMM to myeloma and related disorders
| Prognostic factor . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Bone marrow plasma cells more than 10% | 3.1 (1.6-6.3) | < .01 |
| Abnormal FLC ratio less than 0.125 or more than 8 | 1.9 (1.3-2.7) | < .01 |
| Serum M protein size, more than 30 g/L | 1.9 (1.4-2.6) | < .01 |
| Prognostic factor . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Bone marrow plasma cells more than 10% | 3.1 (1.6-6.3) | < .01 |
| Abnormal FLC ratio less than 0.125 or more than 8 | 1.9 (1.3-2.7) | < .01 |
| Serum M protein size, more than 30 g/L | 1.9 (1.4-2.6) | < .01 |
Risk-stratification models to predict progression of SMM to myeloma or related disorders
| Addition of FLC ratio to known prognostic categories . | Patients, no. . | Relative risk (95% CI) . | P . | Absolute risk of progression at 10 y, % (% corrected for death as competing risk) . |
|---|---|---|---|---|
| High-risk (serum M protein 30 gm/L or more and BMPC 10% or more) | ||||
| FLC ratio 0.125 to 8 | 28 | 1 | .011 | 58.8 (51.3) |
| FLC ratio less than 0.125 or more than 8 | 78 | 2.06 (1.18-3.59) | — | 83.8 (75.2) |
| Intermediate-risk (serum M protein less than 30 gm/L and BMPC 10% or more) | ||||
| FLC ratio 0.125 to 8 | 58 | 1 | .028 | 58.3 (40.4) |
| FLC ratio less than 0.125 or more than 8 | 82 | 1.72 (1.06-2.80) | — | 68.5 (56.8) |
| Low-risk (serum M protein 30 gm/L or more and BMPC less than 10%) | ||||
| FLC ratio 0.125 to 8 | 23 | 1 | .96 | 32.2 (22.8) |
| FLC ratio less than 0.125 or more than 8 | 4 | 1.05 (0.21-5.32) | — | 33.3 (25.0) |
| Addition of FLC ratio to known prognostic categories . | Patients, no. . | Relative risk (95% CI) . | P . | Absolute risk of progression at 10 y, % (% corrected for death as competing risk) . |
|---|---|---|---|---|
| High-risk (serum M protein 30 gm/L or more and BMPC 10% or more) | ||||
| FLC ratio 0.125 to 8 | 28 | 1 | .011 | 58.8 (51.3) |
| FLC ratio less than 0.125 or more than 8 | 78 | 2.06 (1.18-3.59) | — | 83.8 (75.2) |
| Intermediate-risk (serum M protein less than 30 gm/L and BMPC 10% or more) | ||||
| FLC ratio 0.125 to 8 | 58 | 1 | .028 | 58.3 (40.4) |
| FLC ratio less than 0.125 or more than 8 | 82 | 1.72 (1.06-2.80) | — | 68.5 (56.8) |
| Low-risk (serum M protein 30 gm/L or more and BMPC less than 10%) | ||||
| FLC ratio 0.125 to 8 | 23 | 1 | .96 | 32.2 (22.8) |
| FLC ratio less than 0.125 or more than 8 | 4 | 1.05 (0.21-5.32) | — | 33.3 (25.0) |
— indicates not applicable.
Next, we constructed a risk-stratification model based on the risk factors derived from our multivariable model (bone marrow plasmacytosis greater than or equal to 10%; serum M spike greater than or equal to 3 g/dL; and FLC ratio less than 0.125 or greater than 8). There were no significant differences of baseline characteristics among these 3 groups with the exception of age, suppression of uninvolved immunoglobulins, and each of the risk factor constituent variables. The lower-risk group was older, with median ages within the 1–, 2–, and 3–risk factor groups of 67, 63, and 61 years (P = .03) and less likely to have suppression of uninvolved immunoglobulins (35%, 12%, and 7%; P < .001).
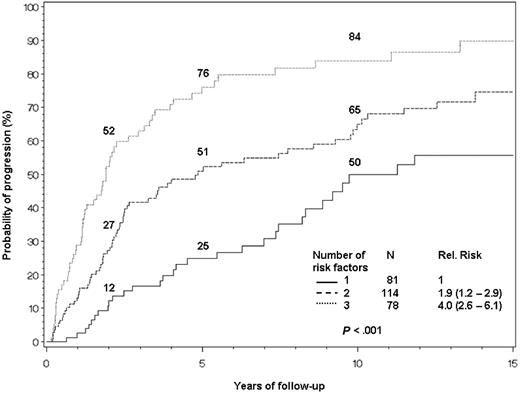
The cumulative probability of progression at 10 years was 50% in patients with one risk factor; 65% in those with 2 risk factors; and 84% in those with 3 risk factors (Figure 3). Corrected for death as a competing risk, the 10-year rates were 35%, 54%, and 75%, respectively (P < .001).
Risk stratification based on bone marrow plasmacytosis, serum M protein, and serum immunoglobulin FLC ratio. Patients are assigned 1 point for meeting each of the following criteria: BMPCs greater than or equal to 10%; serum M protein greater than or equal to 3 g/dL; and serum immunoglobulin FLC ratio either less than 0.125 or more than 8. The median times to progression for 1, 2, or 3 risk factors are 10, 5.1, and 1.9 years, respectively.
Risk stratification based on bone marrow plasmacytosis, serum M protein, and serum immunoglobulin FLC ratio. Patients are assigned 1 point for meeting each of the following criteria: BMPCs greater than or equal to 10%; serum M protein greater than or equal to 3 g/dL; and serum immunoglobulin FLC ratio either less than 0.125 or more than 8. The median times to progression for 1, 2, or 3 risk factors are 10, 5.1, and 1.9 years, respectively.
Discussion
SMM is an important entity because it describes a population of myeloma patients who do not require immediate therapy.1 In addition, these patients need to be differentiated from MGUS, since their risk of progression is approximately 10 times higher. For the purpose of consistency, the International Myeloma Working Group proposed the following uniform definition: the presence of either a serum M-protein value greater than or equal to 3 g/dL or greater than or equal to 10% plasma cells in the bone marrow in the absence of end organ damage.8 We recently described the natural history and risk of progression of SMM defined this way.10 Patients who satisfied the SMM definition by M protein only had a markedly longer median time to progression (19 years) than did patients who met criteria by either bone marrow plasmacytosis only (7.7 years) or by both M protein and bone marrow plasmacytosis (2.2 years). Because the serum immunoglobulin FLC ratio has been shown to be a powerful determinant of prognosis in MGUS, plasmacytoma, immunoglobulin light chain amyloidosis, and active myeloma,11,,–14 we investigated its role in patients with newly diagnosed SMM. Once again, an abnormal ratio proved to be an independent predictor of adverse outcome—in the case of SMM, progression to active MM. Because 90% of our cohort had an abnormal ratio, using “normal” versus “abnormal” was not sufficient, as it had been in patients with MGUS and with solitary plasmacytoma.11,12 Rather, a cut-point of κ to λ of either less than 0.125 (less than 1:8; for patients with clonal λ) or more than 8 (for patients with clonal κ) divided the cohort into 2 groups of roughly similar size. The addition of FLC ratio into our recently reported risk model for patients with SMM10 still yielded 3 risk categories but with a more balanced distribution of patients. Substitution of urinary M protein of 50 mg/24 hours as reported to be a risk factor by Weber et al could not substitute for the FLC ratio in this model.6 The division of patients into high-, intermediate-, and low-risk groups has shifted from 39%, 51%, and 10% of patients, respectively, to 28%, 42%, and 30% with 5-year progression rates of 76%, 51%, and 25%.
The ability to assess risk for a patient with newly diagnosed SMM is important given the heterogeneity of outcomes. The current analysis, which uses 273 of the 276 patients of the original manuscript,10 delves further into the definition of SMM. The small number of patients with high M protein, but less than or equal to 10% BMPCs, precluded demonstration of differential outcomes between the patients with a high FLC ratio and those patients with a borderline abnormal FLC ratio; however, both groups of patients with plasmacytosis greater than 10%—either in isolation or in association with M protein greater than 3 g/dL—had significantly different outcomes based on their FLC ratios.
With our most recent report,10 we noted that unlike MGUS, in which the rate of progression remains constant overtime,9 the overall risk of progression in SMM is greatly influenced by the length of time that the patient has had the diagnosis, with the highest rates of progression in the first several years (Figure 3). This time-dependent differential rate of progression is most notable in the high-risk group, in whom the probability of progression is about 26% per year for the first 2 years but only about 8% per year for the next 3 years. This is in stark contrast to the low-risk group, whose rate of progression is about 6% per year for the each of the first 2 years and about 4% per year henceforth. These observations could be consistent with the hypothesis that some patients classified as SMM are biologically identical to MGUS, and with increasing follow-up the cohort becomes enriched with such patients, resulting in progressively decreasing rates of progression.
Why excess clonotypic FLCs (ie, an abnormal FLC ratio) should predict a worse outcome is unclear, but one could speculate that perhaps these patients are those who have immunoglobulin heavy chain translocations.22 Our definitions of plasma cell disorders have come a long way. First, there was the separation of polyclonal and monoclonal hypergammaglobulinemia.23 Second, there was the realization that there was such a thing as MGUS.24,–26 Third, the concept of smoldering or asymptomatic myeloma became an accepted principle.1,27 Fourth, there was an international consensus on the definitions for the spectrum of MGUS to SMM to active MM.8 Each of these benchmark decisions have been based on observational studies that have incorporated the following4 variables to reach conclusions and recommendations: M protein, bone marrow plasmacytosis, symptom status, and time. We propose that the serum immunoglobulin FLC ratio be introduced as another variable that may better define these entities. It is a simple test that provides information about underlying plasma cell biology. While genetic profiles or specific genetic markers may someday make these variables obsolete,28 that day has not yet arrived. Until it does, the baseline serum immunoglobulin FLC ratio provides valuable prognostic information for each of these entities.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by research grants CA107476 and CA62242 from the National Cancer Institute.
We also thank A. R. Bradwell from the Binding Site for the free light chain reagents and to Carol Shipman for her maintenance of the Dysproteinemia database.
Authorship
A.D., S.V.R., R.A.K., J.A.K., T.M.T., M.A.G., S.K.K., R.F., D.F.J., and L.J.M. participated in the study concept, data analysis, study design, and writing of the report; J.B. and D.L. were involved in data collection, data analysis, and manuscript review; and R.J.C. was involved in performing the FLC assays, data collection, and manuscript review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angela Dispenzieri, Mayo Clinic College of Medicine, 200 First St SW, Rochester, MN 55905; e-mail: dispenzieri.angela@mayo.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal