In the WHO classification, subcutaneous panniculitis-like T-cell lymphoma (SPTL) is defined as a distinct type of T-cell lymphoma with an aggressive clinical behavior. Recent studies suggest that distinction should be made between SPTL with an α/β T-cell phenotype (SPTL-AB) and SPTL with a γδ T-cell phenotype (SPTL-GD), but studies are limited. To better define their clinicopathologic features, immunophenotype, treatment, and survival, 63 SPTL-ABs and 20 SPTL-GDs were studied at a workshop of the EORTC Cutaneous Lymphoma Group. SPTL-ABs were generally confined to the subcutis, had a CD4−, CD8+, CD56−, βF1+ phenotype, were uncommonly associated with a hemophagocytic syndrome (HPS; 17%), and had a favorable prognosis (5-year overall survival [OS]: 82%). SPTL-AB patients without HPS had a significantly better survival than patients with HPS (5-year OS: 91% vs 46%; P < .001). SPTL-GDs often showed (epi)dermal involvement and/or ulceration, a CD4−, CD8−, CD56+/−, βF1− T-cell phenotype, and poor prognosis (5-year OS: 11%), irrespective of the presence of HPS or type of treatment. These results indicate that SPTL-AB and SPTL-GD are distinct entities, and justify that the term SPTL should further be used only for SPTL-AB. SPTL-ABs without associated HPS have an excellent prognosis, and multiagent chemotherapy as first choice of treatment should be questioned.
Introduction
In 1991, Gonzalez et al described a new type of T-cell lymphoma with clinicopathologic features simulating a panniculitis, which was often associated with a hemophagocytic syndrome (HPS) and an aggressive clinical course.1 Under the term subcutaneous panniculitis-like T-cell lymphoma (SPTL), this new condition was included as a provisional entity in the REAL classification2 and in the European Organization for Research and Treatment of Cancer (EORTC) classification for primary cutaneous lymphomas,3 and subsequently as a distinct disease entity in the World Health Organization (WHO) classification.4
In recent years, there has been increasing evidence that, within the group of SPTL, distinction should be made between cases with an α/β T-cell phenotype (SPTL-AB) and cases with a γδ T-cell phenotype (SPTL-GD).5,6 While SPTL-ABs generally have a CD4−, CD8+, CD56− phenotype and a favorable prognosis, SPTL-GDs typically have a CD4−, CD8− T-cell phenotype with frequent coexpression of CD56 and a poor prognosis.7,,,,–12 Based on these observations, in the WHO-EORTC classification for cutaneous lymphomas the term SPTL is used only for SPTL-ABs, whereas SPTL-GDs are included in the group of cutaneous γδ T-cell lymphomas (CGD-TCLs). This group is included as a provisional entity within the broad category of peripheral T-cell lymphoma, unspecified.13
It should be emphasized that this subdivision of SPTL into 2 distinct groups is based on only a few studies and still needs further confirmation. Evaluation of existing literature, which concerns mainly case reports, small case series, and reviews,5,,,,,,–12,14,,,,,,–21 is hampered seriously by lack of complete immunophenotypical data and/or inclusion of malignant lymphomas with predominant subcutaneous involvement other than SPTL.8 This subdivision of SPTL may also have major therapeutic consequences. At present, in most centers, all SPTLs are routinely treated with doxorubicin-based multiagent chemotherapy.21 Whether this is the best treatment for patients with SPTL-AB may, however, be questioned.
The present report describes the results of a workshop of the EORTC Cutaneous Lymphoma Group, in which clinical, histologic, and immunophenotypical data of 63 SPTL-ABs and 20 SPTL-GDs were compared. The aims of this study were (1) to confirm that SPTL-AB and SPTL-GD are indeed distinct entities; (2) to establish more precisely the clinicopathologic features, immunophenotype, response to treatment, prognostic factors, and survival of these 2 groups of SPTL; and (3) to find out which cases should be treated with aggressive therapies and which with nonaggressive (immunosuppressive) therapies.
Methods
A workshop of the EORTC Cutaneous Lymphoma Task Force was held January 19 to 21, 2007, at the Leiden University Medical Center (Leiden, the Netherlands). Participants were experienced hematopathologists, dermatopathologists, and dermatologists from 8 European cutaneous lymphoma centers. Cases solicited for were SPTL, as defined in the WHO classification,4 thus including both SPTL-AB and SPTL-GD. Only cases adequately staged and with follow-up data available were included. Staging included physical examination, complete and differential blood cell counts, serum biochemistry, computed tomography scan of chest and abdomen, and a bone marrow biopsy. Before the meeting, completed data sheets containing information on a large number of clinical and histologic parameters and in most cases routine H&E-stained sections, relevant immunostainings, and unstained sections or paraffin blocks were submitted. If not performed before, additional stainings were completed in the Department of Pathology, Leiden University Medical Center prior to the meeting. Immunostainings had been performed using antibodies against T-cell antigens (CD2, CD3, CD4, CD5, CD7, CD8, CD30, CD56, CD45RO, CD45RA, betaF1, TCRδ-1), B-cell antigens (CD20, CD79a), cytotoxic proteins (granzyme B, TIA-1, perforin), histiocytes (CD68), and the proliferation marker Mib-1. Expression of immunostainings was scored as follows: − indicates less than 25%; −/+, 25% to 50%; +/−, 51% to 75%; and +, more than 75% of the neoplastic cells positive. In most cases, results of in situ hybridization for Epstein-Barr virus (EBV)–encoded small RNA (EBER) and T-cell receptor (TCR) gene rearrangement analyses were available as well.
During the workshop, H&E-stained sections and immunostainings of all submitted cases were studied together behind a 23-headed microscope and classified by consensus using the criteria of the WHO-EORTC classification. A total number of 115 biopsies from 95 cases were available for evaluation. During the workshop, 5 cases were excluded because a diagnosis of benign panniculitis was considered more likely. Another 7 cases were excluded because of insufficient immunophenotyping or incomplete follow-up data. The final study group included 83 cases. Clinical and/or histologic data of 34 of these cases have been part of previous studies.6,–8,11,18,20
Statistical analysis
Overall survival was calculated from the date of histologically confirmed diagnosis until the patient's death or last follow up without an event. Disease-specific survival (DSS) was calculated from the date of diagnosis until death from lymphoma or last follow up without an event. Survival curves were estimated using the method of Kaplan and Meier, and statistical comparison between curves was done by log-rank testing. Relationships between subgroups were examined by Pearson χ2 test or the Fisher exact test, where appropriate. The unpaired t test was used to analyze age differences between groups. All statistical analyses were done with Statistical Product and Services Solutions software, version 12.0.1 (SPSS, Chicago, IL).
Results
Based on clinical, histologic, immunophenotypical, and molecular diagnostic data, 63 cases were classified as SPTL-AB and 20 cases as SPTL-GD following the criteria of the WHO-EORTC classification.13 The main clinical, histologic, and immunophenotypical data of these 2 groups are summarized in Tables 1 and 2 and will be described in more detail in the following paragraphs.
Clinical characteristics and follow-up data
| . | SPTL-AB . | SPTL-GD . | P . |
|---|---|---|---|
| Total patients | 63 (76) | 20 (24) | — |
| Median age, y (range) | 36 (9-79) | 59 (13-79) | .022 |
| Sex | ns | ||
| Male | 21 (33) | 7 (35) | — |
| Female | 42 (67) | 13 (65) | — |
| Ratio M/F | 0.5 | 0.54 | — |
| Presenting skin lesions | — | ||
| Nodules / plaques | 63 (100) | 20 (100) | ns |
| Ulceration | 4 (6) | 9 (45) | <.001 |
| Extent of cutaneous involvement | ns | ||
| Single lesion | 8 (13) | 2 (10) | — |
| Regional | 6 (9) | 3 (15) | — |
| Multifocal | 49 (78) | 15 (75) | — |
| Staging | — | ||
| B-symptoms | 37 (59) | 13 (65) | ns |
| Lab abnormalities | 29 (46) | 11 (55) | ns |
| Abnormal CT scan | 5 (8) | 2 (10) | ns |
| Abnormal bone marrow | 12 (19) | 7 (35) | ns |
| Hemophagocytic syndrome* | 11 (17) | 9 (45) | .004 |
| Treatment | ns | ||
| CHOP or CHOP-like therapy | 31 (49) | 14 (70) | — |
| Immunosuppressive therapy | 24 (38) | 2 (10) | — |
| Radiotherapy | 3 (5) | 1 (5) | — |
| Surgery | 2 (3) | 0 (0) | — |
| No therapy | 2 (3) | 3 (15) | — |
| Unknown | 1 (2) | — | |
| Result initial treatment | ns | ||
| Complete remission | 42 (67) | 6 (30) | — |
| Partial remission | 8 (13) | 7 (35) | — |
| No response | 6 (10) | 2 (10) | — |
| Progressive disease | 6 (10) | 5 (25) | — |
| Status at last follow-up | < .001 | ||
| Alive and well | 39 (62) | 4 (20) | — |
| Alive with disease | 15 (24) | 1 (5) | — |
| Died of lymphoma | 8 (13) | 15 (75) | — |
| Died of other cause | 1 (2) | 0 (0) | — |
| Survival | — | ||
| 5-y overall survival | (82) | (11) | < .001 |
| 5-y disease specific survival | (85) | (11) | < .001 |
| . | SPTL-AB . | SPTL-GD . | P . |
|---|---|---|---|
| Total patients | 63 (76) | 20 (24) | — |
| Median age, y (range) | 36 (9-79) | 59 (13-79) | .022 |
| Sex | ns | ||
| Male | 21 (33) | 7 (35) | — |
| Female | 42 (67) | 13 (65) | — |
| Ratio M/F | 0.5 | 0.54 | — |
| Presenting skin lesions | — | ||
| Nodules / plaques | 63 (100) | 20 (100) | ns |
| Ulceration | 4 (6) | 9 (45) | <.001 |
| Extent of cutaneous involvement | ns | ||
| Single lesion | 8 (13) | 2 (10) | — |
| Regional | 6 (9) | 3 (15) | — |
| Multifocal | 49 (78) | 15 (75) | — |
| Staging | — | ||
| B-symptoms | 37 (59) | 13 (65) | ns |
| Lab abnormalities | 29 (46) | 11 (55) | ns |
| Abnormal CT scan | 5 (8) | 2 (10) | ns |
| Abnormal bone marrow | 12 (19) | 7 (35) | ns |
| Hemophagocytic syndrome* | 11 (17) | 9 (45) | .004 |
| Treatment | ns | ||
| CHOP or CHOP-like therapy | 31 (49) | 14 (70) | — |
| Immunosuppressive therapy | 24 (38) | 2 (10) | — |
| Radiotherapy | 3 (5) | 1 (5) | — |
| Surgery | 2 (3) | 0 (0) | — |
| No therapy | 2 (3) | 3 (15) | — |
| Unknown | 1 (2) | — | |
| Result initial treatment | ns | ||
| Complete remission | 42 (67) | 6 (30) | — |
| Partial remission | 8 (13) | 7 (35) | — |
| No response | 6 (10) | 2 (10) | — |
| Progressive disease | 6 (10) | 5 (25) | — |
| Status at last follow-up | < .001 | ||
| Alive and well | 39 (62) | 4 (20) | — |
| Alive with disease | 15 (24) | 1 (5) | — |
| Died of lymphoma | 8 (13) | 15 (75) | — |
| Died of other cause | 1 (2) | 0 (0) | — |
| Survival | — | ||
| 5-y overall survival | (82) | (11) | < .001 |
| 5-y disease specific survival | (85) | (11) | < .001 |
Data are numbers (%) unless otherwise indicated.
— indicates not applicable; and ns, not significant.
Hemophagocytic syndrome was defined by the criteria of Henter et al.22
Immunophenotype
| . | SPTL-AB . | SPTL-GD (CGD-TCL) . | P . |
|---|---|---|---|
| CD3 | 59/62 (95) | 16/19 (84) | ns |
| CD4 | 0/61 (0) | 1/18 (5) | ns |
| CD8 | 60/63 (95) | 2/20 (10) | < .001 |
| CD30 | 0/45 (0) | 3/19 (16) | .02 |
| CD56 | 0/61 (0) | 12/20 (60) | < .001 |
| Cytotoxic proteins† | 63/63 (100) | 19/19 (100) | ns |
| βF1 | 52/57 (91) | 0/20 (0) | < .001 |
| TCRδ | 0/10 (0) | 6/6 (100) | < .001 |
| Mib-1 | 36/53 (70) | 12/14 (85) | ns |
| EBER | 0/38 (0) | 0/15 (0) | ns |
| Clonal TCRβ | 9/9 (100) | ND | ns |
| Clonal TCRγ | 28/36 (78) | 10/12 (83) | ns |
| . | SPTL-AB . | SPTL-GD (CGD-TCL) . | P . |
|---|---|---|---|
| CD3 | 59/62 (95) | 16/19 (84) | ns |
| CD4 | 0/61 (0) | 1/18 (5) | ns |
| CD8 | 60/63 (95) | 2/20 (10) | < .001 |
| CD30 | 0/45 (0) | 3/19 (16) | .02 |
| CD56 | 0/61 (0) | 12/20 (60) | < .001 |
| Cytotoxic proteins† | 63/63 (100) | 19/19 (100) | ns |
| βF1 | 52/57 (91) | 0/20 (0) | < .001 |
| TCRδ | 0/10 (0) | 6/6 (100) | < .001 |
| Mib-1 | 36/53 (70) | 12/14 (85) | ns |
| EBER | 0/38 (0) | 0/15 (0) | ns |
| Clonal TCRβ | 9/9 (100) | ND | ns |
| Clonal TCRγ | 28/36 (78) | 10/12 (83) | ns |
Data are numbers of cases with more than 50% positive tumor cells/total number of cases studied (%) unless otherwise indicated.
ND indicates not done; and ns, not significant.
Data are expression of granzyme B, TIA-1, and/or perforin.
SPTL with an alpha/beta T-cell phenotype
Clinical characteristics.
The group of SPTL with an alpha/beta T-cell phenotype (SPTL-AB) consisted of 21 males and 42 females. The median age at diagnosis was 36 years (range: 9-79 years). Twelve (19%) of 63 patients were 20 years or younger. The duration of skin lesions before diagnosis ranged from 1 month to more than 10 years (median: 7 months).
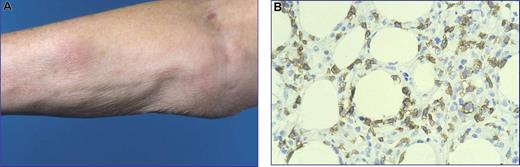
All patients presented with typical nodular skin lesions or deeply seated plaques, which varied in diameter from 1 to more than 20 cm, and sometimes left areas of lipoatrophy after disappearance (Figure 1A). Most patients presented with generalized skin lesions involving the legs (45 cases), the arms (39 cases), and/or the trunk (35 cases), and less commonly the face (16 cases). Fourteen patients had presented with solitary or localized skin lesions. Ulceration was observed in only 4 patients. B symptoms, such as fever, chills, night sweats, and weight loss, had been recorded in 37 of 63 patients. Laboratory abnormalities, mainly anemia, leucopenia, thrombocytopenia or combined cytopenias, and elevated liver function tests, were reported in 29 patients. However, a HPS was noted in only 11 patients, which was fatal in 7 of them. In 5 patients, imaging procedures had revealed lymphadenopathy, hepatosplenomegaly, and/or pleural effusions. Bone marrow cytology and histology had shown hemophagocytosis in 11 patients and a myelodysplastic syndrome in 1 patient. However, staging procedures had not revealed evidence of lymphoma outside the subcutis in any of the 63 patients. Remarkably, 12 patients (19%) had an associated autoimmune disorder, including (systemic) lupus erythematosus (4 patients), juvenile rheumatoid arthritis (2 patients), a combination of Sjögren disease and rheumatoid arthritis, type 1 diabetes mellitus, idiopathic thrombocytopenia, multiple sclerosis, Raynaud disease, and Kikuchi disease (2 patients). Another 4 patients were initially misdiagnosed as lupus erythematosus panniculitis (lupus profundus; LEP), but later reclassified as SPTL, because of the presence of cellular atypia in combination with loss of pan-T-cell markers and/or the presence of clonal TCRγ gene rearrangements.
Subcutaneous panniculitis-like T-cell lymphoma with an α/β T-cell phenotype (SPTL-AB). (A) Nodular skin lesion next to an area of lipoatrophy occurring after disappearance of the skin lesions. (B) Subcutaneous infiltrate with rimming of CD8+ neoplastic T cells around adipocytes (original magnification, ×480). The image in panel B was obtained through a Leica DM600B microscope (Leica, Rijswijk, the Netherlands). Image acquisition was performed with a ProgResC10 camera and software (JenaOptik, Jena, Germany). An HC Plan APO 40×/0.85 objective was used.
Subcutaneous panniculitis-like T-cell lymphoma with an α/β T-cell phenotype (SPTL-AB). (A) Nodular skin lesion next to an area of lipoatrophy occurring after disappearance of the skin lesions. (B) Subcutaneous infiltrate with rimming of CD8+ neoplastic T cells around adipocytes (original magnification, ×480). The image in panel B was obtained through a Leica DM600B microscope (Leica, Rijswijk, the Netherlands). Image acquisition was performed with a ProgResC10 camera and software (JenaOptik, Jena, Germany). An HC Plan APO 40×/0.85 objective was used.
Histology and immunophenotype.
All cases showed a predominantly subcutaneous atypical lymphoid infiltrate, showing typical adipotropism and characteristically involving the fat lobules resembling a lobular panniculitis. Septal involvement was generally mild or absent and appeared secondary. Mild to moderate extension of the atypical infiltrate into the reticular dermis, surrounding and occasionally infiltrating sweat glands and sometimes hair follicles and sebaceous glands, was often observed. However, infiltration of the superficial dermis and epidermis was seen in only 2 cases. Angioinvasion or angiodestruction was uncommon. The neoplastic infiltrate was composed of pleomorphic T cells of variable size with irregular and often hyperchromatic nuclei. In most cases, there was a predominance of small- to medium-sized cells with only scattered large lymphoid cells with clear cytoplasm. Rimming of individual adipocytes by neoplastic T cells was a common feature, although present sometimes focally (Figure 1B). Fat necrosis and karyorrhexis were always present, although to a variable degree. In all cases, the neoplastic T cells were admixed with small reactive lymphocytes and many histiocytes, which were frequently vacuolated because of ingested lipid material. However, multinucleated giant cells were uncommon, and well-defined granulomas were observed in only a minority of cases. Neutrophils and eosinophils were generally absent, apart from accumulating neutrophils in necrotic foci in some cases. In most cases plasma cells were rare. However, in 7 cases considerable proportions of plasma cells were noted. Interestingly, 6 of these 7 cases were either associated with lupus erythematosus (LE) or had been misinterpreted as LE panniculitis in the past.
In all cases, the neoplastic cells had the phenotype of CD3+, CD8+, CD4− T cells, which strongly expressed cytotoxic proteins (granzyme B, TIA-1, perforin) and that showed loss of CD2, CD5, and/or CD7 in 10%, 50%, and 44% of cases, respectively. Staining for CD45RA and CD45RO had been performed in a minority of cases, and consistently showed expression of CD45RO and negative staining for CD45RA. CD30 was always negative, while CD56 was expressed by the minority of neoplastic T cells in only 1 of 61 cases studied. Staining for betaF1 was positive in all cases investigated (57/57) confirming the alpha/beta T-cell phenotype. However, in 5 of 57 cases less than 50% of the CD8+ neoplastic T cells were betaF1 positive. Staining for TCRδ-1 on frozen sections had been performed in only 10 cases, and was always negative. Mib-1 staining showed a high proportion of proliferating cells, which characteristically concentrated around fat cells. In only 6 of 53 cases, less than 25% of the neoplastic T cells stained for Mib-1. In situ hybridization for EBV (EBER) had been performed in 38 cases and was always negative. Clonal rearrangements of the TCR beta and gamma genes had been documented in 9 of 9 and 28 of 36 cases investigated, respectively.
Therapy and follow-up data.
Initial treatment at the time the diagnosis SPTL was made varied widely from only surgery or radiotherapy to doxorubicin-based chemotherapy followed by autologous stem cell transplantation (auto-SCT). In 2 patients, the skin lesions had disappeared spontaneously and no further treatment was given. In 1 patient who died of the complications of HPS, initial treatment was unknown.
Thirty-one patients received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like courses as initial treatment, in 4 of them combined with alemtuzumab (A-CHOP), and in 3 other patients directly followed by an auto-SCT. The results showed a complete response in 19 patients, a partial response in 3 patients, no response in 4 patients, and progressive disease in 5 patients. Of the 4 patients receiving A-CHOP (3 of 4 without HPS) as initial treatment, only 2 reached a complete remission, but 1 of them developed EBV-associated CNS B-cell lymphoma and EBV-associated pharyngeal necrosis. Only 2 of 19 complete responders showed a relapse. Three of 12 patients without complete remission after CHOP therapy went into complete remission following subsequent treatment either with prednisone alone, a combination of prednisone and methotrexate, or following an allogeneic SCT (allo-SCT). At the time of last follow-up, 19 of 31 patients were in complete remission, 8 had ongoing disease, while 4 patients had died, including 3 of 6 patients with HPS and one of the side effects of treatment.
Twenty-four patients had been treated initially with less aggressive therapies including (combinations of) prednisone (19 cases), cyclosporine (5 cases), chlorambucil (3 cases), methotrexate (2 cases), cyclophosphamide (1 case), interferon-alpha (1 case), and gemcitabine (1 case). Sixteen patients had a complete remission, 5 had a partial remission, and 3 showed no response or had progressive disease. Nine of 16 complete responders had a relapse, and 5 of them reached a sustained complete remission upon repeated treatment with prednisone or other immunosuppressive agents. During follow-up, 8 of these 24 patients received CHOP or CHOP-like courses, in 1 of them followed by an auto-SCT, resulting in a complete remission in 3 of 8 cases. At the time of last follow up, 14 of these 24 patients were in complete remission, 6 had ongoing disease, while 4 patients had died, including 3 of 4 patients with associated HPS and 1 of unrelated disease.
Five patients presenting with solitary or localized skin lesions had been treated with radiotherapy (3 cases) or surgery (2 cases). All 5 patients reached complete remission and only 1 of them showed a skin relapse, which was treated successfully with radiotherapy again.
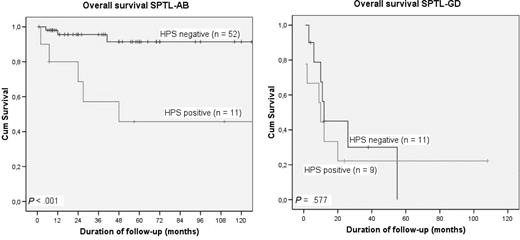
After initial treatment 34 (54%) of 63 patients had developed new skin lesions, but none of them had developed extracutaneous localizations. At the time of last follow-up (median follow-up: 34 months; range: 6-233 months), 39 patients are in complete remission, 15 patients have ongoing skin disease, while 9 patients have died, 8 of the complications of HPS or therapy-related side effects and 1 of unrelated disease. The 5-year OS and DSS of the total group of 63 patients were 82% and 85%, respectively. Patients without HPS had a significantly better 5-year OS (91%) than patients with HPS (46%; P < .001; Figure 2A).
Overall survival of SPTL with and without associated hemophagocytic syndrome (HPS). (A) SPTL-AB with (n = 11) and without (n = 52) HPS; (B) SPTL-GD with (n = 9) and without (n = 11) HPS.
Overall survival of SPTL with and without associated hemophagocytic syndrome (HPS). (A) SPTL-AB with (n = 11) and without (n = 52) HPS; (B) SPTL-GD with (n = 9) and without (n = 11) HPS.
SPTL with a gamma/delta T-cell phenotype (SPTL-GD)
Twenty of 83 cases were classified as SPTL-GD. In 6 of 20 cases, the diagnosis was supported by a positive staining for TCRδ-1 on frozen sections. In the other 14 cases, for which no frozen material was available, definite proof for a gamma/delta T-cell origin was lacking. However, since the neoplastic cells showed a negative staining for betaF1, while admixed reactive T cells were betaF1 positive, a diagnosis of SPTL-GD was considered most likely.13
Clinical characteristics.
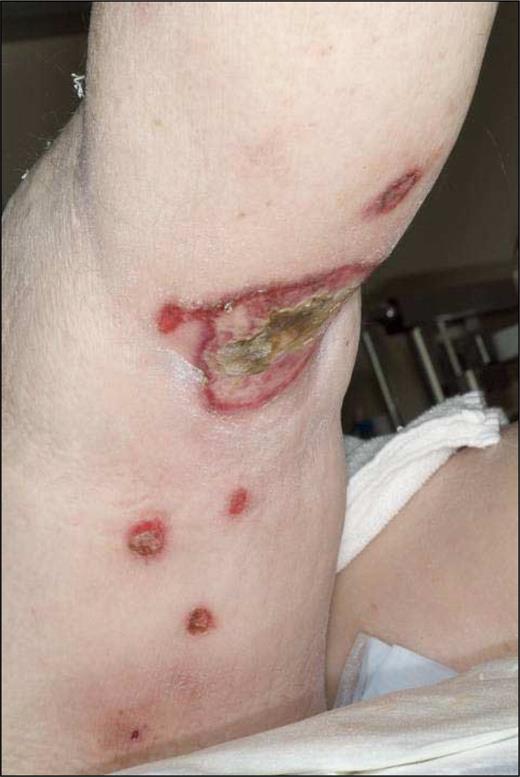
The group of CGD-TCL consisted of 7 males and 13 females. The median age at diagnosis was 59 years (range: 13-79 years). All patients presented with nodular or plaque-like lesions simulating a panniculitis, while 9 patients had ulcerating lesions at presentation (Figure 3). Most patients presented with generalized lesions preferentially affecting the legs (19/20 cases), the arms (13/20 cases), and the trunk (12/20 cases). One of the patients in this group had developed generalized 1- to 2-cm large nodules following treatment with etanercept (anti-TNFα) for more than 3 years for rheumatoid arthritis. All but 6 patients had presented with systemic symptoms, lymphadenopathy, hepatosplenomegaly, cytopenias, and/or elevated liver function tests. Bone marrow examination showed histiocytic hyperplasia, hemophagocytosis, or decreased cellularity in 7 cases, but no evidence of lymphoma. A HPS was diagnosed in 9 of 20 patients, and was fatal in 7 of them.
Subcutaneous panniculitis-like T-cell lymphoma with a γ/δ T-cell phenotype (SPTL-GD). Nodular and ulcerating skin lesions.
Subcutaneous panniculitis-like T-cell lymphoma with a γ/δ T-cell phenotype (SPTL-GD). Nodular and ulcerating skin lesions.
Histology and immunophenotype.
All cases showed a predominantly subcutaneous infiltrate as in SPTL-AB, but in many cases the upper dermis and the epidermis were infiltrated as well by a population of generally medium-sized pleomorphic T cells (Figure 4A,B). In the upper dermis, the neoplastic cells were localized around blood vessels and scattered between collagen bundles, in some cases associated with prominent edema (Figure 4C). Infiltration of the epidermis varied from few scattered cells in the basal layers to a marked lichenoid infiltrate with extensive necrosis of keratinocytes and ulceration. Angioinvasion and angiodestruction were commonly seen. Compared with SPTL-AB, the subcutaneous infiltrates tended to be more monotonous and diffuse, and preferential accumulation around individual fat cells (rimming) was less pronounced. In most cases, a considerable admixture with histiocytes was observed. Apoptosis and necrosis were common and sometimes extensive.
Subcutaneous panniculitis-like T-cell lymphoma with a γ/δ T-cell phenotype (SPTL-GD). (A) Low-power view showing subcutaneous infiltrates, as in SPTL-AB with the addition of perivascular infiltrates in the dermis (H&E staining; original magnification, ×25); (B,C) details of subcutaneous infiltrate showing rimming of fat cells by neoplastic T cells (B), and dermal infiltrates showing angioinvasion (C). Image acquisition obtained as described in Figure 1. An HC FLUOTAR 2.5/0.07 objective was used for panel A; an HC Plan APO objective was used for panels B,C (40×/0.85 for B; 20×/0.70 for C).
Subcutaneous panniculitis-like T-cell lymphoma with a γ/δ T-cell phenotype (SPTL-GD). (A) Low-power view showing subcutaneous infiltrates, as in SPTL-AB with the addition of perivascular infiltrates in the dermis (H&E staining; original magnification, ×25); (B,C) details of subcutaneous infiltrate showing rimming of fat cells by neoplastic T cells (B), and dermal infiltrates showing angioinvasion (C). Image acquisition obtained as described in Figure 1. An HC FLUOTAR 2.5/0.07 objective was used for panel A; an HC Plan APO objective was used for panels B,C (40×/0.85 for B; 20×/0.70 for C).
Immunophenotypically, 17 cases had a CD3+, CD4−, CD8− phenotype; 2 cases, a CD3+, CD4−, CD8+ phenotype; and 1 case, a CD3+, CD4+, CD8− T-cell phenotype. In all cases, strong expression of cytotoxic proteins (granzyme B, TIA-1, perforin) was observed. CD56 was expressed in 12 of 20 cases. In 3 cases, a significant proportion of the neoplastic cells was CD30+. Staining for TCRδ-1 on frozen sections was positive in all 6 cases tested. In all 20 cases, the neoplastic cells were negative for betaF1. From the 14 cases in which TCRδ-1 staining was not performed, but that were betaF1 negative, 6 cases had a CD4−, CD8−, CD56+ phenotype; 6 cases, a CD4−, CD8−, CD56− phenotype; 1 case, a CD4−, CD8+, CD56+ phenotype; and 1 case, a CD4+, CD8−, CD56− phenotype. In situ hybridization for EBV (EBER) had been performed in 16 cases and was consistently negative.
Therapy and follow-up data.
Fourteen of 20 patients were treated with multiagent chemotherapy, generally CHOP or CHOP-like courses, in 3 of them preceded by short courses of prednisone, and in 1 followed by an auto-SCT. In only 3 of 14 cases, including the patients treated with an auto-SCT, a complete remission was reached. Another patient not responding to CHOP reached complete remission following an allo-SCT. In the patient with anti-TNFα–associated SPTL-GD, treatment with prednisone and withdrawal of etanercept resulted in a complete disappearance of all skin lesions. The remaining 5 patients died before proper treatment could be initiated, 1 to 6 months after diagnosis. During follow-up, 7 patients developed visceral disease, involving liver, lungs, kidney, CNS, and oral mucosa. At the time of last follow-up (median follow-up: 12 months; range: 1-108 months), 15 of 20 patients had died of HPS and/or progressive lymphoma, 1 was still alive with progressive disease, and 4 patients were in complete remission 4, 12, 38, and 108 months after diagnosis. The 2-year and 5-year OSs were 31% and 11%, respectively (Figure 2B). In these SPTL-GDs, no significant differences in survival were found between cases with and without HPS, either between CD56+ and CD56− cases (data not shown).
Discussion
In the present study, 83 SPTLs as defined in the WHO classification were reviewed by a multidisciplinary group of hematopathologists, dermatopathologists, and dermatologists collaborating within the EORTC Cutaneous Lymphoma Group. The results of this study confirm that SPTL-AB and SPTL-GD represent distinct clinicopathologic entities.5,,–8 The main distinguishing features between both conditions are summarized in Table 3. Our results justify the terminology and definitions of the new WHO-EORTC classification, in which the term SPTL is used only for the former group, while the latter are included in the group of CGD-TCL, which represents a provisional entity in the broad category of peripheral T-cell lymphoma, unspecified.13
Distinguishing features between SPTL-AB and SPTL-GD
| . | SPTL -AB . | SPTL-GD . |
|---|---|---|
| Immunophenotype | ||
| T-cell receptor | βF1+, TCRδ1− | βF1−, TCRδ1+ |
| T-cell phenotype | CD3+, CD4−, CD8+ | CD3+, CD4−, CD8− |
| Co-expression CD56 (%) | Absent | Common (60) |
| Histological features (architecture) | Subcutaneous | Subcutaneous and epidermal/dermal |
| Clinical features | Nodules and plaques, rarely ulceration, association with auto-immune disorders (20%) | Nodules and plaques, ulceration common |
| HPS (%) | Uncommon (17) | Common (50) |
| 5-y overall survivial, % | 82 | 11 |
| Without HPS | 91 | — |
| With HPS | 46 | — |
| Preferred terminology (WHO-EORTC) | SPTL | CGD-TCL |
| . | SPTL -AB . | SPTL-GD . |
|---|---|---|
| Immunophenotype | ||
| T-cell receptor | βF1+, TCRδ1− | βF1−, TCRδ1+ |
| T-cell phenotype | CD3+, CD4−, CD8+ | CD3+, CD4−, CD8− |
| Co-expression CD56 (%) | Absent | Common (60) |
| Histological features (architecture) | Subcutaneous | Subcutaneous and epidermal/dermal |
| Clinical features | Nodules and plaques, rarely ulceration, association with auto-immune disorders (20%) | Nodules and plaques, ulceration common |
| HPS (%) | Uncommon (17) | Common (50) |
| 5-y overall survivial, % | 82 | 11 |
| Without HPS | 91 | — |
| With HPS | 46 | — |
| Preferred terminology (WHO-EORTC) | SPTL | CGD-TCL |
HPS indicates hemophagocytic syndrome; SPTL, subcutaneous panniculitis-like T-cell lymphoma; CGD-TCL, cutaneous gamma/delta T-cell lymphoma; and —, no difference in survival of patients with or without HPS.
The results of our study show clear-cut differences not only between SPTL-AB and SPTL-GD but also between SPTL-AB with and those without an associated HPS. In contrast, in the group of SPTL-GDs, no difference in survival was found between patients with and patients without HPS.
These observations may have important therapeutic consequences. Initial treatment in SPTL-AB varied widely from only radiotherapy or prednisone to doxorubicin-based chemotherapy, illustrating the current uncertainties how these lymphomas can best be treated. Since the WHO classification from 2001 did not yet distinguish between SPTL-AB and SPTL-GD and describes SPTL as an aggressive type of lymphoma, in most centers doxorubicin-based chemotherapy is the preferred type of treatment, sometimes in combination with alemtuzumab or followed by an auto-SCT.21,23,24 The results of the present and other recent studies indicate that SPTL-ABs have an excellent prognosis, in particular when not complicated by a HPS.8 Of the 52 SPTL-AB patients without HPS only 2 patients died, 1 from therapy-related side effects and 1 of unrelated disease. This observation raises the question whether such patients should be routinely treated with CHOP(-like) therapy or even more aggressive regimens. Our results show that initial treatment with CHOP(-like) courses resulted in a sustained complete remission in 16 (64%) of 25 patients. However, treatment with only prednisone or other immunosuppressive agents gave similar results. Eleven (55%) of 20 patients reached a sustained complete remission, 6 of them upon repeated treatment. In addition, 5 patients presenting with solitary or localized lesions are now in complete remission following only radiotherapy or surgery. We are aware that these results may be biased and that caution is warranted to draw firm conclusions from the present retrospective study including patients from so many different centers. However, our results do not support the view that all SPTL patients should be treated with CHOP(-like) courses or even more aggressively. The results suggest that patients with SPTL-AB without HPS may benefit equally from prednisone or other immunosuppressive agents, also in case of relapse. In rare cases presenting with solitary lesions radiotherapy may be considered.
Regarding SPTL-AB patients with associated HPS, 7 of 11 patients died despite CHOP or CHOP-like therapies as first or second line of treatment in 5 of these 7 patients. Of the 4 patients still alive, 2 patients reached a sustained complete remission (110 and 158 months after diagnosis) following a CHOP-like course or following treatment with prednisone and methotrexate, respectively. The third patient showed no response to A-CHOP, but achieved complete remission after subsequent treatment with prednisone and methotrexate, and is still in complete remission 57 months after diagnosis. The fourth patient did not respond to CHOP and still has progressive disease 7 months after diagnosis. Taken together, these results indicate that CHOP(-like) therapy is not very successful in patients with SPTL with associated HPS. High-dose chemotherapy followed by auto-SCT or allo-SCT has been suggested as an important option in patients with refractory or recurrent SPTL.21,24,25 In our study, 5 SPTL-AB patients underwent an auto-SCT (4 patients) or an allo-SCT (1 patient), and 3 of 5 patients achieved a complete remission. However, all 5 patients did not have associated HPS. Reports on successful auto-SCT or allo-SCT in SPTL-AB patients with HPS are few.26,27
Patients with SPTL-GD also proved highly resistant to multiagent chemotherapy, and had an extremely poor prognosis, irrespective of the presence of a HPS or expression of CD56. Nevertheless, 3 of the 4 patients still alive did not have a HPS. One of these 3 patients went into complete remission after CHOP followed by an auto-SCT, while another did not respond to CHOP courses but is now in complete remission for more than 3 years following an allo-SCT. These observations suggest that in patients with SPTL-GD early stem-cell transplantation may be considered.
In line with previous studies, SPTL-ABs were always negative for EBV.5,7,8 Staining for EBV may therefore be a valuable adjunct in differentiating SPTL from extranodal natural killer (NK)/T-cell lymphoma, nasal type, which may sometimes also present with prominent subcutaneous involvement.7,8 Apart from SPTL-GD and other aggressive T-cell lymphomas, SPTL-AB should be differentiated from lupus erythematosus panniculitis (LEP). The relationship between SPTL and LEP, which may be clinically indistinguishable, is controversial.28,29 In a detailed report on 11 cases of LEP, Massone et al proposed histopathological criteria useful in differentiating between LEP and SPTL-AB, suggesting that these represent distinct entities.29 Useful histopathologic criteria favoring a diagnosis of LEP included epidermal involvement, mucin depositions, the presence of reactive germinal centers, clusters of B cells or considerable numbers of admixed plasma cells, and polyclonal TCRγ gene rearrangement. In contrast, Magro et al emphasized overlapping features between LEP and SPTL and suggested that both conditions form a spectrum of disease.28 The present study group contained 4 SPTL patients with a definitive diagnosis of LE. In addition, at least 4 patients with SPTL had initially been misinterpreted as lupus panniculitis. These numbers might even be higher, since LE history and serology data had not specifically been requested. Interestingly, these patients with a concurrent or prior diagnosis of LE often showed a considerable admixture with CD4+ T cells and plasma cells not observed in the other SPTL cases. In these cases, a diagnosis of SPTL-AB had finally been made, because of the presence of cellular atypia in combination with loss of pan-T-cell markers and/or the presence of clonal TCRγ gene rearrangements. These observations suggest overlapping features between SPTL and LEP in a small proportion of cases, and suggest that all patients with suspected SPTL should be screened for LE. Moreover, in particular in these cases systemic steroids are the preferred mode of treatment, and systemic chemotherapy should not be considered as a first option. A recent report describes a patient with SPTL-GD (CGD-TCL) who presented with skin lesions clinically and histologically resembling LEP, but who had an aggressive clinical course.30 This and other cases31 underline that combination of clinical features, histopathology, immunophenotyping, and molecular analysis as well as repeat biopsies are essential to differentiate between LEP and malignant lymphomas involving the subcutis.
In conclusion, the results of the present study confirm that SPTL-AB and SPTL-GD are distinct entities, and justify that the term SPTL should further be used only for SPTL-AB, while SPTL-GD should now be classified as CGD-TCL. SPTL-AB without associated HPS has an excellent prognosis. There is sufficient reason to question whether CHOP (or in some centers even A-CHOP) should be the first choice of treatment in these patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Dr Brigitte Balme and Dr Stéphane Dalle (Department of Dermatology, Hôpital de L'Hôtel Dieu, Lyon, France), Dr Laurence Lamant (Department of Pathology, Hôpital Purpan, Toulouse, France), Dr Vincent Viraben (Department of Dermatology, Hôpital La Grave, Toulouse, France), and Dr Françoise Huget (Department of Hematology, Hôpital Purpan, Toulouse, France), and Dr Miguel A. Piris and Dr Manual M. Morente (Centro Nacional de Investigaciones Oncológicas, Madrid, Spain) are acknowledged for collecting material and contributing cases and follow-up data to the workshop.
Authorship
Contribution: R.W. designed the study, collected cases, analyzed and interpreted data during and after the workshop, and wrote the paper; P.M.J., L.C., E.B., M.S., C.A., M.R.C.-D., A.C., M.-L.G., S.H., L.J., W.K., C.M., P.L.O.-R., M.P., T.P., J.L.R.P., A.R., N.J.S., M.H.V., J.W., S.W., and C.J.L.M.M. collected cases, analyzed and interpreted data during and after the workshop, and contributed to the preparation of this paper; M.H. and A.R. collected cases, analyzed and interpreted data after the workshop, and contributed to the preparation of this paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rein Willemze, Department of Dermatology, B1-Q-93, Leiden University Medical Center, PO Box 9600; 2300 RC Leiden, the Netherlands; e-mail: rein.willemze@planet.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal