Abstract
The receptor for hyaluronic acid–mediated motility (RHAMM) is an antigen eliciting both humoral and cellular immune responses in patients with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and multiple myeloma (MM). We initiated a phase 1 clinical trial vaccinating 10 patients with R3 (ILSLELMKL), a highly immunogenic CD8+ T-cell epitope peptide derived from RHAMM. In 7 of 10 patients, we detected an increase of CD8+/HLA-A2/RHAMM R3 tetramer+/CD45RA+/CCR7−/CD27−/CD28− effector T cells in accordance with an increase of R3-specific CD8+ T cells in enzyme linked immunospot (ELISpot) assays. In chromium release assays, a specific lysis of RHAMM-positive leukemic blasts was shown. Three of 6 patients with myeloid disorders (1/3 AML, 2/3 MDS) achieved clinical responses: one patient with AML and one with MDS showed a significant reduction of blasts in the bone marrow after the last vaccination. One patient with MDS no longer needed erythrocyte transfusions after 4 vaccinations. Two of 4 patients with MM showed a reduction of free light chain serum levels. Taken together, RHAMM-R3 peptide vaccination induced both immunologic and clinical responses, and therefore RHAMM constitutes a promising target for further immunotherapeutic approaches. This study is registered at http://ISRCTN.org as ISRCTN32763606 and is registered with EudraCT as 2005-001706-37.
Introduction
For patients with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and multiple myeloma (MM), different therapeutic options have been developed including risk-adapted polychemotherapy regimens,1-3 bortezomib,4 lenalidomide,5 5-aza-2′-deoxycytidine,6 and other novel drugs, as well as hematopoietic stem cell transplantation.7-9 Most of the patients with these diseases are not eligible for aggressive treatment options because of their age and comorbidity. Moreover, a high percentage of them relapse after first-line therapy due to the persistence of residual tumor cells. Therefore, there is a fervent need for the development of novel treatment options. Targeted immunotherapies might constitute a synergistic therapeutic approach to eliminate minimal residual disease. Recent advances in the field of tumor immunology have resulted in the identification of a number of leukemia-associated antigens (LAAs): in leukemia patients, specific T-cell responses of cytotoxic T lymphocytes (CTLs) were detected against the Wilms tumor protein WT1, proteinase 3, the receptor for hyaluronic acid–mediated motility (RHAMM), and further LAAs such as PRAME, G250, BCL2, LAMR1, hTERT, survivin, and FLT3-ITD.10-20 For 2 LAAs (WT1 and proteinase 3), clinical peptide vaccination trials have been initiated.21-23
Earlier, we described the expression of RHAMM in more than 80% of AML/MDS, chronic myelogenous leukemia (CML), chronic lymphocytic leukemia (CLL), and MM patients.13,14,24-28 RHAMM is differentially expressed in tumor/leukemia cells, but not in peripheral blood mononuclear cells (PBMCs) or CD34+ bone marrow stem cells of healthy volunteers.24,28 RHAMM elicits both humoral and cellular immune responses in patients with different hematologic malignancies and solid tumors.13,14,24-28 We defined a highly immunogenic CD8+ T-cell epitope derived from RHAMM that was designated R3 (position 165-173: ILSLELMKL). R3-primed CD8+ T cells from AML patients were able to lyse autologous AML blasts expressing RHAMM.13 Because of these favorable characteristics of RHAMM, we initiated this phase 1/2 R3 peptide vaccination trial for HLA-A2 patients with AML, MDS, or MM overexpressing RHAMM to assess the safety and feasibility of this therapeutic approach. All patients in the present study had a residual or a slowly progressive disease, received 4 subcutaneous R3 peptide vaccinations, and were evaluated for the safety of the therapy as well as for their immunologic and clinical response.
Methods
Samples from patients with AML, MDS, and MM
All samples were taken from patients treated in this clinical study approved by the Ethikkommission of the University of Ulm (EudraCT number: 2005-001706-37). Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) from AML patients were prepared by Ficoll (Biochrom, Berlin, Germany) separation and stored for RNA preparation at − 80°C. For cellular assays, Ficoll-separated PBMCs were tested freshly or cryopreserved in AB serum (IKT, Ulm, Germany) containing 10% DMSO (Sigma Aldrich, Steinheim, Germany) and stored in liquid nitrogen.
Study design
Patients with positive HLA-A2 and RHAMM expression on malignant cells but with a limited tumor load were included. RHAMM R3 peptide (300 μg, ILSLELMKL; Merck Biosciences/Clinalpha, Laufingen, Switzerland) emulsified with the incomplete Freund adjuvant (ISA-51, Montanide; Seppic, Paris, France) on day 3 as well as granulocyte-macrophage colony-stimulating factor (GM-CSF, Leukine; Berlex, Richmond, CA) on days 1 to 5 were administrated 4 times subcutaneously at a biweekly interval. The primary aim of this phase 1 clinical trial was to test the safety and feasibility of this peptide vaccination; secondary aims were the evaluation of a specific T-cell immune response to RHAMM R3 peptide and the assessment of the hematologic status before and after R3 peptide vaccination
Inclusion criteria
Patients with AML, MDS, and MM were included. Inclusion criteria were as follows: for AML, up to 25% blasts in the bone marrow (BM); for MDS, up to 20% blasts in the BM (refractory anemia [RA], refractory anemia with excess blasts 1 [RAEB 1], RAEB 2); for MM, partial remission or near complete remission after high-dose chemotherapy with melphalan and autologous stem cell transplantation, immunofixation was still positive, and free light chains in serum and/or urine were detectable. HLA-A2 expression and expression of RHAMM-mRNA in BM or peripheral blood were prerequisites for inclusion. Patients with AML and MM should have received at least one standard therapy for this hematologic malignancy before they were put on peptide vaccination. No administration of chemotherapy or radiotherapy within the last 12 weeks prior to RHAMM-R3 vaccination.
Detection of clinical responses
For all patients, the BM and blood were analyzed before and after vaccination using microscopy and standard fluorescence-activated cell sorting (FACS) analysis.29 Patients with MM were also examined for quantitative immunoglobulins and quantitative free light chains in serum and urine.30 The frequency of erythrocyte and platelet transfusions and the course of differential blood count were documented.
Response criteria were as follows. For patients with AML, the criteria by the World Health Organization (WHO)31 and the International Working Group (IWG)32 were followed as specified: complete remission (CR) indicates reduction of blasts in the BM to less than 5%, in peripheral blood count: hemoglobin level greater than 110 g/L (11 g/dL), neutrophil count of 1500/mm3 (1.5 × 109/L) or more, and platelet count of 100 000/mm3 (100 × 109/L) or more. Partial remission (PR) indicates reduction of blasts in the BM of more than 50%; in peripheral blood count, hemoglobin level greater than 110 g/L (11 g/dL), neutrophil count of 1500/mm3 or more, and platelet count of 100 000/mm3 or more. Stable disease (SD) indicates no CR or PR. Progressive disease (PD) indicates increase of blasts in the BM by more than 50% or increase of WHO classification or progress of transfusion requirements.
For patients with MDS, the criteria by the WHO31 and the IWG33 were followed as specified: CR indicates a complete response was defined as a normocellular BM with less than 5% blasts with normal maturation of all cell lines, with no evidence of dysplasia. In PB, hemoglobin level was greater than 110 g/L (11 g/dL), neutrophil count of 1500/mm3 or more, and platelet count of 100 000/mm3 or more. PR indicates blasts decreased by 50% or more over treatment or a less MDS WHO classification than pretreatment. Hematologic improvement (HI) indicates an improvement was defined as a decrease of at least 50% transfusion requirements, together with at least an improvement of 1 to 2 cell lineages of the peripheral cell counts but not enough to qualify for a PR. SD indicates failure to achieve at least an HI, but no evidence of progression for at least 2 months. PD indicates increase of blasts in bone marrow of more than 50% or increase of WHO classification or progress of transfusion requirements.
For patients with MM, the International Uniform Response Criteria according to Durie et al were applied.34 Stringent complete remission (sCR) indicates CR as defined in the following sentence plus normal free light chain ratio and absence of clonal cells in the BM by immunohistochemistry or immunofluorescence. CR indicates negative immunofixation in the serum and urine. Very good partial response (VGPR) indicates serum and urine M-protein detectable by immunofixation but not on electrophoresis or 90% or greater reduction in serum M-protein plus urine M-protein less than 100 mg per 24 hours. PR indicates 50% or more reduction of serum M-protein and reduction in 24-hour urinary M-protein by 90% or more or to less than 200 mg per 24 hours. SD indicates no CR or PR. PD indicates increase of free light chains in serum or urine or of clonal plasma cells in bone marrow of more than 25%.
Assessment of toxicity of R3-peptide vaccination
Side effects were documented according to Common Terminology Criteria for Adverse Events v3.0 (CTCAE; http://ctep.cancer.gov). Before and 3 weeks after the fourth vaccination physical examination, body weight, ECOG performance score, laboratory tests (kidney and liver function tests, electrophoresis, electrolytes, CRP, LDH, and coagulation tests), chest X-ray, echocardiography, electrocardiography, urine analysis, abdominal sonography, and bone marrow aspiration were performed. For patients with MM, additionally quantitative immunoglobulins and quantitative assessment of free light chains in serum and urine were tested. Before each vaccination, physical examination, laboratory tests (white blood cell [WBC] count, differential blood count, kidney and liver function tests, electrolytes, CRP, LDH, and coagulation tests), and urine analysis were performed. To detect autoimmune reactions, we measured TSH, fT3, fT4, MAK, and TAK for autoimmune thyreoiditis, as well as ANCA, ANA, and rheumatic factor for rheumatic disorders.
Conventional reverse-transcription–polymerase chain reaction
mRNA was prepared from PBMCs or tumor samples using mRNA QuickPrep Micro purification kits (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). Of each mRNA sample, 2.0 μg was subjected to cDNA synthesis (Superscript II; Gibco, Frederick, MD). Polymerase chain reaction (PCR) for RHAMM was performed as described13 using the indicated conditions and ingredients.
HLA-A2 typing
Flow cytometry was performed using an HLA-A2 antibody (BD, Heidelberg, Germany). Patient cells, the T2 cell line as a positive control and the K562 cell line as negative control, were stained with the HLA-A2 antibody. After incubation at 4°C for 20 minutes in the dark and washing twice, stained cells were analyzed by flow cytometry.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISAs) were performed to assess the level of interleukin-2 (IL-2) and interleukin-10 (IL-10) in all patients' sera samples before and 3 weeks after 4 vaccinations using ELISA kits according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). All patients' sera were also subjected to ELISAs for the whole RHAMM protein and the R3 peptide following the method described earlier.35,36
Mixed lymphocyte peptide culture
PBMCs from healthy volunteers or patients were separated by Ficoll and subsequently selected by magnetic beads through a magnetic-activated cell sorting (MACS) column (Miltenyi, Bergisch-Gladbach, Germany). More than 95% purity was reached in the CD8+ fraction as assessed by flow cytometry analysis (data not shown). Mixed lympocyte peptide culture (MLPC) was performed for IMP and R3 as described earlier.13
IFN-γ and granzyme B ELISpot assays
Tetramer staining
The frequency of R3-specific CD8+ T lymphocytes was determined after 8 days of MLPC by staining with anti-CD8 antibody and HLA-A2/R3 tetramer PE. HLA-A2/R3 tetramer PE was synthesized at the Lausanne Branch of the Ludwig Institute for Cancer Research. CD8+ T lymphocytes (0.5-1 × 106) stimulated with irradiated CD8–antigen-presenting cells (APCs) in the presence of the R3 peptide were stained with HLA-A2/R3 tetramer PE 1 μg per test with respect to the peptide–MHC class I component in the dark and incubated for 40 minutes at room temperature. Thereafter, for 4-color staining, 10 μL CD8 PerCP, 10 μL CD45RA FITC, and 5 μL CCR7 APC (BD, Heidelberg, Germany) were added at 4°C for 20 minutes in the dark. As for 6-color staining, the cells were stained with 1 μg HLA-A2/R3 tetramer PE and HLA-A2/WT1 tetramer PerCP per test and incubated for 40 minutes at room temperature in the dark. Thereafter 5 μL CD8 APC-Cy7, 5 μL CD45RA APC (Invitrogen, Carlsbad, CA), 10 μL CCR7 PE-Cy7, and 10 μL CD27 FITC or CD28 FITC (BD, Heidelberg, Germany) were added at 4°C for 20 minutes in the dark. After washing once with PBS, stained cells were fixed with 1% formaldehyde (Sigma Aldrich) and then analyzed by flow cytometry. Whenever possible, at least 100 000 events were collected for analysis. Each sample was run with an appropriate isotype control to define the gate of positive cells. Analysis was performed on tightly gated lymphocytes to exclude dead cells and debris and on CD8+ T lymphocytes to evaluate responses to R3 peptide. Samples were defined as tetramer positive in case of an increase of specific R3-tetramer+/CD8+ T cells of more than 50% (if initial count was ≤ 0.1%), or 25% increase (if initial count was > 0.1%). We participated in an interlaboratory test for tetramer flow cytometric assays.37
Chromium-51 release cytotoxicity assay
RHAMM+/HLA-A2+, RHAMM+/HLA-A2−, and RHAMM-/HLA-A2+ AML blasts, T2 cells pulsed with 20 μg/mL RHAMM-derived peptide R3, and K562 cells were labeled with 51CrO4 (Cr-51) for 2 hours as described earlier.13 After washing, labeled cells were incubated with CD8+ T cells at effector-target ratios of 5:1 to 2.5:1 for 16 hours at 37°C. Radioactivity in the supernatant from all wells of round-bottom 96-well plates (Becton Dickinson, Heidelberg, Germany) was measured by a gamma counter (PerkinElmer, Boston, MA). The percentage of specific lysis was calculated as (Cr-51 release in the test well minus spontaneous Cr-51 release)/(maximum Cr-51 release minus spontaneous Cr-51 release).
Analysis of regulatory T cells (Tregs)
Staining of PBMCs of the patients before, during, and after vaccination was performed using the following fluorescence-labeled monoclonal antibodies: phycoerythrin (PE)–Cy7-conjugated anti-CD4 (BD Biosciences, Heidelberg, Germany), allophycocyanin (APC)–Cy7–conjugated anti-CD25 (BD Biosciences), and intracellular fluorescein isothiocyanate (FITC)–conjugated anti-Foxp3 (eBioscience, Kranenburg, Germany) with the appropriate normal isotype-matched control IgGs. For extracellular staining, cells were incubated for 30 minutes at 4°C with optimal dilution of each antibody. For intracellular staining, the cells were fixed with Reagent A and permeabilized with Reagent B (IntraStain; DakoCytomation, Hamburg, Germany). The cells were analyzed on a FACSAria flow cytometer (Becton Dickinson) using the CellQuest software (Becton Dickinson).
Results
Patient characteristics
All 10 patients included in the present study expressed both RHAMM and HLA-A2 as assessed by conventional PCR and flow cytometry. Cytogenetic and clinical characteristics of these patients are listed in Table 1.
Synopsis of clinical and immunologic characteristics of the 10 vaccinated patients
| No. . | Diagnosis . | Age, y . | WHO classification; karyotype . | Therapy before vaccination . | Status at vaccination . | Period of vaccination . | Level of IL-2 in ELISA . | CD8+ tetra+* . | ELISpot IFNγ . | ELISpot GrB . | Malignant cells, FACS . | Clinical findings . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | ||||||||||
| 1 | AML | 66 | AML with maturation; normal karyotype, NPM1 mutation, FLT3-ITD negativ | VACI 05/2004, PR | PR | 12/04-01/05 | Up | Pos | 0 | 77 | 0 | 5 | 7 | 0.5 | Reduction of blasts in the BM*; relapse at +23 months |
| 2 | MDS | 72 | RAEB I; normal karyotype IPSS Int-1 | None | RAEB I | 02/05-03/05 | Up | Pos | 5 | 16 | 11 | 19 | 8 | 1 | Reduction of blasts in the BM*; normal WBC at +24 months |
| 3 | MM | 45 | IgA kappa; stage IIIA; no del13q14 ß2-MG 5.28 mg/L | DSMM V, autoTX 05+09/2002 | VGPR ß2MG: | 03/05-04/05 | Down | Pos | 5 | 24 | 0 | 9 | 3.5 | 0.5 | Reduction of plasma cells and kappa-LC (+23 months) |
| 4 | AML | 58 | AML with maturation; trisomy 8 | German intergroup arm, HiDAC, CR | Relapse | 04/05-06/05 | Stable | NC | 0 | 0 | 1 | 0 | 5 | 10 | PD |
| 5 | MDS | 80 | RCMD; not done IPSS Int-1 | Weekly erythrocyte and platelet transfusions | RCMD | 05/05-06/05 | Down | nt | 0 | 0 | 32 | 35 | 2 | 1 | NC |
| 6 | MDS | 81 | RA; 45,X,−Y IPSS low risk | Erythrocyte transfusions | RA | 05/05-06/05 | Up | Pos | 0 | 49 | nt | nt | 1.5 | 0.5 | Cessation of transfusions (+22 months) |
| 7 | MM | 67 | IgG kappa, stage IIIA del13q14 ß2-MG 4.5 mg/L | DSMM II, auto TX 07+09/2004 | PD ß2MG: | 06/05-07/05 | Stable | NC | 0 | 20 | 10 | 44 | nt | nt | PD |
| 8 | MM | 51 | IgG kappa stage IIIA; no del13q14 ß2-MG 1.27 mg/L | DSMM V, auto TX | VGPR | 06/05-07/05 | Stable | Pos | 0 | 5 | 29 | 56 | 1.5 | 1 | NC |
| 9 | AML | 44 | AML without maturation; complex karyotype; FLT3-ITD negative, NPM1 wild type | 2xICE, 1xHAM, auto TX | PR | 06/05-08/05 | Down | Pos | 3 | 0 | 0 | 14 | 6 | 7 | NC |
| 10 | MM | 70 | IgG kappa, stage IIIA; no del13q14 ß2-MG 4.4 mg/L | DSMM II, auto TX | VGPR ß2MG: | 07/05-08/05 | Up | Pos | 0 | 84 | 0 | 7 | 0.15 | 0.02 | Reduction of kappa-LC†; but PD at +4 months |
| No. . | Diagnosis . | Age, y . | WHO classification; karyotype . | Therapy before vaccination . | Status at vaccination . | Period of vaccination . | Level of IL-2 in ELISA . | CD8+ tetra+* . | ELISpot IFNγ . | ELISpot GrB . | Malignant cells, FACS . | Clinical findings . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | ||||||||||
| 1 | AML | 66 | AML with maturation; normal karyotype, NPM1 mutation, FLT3-ITD negativ | VACI 05/2004, PR | PR | 12/04-01/05 | Up | Pos | 0 | 77 | 0 | 5 | 7 | 0.5 | Reduction of blasts in the BM*; relapse at +23 months |
| 2 | MDS | 72 | RAEB I; normal karyotype IPSS Int-1 | None | RAEB I | 02/05-03/05 | Up | Pos | 5 | 16 | 11 | 19 | 8 | 1 | Reduction of blasts in the BM*; normal WBC at +24 months |
| 3 | MM | 45 | IgA kappa; stage IIIA; no del13q14 ß2-MG 5.28 mg/L | DSMM V, autoTX 05+09/2002 | VGPR ß2MG: | 03/05-04/05 | Down | Pos | 5 | 24 | 0 | 9 | 3.5 | 0.5 | Reduction of plasma cells and kappa-LC (+23 months) |
| 4 | AML | 58 | AML with maturation; trisomy 8 | German intergroup arm, HiDAC, CR | Relapse | 04/05-06/05 | Stable | NC | 0 | 0 | 1 | 0 | 5 | 10 | PD |
| 5 | MDS | 80 | RCMD; not done IPSS Int-1 | Weekly erythrocyte and platelet transfusions | RCMD | 05/05-06/05 | Down | nt | 0 | 0 | 32 | 35 | 2 | 1 | NC |
| 6 | MDS | 81 | RA; 45,X,−Y IPSS low risk | Erythrocyte transfusions | RA | 05/05-06/05 | Up | Pos | 0 | 49 | nt | nt | 1.5 | 0.5 | Cessation of transfusions (+22 months) |
| 7 | MM | 67 | IgG kappa, stage IIIA del13q14 ß2-MG 4.5 mg/L | DSMM II, auto TX 07+09/2004 | PD ß2MG: | 06/05-07/05 | Stable | NC | 0 | 20 | 10 | 44 | nt | nt | PD |
| 8 | MM | 51 | IgG kappa stage IIIA; no del13q14 ß2-MG 1.27 mg/L | DSMM V, auto TX | VGPR | 06/05-07/05 | Stable | Pos | 0 | 5 | 29 | 56 | 1.5 | 1 | NC |
| 9 | AML | 44 | AML without maturation; complex karyotype; FLT3-ITD negative, NPM1 wild type | 2xICE, 1xHAM, auto TX | PR | 06/05-08/05 | Down | Pos | 3 | 0 | 0 | 14 | 6 | 7 | NC |
| 10 | MM | 70 | IgG kappa, stage IIIA; no del13q14 ß2-MG 4.4 mg/L | DSMM II, auto TX | VGPR ß2MG: | 07/05-08/05 | Up | Pos | 0 | 84 | 0 | 7 | 0.15 | 0.02 | Reduction of kappa-LC†; but PD at +4 months |
Age means age at the time of vaccination therapy. DSMM II/V (protocols II and V of the German Study Group for Multiple Myeloma): ID, IEV, Mel, double-auto/auto-allo-stem cell transplantation. The ELISpot numbers indicated in the table were calculated as follows: before, R3-specific spots minus background spots before vaccination; Max, maximum difference of R3-specific spots minus background spots during or after vaccination; w/o, without.
ID indicates idarubicin/dexamethasone; IEV, ifosfamid/epirubicin/etoposide; Mel, melphalan; LC, serum free light chains; Pos, increase; nt, not tested; response criteria according to Jäger et al36 ; Britten et al37 ; Jager et al38 , and Parmiani et al39 ; CR, complete remission; PR, partial remission; NC, no change; PD, progressive disease; RCMD, refractory cytopenia with multilineage dysplasia; HiDAC, high-dose arabinoside C; VACI, valproic acid, arabinosids C, idarubicin; TX, stem cell transplantation; w/o, without; BM, bone marrow; RA, refractory anemia; RAEB, with excess of blasts; IPSS, International Prognostic Scoring System; VGPR, very good partial response; and ß2MG, beta-2 microglobulin.
See also Table 2 for tetramer assay results in detail.
Assessed 3 weeks after the last vaccination.
Toxicity of peptide vaccination
Ten patients (3 AML, 3 MDS, and 4 MM) were enrolled and completed the course of 4 vaccinations. Only mild side effects such as CTC I° erythema and induration of the skin at the site of injection were observed after peptide vaccination using the RHAMM-R3 peptide. One patient developed an increase in body temperature from 36.5°C to 38.0°C that persisted for several hours after the first vaccine administration, but this symptom was not observed after subsequent vaccinations. There was no other therapy-related toxicity. No clinical signs or laboratory results for vitiligo, autoimmune thyreoiditis, or rheumatic disorders were observed.
Immunologic responses
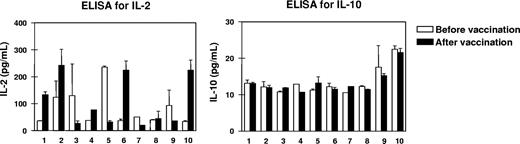
To screen the balance of type 1 and type 2 T-cell cytokines, we measured the levels of IL-2 and IL-10 in the sera of all patients before and 3 weeks after 4 vaccinations. IL-10 levels ranging from 10 to 20 pg/mL were detectable by high-resolution ELISA and remained stable in all patients during the period of vaccination, while IL-2 levels were rather dynamic as shown in Figure 1. High increases in IL-2 starting from levels at 50 pg/mL up to the 5-fold was seen in patients nos. 1, 2, 6, and 10, while IL-2 levels remained stable or even decreased in the rest of the patients.
Changes in the cytokine milieu of the peripheral blood from patients before and after vaccination. To screen type 1 and type 2 T-cell activation, we measured the levels of interleukin-2 and IL-10 (IL-2 and IL-10, respectively) in the sera of all 10 patients before and 3 weeks after the last of 4 RHAMM-R3 peptide vaccinations. While IL-10 levels remained rather low, an up-to-5-fold increase in IL-2 levels as well as stable or decreasing levels for the cytokine were observed. Patient numbers indicated below the columns refer to the patient numbers in Table 1. Error bars represent SD.
Changes in the cytokine milieu of the peripheral blood from patients before and after vaccination. To screen type 1 and type 2 T-cell activation, we measured the levels of interleukin-2 and IL-10 (IL-2 and IL-10, respectively) in the sera of all 10 patients before and 3 weeks after the last of 4 RHAMM-R3 peptide vaccinations. While IL-10 levels remained rather low, an up-to-5-fold increase in IL-2 levels as well as stable or decreasing levels for the cytokine were observed. Patient numbers indicated below the columns refer to the patient numbers in Table 1. Error bars represent SD.
As for cellular immune responses, we detected in the peripheral blood of the 10 patients, a significant increase of specific CD8+ T cells recognizing the R3 peptide in 8 of 10 patients by ELISpot analysis as well as in 7 of 10 patients by tetramer staining. The ELISpot data of these 10 patients for IFNγ and granzyme B secretion are summarized in Table 1 and the tetramer staining results in Table 2. The results of ELISpot assays were considered to be positive when an increase of more than 50% of spots was seen in the course of vaccination. A positive immunologic response for tetramer staining was defined as an increase more than 50% of HLA-A2/R3-tetramer+/CD8+ T lymphocytes less than 0.1% prior to vaccination and with an increase more than 25% of HLA-A2/R3-tetramer+/CD8+ T lymphocytes more than 0.1% prior to vaccination. FACS data combined with ELISpot results of 2 patients are exemplarily shown. Figure 2 presents an increase of the frequency of HLA-A2/R3-tetramer+/CD8+ T lymphocytes from 0.05% to 0.11% with up to 85% of these cells being CD8+/HLA-A2/R3 tetramer+/CCR7−/CD45RA+ effector T cells and a transient increase of the R3-specific secretion of IFNγ during the course of vaccination of this AML patient. Figure 3 displays a 6-color staining for R3-specific CD8+ T lymphocytes with an increase of CD8+/HLA-A2/R3 tetramer+ T lymphocytes from 0.55% to 1.23% after 3 vaccinations and an increase of granzyme B secretion over the time of vaccination and decrease after cessation of vaccination of this patient with MM. In the course of vaccination, the frequency of CD8+/HLA-A2/R3 tetramer+/WT1 tetramer−/CCR7−/CD45RA+/CD27− effector T cells rose up to almost 80% and the CD28− effector T cells increased up to 23%, decreasing, however, 3 weeks thereafter.
Tetramer staining of the 10 vaccinated patients in this trial
| Patient no. . | Before first vaccination . | After first vaccination . | After second vaccination . | After third vaccination . | After fourth vaccination . | Immune response . |
|---|---|---|---|---|---|---|
| 1 | 0.05 | nt | 0.03 | 0.06 | 0.11 | Positive |
| 2 | 0.54 | 0.46 | 0.51 | 0.73 | 0.63 | Positive |
| 3 | 0.46 | 0.65 | 0.42 | 0.7 | nt | Positive |
| 4 | 0.48 | nt | 0.48 | 0.52 | nt | No change |
| 5 | nt | nt | nt | nt | nt | nt |
| 6 | 0.33 | 0.28 | 0.29 | 0.42 | 0.31 | Positive |
| 7 | 0.16 | 0.13 | 0.16 | 0.15 | 0.13 | No change |
| 8 | 0.55 | 0.41 | 0.40 | 1.23 | 0.46 | Positive |
| 9 | 0.06 | 0.14 | 0.13 | 0.09 | 0.11 | Positive |
| 10 | 0.28 | 0.53 | 0.47 | 0.38 | 0.56 | Positive |
| Patient no. . | Before first vaccination . | After first vaccination . | After second vaccination . | After third vaccination . | After fourth vaccination . | Immune response . |
|---|---|---|---|---|---|---|
| 1 | 0.05 | nt | 0.03 | 0.06 | 0.11 | Positive |
| 2 | 0.54 | 0.46 | 0.51 | 0.73 | 0.63 | Positive |
| 3 | 0.46 | 0.65 | 0.42 | 0.7 | nt | Positive |
| 4 | 0.48 | nt | 0.48 | 0.52 | nt | No change |
| 5 | nt | nt | nt | nt | nt | nt |
| 6 | 0.33 | 0.28 | 0.29 | 0.42 | 0.31 | Positive |
| 7 | 0.16 | 0.13 | 0.16 | 0.15 | 0.13 | No change |
| 8 | 0.55 | 0.41 | 0.40 | 1.23 | 0.46 | Positive |
| 9 | 0.06 | 0.14 | 0.13 | 0.09 | 0.11 | Positive |
| 10 | 0.28 | 0.53 | 0.47 | 0.38 | 0.56 | Positive |
Immune response: an initial value less than 0.1% was judged positive if there was a consecutive increase more than 50%; an initial value more than 0.1% was judged positive if there was a consecutive increase more than 25%.
nt indicates not tested, that is, not sufficient cells for tetramer staining (apart from ELISpot assays).
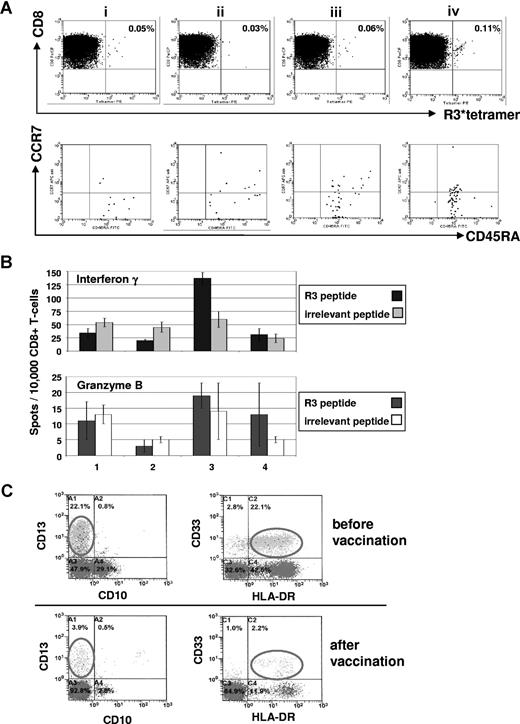
Immunologic and clinical responses of patient no. 1 with acute myeloid leukemia to the course of RHAMM-R3 peptide vaccination. (A) The FACS dot plots show the percentage of HLA-A2/R3-tetramer+/CD8+ T lymphocytes. The frequency of CD8+/HLA-A2/R3-tetramer+ T lymphocytes increased after 4 vaccinations (i represents before; ii, after second; iii, after third; and iv, 2 weeks after fourth/last vaccination). The HLA-A2/R3-tetramer*PE–positive CD8+ T cells were further analyzed for their expression of CCR7 and CD45RA. Most of the cells (70%-85%) revealed to be CD8+/HLA-A2/R3 tetramer+/CCR7−/CD45RA+ effector T cells. (B) The frequency of R3-specific CD8+ T lymphocytes increased during the course of vaccination as assessed by ELISpot assays for IFN-γ and granzyme B release. All assays were performed in triplicate. Error bars indicate the SD. (C) Monitoring of malignant cells correlated with the other findings showing a decrease of CD33+/HLA-DR+ malignant cells during vaccination. Numbers on plots are percentages of all lymphocytes (panel A), or of cells in the blast gate (panel C), respectively.
Immunologic and clinical responses of patient no. 1 with acute myeloid leukemia to the course of RHAMM-R3 peptide vaccination. (A) The FACS dot plots show the percentage of HLA-A2/R3-tetramer+/CD8+ T lymphocytes. The frequency of CD8+/HLA-A2/R3-tetramer+ T lymphocytes increased after 4 vaccinations (i represents before; ii, after second; iii, after third; and iv, 2 weeks after fourth/last vaccination). The HLA-A2/R3-tetramer*PE–positive CD8+ T cells were further analyzed for their expression of CCR7 and CD45RA. Most of the cells (70%-85%) revealed to be CD8+/HLA-A2/R3 tetramer+/CCR7−/CD45RA+ effector T cells. (B) The frequency of R3-specific CD8+ T lymphocytes increased during the course of vaccination as assessed by ELISpot assays for IFN-γ and granzyme B release. All assays were performed in triplicate. Error bars indicate the SD. (C) Monitoring of malignant cells correlated with the other findings showing a decrease of CD33+/HLA-DR+ malignant cells during vaccination. Numbers on plots are percentages of all lymphocytes (panel A), or of cells in the blast gate (panel C), respectively.
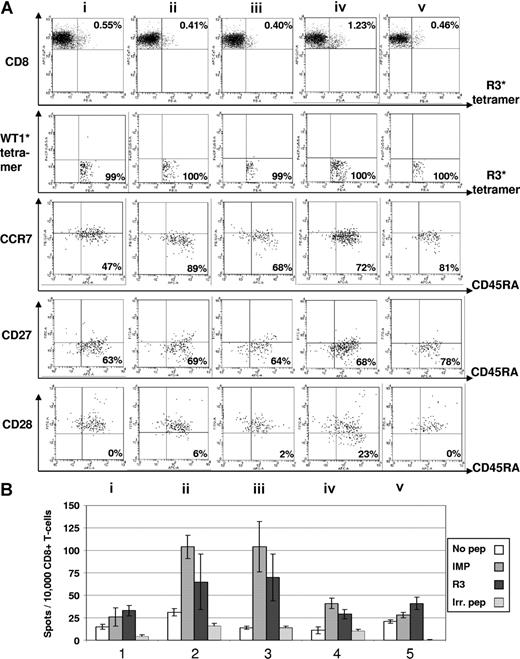
Immunomonitoring for R3-specific CD8+ T lymphocytes in patient no. 8 with multiple myeloma. (A) The dot plots show the percentage of HLA-A2/R3-tetramer+/CD8+ T lymphocytes. Six-color staining for R3-specific CD8+ T lymphocytes revealed an increase of CD8+/HLA-A2/R3-tetramer+ T lymphocytes over 4 vaccinations and a decrease thereafter (i represents before; ii, after first; iii, after second; iv, after third; and v, 3 weeks after fourth/last vaccination). The HLA-A2/R3-tetramer*PE–positive CD8+ T lymphocytes could not be counterstained by WT1-tetramers demonstrating their specificity for the peptide R3. The staining for CCR7 and CD45RA demonstrated an increase of CD8+/HLA-A2/R3 tetramer+/WT1 tetramer−/CCR7−/CD45RA+ effector T cells from 47% to 81%. The frequency of CD8+/HLA-A2/R3 tetramer+/WT1 tetramer−/CCR7−/CD45RA+/CD27− effector T cells rose from 63% up to 78%. The CD8+/HLA-A2/R3 tetramer+/WT1 tetramer−/CCR7−/CD45RA+/CD28− effector T cells increased over 4 vaccinations, but decreased 3 weeks thereafter. Numbers on plots are percentages of all lymphocytes. (B) The results of the ELISpot assay for granzyme B correlated with the FACS data showing an increase and subsequent decrease of R3-specific CD8+ T-lymphocyte function during the course of vaccination. All assays were performed in triplicate. Error bars indicate the SD.
Immunomonitoring for R3-specific CD8+ T lymphocytes in patient no. 8 with multiple myeloma. (A) The dot plots show the percentage of HLA-A2/R3-tetramer+/CD8+ T lymphocytes. Six-color staining for R3-specific CD8+ T lymphocytes revealed an increase of CD8+/HLA-A2/R3-tetramer+ T lymphocytes over 4 vaccinations and a decrease thereafter (i represents before; ii, after first; iii, after second; iv, after third; and v, 3 weeks after fourth/last vaccination). The HLA-A2/R3-tetramer*PE–positive CD8+ T lymphocytes could not be counterstained by WT1-tetramers demonstrating their specificity for the peptide R3. The staining for CCR7 and CD45RA demonstrated an increase of CD8+/HLA-A2/R3 tetramer+/WT1 tetramer−/CCR7−/CD45RA+ effector T cells from 47% to 81%. The frequency of CD8+/HLA-A2/R3 tetramer+/WT1 tetramer−/CCR7−/CD45RA+/CD27− effector T cells rose from 63% up to 78%. The CD8+/HLA-A2/R3 tetramer+/WT1 tetramer−/CCR7−/CD45RA+/CD28− effector T cells increased over 4 vaccinations, but decreased 3 weeks thereafter. Numbers on plots are percentages of all lymphocytes. (B) The results of the ELISpot assay for granzyme B correlated with the FACS data showing an increase and subsequent decrease of R3-specific CD8+ T-lymphocyte function during the course of vaccination. All assays were performed in triplicate. Error bars indicate the SD.
Sera were collected from all patients before and after 4 vaccinations with the HLA class I peptide R3. Neither class G immunoglobulins (IgG) against the whole protein of RHAMM nor specific IgGs against the vaccination peptide R3 could be detected.
Generation of R3-specific cytotoxic CD8+ T lymphocytes
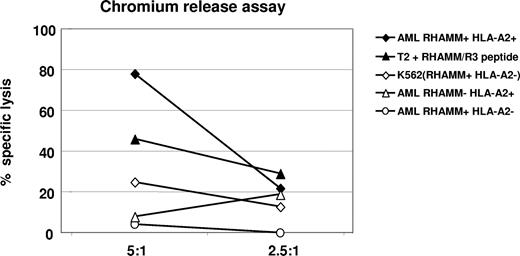
By FACS analysis, we detected an increase of CD8+ effector T cells (Table 2; Figures 2,3) that secreted IFNγ as a marker of activation and released granzyme B (Table 1; Figures 2,3) demonstrating their lytic potential. When we tested these CD8+ T cells in a standard chromium-51 release assay, they were able to lyse 80% of autologous AML blasts at an effector-target (E/T) ratio of 5:1, while allogeneic AML blasts lacking either RHAMM or HLA-A2 expression were not recognized beyond background level. T2 cells pulsed with the R3 peptide were used as a positive control for R3-specific lysis. Absence of lysis of RHAMM-positive, but HLA-A2–negative K562 cells served as a negative control, and demonstrated that the lysis was not mediated through natural killer (NK) cells (Figure 4). Due to restrictions in T cells isolated from the peripheral blood and malignant cells of vaccinated patients, this assay could be performed only for 3 patients, but with similar results. Figure 4 shows a representative result obtained in patient no. 1 with an AML.
Chromium release assay for the verification of R3-specific cytotoxic activity of CD8+ T lymphocytes in patient no. 1 with acute myeloid leukemia after the course of vaccination. An 80% specific lysis of HLA-A2+/R3+ AML blasts by cytotoxic CD8+ T lymphocytes was achieved at a ratio of 5:1 (effector-target cell ratio); 46% of HLA-A2+ T2 cells pulsed with R3 peptide were lysed at this E/T ratio. Allogeneic HLA-A2−/R3+ AML blasts, HLA-A2+/R3− AML blasts, as well as HLA-A2−/R3+ K562 cells were lysed only at background level.
Chromium release assay for the verification of R3-specific cytotoxic activity of CD8+ T lymphocytes in patient no. 1 with acute myeloid leukemia after the course of vaccination. An 80% specific lysis of HLA-A2+/R3+ AML blasts by cytotoxic CD8+ T lymphocytes was achieved at a ratio of 5:1 (effector-target cell ratio); 46% of HLA-A2+ T2 cells pulsed with R3 peptide were lysed at this E/T ratio. Allogeneic HLA-A2−/R3+ AML blasts, HLA-A2+/R3− AML blasts, as well as HLA-A2−/R3+ K562 cells were lysed only at background level.
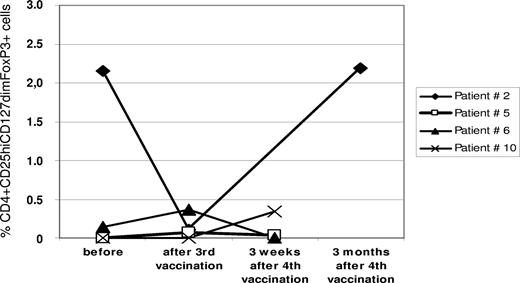
Furthermore, we assessed changes in the frequency of CD4+/CD25hi/CD127dim/FoxP3+ regulatory T cells (Tregs) in the peripheral blood from 4 patients (nos. 2, 5, 6, and 10) before, during, and after R3 peptide vaccination. In all cases, the frequency of Tregs was rather moderate. Only in one patient (no. 2), did we detect a decrease during vaccination followed by an increase after the end of vaccinations (Figure 5).
Changes of regulatory T cells in the peripheral blood. Flow cytometry was performed on PBMCs. For details, see “Methods.” Regulatory T cells (Tregs) were defined as CD4, CD25hi, and FoxP3 coexpressing cells. The graph gives the frequency of Tregs as percentage of all CD4+ T cells in the respective sample at different time points before, during, and after R3 peptide vaccination in 4 patients.
Changes of regulatory T cells in the peripheral blood. Flow cytometry was performed on PBMCs. For details, see “Methods.” Regulatory T cells (Tregs) were defined as CD4, CD25hi, and FoxP3 coexpressing cells. The graph gives the frequency of Tregs as percentage of all CD4+ T cells in the respective sample at different time points before, during, and after R3 peptide vaccination in 4 patients.
Clinical responses to R3 peptide vaccination
Clinical responses were assessed by the examination of peripheral blood and bone marrow samples before and after vaccination. For MM patients, we additionally assessed immunofixation, serum free light chains, and quantitative serum immunoglobulins. Three of 6 patients with myeloid disorders (1 AML, 2 MDS) showed a reduction of CD33+/HLA-DR+ cells in FACS analysis of the bone marrow after 4 vaccinations. One patient with MDS did not need any further erythrocyte substitution after 4 vaccinations. Two patients with MM showed a reduction of plasma cells and of β2-microglobulin in the bone marrow as by FACS analysis and of free light chains in the serum and/or urine. One patient with AML and one patient with MM developed a progress of their disease.
Discussion
In the present study, 10 patients with hematologic malignancies expressing both RHAMM and HLA-A2 were vaccinated with the RHAMM-derived peptide R3 subcutaneously 4 times at a biweekly interval. All patients concluded the course of 4 vaccinations. No adverse events greater than CTC I° skin toxicity could be observed, which is in accordance with the results in numerous peptide vaccination trials performed in patients with solid tumors following the concept of emulsification of the peptide as the core of the vaccine in incomplete Freund adjuvant (IFA) and concomitant administration of granulocyte-macrophage colony-stimulating factor (GM-CSF).38,39 The emulsification in IFA seems to be essential to prevent the peptide from rapid degradation in the subcutis. GM-CSF has been shown to be a potent enhancer of a T-cell response to the vaccination peptide40 and might enhance the maturation of hematopoietic cells. We saw a transient increase in WBC count and platelet count in most of our patients during the time of vaccination. One might speculate that GM-CSF contributes to the maturation of blasts as hematopoietic precursor cells. However, long-lasting effects were seen in the bone marrow, while peripheral blood counts went back to the previous level.
The immunomonitoring of the patients in our study was performed using ELISA, 6-color flow cytometry, and 2-parametric ELISpot assays for the secretion of IFNγ and granzyme B. A good concordance of these 3 readout systems was noted (Tables 1,2). To screen type 1 and type 2 T-cell responses after vaccination, we measured IL-2 and IL-10 levels in the sera of the patients before and 3 weeks after 4 vaccinations. While IL-10 levels remained at a rather low level over the time of vaccination, we detected an increase of IL-2 up to the 5-fold of the initial levels in 4 of 10 patients. Interestingly, these 4 patients showed also a clinical response. R3-specific CD8+ T cells were defined by positive staining with a R3*tetramer and the failure of counterstaining with WT1*tetramer. Seven (70%) of 10 vaccinated patients showed an increase of RHAMM-R3–specific T cells in tetramer assays, 8 (80%) of 10 in ELISpot assays. The number of effectors cells characterized to be CD8+/R3 tetramer+/WT1 tetramer−/CCR7−/CD27−/CD28−/CD45RA+ T cells increased over the time of vaccination (Figures 2,3). Interestingly, a decrease in this subpopulation could be observed in 3 patients after vaccination was stopped (Table 2; Figure 3). Moreover, for CD4+/CD25hi/CD127dim/FoxP3+ regulatory T cells (Tregs), a countercurrent fluctuation could also be observed (Figure 5, patient no. 2). This finding might be an important point to extent the initial series of 4 vaccinations in our present trial to additional boost vaccinations at a longer interval in future trials as described in the case report by Mailänder et al.21 Administration of CD4+ helper T-cell epitopes derived from RHAMM or the rather unspecific CD4+ T-cell stimulator keyhole limpet hemocyanine (KLH) used by the same group21 or other adjuvants such as CPG-rich oligodinucleotides40 might help to induce more long-lasting vaccination results. In chromium-51 release cytotoxicity assays, the lysis of 40% of AML blasts at an E/T ratio of 10:1 could validate the functionality of the effector T cells characterized in flow cytometry and ELISpot analysis in the clinically responding patient. As monitored by ELISA, no increase of RHAMM-specific immunoglobulin class G antibodies could be detected during or after the vaccination. Vaccination with a HLA class I–restricted peptide does not typically result in the induction of humoral immune responses.38,39 Vaccination with the whole protein or an additional class II epitope peptide might overcome that problem.
In this trial, we vaccinated patients with a residual or controlled disease, as in this setting specific T cells were present but not affected by chemotherapy. Such T cells might better cope with a limited tumor load than in an AML patient with a high blast count. Five of 10 patients in this stage showed a blast reduction in the BM (AML/MDS) or a decrease of the serum or urine level of free light chains (MM) 3 weeks after the last peptide vaccination. These positive clinical results are in accordance with observations in other clinical peptide vaccination trials.21-23 Overall, a good correlation between immunologic and clinical responses was observed, which suggests RHAMM constitutes an important factor in the proliferative process of leukemia and myeloma. Involvement of RHAMM in the formation of the mitotic spindle apparatus, the ras-raf signal transduction, and metastasis has been described.41
By targeting a single leukemia-associated antigen by peptide vaccination, one might cover only a part of the total patient cohort. In a recent study,14 we found a better survival in AML patients expressing at least 1 of the 3 antigens G250, PRAME, and RHAMM compared with AML patients without expression of any of these antigens. Based on our encouraging results reported here using monovalent vaccination, we plan now a phase 1/2 clinical polyvalent vaccination trial targeting the 3 antigens G250, PRAME, and RHAMM.
In summary, we demonstrated in the present phase 1 clinical trial the safety and feasibility of a RHAMM-R3 peptide vaccination in patients with hematologic malignancies. Both immunologic and to some extent clinical responses could be observed characterizing RHAMM as a promising target for further immunotherapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Szmaragowska for her excellent technical support.
This work was supported by generous grants from the German José Carreras Leukemia Foundation to J.G., M.W., H.D., and M.S., as well as from the German Research Foundation DFG (GR 2676/1-1) to J.G. and M.S.
National Institutes of Health
Authorship
Contribution: This study was designed by M.S. and J.G; all research was performed by A.S., M.T.R., J.C., K.G., F.F., Y.Y., and M.G; the vaccine preparation was developed by D.E.S; the paper was written by M.S., A.S., and J.G.; M.R., R.F.S., P.L., and M.H. supplied patient material and discussed the paper; P.G. synthesized the tetrameric complexes; and G.R., D.E.S., S.G., D.B., H.S., and H.D. discussed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Schmitt, Department of Internal Medicine III, University of Ulm, Robert-Koch-Str. 8, 89081 Ulm, Germany; e-mail: michael.schmitt@uniklinik-ulm.deM.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal