Abstract
Activation of PI3K is among the earliest signaling events observed in T cells after conjugate formation with antigen-presenting cells (APCs). The relevant PI3K catalytic isoform and relative contribution of the TcR and CD28 to PI3K activity at the immune synapse have not been determined unequivocally. Using a quantitative imaging-based assay, we show that the PI3K activity at the T cell–APC contact area is dependent on the p110δ, but not the p110γ, isoform of PI3K. CD28 enhanced PIP3 production at the T-cell synapse independently of its YMNM PI3K-recruitment motif that instead was required for efficient PKCθ recruitment. CD28 could partially compensate for the lack of p110δ activity during T-cell activation, which indicates that CD28 and p110δ act in parallel and complementary pathways to activate T cells. Consistent with this, CD28 and p110δ double-deficient mice were severely immune compromised. We therefore suggest that combined pharmaceutic targeting of p110δ activity and CD28 costimulation has potent therapeutic potential.
Introduction
Full activation of T cells by antigen requires the integration of signals from several receptors on the T-cell surface that bind ligands on antigen-presenting cells (APCs). The T-cell receptor for antigen (TcR) is associated with CD3 and ζ chains that act as substrates and docking sites for nonreceptor tyrosine kinases. In CD4+ T helper cells (Th), CD4-bound Lck is brought in proximity with the TcR and phosphorylates the CD3 and ζ cytoplasmic immunoreceptor tyrosine-based activation motifs, thus providing docking sites for the ZAP-70 SH2 domains.1 ZAP-70 subsequently phosphorylates the transmembrane adapter protein LAT.2 When phosphorylated, LAT binds directly to the SH2 domains of PLCγ, Grb2, and Gads.3 Gads recruits SLP-76 to the complex and along with it Vav and Itk, each of which have the potential to recruit additional signaling proteins.3,4 These signaling events result in the activation of 2 key lipid-based second messenger signaling pathways catalyzed by PLCγ and PI3K, respectively.5
PLCγ hydrolyzes PtdIns(4,5)P2 (PIP2) to yield the second messenger signaling molecules diacylglycerol (DAG) and inositol(1,4,5)P3 (IP3). DAG recruits both RasGRP and PKCθ to the plasma membrane, and these initiate the Ras-Erk and NF-κB signaling pathways, respectively.6 IP3 is released into the cytosol where it stimulates the release of Ca2+ into the cytoplasm from intracellular stores which stimulates the nuclear translocation of NFAT transcription factors.7
PI3Ks catalyze the conversion of PIP2 to PtdIns(3,4,5)P3 (PIP3), which is resistant to PLC-hydrolysis and acts as a secondary signaling molecule, recruiting PH domain–containing proteins to the plasma membrane.8,9 T cells express 4 different catalytic isoforms of PI3K that have the capacity to generate PIP3.10 The class IA PI3Ks p110α, p110β, and p110δ associate with p85 regulatory subunits and are activated by tyrosine kinases. The class IB p110γ isoform is bound by a p101 or p84 regulatory subunit and is activated by G-protein–coupled receptors. Generation of PIP3 is among the earliest signals that can be observed when T cells respond to stimulation with antigen.11-15 It is still not known how the TcR activates PI3K, although a number of mechanisms are possible, including p85-SH2–mediated binding to YxxM motifs in the transmembrane adapter protein TRIM or to noncanonical phosphotyrosines in LAT, SLP-76, or ZAP-70.2,16-18 p85 may also associate by its SH3 domains with Src kinases.19,20 Finally, the p110 subunit may be activated directly by Ras.21-25
CD28 can bind directly to PI3K by a well-characterized YMNM binding motif in its cytoplasmic domain.26-30 The CD28 ligands CD80 and CD86 can stimulate the association between CD28 and PI3K as well as PIP3 production in T-cell lines.27,31-33 However, both in Jurkat T cells and in primary mouse T cells, CD28 mutants that interfere with the capacity of CD28 to recruit PI3K retain costimulatory capacity in vitro and in vivo.29,34-36 In addition, CD28 costimulation is able to compensate for the lack of PI3K activity under conditions in which CD3-dependent proliferation is impaired.37,38 However, other studies had suggested a key role of PI3K in the context of CD28 costimulation.35,39,40 Therefore, the question of the relative contributions of the TcR and CD28 to PI3K activity at the immune synapse remains unresolved.10,41
We have previously identified p110δ as a key class IA PI3K isoform in T cells.5,37,42 Genetic inactivation of p110δ in the p110δD910A/D910A mice abrogated the capacity of anti-CD3 and anti-CD28 antibodies to stimulate Akt phosphorylation.37,42 Moreover, proliferation of naive T cells, Th1 and Th2 differentiation, and Treg functions were attenuated in p110δD910A/D910A mice.5,37,42-44 However, p110α and p110β are also expressed in primary T cells and have the potential to generate PIP3, at least in vitro.37,42,45 The G-protein–activated PI3K isoform p110γ could potentially also contribute to PIP3 accumulation at the immune synapse. Indeed, p110γ−/− mice have been documented to have impaired T-cell function, and deficiency of both p110γ and p110δ leads to a block in T-cell development at the CD4−CD8− DN3 stage.46-48 In this context it is worth considering that chemokine receptors can form complexes with the TcR.49,50 To date, the activation of p110δ and p110γ in T cells has only been determined indirectly in cells stimulated with antibodies and using the phosphorylation of Akt and other downstream proteins as surrogate markers for PI3K activity.
Here, we use a transgenic model system in which an AktPH-GFP fusion protein51 allows visualization and relative quantification of PIP3 generation in primary live CD4+ mouse T cells responding to stimulation with antigen presented by APCs. We also determined the relative contribution of CD28 and p110δ in the regulation of PKCθ and NF-κB. Finally, we have investigated the consequences of inhibiting both p110δ activity and CD28 costimulation on T-cell immunity in vitro and in vivo.
Methods
Mice
The p110δD910A/D910A (37), p110γ−/− (52), CD28−/− (53), CD28Y170 (29), OT-II TcR (54), and AktPH-GFP (51) mice have all been described previously and had been backcrossed to the C57BL/6 background for 5 to 10 generations. The CD28Y170F mice express a CD28Y170F transgene on the CD28−/− background.29 Compound mutant mice were generated by intercrossing. Mice were maintained under specific pathogen-free conditions. All protocols involving live animals were approved by the United Kingdom Home Office and institutional ethical review committees.
Reagents
IC87114 (55) was a kind gift from Joel Hayflick (ICOS, Seattle, WA). OVA323-339 was synthesized by Southampton Polypeptide, Southampton, United Kingdom. Other chemicals were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise specified.
Antibodies
Antibodies used for fluorescence-activated cell sorting (FACS) and cell sorting were from BD PharMingen (San Diego, CA) or eBioscience (San Diego, CA), unless otherwise noted. The following clones were used: anti-CD3 (2C11), anti-CD28 (37.51), anti-CD4 (L3T4), anti-CD8 (YTS105.18.10), anti-CD69 (H1.2F3), anti-B220 (RA3-6B2), anti-CD49b (DX5), anti-CD80 (16-10A1), anti-CD86 (GL1), anti-MHC class II (M5/114.15.2), anti-Thy1.2 (TS; Sigma-Aldrich), anti-Vα2 (B20.1), and anti-Vβ5 (MR9-4). Antibodies for biochemical studies were as follows: anti-pAkt Ser473, anti-Akt (total) were from Cell Signaling Technology (Danvers, MA). For confocal analysis, anti-TcRβ (H57-597) was from BD Biosciences PharMingen (San Diego, CA), anti-p65/RelA, and anti-PKCθ polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), anti–rabbit Alexa Fluor 405 and anti–rabbit Alexa Fluor 488 were purchased from Invitrogen, Carlsbad, CA.
Cell purification
Naive T cells were purified from lymph nodes by magnetic sorting (Miltenyi Biotec, Auburn, CA). For total CD4+ T-cell purification, non-CD4+ T cells were labeled with FITC-conjugated anti-CD8, anti-CD69, anti-B220, anti-CD49b, and anti-MHC class II, followed by negative selection with anti-FITC magnetic cell sorting beads (Miltenyi Biotec). The resulting cells were more than 95% CD4+. OT-II TcR transgene expression in WT and p110δD910A/D910A backgrounds was similar (as determined by FACS analysis of cells stained anti–TcR-Vα2 and anti–TcR-Vβ5).
OT-II T-cell blasts were obtained by stimulation of cells from spleens with 1μM OVA323-339 peptide. After 6 days of stimulation, CD4+ T cells were purified by magnetic sorting and cultured with 20 ng/mL IL-2 and 1 ng/mL IL-7. Before any experiments, cells were washed to remove IL-2 and IL-7 and rested for 2 hours.
APCs used in this study were B cells purified from spleen cells after T-cell depletion with anti-mouse Thy1.2 (Sigma-Aldrich) and rabbit complement (Cedarlane, Burlington, ON), followed by purification of viable cells using Lympholyte-M (Cedarlane). APCs used in imaging experiments had been incubated overnight in LPS.
Fluorescence microscopy and image analysis
For live cell images, purified OT-II CD4+ T cells were added at a 1:1 ratio to LPS-activated APCs pulsed overnight with 10 μm OVA323-339 peptide. These interactions were imaged in RPMI supplemented with 0.5% FCS and 20mM HEPES pH 7.5 using an objective heater. For PI3K inhibition experiments, T cells were preincubated at 37°C for 15 minutes with 10 μM LY294002 before being added to the APCs. For p110δ-specific inhibition, the drug (IC87114 at 5 μM) was added during the acquisition. Images were taken every 15 seconds. Background noise was minimized by applying the ImageJ fast Fourier transform bandpass filter to the GFP images.
For fixed CD4+ T cell–APC conjugates analysis, APCs were plated on glass coverslips coated with poly(L-lysine) and were incubated at 37°C for 20 minutes, before naive CD4+ T cells were added to the APCs and incubated for 30 minutes. Conjugates were washed once in PBS and fixed in PBS plus 3.7% paraformaldehyde. Cells were permeabilized with 0.2% Triton X-100 in PBS for 4 minutes, blocked with 5% BSA-PBS for 30 minutes, and then stained with the appropriate antibodies. For the analysis of the NF-κB nuclear translocation, nuclei were additionally stained with 7-AAD (Invitrogen). The slides were mounted with fluorescent mounting medium and stored at 4°C before analysis. For 3-dimensional visualization of the PKCθ recruitment, image stacks of 0.3-μM intervals were acquired.
Images were taken with a Zeiss LSM510 META system consisting of a Zeiss Axiovert 200 microscope fitted with a Zeiss Plan-Achromat 63×/1.4 NA oil objective (Carl Zeiss, Jena, Germany). Images were analyzed using the ImageJ software (http://rsb.info.nih.gov/ij/). For the quantification of the fluorescence of the GFP at the membrane in fixed cells, we used a 2-color approach: in addition to the GFP, the CD4+ T cells were stained for TcR expression. The TcR staining was then used to create a mask corresponding to the membrane of the CD4+ T cells. The rest of the cell was considered as the “nonmembrane” region. The mask created was then used to determine the average intensity of fluorescence of GFP at the membrane, and this was compared with the average intensity of fluorescence of the nonmembrane region.
Proliferation assay
Proliferation assays were done in RPMI with 10% FCS, 5 × 10−5M β-mercaptoethanol, and antibiotics.
For anti-CD3 + anti-CD28, lymph node cells were stimulated at 2 × 105 per well with anti-CD3 (0.1 μg/mL) and anti-CD28 (1/100 supernatant from 37.51 hybridoma cultures). After 3 days, the cells were pulsed with [3H]-Thymidine for 6 hours.
For OT-II, purified CD4+ OT-II T cells were incubated with irradiated T cell–depleted spleen cells (both at 5 × 104 per well) and OVA323-339 peptide at the indicated concentrations. After 3 days, the cells were pulsed with [3H]-Thymidine for 6 hours.
Immunization
Each mouse was immunized with 100 μg DNP-KLH (Calbiochem, San Diego, CA) absorbed onto alum (Serva, Garden City Park, NY) on days 0 and 78. Blood was taken 11 and 12 days after the first and second immunizations, respectively, and serum was obtained. Antibody titers directed against DNP-BSA were determined using the SBA clonotyping system-HRP (Southern Biotechnology Associates, Birmingham, AL). The EC50 values were calculated from serial dilutions of serum using the GraphPad Prism program (GraphPad Software, San Diego, CA).
In vivo killing assay
Donor lymphocytes from C57BL/6 (B6) or (C57BL/6 × Cba/1) F1 mice were differentially labeled with 1.5 μM or 0.15 μM CFSE, respectively, and injected into recipient mice on the C57BL/6 background (5 × 106 cells per recipient). The proportion of F1 to B6 donors were analyzed in blood taken from the tail vein on days 1 to 4 after transfer. The relative killing efficiency in each mouse was normalized so that the ratio on day 1 was 1:1.
Statistical analysis
The data included in the analysis of the fluorescence at the plasma membrane of AktPH-GFP start 3 time windows before the contact between the cells up to 406 seconds. The time at which the plateau was reached was determined graphically for each individual mouse, and the values included in the calculation of the mean fluorescence for each mouse correspond to the 15 subsequent frames. This number was chosen because it corresponds to the minimum plateau length for CD28Y170F mice which took the longest to stabilize. The choice was also made to provide balanced samples more suitable for statistical analysis. On the basis of the mean fluorescence values for each animal, data were tested for normality, and 2 cells (WT + LY294002) were consistently identified as outliers. The natural log of the fluorescent ratios obtained from 15 plateau measurements (shown in Figure 1C) was calculated. Statistical analysis of the ratios between membrane and cytosol fluorescence in Figure 1C and Figure 2B and C was analyzed by one-way ANOVA followed by Tukey multiple comparison test using GraphPad Prism software (GraphPad Software).
Results
Regulation of PIP3 production at the immune synapse
The phosphorylation of PIP2 at the plasma membrane by PI3K produces PIP3 that acts as a docking site for the PH domain of Akt. Thus, the translocation of the Akt PH domain, fused to GFP, allows real-time visualization of PIP3 generated at the immune synapse. Therefore, to monitor the relative contributions of p110δ, p110γ, and CD28 to PIP3 metabolism at the immune synapse, we crossed p110δD910A/D910A, p110γ−/−, CD28−/−, and CD28Y170 mice to mice expressing OVA323-339–specific OT-II TcR and AktPH-GFP transgenes. The AktPH-GFP transgene did not interfere with T-cell development, Akt phosphorylation, or T-cell proliferation56 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). CD4+ T-cell blasts from each of these mouse lines were incubated with WT APCs expressing MHC II-Ab bearing the OVA323-339 peptide. AktPH-GFP accumulation in the T cells at the contact areas with the APCs was monitored in real time, and the ratio of AktPH-GFP at the contact area relative to the rest of the cell was determined.
PIP3 accumulated at the contact area between the T cells and the APCs within seconds of conjugate formation and remained concentrated at the contact area for the duration of the recordings (usually 10 minutes; Figure 1A,B). Figure 1B shows the average intensity of PIP3 for the different conditions over time, whereas Figure 1C shows the average of 15 measurements for each cell once they had reached a plateau level of activation (1-2 minutes after conjugate formation). Pretreatment with LY294002, a pan-PI3K inhibitor, reduced AktPH-GFP translocation nearly to base level (ie, where the ratio = 1) in the majority of T cells.
p110δ is essential for PIP3 accumulation to the plasma membrane. (A) Images showing fluorescence and transmitted light from a movie of AktPH-GFP+ WT, p110δD910A/D910A, p110γ−/−, CD28−/−, and CD28Y170F CD4+ T-cell blasts interacting with LPS-activated APCs presenting OVA323-339 peptide. In some experiments, WT cells were pretreated with PI3K inhibitor (LY294002) for 15 minutes before stimulation. (B) Quantification of AktPH-GFP redistribution in WT (n = 11), LY294002 pretreated WT (n = 8), p110δD910A/D910A (n = 9), p110γ−/− (n = 12), CD28−/− (n = 9), and CD28Y170F (n = 7) CD4+ T cells. A few LY294002-treated T cells showed apparently normal AktPH-GFP accumulation (Figure S3). It is possible that a subset of T cells express sufficient levels of multidrug resistance protein transporter and could potentially thus evade inhibition.81,82 Therefore, to establish a realistic baseline for minimal PI3K activity, these outliers were excluded from the analysis, but they are shown in Figure S3. The recruitment of AktPH-GFP at the plasma membrane is expressed as a ratio between the fluorescence measured at the contact area and the average fluorescence within the cell. Fluorescence was measured on at least 2 independent days. (C) Average ratio of PIP3 at membrane during the plateau phase of the response (approximately 40-400 seconds). The mean fluorescence values were determined as described in “Methods” (**P < .01, ***P < .001). (D) Acute inhibition of PI3K activity with the p110δ-selective inhibitor IC87114. Two T cell–B cell conjugates are shown after the addition of IC87114 as indicated by arrows. PIP3 depletion from the membrane occurred rapidly in both cases.
p110δ is essential for PIP3 accumulation to the plasma membrane. (A) Images showing fluorescence and transmitted light from a movie of AktPH-GFP+ WT, p110δD910A/D910A, p110γ−/−, CD28−/−, and CD28Y170F CD4+ T-cell blasts interacting with LPS-activated APCs presenting OVA323-339 peptide. In some experiments, WT cells were pretreated with PI3K inhibitor (LY294002) for 15 minutes before stimulation. (B) Quantification of AktPH-GFP redistribution in WT (n = 11), LY294002 pretreated WT (n = 8), p110δD910A/D910A (n = 9), p110γ−/− (n = 12), CD28−/− (n = 9), and CD28Y170F (n = 7) CD4+ T cells. A few LY294002-treated T cells showed apparently normal AktPH-GFP accumulation (Figure S3). It is possible that a subset of T cells express sufficient levels of multidrug resistance protein transporter and could potentially thus evade inhibition.81,82 Therefore, to establish a realistic baseline for minimal PI3K activity, these outliers were excluded from the analysis, but they are shown in Figure S3. The recruitment of AktPH-GFP at the plasma membrane is expressed as a ratio between the fluorescence measured at the contact area and the average fluorescence within the cell. Fluorescence was measured on at least 2 independent days. (C) Average ratio of PIP3 at membrane during the plateau phase of the response (approximately 40-400 seconds). The mean fluorescence values were determined as described in “Methods” (**P < .01, ***P < .001). (D) Acute inhibition of PI3K activity with the p110δ-selective inhibitor IC87114. Two T cell–B cell conjugates are shown after the addition of IC87114 as indicated by arrows. PIP3 depletion from the membrane occurred rapidly in both cases.
p110δD910A/D910A T cells showed greatly reduced AktPH-GFP recruitment to the T-B contact area, suggesting that p110δ is the main PI3K isoform that produces PIP3 at the immune synapse (Figure 1A,B). Although moderate recruitment of PIP3 could be observed in some p110δD910A/D910A T cells, the difference between T cells treated with LY294002 and p110δD910A/D910A T cells did not reach statistical significance in these experiments (Figure 1C). Addition of IC87114, a small molecule inhibitor with high selectivity for p110δ, (55 ) after conjugate formation returned PIP3 levels to baseline, showing that continuous p110δ activity at the contact area is required to sustain elevated PIP3 levels (Figure 1D). p110γ−/− T cells showed a more variable but not significantly reduced AktPH-GFP recruitment (Figure 1A-C). By contrast, p110γ but not p110δ was critical for AktPH-GFP recruitment to the plasma membrane induced by the chemokine CXCL-12 (Figure 2A,B).
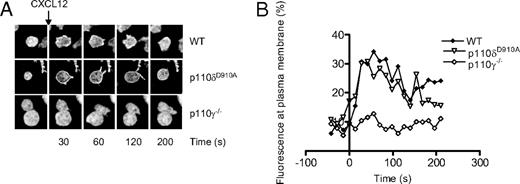
CXCL12-induced (500 ng/mL) accumulation of PIP3 at the plasma membrane of CD4+ T-cell blasts is dependent on p110γ but not p110δ. (A) Representative images showing the translocation of the AktPH-GFP probe to the plasma membrane. (B) Graphical representation of the percentage of total fluorescence at the plasma membrane as function of time. Data are representative of 2 independent experiments (WT, n = 6; p110δD910A/D910A, n = 4; p110γ−/−, n = 5).
CXCL12-induced (500 ng/mL) accumulation of PIP3 at the plasma membrane of CD4+ T-cell blasts is dependent on p110γ but not p110δ. (A) Representative images showing the translocation of the AktPH-GFP probe to the plasma membrane. (B) Graphical representation of the percentage of total fluorescence at the plasma membrane as function of time. Data are representative of 2 independent experiments (WT, n = 6; p110δD910A/D910A, n = 4; p110γ−/−, n = 5).
In CD28−/− T cells, PIP3 accumulation was observed early in the response, but was not sustained, nor did it reach WT levels (Figure 1A,B). Surprisingly, CD28Y170F T cells reached a plateau level of PIP3 accumulation equivalent to that of WT T cells, albeit with somewhat delayed kinetics (Figure 1A-C). These results indicate that CD28 can promote sustained PIP3 generation at the immune synapse independently of its capacity to bind p85/p110δ directly. The imaging-based experiments were corroborated by biochemical analysis because we found that Akt was robustly phosphorylated in CD28−/− and CD28Y170F T cells using anti-CD3 and anti-CD28, whereas, as previously reported,37,42 virtually no Akt phosphorylation was detected in p110δD910A/D910A T cells (Figure S1C,D). We conclude from these experiments that p110δ is the main PI3K isoform responsible for generating PIP3 at the immune synapse and that p110δ can be activated without being recruited to Y170 in the CD28 cytoplasmic domain.
Regulation of PKCθ and NF-κB
We next investigated how reduced PIP3 production at the synapse might contribute to the regulation of PLCγ-dependent signaling. During the formation of an immune synapse, production of DAG by PLCγ leads to the recruitment of PKCθ that localizes in the center of the contact area between the T cell and the APC.57 CD28 and PI3K have been suggested to influence the recruitment of PKCθ to the immune synapse.58 We therefore examined PKCθ localization in the various mutant T cells to determine the involvement of CD28 and p110δ during the formation of the immune synapse. At the same time we observed PIP3 recruitment using the AktPH-GFP probe. In contrast to the experiments presented in Figure 1, these experiments were done using naive T cells that had been incubated with peptide-loaded APCs for 30 minutes and then fixed. After fixation, the cells were stained with antibodies to the TcR and PKCθ (Figure 3A). These experiments were consistent with those presented in Figure 1 by revealing that p110δ, but not p110γ, was required for membrane accumulation of PIP3 and by showing that PIP3 recruitment was defective in CD28−/−, but not CD28Y170F T cells (Figure 3A,B). Thus, the lack of requirement for the CD28 YMNM motif to activate PI3K is true both in naive and in previously activated T cells. In addition, these experiments showed that neither of the PI3K mutants showed defect in PKCθ accumulation at the plasma membrane (Figure 3C). However, the recruitment of PKCθ was attenuated in CD28−/− T cells and to a lesser extent in CD28Y170F T cells (Figure 3C). Moreover, CD28−/− and CD28Y170F T cells showed a more diffuse localization of PKCθ within the immune synapse than in conjugates with WT or p110δD910A/D910A T cells (Figure 3D). Thus, CD28 contributes to PKCθ localization by a mechanism that is at least partially dependent on the YMNM motif, but which is independent of p110δ activity.
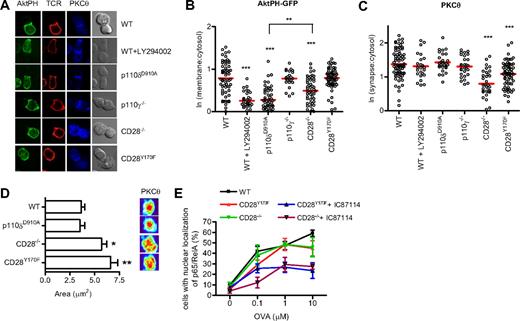
CD28, but not p110δ, regulates PKCθ localization at the synapse. (A) Conjugates between naive WT, WT + LY294002, p110δD910A/D910A, p110γ−/−, CD28−/−, or CD28Y170F CD4+ T cells and OVA323-339–pulsed APCs were stained with antibodies against PKCθ and the TcRβ. Representative images of the distribution of AktPH-GFP, TcR, and PKCθ during conjugate formation are shown. (B) Quantification of AktPH-GFP recruitment at the plasma membrane (WT, n = 72; WT + LY294002, n = 31; p110δD910A/D910A, n = 53; p110γ−/−, n = 23; CD28−/−, n = 51; CD28Y170F, n = 62), determined as described in “Methods” (**P < .01, ***P < .001). (C) Recruitment of PKCθ to the contact area (WT, n = 76; WT + LY294002, n = 24; p110δD910A/D910A, n = 28; p110γ−/−, n = 28; CD28−/−, n = 33; CD28Y170F, n = 67), expressed as the ratio between the fluorescence at the contact area and the fluorescence within the cell. Only the means of the values for the CD28−/− and CD28Y170F values were significantly different from the WT values (***P < .001). (D) A 3-dimensional reconstruction of the contact area was done and representative pictures of the interface projection and the mean area (± SEM, *P < .05, **P < .001, Student 2-tailed t test; WT, n = 38; p110δD910A/D910A, n = 18; CD28−/−, n = 23; CD28Y170F, n = 21) occupied by PKCθ at the immune synapse are shown. Data are representative of 2 independent experiments. (E) The YMNM motif of CD28 is not required for NF-κB nuclear translocation. Conjugates between WT, CD28−/−, or CD28Y170F CD4+ T cells and OVA323-339–pulsed APCs were stained for p65 NF-κ, and nuclei were stained with 7-AAD. The conjugates were scored for the frequency of nuclear localization of NF-κB. The p110δ inhibitor IC87114 blocked NF-κB nuclear translocation in CD28−/− and CD28Y170F T cells. Data represent mean (± SEM) with more than 30 cells analyzed in each of 3 experiments.
CD28, but not p110δ, regulates PKCθ localization at the synapse. (A) Conjugates between naive WT, WT + LY294002, p110δD910A/D910A, p110γ−/−, CD28−/−, or CD28Y170F CD4+ T cells and OVA323-339–pulsed APCs were stained with antibodies against PKCθ and the TcRβ. Representative images of the distribution of AktPH-GFP, TcR, and PKCθ during conjugate formation are shown. (B) Quantification of AktPH-GFP recruitment at the plasma membrane (WT, n = 72; WT + LY294002, n = 31; p110δD910A/D910A, n = 53; p110γ−/−, n = 23; CD28−/−, n = 51; CD28Y170F, n = 62), determined as described in “Methods” (**P < .01, ***P < .001). (C) Recruitment of PKCθ to the contact area (WT, n = 76; WT + LY294002, n = 24; p110δD910A/D910A, n = 28; p110γ−/−, n = 28; CD28−/−, n = 33; CD28Y170F, n = 67), expressed as the ratio between the fluorescence at the contact area and the fluorescence within the cell. Only the means of the values for the CD28−/− and CD28Y170F values were significantly different from the WT values (***P < .001). (D) A 3-dimensional reconstruction of the contact area was done and representative pictures of the interface projection and the mean area (± SEM, *P < .05, **P < .001, Student 2-tailed t test; WT, n = 38; p110δD910A/D910A, n = 18; CD28−/−, n = 23; CD28Y170F, n = 21) occupied by PKCθ at the immune synapse are shown. Data are representative of 2 independent experiments. (E) The YMNM motif of CD28 is not required for NF-κB nuclear translocation. Conjugates between WT, CD28−/−, or CD28Y170F CD4+ T cells and OVA323-339–pulsed APCs were stained for p65 NF-κ, and nuclei were stained with 7-AAD. The conjugates were scored for the frequency of nuclear localization of NF-κB. The p110δ inhibitor IC87114 blocked NF-κB nuclear translocation in CD28−/− and CD28Y170F T cells. Data represent mean (± SEM) with more than 30 cells analyzed in each of 3 experiments.
PKCθ is essential for the nuclear translocation of NF-κB.59 We have previously shown that the nuclear translocation of p65 NF-κB is normal in p110δD910A/D910A T cells stimulated with peptide antigen.42 To our surprise, p65 NF-κB translocation was also readily observed in the absence of CD28 signaling (Figure 2E). However, when CD28−/− or CD28Y170F T cells were incubated with the p110δ-specific inhibitor IC87114, p65 NF-κB nuclear translocation was attenuated by approximately 50%. The latter is consistent with our previously observed defect in anti-CD3 but not anti-CD3 plus anti-CD28–stimulated NF-κB translocation in p110δD910A/D910A T cells.42 These results show that p110δ and CD28 cooperatively regulate p65 NF-κB translocation to some extent, but neither input signal is essential for p65 NF-κB nuclear translocation.
CD28 and p110δ complement each other to provide full T-cell stimulation
Our results indicate that CD28 is not essential for p110δ activation and that CD28 and p110δ, at least in part, control parallel signaling pathways. A prediction from these observations is that dual inhibition of CD28 and p110δ should act synergistically. However, if CD28 were a key regulator of p110δ activation, then the prediction would be that the inhibition of p110δ would not potentiate inhibition of CD28 signaling alone. To test these predictions, we generated p110δD910A/D910ACD28−/− and p110δD910A/D910ACD28Y170F double mutant mice. In response to anti-CD3 and anti-CD28 stimulation, the p110δD910A/D910A, CD28Y170F, or p110δD910A/D910ACD28Y170F mutant T cells showed intact proliferative responses (Figure 4A). These results exclude the possibility that p110α or p110β could be recruited to CD28 Y170 to compensate for the lack of p110δ activity and promote proliferation in p110δD910A/D910A T cells. Consistent with previous results,37 in response to anti-CD3 alone, p110δ was required for optimal proliferation (compare the response of CD28−/− T cells with p110δD910A/D910ACD28−/− T cells). We have previously shown that proliferation of p110δD910A/D910A T cells in response to peptide presented by APCs is impaired, suggesting that the natural ligand for CD28, in contrast to antibodies directed against CD28, is insufficient to rescue proliferation to WT levels in the absence of p110δ activity.37,42 By contrast, CD28Y170F T cells proliferate normally regardless of the stimulus.29,60 In p110δD910A/D910ACD28−/− double-deficient T cells, the proliferative response was abolished (Figure 4B). However, the expression of the CD28Y170F transgene partially rescued proliferation of p110δD910A/D910ACD28−/− T cells stimulated with 1 μm, but not 0.1 μm, peptide (Figure 4B). Thus, the CD28 YMNM motif may compensate in part for the lack of p110δ activity under conditions of weak TcR stimulation, but it is not required in p110δ-sufficient T cells.
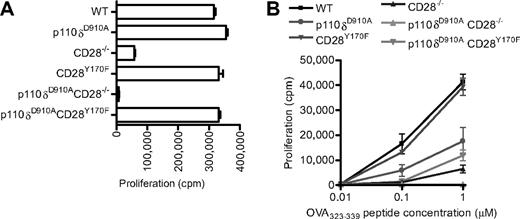
Proliferation of CD28 and p110δ double mutant T cells. (A) Anti-CD3– and anti-CD28–dependent proliferation of WT, p110δD910A/D910A, CD28−/−, CD28Y170F, p110δD910A/D910ACD28−/−, and p110δD910A/D910ACD28Y170F lymph node T cells stimulated with 0.1 μg/mL anti-CD3 and with anti-CD28 37.51 hybridoma supernatant (1/100). (B) Proliferation of T cells from the mutants OT-II+ transgenic WT, p110δD910A/D910A, CD28−/−, CD28Y170F, p110δD910A/D910ACD28−/−, and p110δD910A/D910ACD28Y170F CD4+ T cells in response to 0.01μM, 0.1μM, or 1.0μM OVA323-339 peptide.
Proliferation of CD28 and p110δ double mutant T cells. (A) Anti-CD3– and anti-CD28–dependent proliferation of WT, p110δD910A/D910A, CD28−/−, CD28Y170F, p110δD910A/D910ACD28−/−, and p110δD910A/D910ACD28Y170F lymph node T cells stimulated with 0.1 μg/mL anti-CD3 and with anti-CD28 37.51 hybridoma supernatant (1/100). (B) Proliferation of T cells from the mutants OT-II+ transgenic WT, p110δD910A/D910A, CD28−/−, CD28Y170F, p110δD910A/D910ACD28−/−, and p110δD910A/D910ACD28Y170F CD4+ T cells in response to 0.01μM, 0.1μM, or 1.0μM OVA323-339 peptide.
We next investigated the genetic interactions between CD28, CD28Y170F, and p110δ in vivo. Each of the 6 lines described in Figure 4A were immunized with KLH-DNP adsorbed onto alum and rechallenged 78 days later. DNP-specific antibody titers were measured 11 or 12 days after each immunization. This experiment confirmed our previous reports,29,37 showing defective primary immune humoral immune responses in p110δD910A/D910A mice and in CD28−/− mice, with antibody responses in CD28Y170F mice being intact (Figure 5A). The antibody responses of CD28−/−p110δD910A/D910A mice were dramatically reduced, whereas p110δD910A/D910ACD28Y170F mice showed equivalent immune responses to p110δD910A/D910A mice. During recall responses, the antibody titers from WT, CD28−/−, and CD28Y170F mice were enhanced by 2 orders of magnitude, whereas p110δD910A/D910A mice did not show enhanced secondary responses. Although the impaired humoral immune response is caused by defective antigen receptor signaling in p110δD910A/D910A B cells, humoral immune responses are also attenuated in mice in which PI3K signaling has been selectively eliminated in the T-cell lineage.38 We conclude that p110δ and CD28 complement each other during the regulation of humoral immune responses and that p110δ, but not CD28, is essential for enhanced recall responses.
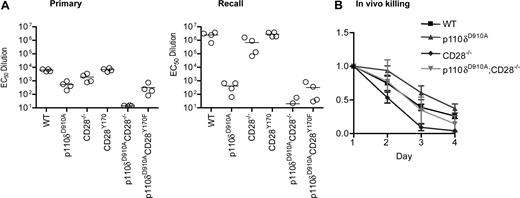
Impaired humoral immune responses in p110δD910A/D910A CD28−/− mice. (A) WT, p110δD910A/D910A, CD28−/−, CD28Y170F, p110δD910A/D910A CD28−/−, and p110δD910A/D910ACD28Y170F mice were immunized with DNP-coupled KLH adsorbed onto alum. The mice were rechallenged with the same dose of antigen 78 days later. Anti-DNP–specific antibodies were detected by enzyme-linked immunoabsorbent assay. Each dot represents one mouse. (B) CD28 and p110δ are indispensable for efficient killing of allogeneically mismatched lymphocytes in vivo. Recipient mice on the B6 background (H-2b) were injected with a mixture of 5 × 106 CFSEhigh B6 lymphocytes and CFSElow CB6 F1 lymphocytes (H-2b/k). The ratio of CFSElow to CFSEhigh was normalized to 1 on day 1, and the average from 3 recipients from each genotype is shown (± SD).
Impaired humoral immune responses in p110δD910A/D910A CD28−/− mice. (A) WT, p110δD910A/D910A, CD28−/−, CD28Y170F, p110δD910A/D910A CD28−/−, and p110δD910A/D910ACD28Y170F mice were immunized with DNP-coupled KLH adsorbed onto alum. The mice were rechallenged with the same dose of antigen 78 days later. Anti-DNP–specific antibodies were detected by enzyme-linked immunoabsorbent assay. Each dot represents one mouse. (B) CD28 and p110δ are indispensable for efficient killing of allogeneically mismatched lymphocytes in vivo. Recipient mice on the B6 background (H-2b) were injected with a mixture of 5 × 106 CFSEhigh B6 lymphocytes and CFSElow CB6 F1 lymphocytes (H-2b/k). The ratio of CFSElow to CFSEhigh was normalized to 1 on day 1, and the average from 3 recipients from each genotype is shown (± SD).
We next examined the role of CD28 and p110δ in cytotoxic immune responses. To this end, recipient mice on the C57BL/6 (B6) background (H-2b) were injected with differentially labeled lymph node cells from a C57BL/6 or a C57BL6 × Cba/1 (F1) donor (H-2b/k). The proportion of circulating F1 cells to B6 cells was monitored during the next 4 days. p110δD910A/D910A mice rejected the F1 cells more slowly than did WT hosts, whereas CD28−/− mice showed accelerated rejection consistent with a nonessential role for CD28 in CD8 T cell–dependent killing of allogeneic grafts61,62 (Figure 5B). p110δD910A/D910ACD28−/− mice rejected the F1 cells at a rate that was indistinguishable from WT mice, which also could be considered intermediate between that of p110δD910A/D910A and CD28−/− mice. We conclude that CD28 and p110δ are less important for CD8 T cell–dependent cytotoxic immune responses than they are for humoral immune responses.
Discussion
In this study, we have used an imaging approach to visualize PI3K signaling in single T cells in response to antigen stimulation. This approach circumvents some of the potential artifacts associated with antibody stimulation of T cells and presents a more dynamic view of PI3K activity in real time. Moreover, by measuring the ratio of the fluorescence near the membrane to that in the cytosol, we were able to assess the relative PI3K signaling strength in different PI3K or CD28 mutant T cells. Using this approach, we show that p110δ is the main PI3K isoform responsible for accumulation of PIP3 at the immune synapse and that p110γ is required for chemokine- but not antigen-dependent PI3K activity. In contrast to a previous report,46 we did not observe defects in proliferation or cytokine secretion in p110γ−/− CD4+ T cells (data not shown). Moreover, pharmacologic inhibition of p110δ, but not of p110γ, led to reduced proliferation and cytokine secretion by CD4+ T cells.63 The previously reported defective activation of p110γ−/− T cells may have been secondary to an undefined genetic lesion that also lead to colorectal carcinomas, a phenotype that has not been observed in other p110γ−/− strains (including the one used in this study).64,65 In addition, dendritic cell migration is impaired in p110γ−/− mice which may have contributed to impaired T-cell responses in vivo.66 Nonetheless, T-cell development is largely normal in p110δD910A/D910A mice and p110γ−/− mice, whereas p110δp110γ double-knockout mice have a pronounced defect in TcRβ-dependent selection of double-negative thymocytes.47,48 These results suggest that the functional redundancy between p110δ and p110γ is more pronounced during thymocyte selection than during activation of mature T cells and implicates the contribution of an undefined G-protein–coupled receptor at this thymic selection stage.
Although CD28 also contributes to PI3K activity and PIP3 accumulation at the synapse, direct interaction between PI3K and CD28 via the YMNM motif was not required. One possibility could be that CD28 could interact with PI3K by a distinct motif. Indeed, the CD28 cytoplasmic domain contains 4 tyrosines, and it has been suggested the most C-terminal tyrosine (with a YRS motif) contributes to the CD28-p85 association.67 However, this tyrosine is thought to stabilize the interaction between CD28 and p85 bound to the YMNM motif rather than binding independently to PI3K. Thus, a Y to F mutation of the YMNM motif completely abrogated CD28-dependent PI3K activity, whereas a mutation of the YRS delayed and attenuated the PI3K response.27,67
In addition to the tyrosine-based motifs, the cytoplasmic domain of CD28 contains 2 PxxP motifs that can interact with SH3 domains.68-72 In particular, CD28 costimulation has been shown to sustain Lck activity in the immune synapse by an interaction involving the C-terminal PxxP motif in the cytoplasmic domain of CD28.73 In CD4+ T cells, Lck is recruited to the immune synapse by CD4; however, the association of CD4 with the immune synapse is transient, and, in the absence of CD28, Lck activity is not sustained.73 Consistent with these results, mice with mutations in the C-terminal PxxP motif in CD28 show impaired costimulation in vitro and in vivo.34,74 Because the CD28Y170F T cells showed intact accumulation of PIP3 at the immune synapse, the capacity of CD28 to sustain Lck activity is a plausible mechanism for how CD28 maintains PI3K signaling, the assumption being that Lck phosphorylates other docking sites for p85-p110δ at the immune synapse. CD28−/− T cells have been shown capable of initiating but not sustaining intracellular Ca2+ flux after activation, which is similar to what we observed for PIP3 accumulation.75 Thus, the reduced PIP3 accumulation at the immune synapse in CD28−/− T cells probably reflects a more general role for CD28 in enhancing TcR signaling than a specific role for CD28 in regulating PI3K activation.76
Our results do not exclude a role for CD28 in the direct recruitment of p85-p110δ or for Grb2 of Gads under different circumstances. Indeed, we found a requirement for the YMNM motif in concentrating the accumulation of PKCθ at the immune synapse. Inactivation of p110δ did not affect PKCθ localization, suggesting that PKCθ localization may be regulated by Grb2 or Gads that binds to the same tyrosine. Superagonistic antibodies against CD28 stimulated T-cell responses that were dependent on the YMNM motif but not on PI3K77 (data not shown). We therefore propose that the CD28-Grb2/Gads association can regulate PKCθ and other pathways normally regulated by the TcR, but that this capacity of CD28 only becomes functionally relevant when CD28 signaling occurs in the absence of agonist-induced TcR signaling.77 The CD28 YMNM motif can also influence T-cell trafficking to nonlymphoid organs in vivo.60
Our results showing synergy between CD28 and p110δ have potential clinical relevance, because inhibition of CD28 and PI3K signaling is achievable pharmaceutically. CTLA4Ig (abatacept) has recently received FDA approval for the treatment of rheumatoid arthritis, and the related inhibitor belatacept is in clinical trials for transplantation.78 However, CD28 blockade alone is unlikely to be sufficient to prevent autoimmunity or graft rejection in most cases. More recently, p110δ-selective small molecule inhibitors have been developed as candidate drugs to treat autoimmunity and inflammation.55,79,80 Our data suggest that dual inhibition of CD28 and p110δ may achieve more potent alleviation of pathologic immune responses than can be achieved with either inhibitor alone.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Anne Segonds-Pichon for expert statistical analysis, Simon Walker for assistance with microscopy, and members of the small animal barrier unit for animal husbandry and immunizations. We thank Dalya Soond, Martin Turner, Jacques Nunès, and Bart Vanhaesebroeck for their constructive comments on the manuscript.
This work was supported by grants from the Biotechnology and Biological Sciences Research Council (BBSRC; JF19128 and BBC5098901) and from the Juvenile Diabetes Research Foundation (5-2006-229).
Authorship
Contribution: F.G. designed and performed research and analyzed and interpreted data; D.T.P. and J.L.E. performed research; E.H., R.R., and T.S. contributed vital reagents; K.O. designed and performed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: One of the authors (K.O.) is employed as consultant by PIramed. The other authors declare no competing financial interests.
Correspondence: Klaus Okkenhaug, Laboratory of Lymphocyte Signaling and Development, Babraham Institute, Babraham Research Campus, Cambridge CB22 3AT, United Kingdom; e-mail: klaus.okkenhaug@bbsrc.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal