Abstract
Decoy receptor 3 (DcR3) is a soluble decoy receptor belonging to the tumor necrosis factor receptor (TNFR) superfamily, and its expression is not only up-regulated in cancer cells derived from various cell lineages, but also correlates with overall survival of patients with cancer. It has been shown that DcR3 sensitize cells of hematopoietic origin to TNF-related apoptosis-inducing ligand (TRAIL)–induced apoptosis; therefore, we asked whether DcR3 down-regulated host immunity by inducing immune cell apoptosis. We demonstrate that DcR3 induces dendritic cell (DC) apoptosis by activating PKC-δ and JNK subsequently to up-regulate DR5 to recruit Fas-associated death domain (FADD) to propagate the apoptotic signals. The association of FADD with DR5 results in the formation of death-inducing signaling complex (DISC) to trigger the downstream apoptotic signaling cascade. PKC-δ is activated via cross-linking of heparan sulfate proteoglycan (HSPG) on DCs, because recombinant protein containing the heparin-binding domain (HBD) of DcR3 and the Fc portion of IgG1, the HBD.Fc fusion protein, is also able to trigger DC apoptosis. This provides the first evidence that cross-linking of HSPG on DCs can activate PKC-δ to induce DC apoptosis via the formation of DR5 DISC, and elucidates a novel mechanism of DcR3-mediated immunosuppression.
Introduction
Decoy receptor 3 (DcR3) is a soluble tumor necrosis factor receptor (TNFR) overexpressed in various tumors, including lung cancers,1 gastrointestinal tract tumors,2 virus-associated lymphomas,3 malignant gliomas,4 and pancreatic cancers.5 DcR3 suppresses the activation and differentiation of dendritic cells (DCs)6 and macrophages,7 and enhances osteoclast differentiation8 and angiogenesis.9 Moreover, DcR3 is shown to sensitize cells to TNF-related apoptosis-inducing ligand (TRAIL)–induced apoptosis.10 Moreover, almost all serum DcR3+ individuals (98.8%; 82 of 83 patients) had malignancy,11 and DcR3 overexpression was found to be associated with shortened duration of overall survival in patients with cancer.12 Therefore, DcR3 is an important immunosuppressive factor in promoting tumor growth in patients with cancer.
DCs are the most potent antigen-presenting cells capable of priming tumor-specific T cells. The sentinel lymph node (SLN) is the first draining lymph node from the area in which a tumor is located, and the numbers of DC are reduced in SLNs from some patients with cancer.13 In our previous study, we found the total numbers of CD14+ monocyte-derived DCs decreased significantly when incubated with DcR3.6 Thus, we are interested to know whether DcR3 is one of the tumor-associated factors that affect DC survival, as well as to dissect the signaling cascades leading to DC apoptosis.
Apart from neutralizing endogenous TL1A to promote angiogenesis, the pleiotropic effects of DcR3 described here are independent of its interaction with 3 known ligands—FasL, LIGHT, and TL1A. Moreover, recombinant protein comprising the heparin-binding domain (HBD) of DcR3 and Fc portion of human IgG1 (HBD.Fc), which is unable to interact with LIGHT, FasL, or TL1A, has same effect as DcR3.Fc to modulate macrophage differentiation and to activate PKC.14,15 This indicates that DcR3-triggered signaling cascade is mediated by interaction with the heparan sulfate proteoglycans (HSPGs) on cell surfaces.
PKC is a family of serine/threonine kinases grouped into 3 subfamilies based on their structures and cofactors required for activation: the conventional (α, βI, βII, and γ), the novel (δ, ϵ, η, and θ), and the atypical (ζ and λ/ι) isoforms.16 The conventional PKCs are activated by diacylglycerol and phosphatidylserine in the presence of Ca2+. The novel PKCs are activated by diacylglycerol and phosphatidylserine but are independent of Ca2+, and the atypical PKCs appear to respond only to phosphatidylserine. It is interesting to note that the selective inhibitor of PKC-δ, rottlerin, and a dominant-negative mutant of PKC-δ attenuate apoptosis induced by phorbol ester, H2O2, UV radiation, taxol and etoposide,17,18 indicating that PKC-δ is involved in cell apoptosis. Previously, we have shown that DcR3.Fc (3 μg/mL) modulates CD14+ monocyte–derived DC differentiation.6 Recently, we further observed that a higher concentration of DcR3.Fc (10 μg/mL) induces DC apoptosis. Therefore, we compared the effects of DcR3.Fc and HBD.Fc on the survival of DCs, and investigated whether PKC was involved in DcR3-mediated DC apoptosis.
Here, we report that both DcR3.Fc and HBD.Fc induced DC apoptosis by activating JNK to up-regulate DR5 and trigger death- signaling cascades. Moreover, the PKC-δ–specific inhibitor rottlerin suppressed JNK activation and blocked DcR3-induced DC apoptosis. Since recombinant HBD.Fc has a similar effect as DcR3.Fc, this indicates that cross-linking of HSPG on DCs can activate PKC-δ specifically to induce DC apoptosis. This elucidates a novel mechanism of DcR3 to regulate cell apoptosis, and cross-linking of HSPG on DCs might become a novel strategy for immunosuppression in the future.
Methods
Antibodies and reagents
The sources of mAbs are as follows: anti-DR4, anti-DR5, anti-JNK1, anti–phospho-JNK, and anti-caspase-3 (R&D Systems, Minneapolis, MN); anti-Fas (eBioscience, San Diego, CA); anti–caspase-8, anti-Bid, and anti–poly(ADP-ribose) polymerase (PARP; Cell Signaling Technology, Beverly, MA); and anti–caspase-10 (MBL, Naka-Ku Nagoya, Japan), anti–Fas-associated death domain (FADD), antiactin, and anti–PKC-δ antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The kinase inhibitors (SP600125, PD98059, SB203580, rottlerin, Ro-32-0432, safingol, Ro-31-8220, GF-109203X, HBDDE, Gö 6976, Gö 6983, LY333531, and LY379196), caspase inhibitors (Z-VAD-FMK, Z-VDVAD-FMK, Z-DEVD-FMK, Z-IETD-FMK, and Z-AEVD-FMK), and caspase substrates MCA-IETD.APK 2,4-dinitrophenyl (DNP), MCA-AEVD.APK (DNP), MCA-VDVAD.APK (DNP), and MCA-DEVD.APK (DNP) were purchased from Calbiochem (San Diego, CA), while M-CSF, IL-4, TRAIL, and FasL were purchased from R&D Systems. All other chemical reagents, unless specified, were purchased from Sigma-Aldrich (St Louis, MO).
Cell cultures
Human peripheral blood (PBL) samples were obtained from healthy donors (Red Cross society, Taiwan) according to institutional guidelines of the National Yang-Ming University (Taipei, Taiwan). Monocyte-derived DCs6 and macrophages7 were cultured from CD14+ monocytes from human PBL as described, and were cultured in RPMI 1640 (Life Technologies, Gaithersburg, MD) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA) at 37°C in 5% (vol/vol) CO2 as previously described.6,7
Detection of apoptotic cells
The morphology of apoptotic DCs was analyzed by incubating cells with 0.1% trypan blue and 0.2 μg/mL Hoechst 33258 in phosphate-buffered saline (PBS) for 3 minutes at room temperature, and viable cells in 10 randomly chosen fields were counted at 200-fold magnification using phase-contrast and immunofluorescent microscopy (DIAPHOT 300, Nikon, Tokyo, Japan) with a CoolSNAP digital camera (RS Photometrics, Tucson, AZ), and using Plan 20×/0.4 NA and Plan Fluor 20×/0.5 NA lenses. All images were performed using RS Image software (version 1.9.1; Roper Scientific, Trenton, NJ) and were processed using Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA). For sub-G1 peak analysis, cells were fixed with absolute ethanol at 4°C for 30 minutes, followed by incubation with propidium iodide (PI) staining solution (0.01 mg/mL PI, 0.1% Triton X-100, and 5 mM EDTA in PBS) containing DNase-free RNase (0.1 mg/mL). To quantify apoptotic cells, cells were incubated with annexin V–PE/7-AAD and PI according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). Apoptotic cells were detected by flow cytometer (FACSCalibur; BD Biosciences, Mountain View, CA). Alternatively, cell viability was assessed by monitoring reduction of MTT. Absorbance of reduced MTT was measured after 18 hours of incubation in the dark. Data are presented as cell activity relative to control samples. Viability was determined by the formula [(A570nm/A630nm)sample/(A570nm/A630nm)control] × 100%.
Surface staining and mitochondrial membrane potential
The surface markers of monocyte-derived DCs were determined by flow cytometry analysis as previously described.6 To measure mitochondrial membrane potential, cells were incubated in complete medium containing with JC-1 (Molecular Probes, Eugene, OR) for 30 minutes at 37°C in the dark, followed by flow cytometric analysis.
RNA isolation and real-time polymerase chain reactions
Total RNA was isolated by TRIzol Reagent (Invitrogen). RNA (1 μg) was reverse transcribed into single-stranded cDNA by using avian myeloblastosis virus reverse transcriptase (Stratagene, La Jolla, CA). Polymerase chain reactions (PCRs) were performed using the following primers: FAS (forward, 5′-ACTCTACTGTATGTGAACACT-3′; reverse, 5′-TTGGGTACTTAGCATGCCACTG-3′); TNFRI (forward, 5′-ATTTGCTGTACCCAAGTGCCACAAAGGAACC-3′; reverse, 5′-GTCGATTTCCCACAAACAATGGAGTAGAGC-3′); DR4 (forward, 5′-CAAGACCTTCAAGTTTGTCGTCGTC-3′; reverse, 5′-TGCTGCACTTCCGGCACATCTC-3′); DR5 (forward, 5′-ACAGGGTGTCCCAGAGGGATGG-3′; reverse, 5′-CTGGAACCAGCAGCCTCCTCCTC-3′); and actin (forward, 5′-GACTACCTCATGAAGATCCT-3′; reverse, 5′-CCACATCTGCTGGAAGGTGG-3′). Real-time polymerase chain reaction (RT-PCR) on the LightCycler (Roche Diagnostics, Palo Alto, CA) was performed in glass capillaries in a total volume of 20 μL. RT-PCR was performed with an initial denaturation step of 15 minutes at 95°C, followed by 50 cycles of 20-second denaturation at 94°C, 60 seconds of annealing at 57°C, and 30 seconds of elongation time at 72°C. GAPDH (forward, 5′-GAAGGTGAAGGTCGGAGTC-3′; reverse, 5′- GAAGATGGTGATGGGATTTC-3′) were used as reference primers. Relative quantification was performed using the Light Cycler software.
Analysis of caspase activity
Cell extracts were prepared by freezing and thawing in lysis buffer as previously described.19 Cell lysates (50 μg) were diluted in 500 μL ICE buffer (100 mM HEPES-KOH buffer [pH 7.5], 10% sucrose, 0.1% CHAPS, 10 mM dithiothreitol, and 0.1 mg/mL ovalbumin) with 20 μM fluorescent substrates: MCA-IETD.APK (DNP), MCA-AEVD.APK (DNP), MCA-VDVAD.APK (DNP), and MCA-DEVD.APK (DNP) (R&D Systems) and incubated at 30°C for 60 minutes. Fluorescence intensity was measured using a fluorescence spectrophotometer (Hitachi, Tokyo, Japan).
Measurement of PKC-δ activity
Cell extracts (300 μg) were incubated with anti–PKC-δ antibody, followed by protein A–Sepharose CL 4B beads (GE Healthcare, Little Chalfont, United Kingdom) to pull down PKC-δ. The immunocomplexes were resuspended in 25 μL kinase buffer containing 5 μg myelin basic protein (Sigma-Aldrich) with 20 μCi (0.74 MBq) [γ-32P] ATP at 30°C for 20 minutes before addition of SDS sample buffer to terminate the reaction. The phosphorylated myelin basic protein by PKC-δ was visualized by autoradiography and quantified by densitometer (GE Healthcare).
Immunoblot and immunoprecipitation analysis
Cell lysates were prepared for immunoblotting as described previously.19 For DR5 DISC analysis, DcR3.Fc-treated cells were lysed in lysis buffer (1% NP-40, 20 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 10% glycerol). Cell lysates were centrifuged at 10 000g for 10 minutes at 4°C, and supernatants were incubated for 16 hours with Sepharose beads (GE Healthcare) adsorbed with anti-DR5 antibody. Beads were then washed 4 times in cold lysis buffer, and immunoprecipitates were fractionated on SDS-PAGE before being subjected to immunoblotting.
Statistical analysis
The Student t test (paired data) was used to analyze the statistical significance of differences.
Results
Apoptosis of DCs induced by DcR3.Fc and HBD.Fc
HSPGs have been shown to be ligands for DcR3, and knockdown of syndecan-2 and CD44v3, 2 abundant HSPGs on macrophages and DCs, abolished DcR3-mediated PKC activation in macrophages.14 To compare the effect of DcR3.Fc and HBD.Fc on DC apoptosis, CD14+ monocyte–derived DCs or macrophages were incubated with DcR3.Fc and HBD.Fc. More than 50% (DcR3.Fc-treated) and 30% (HBD.Fc-treated) of the DCs' nuclei were stained blue (trypan blue) with characteristic apoptotic bodies (Hoechst 33258), while fewer than 2% of IgG1-treated DCs displayed apoptotic morphology (Figure 1A). In contrast, macrophages did not respond to DcR3.Fc-mediated cell apoptosis under the same conditions (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The apoptosis of DCs was further confirmed by sub-G1 peak analysis (Figure 1B). To investigate the kinetics of cell death, cells were subjected to annexin V/7-AAD staining to detect early apoptotic (annexin V+), late apoptotic/necrotic (annexin V+/7-AAD+), and necrotic (7-AAD+) cells. Compared with IgG1, a significant percentage of early apoptotic and late apoptotic/necrotic cells were observed when the DCs were incubated with DcR3.Fc or HBD.Fc (Figure 1C). The kinetics of early and late apoptotic cells and necrotic cells after DcR3.Fc and HBD.Fc treatment for 18, 36, and 72 hours are summarized in Figure 1D. Since HBD.Fc induces apoptosis in DCs to an extent similar to that of DcR3.Fc, this suggests that DcR3.Fc- and HBD.Fc-induced apoptosis maybe mediated through interaction with HSPGs, rather than by neutralizing FasL, LIGHT, or TL1A.
DcR3-induced cell death is via the HBD. (A) Morphology of DcR3.Fc- or HBD.Fc-treated DCs. A total of 5 × 105 CD14+ monocytes were incubated with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of 10 μg/mL Fc-fusion proteins or IgG1 for 6 days to differentiate them into DCs. Apoptotic cells were identified by adding trypan blue/PBS (0.1%) and Hoechst 33258 (1μg/mL) for 3 minutes, and observing them under phase-contrast and fluorescence microscopy, respectively. (B,C) Analysis of apoptotic cells by sub-G1 peak (B) and annexin V–PE (FL-2) and 7-AAD (FL-3) staining (C). 5 × 105 monocyte-derived DCs (moDCs) were cultured in the presence of 10 μg/mL Fc-fusion proteins or IgG1 during the differentiation to DCs, followed by incubation with PI (0.01 mg/mL) or annexin V–PE/7-AAD (after 36 hours of Fc-fusion protein treatment), and analyzed by flow cytometry. The numbers in the quadrants represent the percentage from total cell population. (D) Kinetics of dead and apoptotic cells analyzed by annexin V–PE/7-AAD staining. *P < .05 compared with control groups at each time point. Data are representative of 3 independent experiments. Error bars represent SD of the mean..
DcR3-induced cell death is via the HBD. (A) Morphology of DcR3.Fc- or HBD.Fc-treated DCs. A total of 5 × 105 CD14+ monocytes were incubated with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of 10 μg/mL Fc-fusion proteins or IgG1 for 6 days to differentiate them into DCs. Apoptotic cells were identified by adding trypan blue/PBS (0.1%) and Hoechst 33258 (1μg/mL) for 3 minutes, and observing them under phase-contrast and fluorescence microscopy, respectively. (B,C) Analysis of apoptotic cells by sub-G1 peak (B) and annexin V–PE (FL-2) and 7-AAD (FL-3) staining (C). 5 × 105 monocyte-derived DCs (moDCs) were cultured in the presence of 10 μg/mL Fc-fusion proteins or IgG1 during the differentiation to DCs, followed by incubation with PI (0.01 mg/mL) or annexin V–PE/7-AAD (after 36 hours of Fc-fusion protein treatment), and analyzed by flow cytometry. The numbers in the quadrants represent the percentage from total cell population. (D) Kinetics of dead and apoptotic cells analyzed by annexin V–PE/7-AAD staining. *P < .05 compared with control groups at each time point. Data are representative of 3 independent experiments. Error bars represent SD of the mean..
DcR3 activates caspase-8–mediated apoptotic signaling pathway
To dissect the potential role of caspases in DcR3-mediated DC apoptosis, DCs were pretreated with various caspase inhibitors before addition of DcR3.Fc. As shown in Figure 2A, DcR3.Fc-treated cell death was inhibited by the general caspase inhibitor (Z-VAD-FMK), and by caspase-specific inhibitors, including Z-VDVAD-FMK (caspase-2 inhibitor), Z-DEVD-FMK (caspase-3 inhibitor), Z-IETD-FMK (caspase-8 inhibitor), and Z-AEVD-FMK (caspase-10 inhibitor; Figure 2A top panel). This suggested that caspases are crucial in DcR3.Fc-mediated cell death. To further confirm this observation, caspase substrates were used to quantify caspase activities (Figure 2A bottom panels). The activities of caspase-8 and caspase-10 increased at 12 and 36 hours after DcR3.Fc treatment. Moreover, caspase-2, which has been reported to process procaspase-8 to sensitize TRAIL-mediated apoptosis,20 was also activated with similar kinetics. Caspase-3 was slightly activated at 12 hours, and reached peak at 36 hours after treatment (Figure 2A,B bottom panels). It is interesting to note that activation of caspase-8 correlated with the cleavage of Bid, a caspase-8 substrate, at 12 hours after treatment (Figure 2B top panel). Moreover, the kinetics of caspase-3 activation correlated with cleavage of the caspase-3 substrate, PARP (Figure 2B bottom panel). This demonstrated that caspases play essential roles in DcR3.Fc-mediated cell death.
Caspase activities and mitochondrial functions in DcR3.Fc-treated DCs. (A) Top panel shows effect of caspase inhibitor on DcR3-mediated apoptosis. CD14+ monocytes (5 × 105) were pretreated with caspase inhibitors (general caspase, 50 μM Z-VAD-FMK; caspase-2, 50 μM Z-VDVAD-FMK; caspase-3, 50 μM Z-DEVD-FMK; caspase-8, 50 μM Z-IETD-FMK; and caspase-10, 50 μM Z-AEVD-FMK) for 1 hour before incubation with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of 10 μg/mL Fc-fusion proteins or IgG1. Cell viability was determined by incubation with annexin V–PE/7-AAD and analyzed by flow cytometry. *P < .05 compared with the control group. Bottom panels show determination of caspase activities using fluorescent substrates. Caspase activities in cell lysates were determined by incubation with fluorescent probes MCA-IETD.APK (DNP), MCA-AEVD.APK (DNP), MCA-VDVAD.APK (DNP), and MCA-DEVD.APK (DNP) to measure the activities of caspase-8 (top left), caspase-10 (top right), caspase-2 (bottom left) and caspase-3 (bottom right), respectively. *P < .05 compared with nontreated group. (B) Activation of caspases in DcR3.Fc-treated DCs. Cells were incubated with DcR3.Fc (10 μg/mL) for different time intervals before harvesting for Western blot analysis. Blots were probed with respective mAb to detect activated caspase-3 or caspase-8 and cleaved Bid or PARP. Vertical lines have been inserted to indicate a repositioned gel lane. (C) Determination of mitochondria membrane potential by incubation of DcR3-treated DCs with JC-1 (10 μg/mL) for 30 minutes before flow cytometric analysis (FL2 MFI). (D) Analysis of apoptotic cells by MTT at different time intervals. A total of 5 × 104 monocytes were incubated with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of (10 μg/mL) Fc-fusion proteins or IgG1, followed by addition of MTT for 4 hours and SDS for another 16 hours. MTT formazan was measured as described in “Methods.” Error bars represent SD of the mean.
Caspase activities and mitochondrial functions in DcR3.Fc-treated DCs. (A) Top panel shows effect of caspase inhibitor on DcR3-mediated apoptosis. CD14+ monocytes (5 × 105) were pretreated with caspase inhibitors (general caspase, 50 μM Z-VAD-FMK; caspase-2, 50 μM Z-VDVAD-FMK; caspase-3, 50 μM Z-DEVD-FMK; caspase-8, 50 μM Z-IETD-FMK; and caspase-10, 50 μM Z-AEVD-FMK) for 1 hour before incubation with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of 10 μg/mL Fc-fusion proteins or IgG1. Cell viability was determined by incubation with annexin V–PE/7-AAD and analyzed by flow cytometry. *P < .05 compared with the control group. Bottom panels show determination of caspase activities using fluorescent substrates. Caspase activities in cell lysates were determined by incubation with fluorescent probes MCA-IETD.APK (DNP), MCA-AEVD.APK (DNP), MCA-VDVAD.APK (DNP), and MCA-DEVD.APK (DNP) to measure the activities of caspase-8 (top left), caspase-10 (top right), caspase-2 (bottom left) and caspase-3 (bottom right), respectively. *P < .05 compared with nontreated group. (B) Activation of caspases in DcR3.Fc-treated DCs. Cells were incubated with DcR3.Fc (10 μg/mL) for different time intervals before harvesting for Western blot analysis. Blots were probed with respective mAb to detect activated caspase-3 or caspase-8 and cleaved Bid or PARP. Vertical lines have been inserted to indicate a repositioned gel lane. (C) Determination of mitochondria membrane potential by incubation of DcR3-treated DCs with JC-1 (10 μg/mL) for 30 minutes before flow cytometric analysis (FL2 MFI). (D) Analysis of apoptotic cells by MTT at different time intervals. A total of 5 × 104 monocytes were incubated with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of (10 μg/mL) Fc-fusion proteins or IgG1, followed by addition of MTT for 4 hours and SDS for another 16 hours. MTT formazan was measured as described in “Methods.” Error bars represent SD of the mean.
It has been reported that Bid relays the apoptotic signal from the cell surface to the mitochondria. After cleavage, truncated Bid translocates to mitochondria, disrupts the mitochondrial membrane potential (Ψm), and thereby triggers cytochrome c release.20 To investigate the mitochondrial function during DcR3.Fc and HBD.Fc treatment, JC-1 staining or MTT assay were performed. After DcR3.Fc or HBD.Fc treatment, the mean fluorescence intensity (MFI) of FL2 channel (aggregated JC-1) was down-regulated, indicating disruption of the Ψm (Figure 2C). An MTT assay was used to investigate further the kinetics of apoptosis over 72 hours. We found that the number of apoptotic cells increased sharply from 24 hours to 72 hours when cells were treated with either DcR3.Fc or HBD.Fc (Figure 2D). The sequential activation of caspases and down-regulation of membrane potential suggests that activation of the apoptosis-initiating caspases (caspase-2, caspase-8, and caspase-10) leads to disruption of the mitochondrial membrane potential and activation of caspase-3.
PKC and MAPK are involved in DcR3-mediated cell death
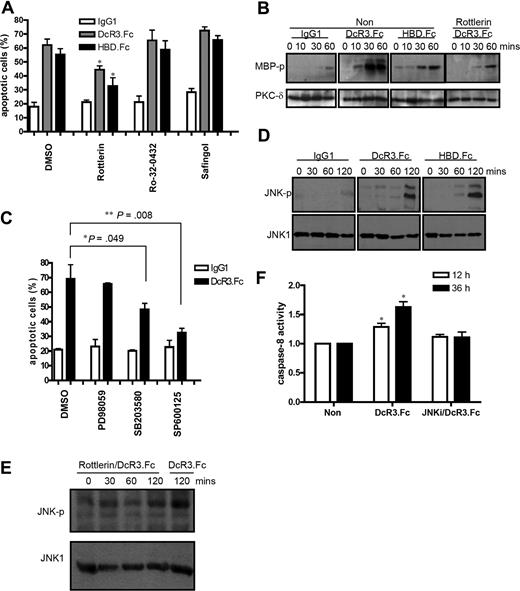
Cross-linking of syndecan 2 and CD44v3, 2 abundant HSPGs on macrophages and DCs, by DcR3.Fc and HBD.Fc has been shown to activate PKC to induce cell adhesion in myeloid cells.14 To investigate whether PKC is involved in DcR3-mediated DC apoptosis, DCs were coincubated with various PKC inhibitors in the presence of DcR3.Fc or HBD.Fc. As shown in Figure 3A, the PKC-δ–selective inhibitor rottlerin suppressed DC apoptosis, while Ro-32-0432 (PKC-α inhibitor) and a broad-spectrum PKC inhibitor (safingol) had no protective effect. In vitro kinase assay further demonstrated that DcR3.Fc and HBD.Fc were able to activate PKC-δ (Figure 3B). This observation suggested that cross-linking of HSPG could activate PKC-δ to trigger a downstream apoptotic signal in DCs.
PKC and JNK are involved in DcR3.Fc-induced DC apoptosis. (A) CD14+ monocytes (5 × 105) were pretreated with PKC inhibitors for 1 hour before incubation with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of 10 μg/mL Fc-fusion proteins or IgG1. Apoptotic cells were determined by annexin V–PE/7-AAD staining. *P < .05 compared with control group. PKC inhibitors were safingol (5 μM), rottlerin (5 μM), and Ro-32–0432 (50 nM). (B) In vitro kinase assay of PKC-δ activity in DcR3.Fc-treated DCs. Total cell lysates (300 μg) from different treatments were immunoprecipitated with an antibody against PKC-δ, and in vitro kinase assay was performed in the absence or presence of rottlerin using myelin basic protein (MBP) as substrate. Cell lysates were fractionated on SDS-PAGE, and the phosphorylated MBP was detected by autoradiography. (C) After pretreatment with kinase inhibitors PD98059 (50 μM), SB203580 (30 μM), and SP600125 (20 μM) for 1 hour, apoptotic cells were determined by annexin V–PE/7-AAD staining *P < .05; **P < .01 compared with DcR3.Fc without inhibitor treatment. (D,E) JNK phosphorylation in IgG1-, DcR3.Fc-, or HBD.Fc-treated DCs in the absence or presence of the PKC-δ inhibitor rottlerin. Cell lysates were fractionated on SDS-PAGE and blotted onto nitrocellulose paper before being probed with anti–phospho-JNK1 mAb. (F) The caspase-8 activity in cell lysates was determined using fluorescent caspase substrates. JNKi indicates JNK inhibitor SP600125 (20 μM). Error bars represent SD of the mean.
PKC and JNK are involved in DcR3.Fc-induced DC apoptosis. (A) CD14+ monocytes (5 × 105) were pretreated with PKC inhibitors for 1 hour before incubation with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of 10 μg/mL Fc-fusion proteins or IgG1. Apoptotic cells were determined by annexin V–PE/7-AAD staining. *P < .05 compared with control group. PKC inhibitors were safingol (5 μM), rottlerin (5 μM), and Ro-32–0432 (50 nM). (B) In vitro kinase assay of PKC-δ activity in DcR3.Fc-treated DCs. Total cell lysates (300 μg) from different treatments were immunoprecipitated with an antibody against PKC-δ, and in vitro kinase assay was performed in the absence or presence of rottlerin using myelin basic protein (MBP) as substrate. Cell lysates were fractionated on SDS-PAGE, and the phosphorylated MBP was detected by autoradiography. (C) After pretreatment with kinase inhibitors PD98059 (50 μM), SB203580 (30 μM), and SP600125 (20 μM) for 1 hour, apoptotic cells were determined by annexin V–PE/7-AAD staining *P < .05; **P < .01 compared with DcR3.Fc without inhibitor treatment. (D,E) JNK phosphorylation in IgG1-, DcR3.Fc-, or HBD.Fc-treated DCs in the absence or presence of the PKC-δ inhibitor rottlerin. Cell lysates were fractionated on SDS-PAGE and blotted onto nitrocellulose paper before being probed with anti–phospho-JNK1 mAb. (F) The caspase-8 activity in cell lysates was determined using fluorescent caspase substrates. JNKi indicates JNK inhibitor SP600125 (20 μM). Error bars represent SD of the mean.
Mitogen-activated protein kinases (MAPKs) have been reported to regulate apoptosis and are involved in DcR3.Fc-induced signals.8 Among the MAPK inhibitors tested, the JNK inhibitor (SP600125) was the most potent (P = .008) in inhibiting DcR3.Fc-mediated cell death, while the p38 MAPK inhibitor (SB203580) also had a mild (P = .049) inhibitory effect (Figure 3C). This was in accord with the observation that JNK was phosphorylated by 120 minutes after treatment (Figure 3D). Moreover, JNK phosphorylation was suppressed by the PKC-δ inhibitor rottlerin (Figure 3E), indicating that JNK activity was regulated by PKC-δ.
Since caspase activation occurred later than JNK phosphorylation, we further asked whether caspase activation is regulated by JNK. As shown in Figure 3F, caspase-8 activation was inhibited by the JNK inhibitor SP600125. From these observations, we concluded that DcR3.Fc and HBD.Fc activated PKC-δ and JNK sequentially, and that caspase activation is downstream of JNK activation.
Up-regulation of DR5 and FAS expression by DcR3.Fc and HBD.Fc
Even though caspase-8 was activated after DcR3.Fc or HBD.Fc treatment, we could not detect the expression of FasL, TNF, and TRAIL in DCs. It has been shown that caspases can be activated by overexpression and self-aggregation of death domain–containing receptors21 ; thus, we examined whether DcR3.Fc-mediated DC apoptosis is triggered via ligand-independent activation of death domain–containing receptors, such as DR4 (TRAIL receptor-1), DR5 (TRAIL receptor-2), TNFRI, and Fas. RT-PCR analysis demonstrated that DcR3.Fc induced expression of DR5, which was detectable at 6 hours and lasted for at least 36 hours, while FAS up-regulation was first detectable at 18 hours and peaked at 36 hours (Figure 4A). In contrast, TNFRI and DR4 were constitutively expressed in DCs, and were not further up-regulated by DcR3.Fc (Figure S2). Kinetic study by flow cytometric analysis demonstrated that the up-regulation of DR5 on DCs by DcR3.Fc and HBD.Fc was detectable at 8 hours and lasted for at least 72 hours. However, in contrast to RT-PCR analysis, Fas was constitutively expressed and was not further up-regulated by either DcR3.Fc or HBD.Fc (Figure 4B). To further understand whether up-regulation of DR5 lead to cell apoptosis, we analyzed the formation of a DISC by immunoprecipitating DR5 to detect the presence of FADD and activated caspase-8 in the immuno-complex. The kinetic study demonstrated that both FADD and activated caspase-8 were detected in the DISC 9 hours and 12 hours after DcR3.Fc treatment (Figure 4C), and this demonstrated that DcR3.Fc–up-regulated DR5 recruits FADD, which then activates caspase-8.
Expression of DR5 and Fas in DcR3.Fc-treated DCs. (A) Expression of DR5 and FAS in DCs was determined by quantitative RT-PCR. Levels of the housekeeping gene GAPDH mRNA were used as internal controls for normalization of RNA quantity and quality differences in all samples. *P < .05 compared with nontreated group. (B) Surface expression of DR5 and Fas by flow cytometry. Cells were incubated with PE-conjugated anti-DR5 or PE-conjugated anti-Fas mAb before FACScan analysis. Shaded histograms indicate isotype control Abs; open histograms, specific Abs. (C) Time course of the formation of DR5 DISC. Monocytes (3 × 107) were incubated with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of 10 μg/mL Fc-fusion proteins or IgG1 for different time intervals. Cells were lysed, and the assembled DISCs were immunoprecipitated with Sepharose beads adsorbed with anti-DR5 antibody and analyzed by Western blotting using antibodies to FADD and caspase-8. (D) Effect of MAPK inhibitors on the expression of DR5 and Fas. Monocytes (5 × 105) were pretreated with kinase inhibitors for 1 hour before incubation with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of 10 μg/mL Fc-fusion proteins or IgG1, for 12 hours and 72 hours. Shaded histograms indicates isotype control Abs; open histograms, specific Abs. Error bars represent SD of the mean.
Expression of DR5 and Fas in DcR3.Fc-treated DCs. (A) Expression of DR5 and FAS in DCs was determined by quantitative RT-PCR. Levels of the housekeeping gene GAPDH mRNA were used as internal controls for normalization of RNA quantity and quality differences in all samples. *P < .05 compared with nontreated group. (B) Surface expression of DR5 and Fas by flow cytometry. Cells were incubated with PE-conjugated anti-DR5 or PE-conjugated anti-Fas mAb before FACScan analysis. Shaded histograms indicate isotype control Abs; open histograms, specific Abs. (C) Time course of the formation of DR5 DISC. Monocytes (3 × 107) were incubated with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of 10 μg/mL Fc-fusion proteins or IgG1 for different time intervals. Cells were lysed, and the assembled DISCs were immunoprecipitated with Sepharose beads adsorbed with anti-DR5 antibody and analyzed by Western blotting using antibodies to FADD and caspase-8. (D) Effect of MAPK inhibitors on the expression of DR5 and Fas. Monocytes (5 × 105) were pretreated with kinase inhibitors for 1 hour before incubation with GM-CSF (200 ng/mL) and IL-4 (10 ng/mL) in the presence of 10 μg/mL Fc-fusion proteins or IgG1, for 12 hours and 72 hours. Shaded histograms indicates isotype control Abs; open histograms, specific Abs. Error bars represent SD of the mean.
It has been shown that JNK is involved in DR5 up-regulation by bile acid.22 Since JNK activation (2 hours after treatment) occurred earlier than up-regulation of DR5 (8 hours after treatment), we examined whether JNK and other MAPKs are involved in DcR3-mediated DR5 up-regulation. As shown in Figure 4D, the JNK inhibitor (SP600125) suppressed the up-regulation of DR5, while both the p38 MAPK inhibitor (SB203580) and ERK inhibitor (PD98059) had no effect on DR5 up-regulation (Figure 4D). This observation demonstrates that DcR3-induced DR5 expression is regulated by JNK. Therefore, we concluded that DcR3.Fc activates JNK to up-regulate the expression of DR5, which then recruits FADD and activates caspase-8 to trigger apoptotic signaling cascades in DCs.
DcR3-mediated apoptosis is enhanced by exogenous TRAIL
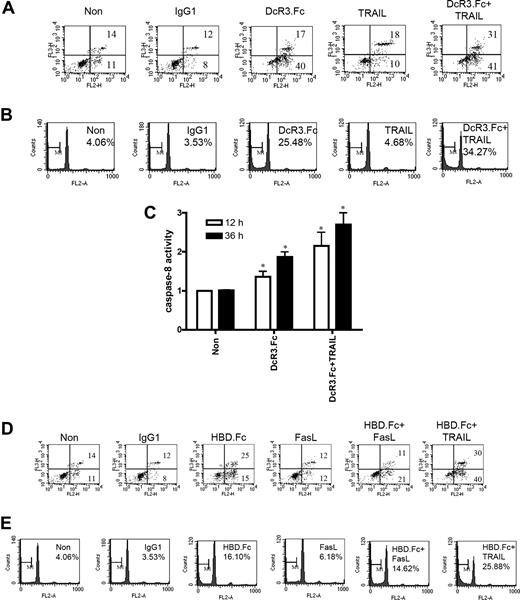
Since DcR3.Fc and HBD.Fc up-regulated DR5 expression in DCs, we asked whether exogenous TRAIL (DR5 ligand) could further enhance DC apoptosis. To address this question, DCs were treated with DcR3.Fc alone, recombinant TRAIL alone, or a combination of DcR3.Fc and TRAIL. Without DcR3.Fc treatment, the percentage of DC apoptosis induced by TRAIL (28%) was similar to that induced by FasL (24%). Interestingly, DcR3.Fc-induced apoptosis (57%) was further increased by exogenous TRAIL (72%) as revealed by annexin V/7-AAD double staining (Figure 5A). PI staining to detect the percentage of apoptotic cells in sub-G1 peak (Figure 5B) further confirmed this observation. Enhancement of DcR3.Fc/TRAIL-induced DC apoptosis correlated with the augmentation of caspase-8 activity (Figure 5C), and this further confirmed the essential role of DR5 DISC formation in DcR3-induced apoptosis. To further ask whether FasL can enhance DC apoptosis, the DCs were incubated with FasL or TRAIL in conjunction with HBD.Fc (because DcR3.Fc would bind FasL and neutralize FasL activity). Unlike TRAIL, FasL failed to enhance HBD.Fc-mediated DC apoptosis (Figure 5D,E). Therefore, we concluded that up-regulation of DR5, but not Fas, induces the formation DR5 DISC which, thereby, increases DC susceptibility to TRAIL-mediated apoptosis.
DcR3.Fc and TRAIL synergistically induce DC apoptosis. (A,B) Analysis of apoptotic cells cotreated with DcR3.Fc and TRAIL. DCs (5 × 105) were incubated with Fc-fusion proteins (10 μg/mL) in conjunction with TRAIL (100 ng/mL) for 36 hours. Cell viability was determined by annexin V/7-AAD double staining (A) or PI staining (B). (C) Cell lysates of DCs incubated with TRAIL and DcR3.Fc for 12 hours or 36 hours were incubated with fluorescent probes MCA-IETD.APK (DNP) or MCA-AEVD.APK (DNP) to determine caspase-8 activity. *P < .05 compared with control group. Error bars represent SD of the mean. (D,E) Analysis of apoptotic cells cotreated with HBD.Fc and TRAIL or FasL. The experiment is performed as described in panels A and B except that DcR3.Fc was replaced with HBD.Fc. Fas ligand was added at 100 ng/mL. The numbers in the quadrants represent the percentage from total cell population.
DcR3.Fc and TRAIL synergistically induce DC apoptosis. (A,B) Analysis of apoptotic cells cotreated with DcR3.Fc and TRAIL. DCs (5 × 105) were incubated with Fc-fusion proteins (10 μg/mL) in conjunction with TRAIL (100 ng/mL) for 36 hours. Cell viability was determined by annexin V/7-AAD double staining (A) or PI staining (B). (C) Cell lysates of DCs incubated with TRAIL and DcR3.Fc for 12 hours or 36 hours were incubated with fluorescent probes MCA-IETD.APK (DNP) or MCA-AEVD.APK (DNP) to determine caspase-8 activity. *P < .05 compared with control group. Error bars represent SD of the mean. (D,E) Analysis of apoptotic cells cotreated with HBD.Fc and TRAIL or FasL. The experiment is performed as described in panels A and B except that DcR3.Fc was replaced with HBD.Fc. Fas ligand was added at 100 ng/mL. The numbers in the quadrants represent the percentage from total cell population.
Discussion
In this study, we demonstrated that both DcR3.Fc and HBD.Fc induced DC apoptosis by activating PKC-δ to trigger the downstream apoptotic signaling cascade. This further confirms that the pleiotropic effects of DcR3.Fc are mediated by cross-linking HSPGs instead of by neutralizing FasL, TL1A, or LIGHT—3 members of the TNF superfamily (Figure 6). Interestingly, neither DcR3.Fc nor HBD.Fc induced apoptosis in macrophages, and this is in accord with previous observations that DcR3.Fc and HBD.Fc activate PKC to induce actin reorganization in macrophages.7,14,15 Since PKC-δ is well expressed in both macrophages and DCs, this indicates that PKC-δ may play a differential role in macrophages and DCs. This argument is supported by the observation that the role played by PKC-δ to either control cellular proliferation or to induce apoptosis is dependent on cell lineages and stimulation signals (as reviewed by Griner and Kazanietz23 ). To further dissect the potential role of other PKC isoforms in DcR3-induced apoptosis, various PKC isoform–specific inhibitors, including HBDDE (PKC-α and γ inhibitor), Gö 6976 (classical PKC inhibitor), LY333531 (PKC-β inhibitor), and LY379196 (PKC-βII inhibitor) were used to address this question. However, none of these inhibitors can increase the survival rate of DcR3-treated DCs (Figure S3). This further confirms the important role of PKC-δ in DcR3-mediated DC apoptosis. Since different PKC isoforms can either promote cell survival or induce cell death in different lineages,16 a pan-PKC inhibitor may block both pro- and antiapoptotic PKC isoforms; thus, it would be difficult to observe the protective effect of pan-PKC inhibitor, such as safingol, on DcR3-induced apoptosis (Figure 3A). This argument is further supported by the observation that other broad-spectrum PKC inhibitors (including Ro-31-8220, GF-109203X, and Gö 6983), also fail to protect DCs from DcR3-mediated apoptosis (Figure S3).
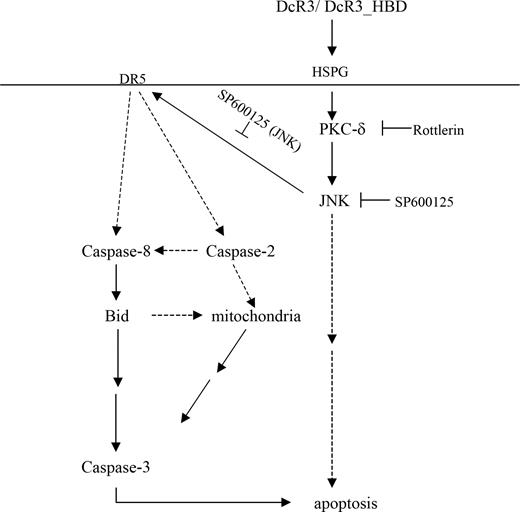
Schematic of DcR3-induced DC apoptosis. DcR3 binds to HSPG to activate PKC-δ and subsequently JNK. Activation of JNK up-regulates DR5 expression to induce ligand-independent activation of the downstream apoptotic signaling pathway via formation of DR5 DISC. The upstream caspase-2 and caspase-8 cleave Bid, which in turn disrupts the mitochondrial membrane potential and activates caspase-3 to induce DC apoptosis.
Schematic of DcR3-induced DC apoptosis. DcR3 binds to HSPG to activate PKC-δ and subsequently JNK. Activation of JNK up-regulates DR5 expression to induce ligand-independent activation of the downstream apoptotic signaling pathway via formation of DR5 DISC. The upstream caspase-2 and caspase-8 cleave Bid, which in turn disrupts the mitochondrial membrane potential and activates caspase-3 to induce DC apoptosis.
It has been reported that PKC-δ activation is upstream of JNK activation in DNA damage-induced apoptosis.17 This is in accord with our observation that JNK activation (60 minutes after DcR3.Fc treatment) occurs later than PKC activation (10 minutes after DcR3.Fc treatment; Figure 3D), and JNK phosphorylation is suppressed by the PKC-δ inhibitor rottlerin (Figure 3E). Moreover, JNK has been reported to be involved in DR5 up-regulation by bile acid,24 buthionine sulfoximine,25 and methyl-2-cyano-3,12-dioxooleana-1 treatment,26 even though the underlying mechanism is still to be determined. In this study, we demonstrate that the cross-linking of HSPGs by HBD.Fc and DcR3.Fc up-regulates DR5 expression to induce the formation of DR5 DISC. Since JNK inhibitor down-regulates DR5 expression (Figure 4D) and inhibits caspase-8 activity (Figure 3F), this demonstrates that JNK-mediated apoptosis occurs through DR5 DISC formation in DcR3.Fc-treated DCs. The role of DR5 in DC apoptosis is further supported by the observation that DcR3.Fc increases DC susceptibility to exogenous TRAIL-induced apoptosis (Figure 5A,B). Since DcR3.Fc neither up-regulates the expression of Fas and TNFRI nor increases DC susceptibility to FasL- and TNF-induced apoptosis, this further confirms the argument that cross-linking of HSPGs induces DC apoptosis via DR5 DISC formation, and is independent of other death domain–containing receptors, such as Fas and TNFRI.
In humans, there are 5 TRAIL-interacting receptors, including TRAIL-R1 (DR4), TRAIL-R2 (DR5), TRAIL-R3 (DcR1; TRID), TRAIL-R4 (DcR2; TRUNDD), and osteoprotegerin (OPG). TRAIL-R1 and TRAIL-R2 possess an intracellular tail containing a conserved motif known as the death domain to transduce apoptotic signals, while TRAIL-R3, TRAIL-R4, and OPG are regarded as decoy receptors to neutralize TRAIL. In mice, there is only one TRAIL-R (also known as murine DR5 [mDR5] or murine TRAILR-2 [mTRAILR2]). The amino acid sequence of mDR5 is most similar to human DR5, and like its human homolog, is capable of signaling apoptosis in transformed cells after either overexpression or ligation by TRAIL.27 Therefore, DR5 is highly conserved, while TRAIL-R2, TRAIL-R3, and TRAIL-R4 appear to have evolved later. In human DCs, DR4 is constitutively expressed, while DR5 is up-regulated by DcR3 in DCs; thus, DR5 seems to play a more prominent role than DR4 in DC apoptosis. Because mice do not have DcR3 in the genome, and the regulation of mDR5 has not been investigated, it would be interesting to investigate whether DcR3 also up-regulates mDR5 and induces murine DC apoptosis. It is well known that cancer cells use several mechanisms to evade the host immune system, such as loss of tumor antigen, alteration of HLA class I antigen, lack of costimulation signal, induction of immunosuppressive T cells, and secretion of immunosuppressive cytokines.28 Moreover, tumor cells inhibit the maturation of DCs and induction of regulatory T cells by secreting IL-10 and transforming growth factor-β (TGF-β).29-33 DcR3, which is expressed in 25% to 70% of human cancer cells derived from different lineages,1-5 is a novel immunosuppressive factor secreted by tumor cells to down-regulate the activation of DCs6 and macrophages,7 and to induce DC apoptosis. However, unlike IL-10 and TGF-β, DcR3 is absent in the murine genome. Thus, the discrepancy between the therapeutic effects of potential anticancer drugs observed in murine model systems and those seen in clinical trials may be due to the neglect of the possible role of DcR3, which is detectable in tumor cells as well as in the sera of patients with cancer. Transgenic mice overexpressing DcR3, or inoculation of tumor cells stably expressing DcR3, may be better model systems to monitor the efficacy of anticancer drugs.
The expression of DcR3 in cancer cells seems to be correlated with poor prognosis in patients with cancer. We randomly selected 100 specimens of colon adenocarcinoma, and followed up the survival rate of patients based on the presence or absence of DcR3. The long-term survival rate of the DcR3+ group is shorter than DcR3− group under the same treatment protocol significantly (P < .001; W.-S.W., unpublished data, November 2007), and this suggests that DcR3 might become a valuable marker to monitor cancer progression in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Wan-Wan Lin, Dr Ming-Zong Lai, and See-Wen Chen for providing reagents and technical assistance, and Dr Colleen Fearns for critical review of this article.
This work was supported mainly by grant NSC 95-2320-B-010-040-MY3 from the National Sciences Council; grant V96S5-001 from Taipei Veterans General Hospital; and grants 94 M002-1 and AS-97-FP-L06-5 from Academia Sinica, Taiwan.
Authorship
Contribution: R.-I.Y. designed, performed, and analyzed experiments and wrote the manuscript; P.-M.C. and W.-S.W. analyzed clinical data; Y.-C.C., T.-L.H., C.-Y.Y., and C.-T.L. performed experiments and contributed to writing the paper; and S.-L.H. designed and analyzed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shie-Liang Hsieh, Institute of Microbiology and Immunology, National Yang-Ming University, Shih-Pai, Taipei 11221, Taiwan; e-mail: slhsieh@ym.edu.tw.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal