Donor cell expression of C3 enhances the alloimmune response and is associated with the fate of transplantation. To clarify the mechanism for enhancement of the immune response, we have explored the role of C3a receptor (C3aR)–ligand interaction on murine bone marrow dendritic cells (DCs). We show that DCs either lacked receptor for C3a (a C3 cleavage product) or were treated with C3aR antagonist, elicited defective T-cell priming against alloantigen expressed on the DCs. This was associated with reduced surface expression of major histocompatibility complex (MHC) and costimulatory molecules on the DCs, and with defective priming in skin allograft rejection. In addition, DCs lacking factor B were unable to generate potent T-cell responses against donor antigen, whereas lack of C4 had no detectable effect, suggesting a role for the alternative pathway contributing to allostimulation. Furthermore, therapeutic complement regulator can down-regulate DC allostimulatory function. These findings suggest that the capacity of DCs for allostimulation depends on their ability to express, activate, and detect relevant complement components leading to C3aR signaling. This mechanism, in addition to underpinning the cell-autonomous action of donor C3 on allostimulation, has implications for a wider range of immune responses in self-restricted T-cell priming.
Introduction
The complement system consists of a set of soluble and cell surface proteins, including components, receptors, and regulators. The complement system not only plays a major role in innate immunity but also forms an important link between innate and adaptive arms of immune response.1,,–4 C3 is the most abundant component of the complement cascade and the convergent point for all 3 major pathways of complement activation, namely, the classic pathway, the alternative pathway, and the lectin pathway. Cleavage of C3 generates a set of effector molecules with diverse biologic functions. Small molecules, such as the anaphylatoxins C3a and C5a, and the large fragment of C3, C3b and its derivatives (eg, iC3b, C3dg) interact with their respective receptors, such as C3aR, C5aR, CR1-CR4, and the newly identified receptor CRIg,5 which lead to opsonophagocytosis, inflammation, and cell activation. The formation of C5b-9 on cells causes the direct killing of pathogens and cell activation.
The liver is the primary site for the synthesis of most circulating complement proteins. However, extrahepatic synthesis of a wide range of soluble proteins of the complement system, including C3, occurs in a variety of organs/tissues and cells, either constitutively or response to various stimuli (ie, infectious or noninfectious).6,7 In addition, a large body of data has shown a link between up-regulation of local synthesis of complement and the progression of inflammatory or immune disease, which highlights the importance of local production of complement in both physiologic and pathologic settings, including transplant rejection.8,–10
After the study in mouse kidney transplantation showing that donor kidney synthesis of C3 plays a contributory role in acute allograft rejection,11 a study in human kidney transplantation has found that donor C3 polymorphism associates with late graft failure.12 These observations suggest that donor tissue expression of C3 influences the alloimmune response and the fate of transplantation. Recent work has provided evidence that the capacity of donor antigen presenting cells (APCs; ie, macrophages and dendritic cells [DCs]) to stimulate alloreactive T cells is strongly dependent on cell-autonomous C3, absent production resulting in impaired antidonor Th1 responses and expansion of regulatory T cells.13,14 How complement, particularly local production of complement, modulates donor APC function was, however, unclear, and this seemed critical to our understanding of and ability to exploit the complement system in cell and tissue transplantation.
DCs, the most potent APCs, reside in most peripheral tissues, particularly at the sites of interstitial space and interface with environment, where they are stimulated by danger signals (pathogens and/or tissue injury) and become competent APCs and T-cell activators.15 Although it is well known that DCs can detect and respond to pathogen factors through pattern recognition receptors (eg, TLRs), little is known about whether DCs can also detect and respond to endogenous molecules, such as complement effector products, widely generated in both pathogen or nonpathogen related inflammatory conditions.
In the transplant setting, donor alloantigen expressed on transplanted tissues and cells is detected directly by the recipient T cells, in contrast to the way other foreign antigens are necessarily taken up by recipient APCs that trigger the T-cell response. The biology of donor APCs, in particular DCs, is therefore paramount in regulating the recipient immune response that contributes to allograft rejection. In this study, we set out to investigate the capability of donor DCs to generate complement effector products and explore the mechanisms by which local production and activation of complement up-regulates the allostimulatory function of DCs.
Thus, we studied the complement gene expression profile in murine bone marrow (BM) DCs and determined if C3a can be generated locally. We investigated the influence of C3a-C3aR interaction on DC activation, and determined if C3a-C3aR interaction modulates donor DC function in a mouse transplant model (H-2 disparate) in vitro and in vivo. We also assessed if C3a-C3aR interaction in donor tissue which containing DCs could have effect on allograft rejection. Finally, we explored the possibility that therapeutic complement regulator targeted to the DC surface could down-regulate DC allostimulatory function.
Methods
Reagents
Reagents are shown in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Mice
Homozygous C3−/−, C4−/−, factor B−/−, C3aR−/− mice were derived by homologous recombination in embryonic stem cells and backcrossed on to C57BL/6H-2b (for C3−/−, C4−/−, and fB−/− mice) or BALB/cH-2d (for C3aR−/− mice) parental strain for at least 10 generations.16,,–19 WT mice, including C57BL/6H-2b and BALB/cH-2d, were purchased from Harlan UK (Bicester, United Kingdom). Male mice (6-7 weeks old) were used in all experiments. All animal procedures were carried out within the Animals (Scientific Procedures) Act, 1986 (United Kingdom).
Preparation of DCs
BM DCs were generated as described previously with minor modifications.20 In brief, BM cells were harvested from mouse femurs and tibias and cultured in DC medium (RPMI 1640 medium containing 5% fetal calf serum, 50 μM β-mercaptoethanol, 50 μg/mL gentamicin, 2.5 μg/mL Fungizone, 20 ng/mL granulocyte-macrophage colony-stimulating factor). Culture medium was replaced every other day. On day 4 or 6, DCs were collected and enriched with CD 11c microbeads. In some experiments, purified day 6 DCs were cultured for another 24 hours in the presence or absence of lipopolysaccharide (LPS; 1 μg/mL). In some experiments, C3aR antagonist (5-500 nM) or C3aR agonist (4-20 nM) or membrane-localizing complement regulator (APT070, 20 μg/mL) or control molecule for APT070 (APT542, 1.6 μg/mL, the equivalent molar concentration to APT070) was added to DC culture medium from the beginning of BM cell culture and with repeated addition every 2 days. In each experiment, DCs were prepared from 3 to 5 mice and pooled together for analysis.
Preparation of T cells
Naive CD4 T cells were prepared from spleens of WT BALB/c or C57BL/6 mice using Spin-Sep Enrichment Cocktail Kit (Stem Cell Technologies UK, London, United Kingdom). After the isolation, the purity of the T cell preparation was routinely more than 90%, as determined by flow cytometry.
Gene microarray
Total RNA prepared from BM DCs (LPS stimulated for 24 hours) was used for microarrays. The microarrays were performed by Genomics Center, King's College London, using the GeneChip Mouse Expression Set 430 (Affymetrix, San Diego, CA).
Reverse transcribed-quantitative polymerase chain reaction
Reverse transcribed-quantitative polymerase chain reaction (qPCR) was performed with an MJ Research PTC-200 Peltier Thermal Cycler and DyNAmo HS SYBR Green qPCR kit (MJ BioWorks, Espoo, Finland), according to the manufacturer's instructions. The PCR primer sequences and product sizes are shown in Table S1.
Conventional RT-PCR
Total RNA and subsequent cDNA were prepared from BM DCs as previously described.21 PCR was carried out with 2 μL cDNA (reflecting 0.2 μg total RNA), 12.5 pmol of each 3′ and 5′ primer pair for each testing gene, in 25 μL reaction buffer. The PCR cycle consisted of 1 minute at 94°C, 1 minute at 62°C, and 1 minute at 72°C. Amplified PCR products were visualized after electrophoresis on 1.5% agarose containing ethidium bromide. Primers for 18sRNA were also added in every PCR reaction as an internal control.
Analysis of alloreactive T-cell response in vitro
Irradiated (2000 cGy) DCs (5 × 104/well) and purified alloreactive CD4 T cells (2 × 105/well) were cocultured in T cell culture medium (RPMI-1640 containing 10% heat inactivated fetal calf serum, 50 μM 2-mecaptoethanol, 50 μg/mL gentamicin, 2.5 and μg/mL Fungizone). The supernatants were collected at day 5 of coculture and analyzed for interferon-γ (IFN-γ) using enzyme-linked immunosorbent assay (ELISA). 3H-thymidine up take was performed after 3 days of coculture.
Analysis of immune response in vivo
Allogeneic mice (C57BL/6) received an intraperitoneal injection of 5 × 105 irradiated donor DCs (BALB/c). After 10 days, mice were killed. Splenocytes and CD4 T cells were prepared from each mouse and used for measuring allospecific T-cell reactivity ex vivo by mixed lymphocyte reaction. Briefly, splenocytes (2 × 105/well) or CD4 T cells (105/well) from immunized mice were cocultured with splenocytes (2 × 105/well) from donor strain (BALB/c) in T-cell culture medium for up to 5 days. T-cell responses were measured by IFN-γ production and 3H-thymidine uptake.
3H-thymidine incorporation assay
The cocultures were set up in 96-well round-bottom plates for 72 hours. 3H-thymidine (1 μCi/well) was added during the last 8 hours. The amount of 3H-thymidine incorporation was measured by liquid scintillation (1205 BS Betaplate; Wallac, Waltham, MA). Controls (ie, stimulator alone and responder cells alone) were included in each experiment and gave consistently low backgrounds.
Flow cytometry
Before staining with labeled antibodies, DCs were preincubated with Fc-blocking antibody. DCs (2 × 105) were stained with phycoerythrin-conjugated antibody or the appropriate isotype control antibody, at 4°C for 30 minutes followed by washing 3 times in 2 mL of PBS containing 1% BSA. The cells were then fixed in 400 μL of 1% paraformaldehyde in PBS. The stained cells were analyzed using flow cytometry.
ELISA
Sandwich ELISA was performed using OptEIA ELISA set (BD Biosciences, Oxford, United Kingdom) for mouse IFN-γ, IL-10, and IL-12, and a pair of monoclonal antibodies for C3a, according to the manufacturer's instructions. The zymosan-activated mouse serum was used as positive control in C3a ELISA.
Skin grafting
Tail skin (∼1.5 cm2) from donor animals was grafted onto the left flank of recipients under isoflurane anesthetic. The graft site was covered with paraffin-embedded gauze and a dressing of dry gauze and a clear adhesive tape. The dressing was removed on day 5, and the grafts were assessed visually on a daily basis for signs of rejection in a blinded fashion. The time to rejection was determined as the day on which more than 80% of the graft area was necrotic.
Statistical analysis
ELISA was performed in 4 to 6 replicate wells of the culture or coculture per sample. T-cell proliferation assays were performed in 6 to 8 replicate wells of the coculture per sample. Results were expressed as means plus or minus SEM and subjected to statistical analysis. Student t test or 2-way ANOVA was used where appropriate to determine significant differences between samples. Log rank test was used to analyze the data of skin grafting.
Results
BM DCs express a range of complement components, regulators, and receptors and C3a is generated locally
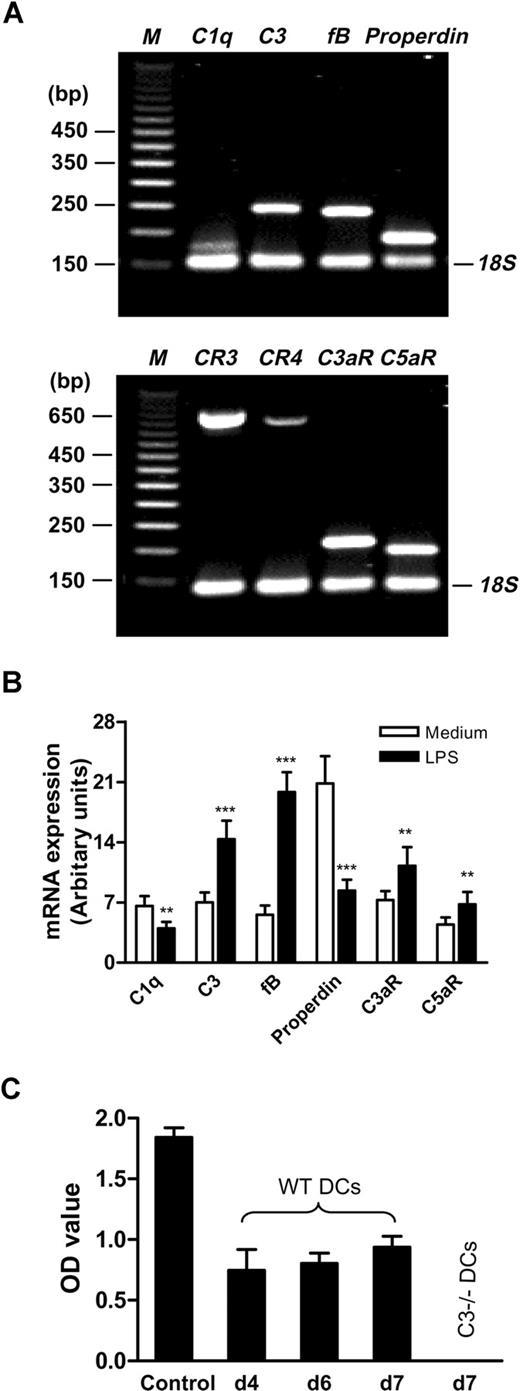
As circulating complement proteins, particularly large molecules like C3 (180 kDa), may not be able to cross into the interstitial space where DCs reside,22 it is important to determine whether DCs and/or adjacent cells in their natural locality to express complement components, regulators, and receptors and to determine whether C3a can be generated locally. To investigate this initially, we performed comprehensive analysis of the gene expression for complement components, regulators, and receptors in murine BM DCs (LPS stimulated). Using gene array and qPCR, we detected several of the complement components and cofactor that are needed to cleave C3 (ie, C1q, C3, factor B (fB), properdin) as well as complement receptors (C3aR, C5aR, CR3, CR4). However, the components for the classic pathway (ie, C2, C4) and for the terminal pathway (ie, C5, C6, C8, C9) were not detected. Interestingly, several of the complement inhibitory regulators, including factor H (fH), CR1/2, DAF (CD55) and MCP (CD46), were not detected, although rodent complement receptor-related protein (Crry) and CD59 were found (Table 1). For some of these positively detected molecules (ie, C1q, C3, fB, properdin, CR3, CR4, C3aR, and C5aR), we performed conventional RT-PCR and further confirmed their expression in DCs (Figure 1A). In addition, we performed qPCR to examine the differential expression of these molecules in DCs with and without LPS stimulation. We found that, even without LPS stimulation, DCs are still able to express these molecules. LPS stimulation significantly increases the level of C3, fB, C3aR, and C5aR expression but decreased the level of C1q and properdin expression (Figure 1B).
BM DCs express a range of complement components, regulators, and receptors and C3a is generated locally
| Complement . | Gene array (intensity) . | Real-timeRT-PCR . |
|---|---|---|
| Alternative pathway | ||
| C3 | + (700) | + |
| fB | + (900) | + |
| Poperdin | + (500) | + |
| Classical pathway | ||
| C1q | + (500) | + |
| C2 | − | − |
| C4 | − | − |
| Terminal pathway | ||
| C5 | N/A | − |
| C6 | − | − |
| C8 | − | − |
| C9 | − | − |
| Inhibitory regulators | ||
| DAF | − | − |
| MCP | − | − |
| CR1/2 | − | − |
| fH | − | − |
| Crry | + (160) | + |
| CD59 | + (70) | + |
| Receptors | ||
| CR3 | + (380) | + |
| CR4 | + (430) | + |
| C3aR | + (480) | + |
| C5aR | + (50) | + |
| Complement . | Gene array (intensity) . | Real-timeRT-PCR . |
|---|---|---|
| Alternative pathway | ||
| C3 | + (700) | + |
| fB | + (900) | + |
| Poperdin | + (500) | + |
| Classical pathway | ||
| C1q | + (500) | + |
| C2 | − | − |
| C4 | − | − |
| Terminal pathway | ||
| C5 | N/A | − |
| C6 | − | − |
| C8 | − | − |
| C9 | − | − |
| Inhibitory regulators | ||
| DAF | − | − |
| MCP | − | − |
| CR1/2 | − | − |
| fH | − | − |
| Crry | + (160) | + |
| CD59 | + (70) | + |
| Receptors | ||
| CR3 | + (380) | + |
| CR4 | + (430) | + |
| C3aR | + (480) | + |
| C5aR | + (50) | + |
BM DCs were prepared from WT C57BL/6 mice. mRNA arrays and real-time RT-quantitative PCR were performed in DCs with 24-hour LPS stimulation. mRNA microarrays were performed in DCs from 2 individual mice, and similar results were obtained. A representative intensity of array signals is shown in brackets.
N/A indicates C5 information not available; +, present; and −, not present.
BM DCs express a range of complement components, regulators, and receptors and C3a is generated locally. BM DCs were prepared from WT C57BL/6 mice. (A) Conventional RT-PCR was performed in DCs with 24-hour LPS stimulation. The typical agarose gels show the PCR products for C1q, C3, fB, properdin, and 18S (internal control, upper gel), and CR3, CR4, C3aR, C5aR, and 18S (lower gel). The 50-bp DNA markers (M) are shown along side the gels. (B) qPCR was performed in DCs with or without 24-hour LPS stimulation. (C) Detection of C3a in the supernatants from different stages (days 4, 6, and 7) of WT DC culture by ELISA. Activated mouse serum (1 of 10 000 dilution) was used as C3a positive control, and the supernatant from C3−/− DC culture at day 7 was used as a negative control. All results are representative of at least 3 independent experiments. Data in panels B and C are means plus or minus SEM (n = 9). Data were analyzed by Student t test. P values are for comparisons between no LPS and LPS treatment (**P < .001; ***P < .0001).
BM DCs express a range of complement components, regulators, and receptors and C3a is generated locally. BM DCs were prepared from WT C57BL/6 mice. (A) Conventional RT-PCR was performed in DCs with 24-hour LPS stimulation. The typical agarose gels show the PCR products for C1q, C3, fB, properdin, and 18S (internal control, upper gel), and CR3, CR4, C3aR, C5aR, and 18S (lower gel). The 50-bp DNA markers (M) are shown along side the gels. (B) qPCR was performed in DCs with or without 24-hour LPS stimulation. (C) Detection of C3a in the supernatants from different stages (days 4, 6, and 7) of WT DC culture by ELISA. Activated mouse serum (1 of 10 000 dilution) was used as C3a positive control, and the supernatant from C3−/− DC culture at day 7 was used as a negative control. All results are representative of at least 3 independent experiments. Data in panels B and C are means plus or minus SEM (n = 9). Data were analyzed by Student t test. P values are for comparisons between no LPS and LPS treatment (**P < .001; ***P < .0001).
Because some gene expression may be decreased during maturation of DCs, it is possible the negative detected genes may be expressed in early stages of DC cultures. To investigate this, we performed RT-PCR on day 4 and day 6 DCs. We found that mRNAs for DAF, MCP, CR1, fH, C2, C4, C5, C6, C8, and C9 (their mRNAs were not detected in day 7-DCs) were still either negative or very weakly expressed in both day 4 and day 6 DCs. The data for day 6-DCs are shown in Figure S1. We have performed protein assays for several of positively detected genes, in addition to C3, using Western blot or flow cytometry. Our results showed that proteins for C1q, fB, Crry, CR3, CR4, and C5aR were positively detected in DC cultures (Figure S2).
Taken together, our results indicate that murine BM DCs can constitutively express a range of complement components, regulators, and receptors, and the expressions are differentially regulated by LPS stimulation. Complement components and cofactors expressed in DCs mainly involve the alternative pathway of complement activation. Most of complement inhibitory regulators are not expressed in DCs.
To address if activation of C3 occurs locally to generate C3a, we collected the culture supernatants from different stages (days 4, 6, and 7) of WT DC culture and measured C3a using ELISA. C3a was clearly detected in all these culture supernatants but was not detected in the C3−/− DC cultures (Figure 1C). These results indicate that C3a can be generated locally in the DC milieu and suggest the possibility that C3a interacts with C3aR on DCs and, thus, activates DCs.
C3a-C3aR interaction is required for DC activation
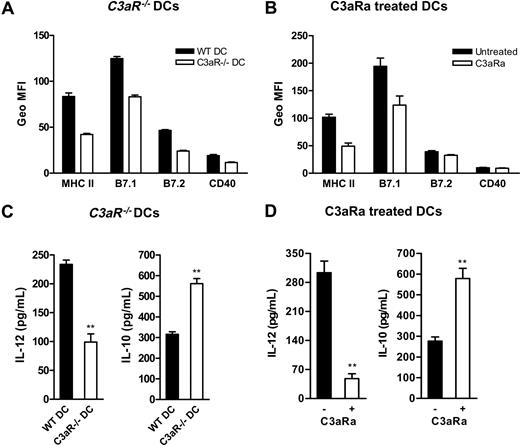
Before determining if C3a-C3aR interaction could modulate DC allostimulatory function, we studied the influence of C3a-C3aR interaction on DC activation. DCs (without LPS stimulation) either prepared from C3aR−/− mice or WT DCs treated with C3aR antagonist (C3aRa) or control DCs (untreated WT DCs) were analyzed for surface expression of major histocompatibility complex (MHC) class II and costimulatory molecules using flow cytometry. We found that C3aR−/− DCs and C3aRa treated WT DCs have reduced surface expression of MHC class II, B7.1, B7.2, and CD40 compared with untreated WT DCs (Figure 2A-B). In addition to surface molecule expression, we also assessed DC cytokine production in response to LPS stimulation. We found that, on stimulation with LPS, C3aR−/− DCs and C3aRa-treated DCs release lower amount of IL-12 (a potent inducer of IFN-γ production leading to the development of Th1 responses) but higher IL-10 (an anti-inflammatory cytokine) than untreated WT DCs (Figure 2C-D). These results suggest that, in the absence of C3a-C3aR interaction, DCs exhibit a less activated and more tolerogenic phenotype.
C3a-C3aR interaction is required for DC activation. (A,B) Flow cytometric analysis of expression of MHC and costimulatory molecules on cultured 7-day BM DCs that include C3aR−/− DCs and WT control DCs (A) and C3aRa treated and untreated WT DCs (B). A representative of 3 independent experiments is shown. (C,D) DC cytokine production in response to LPS stimulation. The purified 6-day BM DCs (106/mL), including C3aR−/− DCs and their WT control DCs (C), and C3aRa treated and untreated WT DCs (D), were further cultured for 24 hours in the presence of LPS. The supernatants were analyzed for IL-12 and IL-10 production using ELISA. Data are means plus or minus SEM (n = 4). Data were analyzed by Student t test (**P < .004). A representative of 5 independent experiments is shown.
C3a-C3aR interaction is required for DC activation. (A,B) Flow cytometric analysis of expression of MHC and costimulatory molecules on cultured 7-day BM DCs that include C3aR−/− DCs and WT control DCs (A) and C3aRa treated and untreated WT DCs (B). A representative of 3 independent experiments is shown. (C,D) DC cytokine production in response to LPS stimulation. The purified 6-day BM DCs (106/mL), including C3aR−/− DCs and their WT control DCs (C), and C3aRa treated and untreated WT DCs (D), were further cultured for 24 hours in the presence of LPS. The supernatants were analyzed for IL-12 and IL-10 production using ELISA. Data are means plus or minus SEM (n = 4). Data were analyzed by Student t test (**P < .004). A representative of 5 independent experiments is shown.
C3aR−/−- or C3aRa-treated DCs elicit reduced allospecific T-cell responses in vitro
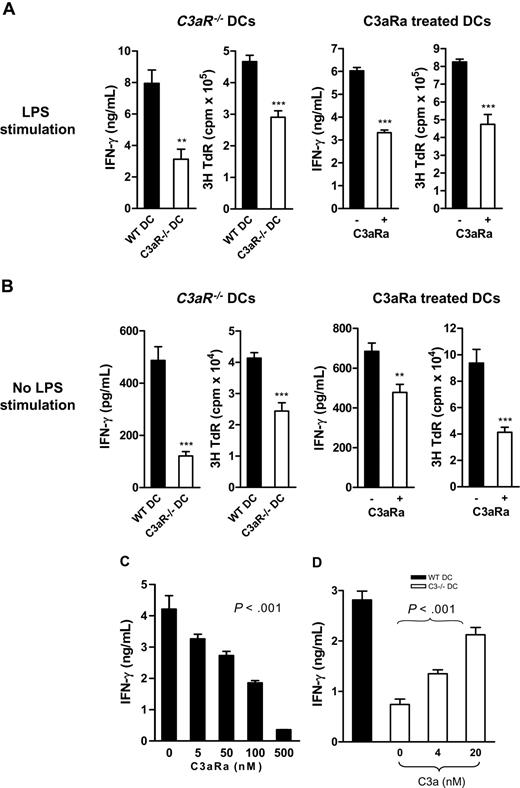
We next determined if C3a-C3aR interaction modulates DC function in alloreactive T-cell stimulation in vitro (allostimulatory function). We prepared DCs with or without LPS stimulation, including C3aR−/− DCs and their control WT DCs, C3aRa-treated, and untreated WT DCs. The irradiated DCs were then used to stimulate naive alloreactive CD4 T cells. T-cell responses were measured by IFN-γ production and thymidine uptake. Under both conditions, with and without LPS stimulation, C3aR−/− DCs and C3aRa-treated DCs elicited a reduction of allospecific T-cell responses, as measured by reduced thymidine uptake and IFN-γ production, compared with untreated WT DCs (Figure 3A-B). In a separate experiment, we examined the dose-response to C3aRa of DC allostimulatory function and found that C3aRa treatment down-regulated DC potency for stimulating alloreactive T cells in a dose-dependent manner at 5 to 500 nM (Figure 3C).
C3aR−/− or C3aRa treated DCs elicit reduced allospecific T-cell responses in vitro. (A-C) Irradiated BM DCs (BALB/c) were cocultured with naive alloreactive CD4 T cells (C57BL/6). T-cell responses were measured by IFN-γ production and/or thymidine uptake. (A) LPS stimulated DCs (ie, C3aR−/− DCs and their WT control DCs, and C3aRa treated and untreated WT DCs). (B) No LPS stimulated DCs (ie, C3aR−/− DCs and their WT control DCs, and C3aRa treated and untreated WT DCs). (A-B) Data are shown as mean plus or minus SEM (n = 4, for ELISA; or n = 6, for thymidine uptake). Data were analyzed by Student t test (**P < .006; ***P < .001). A representative of 4 independent experiments is shown. (C) WT DCs treated with different dose of C3aRa (LPS stimulated for 24 hours). (D) Irradiated C3−/− DCs (C57BL/6) that were treated with or without C3a (LPS stimulated for 24 hours) were cocultured with naive alloreactive CD4 T cells (BALB/c). Untreated WT DCs were included as comparison. (C-D) Data are means plus or minus SEM (n = 4). Data were analyzed by one-way ANOVA. A representative of 2 independent experiments is shown.
C3aR−/− or C3aRa treated DCs elicit reduced allospecific T-cell responses in vitro. (A-C) Irradiated BM DCs (BALB/c) were cocultured with naive alloreactive CD4 T cells (C57BL/6). T-cell responses were measured by IFN-γ production and/or thymidine uptake. (A) LPS stimulated DCs (ie, C3aR−/− DCs and their WT control DCs, and C3aRa treated and untreated WT DCs). (B) No LPS stimulated DCs (ie, C3aR−/− DCs and their WT control DCs, and C3aRa treated and untreated WT DCs). (A-B) Data are shown as mean plus or minus SEM (n = 4, for ELISA; or n = 6, for thymidine uptake). Data were analyzed by Student t test (**P < .006; ***P < .001). A representative of 4 independent experiments is shown. (C) WT DCs treated with different dose of C3aRa (LPS stimulated for 24 hours). (D) Irradiated C3−/− DCs (C57BL/6) that were treated with or without C3a (LPS stimulated for 24 hours) were cocultured with naive alloreactive CD4 T cells (BALB/c). Untreated WT DCs were included as comparison. (C-D) Data are means plus or minus SEM (n = 4). Data were analyzed by one-way ANOVA. A representative of 2 independent experiments is shown.
To confirm the action of C3a on DC allostimulatory function, we used C3a to stimulate C3−/− DCs (which express C3aR but are incapable of generating C3a) and then assessed the allostimulatory function of the treated DCs. Our results showed that addition of C3a increased DC ability to stimulate alloreactive T cells, as measured by IFN-γ production, in a dose-dependent manner (Figure 3D).
C3aR−/−- or C3aRa-treated DCs elicit reduced alloreactive T-cell response in vivo
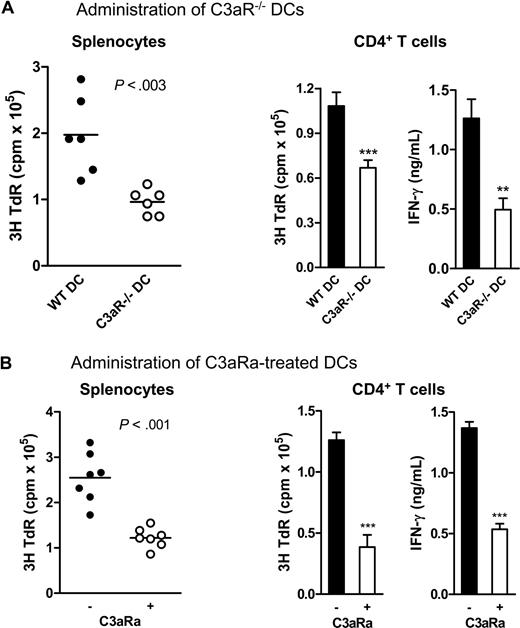
In addition to in vitro studies, we also studied the influence of C3a-C3aR interaction on DCs' capacity to stimulate alloreactive T cells in vivo using a protocol we previously described.13,14 We cultured 7-day DCs (ie, C3aR−/− DCs, C3aRa-treated WT DCs, and untreated WT DCs) and injected the cells intraperitoneally into allogeneic mice. After 10 days, the splenocytes and CD4T cells from these mice were used for measuring the allospecific T-cell reactivity ex vivo by mixed lymphocyte reaction. In this case, when primed with allogeneic DC, the recipient T-cell response in the early phase mainly reflects direct presentation of alloantigen on the donor DC, as opposed the indirect presentation of processed alloantigen by recipient APC.23,24 We found that mice immunized by C3aR−/− or C3aRa-treated DCs exhibited a striking reduction in allospecific T-cell responses, in both splenocytes and CD4 T cell preparations, as measured by reduced thymidine uptake and IFN-γ production, than the mice immunized by untreated WT DCs (Figure 4).
C3aR−/− or C3aRa treated DCs elicit reduced alloreactive T-cell response in vivo. Irradiated donor DCs (BALB/c) were administered to allogeneic recipient mice (C57BL/6) by intraperitoneal injection (6 or 7 mice in each group). After 10 days, splenocytes or CD4 T cells from these mice were restimulated ex vivo with donor splenocytes. Each dot represents a single animal and is shown as mean of 4 to 6 replicate wells of the ex vivo culture. (A) C3aR−/− DCs and WT control DCs. (B) C3aRa treated and untreated WT DCs. Data were analyzed by Student t test (***P < .001; **P < .003). A representative of 3 independent experiments is shown. The horizontal bars in Figure 4A and B are the mean(s) for all data points in the graphs. The points are about 30 in Figure 4A (left panel) and 35 in Figure 4B (left panel). Error bars represent SEM.
C3aR−/− or C3aRa treated DCs elicit reduced alloreactive T-cell response in vivo. Irradiated donor DCs (BALB/c) were administered to allogeneic recipient mice (C57BL/6) by intraperitoneal injection (6 or 7 mice in each group). After 10 days, splenocytes or CD4 T cells from these mice were restimulated ex vivo with donor splenocytes. Each dot represents a single animal and is shown as mean of 4 to 6 replicate wells of the ex vivo culture. (A) C3aR−/− DCs and WT control DCs. (B) C3aRa treated and untreated WT DCs. Data were analyzed by Student t test (***P < .001; **P < .003). A representative of 3 independent experiments is shown. The horizontal bars in Figure 4A and B are the mean(s) for all data points in the graphs. The points are about 30 in Figure 4A (left panel) and 35 in Figure 4B (left panel). Error bars represent SEM.
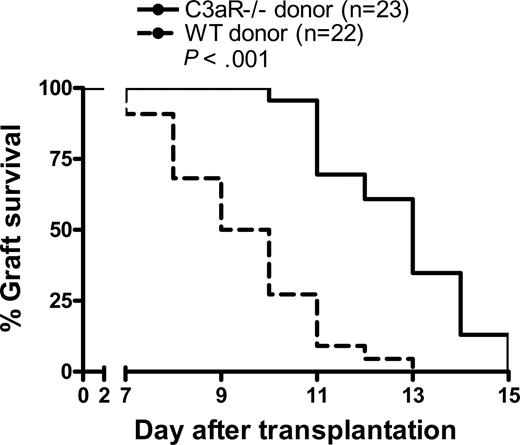
C3aR−/− skin grafts survive longer than WT skin grafts
The allospecific T-cell response is thought to be the major mechanism that causes cellular rejection in transplantation. We have shown that C3a-C3aR interaction has significant impact on the allostimulatory function of donor DCs, which leads to the enhanced allospecific T-cell responses in vitro and ex vivo. The next question we asked was if C3a-C3aR interaction in donor tissue, which contain DCs and other APCs, could have effect on allograft rejection. To investigate this, we transplanted skin grafts from C3aR−/− donor mice or WT control mice into allogeneic WT recipients. We observed that the skin tissues from C3aR−/− mice survived significantly longer than WT skin allografts (median survival, C3aR−/− 13 days vs WT 9.5 days), despite the stringency of fully H-2 disparate skin grafting (Figure 5). Our results indicate that C3aR expression on donor tissues accelerates allograft rejection.
C3aR−/− skin grafts survive for longer than WT skin grafts. Skin tissues from C3aR−/− (n = 23) or WT control mice (n = 22; BALB/c) were transplanted into allogeneic recipient mice (C57BL/6). Data were analyzed by log rank test.
C3aR−/− skin grafts survive for longer than WT skin grafts. Skin tissues from C3aR−/− (n = 23) or WT control mice (n = 22; BALB/c) were transplanted into allogeneic recipient mice (C57BL/6). Data were analyzed by log rank test.
The alternative pathway of complement activation underlies complement-mediated enhancement of DC function in allostimulation
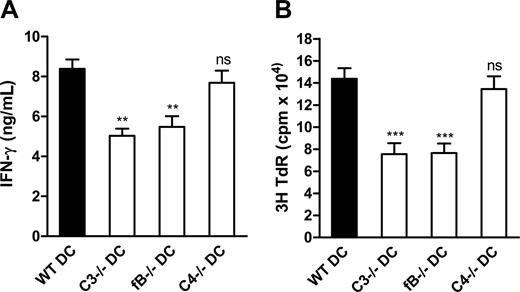
Cleavage of C3 potentially occurs through any of the 3 known activation pathways. Identifying which of these pathways underlies complement-mediated enhancement of DC function in allostimulation may provide clues about the initiating factors that trigger complement activation and may suggest more precise means of intervention without disrupting other functions of complement. Therefore, we studied the influence of DC production of key components (ie, C3, fB, C4) for each pathway on DC allostimulation function in vitro. To study this, we prepared 7-day DCs from C3−/−, C4−/−, and fB−/− mice and WT control mice, and assessed their capacity to stimulate alloreactive T cells in vitro. We found that DCs from C3−/− or fB−/− mice elicited weaker T-cell responses, as measured by reduced thymidine uptake and IFN-γ production, compared with DCs from WT mice; however, DCs from C4−/− mice have no defective function in allostimulation (Figure 6). These findings suggest that, in vitro, the alternative pathway of complement activation is an important trigger of complement-mediated enhancement of DC function in allostimulation.
The alternative pathway of complement activation primarily underlies the complement-dependent enhancement of DC function in allostimulation in vitro. Irradiated BM DCs (LPS stimulated) from C3−/− or fB−/− or C4−/− mice and WT control mice (C57BL/6) were cocultured with naive alloreactive CD4 T cells (BALB/c) in T cell culture medium. (A) IFN-γ production. (B) 3H-thymidine uptake. Data are shown as mean plus or minus SEM (n = 6, for ELISA; or n = 8, for thymidine uptake). Data were analyzed by Student t test (**P < .007; ***P < .001; ns, no significant difference). A representative of 4 independent experiments is shown.
The alternative pathway of complement activation primarily underlies the complement-dependent enhancement of DC function in allostimulation in vitro. Irradiated BM DCs (LPS stimulated) from C3−/− or fB−/− or C4−/− mice and WT control mice (C57BL/6) were cocultured with naive alloreactive CD4 T cells (BALB/c) in T cell culture medium. (A) IFN-γ production. (B) 3H-thymidine uptake. Data are shown as mean plus or minus SEM (n = 6, for ELISA; or n = 8, for thymidine uptake). Data were analyzed by Student t test (**P < .007; ***P < .001; ns, no significant difference). A representative of 4 independent experiments is shown.
Therapeutic manipulation of C3 at cell surface reduces DC activation and allostimulatory function
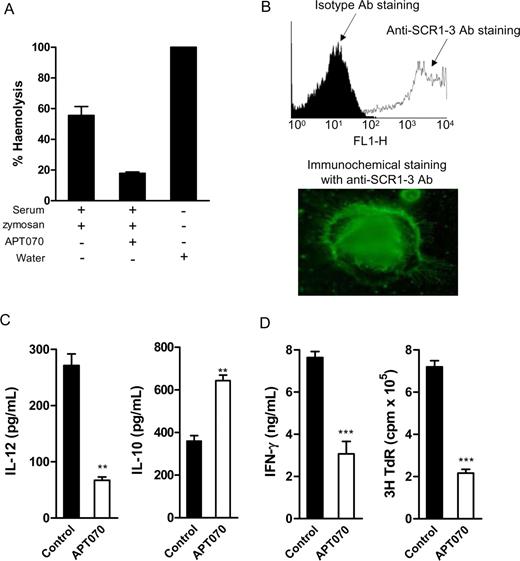
The data presented above imply that complement activation occurring at the surface of APCs enhances the allostimulatory capacity of APCs, leading to a more efficient antidonor T-cell response. We thus proposed that therapeutic over expression of complement regulator would lower the T-cell stimulatory capacity of APCs in favor of T-cell suppression/tolerance induction. To test this possibility, we applied a membrane-localizing complement regulator (APT 070) in our mouse alloimmune model in vitro. APT070 consists of 3 chemically linked parts, including a membrane binding domain (poly-L-lysine, a positively charged peptide), a membrane-inserting domain (mirostyl, a hydrophobic peptide), and the functional portion of human CR1. This feature allows APT070 to effectively bind to cell membrane and inhibit complement activation on the cell surface.
Although therapeutic studies using APT070 have proved effective in several experimental disorders associated with local complement activation in human and rat,25,,–28 this compound has not been extensively studied in the mouse. Therefore, we first assessed if APT070 can inhibit murine complement activation and binding to murine BM DCs. Using a hemolytic assay (Document S1), we showed that APT070 (40 μg/mL) inhibits more than 75% of complement-mediated cell lysis (Figure 7A). Using flow cytometry and immunochemical staining with mouse antihuman SCR1-3 mAb (methods were shown in the supplemental material), we showed that APT070 efficiently binds to DCs (Figure 7B).
Modulation of DC function by C3 inhibitor (APT070). (A) Inhibition of mouse complement activity by APT070 (hemolytic assay). Values are means plus or minus SEM of triplicate determination for each condition. (B) APT070 bound to cultured BM DCs, which was detected by flow cytometry and immunochemical staining with antihuman CR1-3mAb. Slides were viewed with a Leitz DIAPLAN microscope using a PL Fluotar lens (Leitz, Wetzlar, Germany) with 100×/1.32 NA oil immersion and Permafluor mountant medium (Thermo Electron, Cheshire, United Kingdom). Images were acquired using a Nikon DXM1200 digital camera and processed with LUCIA imaging software version 4.71 (Jencons-PLS, Leighton Buzzard, United Kingdom) and Adobe Photoshop version 6.0 (Adobe Systems, San Jose, USA). (C) IL-12 and IL-10 production in response to LPS stimulation in APT070 or control molecule treated DCs (ELISA). Data are shown as means plus or minus SEM (n = 4). Data were analyzed by Student t test (**P < .005). A representative of 4 independent experiments is shown. (D) Allospecific T-cell responses to the stimulation of APT070 treated or control molecule treated DCs. Irradiated DCs (C57BL/6) and naive alloreactive CD4 T cells (BALB/c) were cocultured in T cell culture medium. Data are means plus or minus SEM (n = 4, for ELISA; or n = 6, for thymidine uptake). Data were analyzed by Student t test (***P < .001). A representative of 4 independent experiments is shown.
Modulation of DC function by C3 inhibitor (APT070). (A) Inhibition of mouse complement activity by APT070 (hemolytic assay). Values are means plus or minus SEM of triplicate determination for each condition. (B) APT070 bound to cultured BM DCs, which was detected by flow cytometry and immunochemical staining with antihuman CR1-3mAb. Slides were viewed with a Leitz DIAPLAN microscope using a PL Fluotar lens (Leitz, Wetzlar, Germany) with 100×/1.32 NA oil immersion and Permafluor mountant medium (Thermo Electron, Cheshire, United Kingdom). Images were acquired using a Nikon DXM1200 digital camera and processed with LUCIA imaging software version 4.71 (Jencons-PLS, Leighton Buzzard, United Kingdom) and Adobe Photoshop version 6.0 (Adobe Systems, San Jose, USA). (C) IL-12 and IL-10 production in response to LPS stimulation in APT070 or control molecule treated DCs (ELISA). Data are shown as means plus or minus SEM (n = 4). Data were analyzed by Student t test (**P < .005). A representative of 4 independent experiments is shown. (D) Allospecific T-cell responses to the stimulation of APT070 treated or control molecule treated DCs. Irradiated DCs (C57BL/6) and naive alloreactive CD4 T cells (BALB/c) were cocultured in T cell culture medium. Data are means plus or minus SEM (n = 4, for ELISA; or n = 6, for thymidine uptake). Data were analyzed by Student t test (***P < .001). A representative of 4 independent experiments is shown.
We next determined if APT070 treatment modulates the function of DC and their capacity to stimulate alloreactive T cells. Using the same protocol for C3aRa or C3a treatment, we added APT070 or a control molecule (the membrane-localizing peptide, APT542) into the DC culture medium, from the beginning of BM cell culture and with repeated addition every 2 days. After the treatment, we assessed DC cytokine production in response to LPS stimulation and DC function in alloreactive T-cell stimulation. Compared with control molecule treated DCs, APT070-treated DCs exhibited a tolerogenic cytokine profile with a lower IL-12 and higher IL-10 production (Figure 7C). When cocultured with naive allogeneic CD4 T cells, APT070-treated DC elicited significantly lower T-cell responses measured by IFN-γ production and thymidine uptake (Figure 7D).
Discussion
Our study demonstrates 3 novel aspects of complement function, with regard to its emerging role in immune regulation. First, our results provide evidence that triggering of the receptor for the anaphylatoxin C3a on donor APCs enhances their capacity to stimulate alloreactive T cells. Second, the integration of DC function to produce C3, to cleave off C3a spontaneously and to react directly to C3a, described in our study, provide one possible explanation for the effect of APC-derived C3 on T-cell priming, which we and others have earlier reported.11,13,14,29 Third, our results implicate a role for the alternative pathway of complement activation in triggering the effect of cell-autonomous complement on DC function.
DCs reside in the interstitial space where it seems there is only limited access to complement components from the circulation.22 Therefore, if tissue-resident APCs do not encounter sufficient circulating components at tissue interstitial spaces, local production and activation of complement in the DC environment and expression of relevant complement receptor(s) on DCs are a prerequisite for complement-mediated enhancement of DC function. Our data showed that cultured murine BM DCs with or without LPS stimulation express a range of complement components, regulators, and receptors, indicating that production of complement is an important feature of DCs under the both physiologic and pathologic conditions. Predominant expression of C3, fB and properdin, and relative lack of expression of inhibitory regulators and components that involve the classical pathway and the terminal pathway suggest that DC expression favors the activation of complement through the alternative pathway. Consistent with a predominant role for the alternative pathway, we also observed that DCs (in this study) and macrophages (in our previous study) prepared from mice lacking factor B generated a poor alloreactive CD4 T-cell response.13 In addition, Heeger et al found that splenocytes prepared from mice deficient in factor D (an activator of the alternative pathway) elicited a reduced T-cell response.29 In contrast, C4-deficient DCs (in this study) and macrophages (our previous study) appear to function normally in alloreactive T-cell stimulation, and C4-deficient kidney allografts are rejected normally.13,30 These results suggest that, in vivo, factors capable of triggering the alternative pathway with subsequent cleavage of C3 would be sufficient to enhance APC function in T-cell priming.
The cleavage of C3 leads to the generation of complement effector products, among which C3a and C5a are potent pro-inflammatory mediators and cell activators, exerting their functions through binding to specific receptors with nanomolar affinity.31,32 Engagement of C3aR causing cell activation has been reported in many types of cells (eg, mast cells, macrophages, neutrophils, endothelial cells, and epithelial cells). Recent studies suggest that C3a and C5a have a much stronger influence on inflammatory and immunologic diseases than hitherto known.33,–35 Furthermore, in addition to their known effector functions in the innate response, evidence for a new role of C3a and C5a in the regulation of adaptive immunity has emerged suggesting that C3aR (or C5aR)-ligand interactions could potentially influence APC activation and modulate APC function.36,–38
Our data show that, in the absent of C3a-C3aR interaction, due either to defective expression of C3aR or antagonism of C3a function, DCs exhibit reduced immunogenicity, manifest as a less activated phenotype and lowered ability to stimulate alloreactive T-cell responses in vitro and in vivo. Conversely, stimulation of C3aR with receptor ligand (C3a) increased the DC ability to stimulate allospecific T-cell response. Thus, our data strongly support the C3a-C3aR interaction as a mechanism by which local production and activation of C3 enhances DC function in alloreactive T-cell stimulation.
In addition to C3a, we have considered whether other receptor-mediated (C3b, iC3b, C3dg, C5a) and non–receptor-mediated (C5b-9) mechanisms could modulate DC function. However, the gene expression studies reported here failed to identify some of the relevant receptors (CR1 and CR2) in our DC system, confirming published data in murine macrophages and DCs.39,40 Therefore, it is unlikely that the larger cleavage products, C3b/C3dg, would have a direct effect on DC activation through CR1 and CR2. Although DCs express CR3 and CR4, their main function involves mediating phagocytosis of pathogen and altered self-materials rather than activating cells. Similarly, we have considered the terminal pathway products C5b-9 and C5a; but because these components are not expressed in our system, we focused on C3 and its activated product C3a. Nonetheless, it remains possible that local production of complement by other cells in the DC environment, such as monocytes/macrophages and endothelial and epithelial cells, may generate terminal pathway components, including C5. In addition, C5aR expression on human and mouse DCs suggests that C5a generated at the DC environment could act on DCs and modulate its function. Indeed, our preliminary studies on C5a and C5aR interaction have shown that C5a can be detected in DC culture supernatant; however, compared with WT DCs, nonstimulated C5aR−/− DCs do not have reduced capacity for allostimulation (W.Z., Q.P., K.L., K.A., C.A.F., R.A.G.S., B.L., S.H.S., unpublished data, August 2007). We are currently exploring the role of C5aR after the manipulation of DCs. Our data for C3aR demonstrate that locally generated C3a can provide positive signals to DCs contributing to T-cell priming. However, several studies have shown that complement effector products, such as iC3b and C5a, can also provide negative signals to APCs contributing to the down-regulation of adaptive immune responses.37,41,,–44 It can therefore be speculated that complement activation products, driven by the production of locally produced C3, through receptor-dependent and -independent mechanisms, may have a complex action on APCs. This might depend on the type of complement activation products and type of APCs, consequently up-regulating or suppressing antigen-specific T-cell responses.
It is thought that alloantigen recognition is mainly initiated by T-cell receptor interacting with the endogenous peptide/MHC complex, and T-cell receptor reactivity focuses more on the MHC itself and less on the peptide.45,46 Therefore, the allostimulatory function of APCs may largely depend on expression of MHC molecules. In addition, allostimulatory capacity of APCs is also regulated by expression of costimulatory molecules. In this study, we observed reduced surface expression of MHC class II and several costimulatory molecules (ie, B7.1, B7.2, and CD40) in both C3aR−/− DCs and C3aR antagonist-treated DCs, and these defects were associated with lowered allospecific T-cell stimulation. Thus, our results suggest that C3aR mediated enhancement of DC function in allostimulation could be through modulating DC activation and subsequent expression of MHC class II and costimulatory molecules. In addition, work on conventional antigen recognition has shown that exogenous antigen uptake and presentation (ie, protein, peptide) are dependent on local production of C3 and C3aR signaling.7 Because allostimulation may also involve the process of conventional antigen recognition, the up-regulation of such a process by complement may have impact on DC capacity to stimulate alloreactive T cells.
To study the relevance of our findings in cultured DCs to graft rejection, we have focused on the impact of C3aR signaling on donor cells. Because donor-derived APCs are an important constituent of solid organ transplants47 and play a crucial role in the initiation and maintenance of the allospecific T-cell alloresponse,23 it is possible that local production and activation of C3 generate C3a, which bind to C3aR on donor APCs, causing cell activation and increasing their allostimulatory capability, subsequently eliciting increased T-cell responses against the donor tissue. In support of this, our data showed that donor skin tissues from C3aR−/− mice, when transplanted to WT allogeneic mice, survive significantly longer than WT donor skin tissues. This postulated effect of donor complement on passenger leukocyte migration merits further investigation.
To explore the therapeutic potential, in addition to C3aRa, we applied a membrane-localizing complement regulator (APT 070) in our in vitro model. The results showed a comparable effect of APT070 treatment with lack or therapeutic antagonism of C3a receptor, in terms of the competency of the DC activation profile or its function in T-cell stimulation. One possible explanation is that membrane-localized functional domains of CR1 (complement regulator 1) of APT070 bound to locally generated C3b prevents the formation of C3 convertase and yields inactive C3b (iC3b). As a result of inhibition of complement activation at C3 level on DC surface, APT070 treatment may not only reduce the generation of positive signals to DCs including C3a but may also promote the formation of iC3b, which may provide negative signals to DCs.41,,–44 Unlike systemically administered C3aRa or soluble complement system regulatory molecules, APT070 can be localized and retained in organs by perfusion or administration before transplantation.27,28 Thus, therapeutic overexpression of complement regulator on APC may be useful for lowering the alloreactive T-cell stimulatory capacity of APCs in favor of immune suppression/tolerance in a clinically feasible manner.
In conclusion, the work presented here illuminates a further level of integration between the innate immune system and the adaptive arm of the immune response. The DC appears to express a fully functional set of complement components and receptors, which presumably offer an advantage in the encounter against pathogens and the generation of a potent effector response. However, when expressed alongside alloantigen, the production of complement and downstream generation of C3a appear to contribute to more aggressive rejection, mediated in part by up-regulation of donor antigen and costimulatory molecules on APC and more efficient T-cell stimulation against the donor organs. The involvement of the alternative pathway, as suggested here and in previous work, and subsequently of C3a, offers the possibility of more selective approaches to inhibiting the immune system in allotransplantation.
The online version of this article contains a data supplement.
Presented in abstract form at the American Transplantation Congress, San Francisco, CA, May 6, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Alistair Noble for scientific discussions, Professor M. Carroll for providing the C3 and C4 knockout mice, and Dr R. Wetsel for providing the fB knockout mice.
This work was supported by Medical Research Council of the United Kingdom and the Guy's & St Thomas' Kidney Patients' Association, United Kingdom.
Authorship
Contribution: W.Z. and Q.P. designed the research and wrote the paper; Q.P., K.L., K.A., and C.A.F. performed research and analyzed data. R.A.G.S. contributed APT070 and related reagents and expertise on membrane localization; B.L. provided C3aR-deficient mice and related information; and S.H.S. helped with research design and manuscript discussion.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wuding Zhou, MRC Centre for Transplantation and Department of Nephrology and Transplantation, 5th Floor, Thomas Guy House, Guy's Hospital, London SE1 9RT, United Kingdom; e-mail: wuding.zhou@kcl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal