Treatments for myeloma have expanded in the last decade, but it is not clear if the introduction of novel therapies and the increased use of high-dose therapy have translated into better outcome for patients with myeloma. We examined the outcome of 2 groups of patients seen at a single institution, one from time of diagnosis and the other from the time of relapse, to examine the survival trends over time. Among 387 patients relapsing after stem-cell transplantation, a clear improvement in overall survival from the time of relapse was seen, with those relapsing after 2000 having a median overall survival of 23.9 versus 11.8 months (P < .001) for those who relapsed prior to this date. This improvement was independent of other prognostic factors. Patients treated with one or more of the newer drugs (thalidomide, lenalidomide, bortezomib) had longer survival from relapse (30.9 vs 14.8 months; P < .001). In a larger group of 2981 patients with newly diagnosed myeloma, those diagnosed in the last decade had a 50% improvement in overall survival (44.8 vs 29.9 months; P < .001). In this study, we demonstrate improved outcome of patients with myeloma in recent years, both in the relapsed setting as well as at diagnosis.
Introduction
Multiple myeloma (MM), a neoplasm of plasma cells, affects 1 to 5 per 100 000 individuals each year worldwide with a higher incidence in the West.1 It is the second most common hematologic malignancy in the United States, and it is estimated that there will be 19 900 new diagnoses and 10 790 deaths due to myeloma in 2007.2 The median survival of patients with MM was less than a year before introduction of alkylating agents, and the introduction of melphalan in the 1960s resulted in improved survival.3,4 A timeline of major therapeutic advances in multiple myeloma is outlined in Table 1. In the 1980s, introduction of high-dose chemotherapy and stem-cell rescue (ASCT) was introduced, and randomized trials since have demonstrated a survival advantage for this modality compared with conventional chemotherapy (CCT).5,–7 The introduction of thalidomide represented a major milestone in the treatment of myeloma, and the subsequent availability of its analog lenalidomide and the proteasome inhibitor bortezomib have expanded the therapeutic armamentarium for myeloma.8,,,–12 Incorporation of these novel agents has resulted in a paradigm shift in the treatment of myeloma, with their use earlier in the disease course.13 While the new drugs have allowed successful salvage of relapsed disease, it is not clear if the survival of patients has improved during the last few years. We examined patients seen at our institution over a 36-year period (1971-2006) to determine whether there has been an improvement in survival of myeloma patients seen during this time period.
Major milestones in therapeutic options for myeloma
| . | Milestone . | Notes . |
|---|---|---|
| 1962 | Melphalan-prednisone (MP)4 | Introduction of melphalan in the 1960s was associated with improved survival.4 More intense chemotherapy regimens increased response rates, but with no improvement in survival compared to melphalan and prednisone.30 |
| 1996 | Autologous SCT6,7 | Several randomized trials demonstrated a survival advantage for this modality compared to conventional chemotherapy (CCT).5,–7 However, other trials either have failed to demonstrate an overall survival advantage or have demonstrated equivalent benefit from early or late ASCT.31,,–34 |
| 1999 | Thalidomide (Thalomid)8,9 | Thalidomide has demonstrated improved response rates and progression-free survival rates compared to dexamethasone alone. When added to MP, it improves survival compared to MP alone. |
| 2003 | Bortezomib (Velcade)12,35 | Bortezomib has improved survival compared to high-dose dexamethasone in patients with relapsed myeloma. |
| 2003 | Tandem autologous SCT36 | Tandem SCT has improved survival compared with single transplantation, albeit in those failing to achieve a very good partial response to first transplantation. |
| 2005 | Lenalidomide (Revlimid)10,26 | Lenalidomide and dexamethasone have improved survival compared with dexamethasone in relapsed myeloma in phase III trials. |
| . | Milestone . | Notes . |
|---|---|---|
| 1962 | Melphalan-prednisone (MP)4 | Introduction of melphalan in the 1960s was associated with improved survival.4 More intense chemotherapy regimens increased response rates, but with no improvement in survival compared to melphalan and prednisone.30 |
| 1996 | Autologous SCT6,7 | Several randomized trials demonstrated a survival advantage for this modality compared to conventional chemotherapy (CCT).5,–7 However, other trials either have failed to demonstrate an overall survival advantage or have demonstrated equivalent benefit from early or late ASCT.31,,–34 |
| 1999 | Thalidomide (Thalomid)8,9 | Thalidomide has demonstrated improved response rates and progression-free survival rates compared to dexamethasone alone. When added to MP, it improves survival compared to MP alone. |
| 2003 | Bortezomib (Velcade)12,35 | Bortezomib has improved survival compared to high-dose dexamethasone in patients with relapsed myeloma. |
| 2003 | Tandem autologous SCT36 | Tandem SCT has improved survival compared with single transplantation, albeit in those failing to achieve a very good partial response to first transplantation. |
| 2005 | Lenalidomide (Revlimid)10,26 | Lenalidomide and dexamethasone have improved survival compared with dexamethasone in relapsed myeloma in phase III trials. |
Methods
We examined 2 cohorts of patients seen at Mayo Clinic with a diagnosis of MM. The first cohort consisted of 387 patients who were examined for potential improvement in survival following first relapse after ASCT. These patients were grouped into 2, based on a relapse date of December 31, 2000, which was chosen because of the availability of thalidomide and bortezomib as well as subsequent availability of lenalidomide through clinical trials after this date. Information regarding use of these novel agents (thalidomide, lenalidomide and/or bortezomib) following relapse was obtained from the patient database and examination of medical records. These patients also were studied further by dividing them into several groups at 2-year intervals.
The second group of patients consisted of 2981 patients with newly diagnosed MM seen between January 1971 and December 2006 and was used to examine the trends in overall survival from diagnosis during this time period. These included patients who were seen at the clinic within a month of their diagnosis. They were initially divided into 2 groups, those diagnosed within the last decade (January 1, 1997, to December 31, 2006) and the rest seen on or before December 31, 1996. These patients also were divided by their date of diagnosis into 6 groups at intervals of 6 years each from January 1971 to December 2006. Patients in the first group enabled us to examine the impact of novel agents on the ability to provide effective salvage for patients failing stem-cell transplantation. In contrast, changes in the outcome of patients in the second group would potentially be impacted by improvements in initial therapy as well as availability of salvage therapies. Data regarding these patients were extracted from prospectively maintained databases and review of medical records. Follow-up information on these patients are collected prospectively and entered at the time of each visit. For patients followed up at other institutions, annual follow-up letters are sent to patients to inquire regarding their disease status.
Kaplan-Meier analysis was used for analyzing overall survival, and differences between groups were tested for statistical significance using the 2-tailed log-rank test.14 Survival curves for the larger group were generated with all patients surviving beyond 10 years censored at that time. Multivariate analysis of factors affecting survival was carried out using a Cox proportional hazards model. All patients had consented to the use of their medical records, and the study was conducted in accordance with the institutional guidelines with approval of the Institutional Review Board of the Mayo Clinic and in accordance with the Declaration of Helsinki.
Results
Three-hundred eighty-seven patients who had relapsed after ASCT were included in the study. There were 245 males (63%); median (range) age at transplantation was 57 years (33-75), and the median time to transplantation was 8 months (1-90 months) from diagnosis. The median time to relapse was 13 months (1 month-10 years) from ASCT, and 167 patients (43%) had relapsed within 12 months of transplantation. One-hundred forty-one (36%) were alive at the time of analysis, and the median follow-up for these patients was 47 months (8-145 months) from transplantation and 21 months (0-86 months) from posttransplantation relapse, respectively. Among these, 98 relapses (25%) were prior to December 31, 2000, and the rest after this date. The median overall survival from relapse was 11.8 months (95% CI; 8.7, 14.9) for those relapsing in the early time period compared with 23.9 months (95% CI; 19.8, 27.6) for those relapsing in the later time period. The 2-year estimated survivals were 24% and 49% in the 2 groups, respectively. Patients in the earlier group were less likely to have had an early transplantation (within 12 months of diagnosis), more likely to have had relapsed disease at transplantation, and had more regimens prior to their transplantation than the latter group. However, in a multivariate analysis, the shorter survival following relapse from SCT seen in those relapsing in the earlier time period was independent of the disease status at time of ASCT (in response, primary refractory or in relapse), timing of SCT (early vs late), number of treatment regimens prior to SCT, as well as the response duration to the transplant.
The outcome also was examined over 2-year intervals according to the date of relapse (Figure 1A). A progressive improvement in the overall survival from the time of relapse was observed during the past decade. Among this patient group, 161 (41%) had at some point after their relapse received bortezomib, thalidomide, or lenalidomide. These included 69, 111, and 36 patients, each who had treatment with bortezomib, thalidomide, or lenalidomide, respectively. The median overall survival for the 161 patients was 30.9 months (95% CI; 23.6, 38.2) compared with 14.8 months (95% CI; 11.3, 18.4; P < .001) for patients not treated with the novel agents (Figure 1B). As expected, more patients in the latter years had been exposed to the new drugs as part of salvage treatment regimens. This difference remained significant even when the patients were stratified by the time period of relapse.
Overall survival from time of relapse after ASCT. (A) The Kaplan-Meier curves for overall survival from the time of posttransplantation relapse. The patients are grouped into 2-year intervals based on the date of relapse. (B) The Kaplan-Meier curves for the overall survival from the time of posttransplantation relapse grouped by whether they were treated subsequently with one or more of the newer drugs (thalidomide, bortezomib, or lenalidomide). Survival curves were compared using log-rank test.
Overall survival from time of relapse after ASCT. (A) The Kaplan-Meier curves for overall survival from the time of posttransplantation relapse. The patients are grouped into 2-year intervals based on the date of relapse. (B) The Kaplan-Meier curves for the overall survival from the time of posttransplantation relapse grouped by whether they were treated subsequently with one or more of the newer drugs (thalidomide, bortezomib, or lenalidomide). Survival curves were compared using log-rank test.
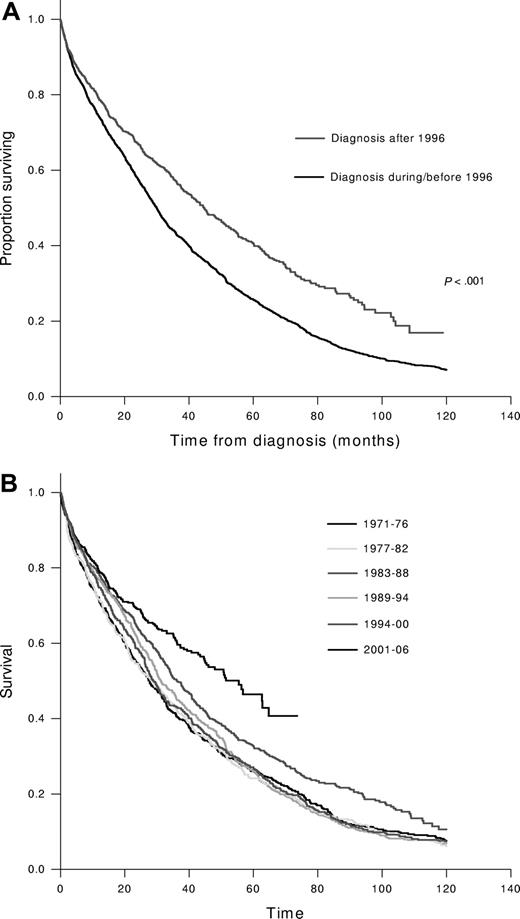
We then examined the outcome of 2981 patients with newly diagnosed MM seen during a 36-year period from January 1971 to December 2006. The median age at diagnosis was 66 years (21-97), and 1770 (59%) were males. The median follow-up for the entire group was 27 months (0-29 years), and at the time of analysis, 558 patients (19%) were alive, with a median follow-up of 33 months (0-29 years). Among these, 1051 (35%) patients were diagnosed during the past 10 years, and the rest were diagnosed between January 1971 and December 1996. The median survival for those diagnosed in the last decade was 44.8 months (95% CI; 39.6, 50) compared with 29.9 months (95% CI; 28.3, 31.6); P < .001 for those diagnosed earlier (Figure 2A). This improved survival was seen in every stage of the International Staging System (ISS) stage with 5-year survival estimates for the later group compared with the earlier group being 52% versus 37%, 42% versus 28%, and 28% versus 14% for ISS stages I, II, and III, respectively (P < .01 for all comparisons). We also divided the patients into 6 groups, each diagnosed over a 6-year interval, from 1971 to 2006. No differences were observed in the median age at diagnosis among the 6 time periods studied. While no change was seen in the median survival during the first four 6-year periods, there was a trend toward improvement during 1995-2000, and a statistically significant improvement in survival was seen during the past 6 years (Figure 2B). We also examined the impact of age and sex on the survival trends. The median overall survival was significantly better for patients 65 or younger at the time of diagnosis (42 months vs 28 months, P < .001). The improvement in survival seen in the last decade among newly diagnosed patients was predominantly among those younger than 65 years (60 months vs 33 months) compared with older patients (32 months vs 26 months). In addition, female patients had slightly better survival from diagnosis (35 months vs 32 months; P = .03), but both sexes had comparable improvement in survival during the past decade.
Overall survival from diagnosis of multiple myelomas. (A) The Kaplan-Meier curves for overall survival from diagnosis. The groups were divided based on the date of diagnosis: within last decade (after December 31, 1996) versus on or before December 31, 1996. (B) The Kaplan-Meier curves for overall survival from the time of diagnosis grouped into 6-year intervals based on the date of diagnosis.
Overall survival from diagnosis of multiple myelomas. (A) The Kaplan-Meier curves for overall survival from diagnosis. The groups were divided based on the date of diagnosis: within last decade (after December 31, 1996) versus on or before December 31, 1996. (B) The Kaplan-Meier curves for overall survival from the time of diagnosis grouped into 6-year intervals based on the date of diagnosis.
Discussion
There has been a paradigm shift in the treatment of MM in the past decade, and while it appears to be incurable with current approaches, considerable progress has been made. While supportive care strategies have improved, this progress is largely due to introduction of ASCT and new therapeutic agents including thalidomide, lenalidomide, and bortezomib. In the setting of MM, the impact of new therapies is likely to be seen earlier in the relapsed setting given that the initial trials are usually carried out in this group. However, the full impact of new drugs may not be appreciated in relapsed setting due to more aggressive disease biology. Introduction of novel therapies early in the course of MM may have an impact beyond that related to disease response as they may improve the natural course of disease. Given this scenario, we examined 2 cohorts of patients, one from diagnosis and the other from the time of relapse after ASCT. We studied patients undergoing ASCT for several reasons. They encompass a group of patients evaluated in a uniform manner, were followed more frequently with time of relapse and criteria for relapse more strictly defined, and were followed at our institution for most of their care and the ascertainment of subsequent therapies (such as exposure to new drugs) more accurate. We believe the data from these 2 groups complement each other, enabling more definite conclusions.
The introduction of thalidomide represented a major advance in the management of MM, with the initial trials demonstrating responses in nearly one-third of patients with relapsed, refractory disease.15 Since then several studies of thalidomide and other novel agents have provided an early estimate of the impact of these therapies, given previous data demonstrating a median survival of 17 months from the time of first relapse of myeloma.16,,,,–21 Phase III randomized trials of bortezomib and lenalidomide have demonstrated a survival benefit in patients with relapsed disease.22,23 In the Assessment of Proteasome Inhibition for Extending Remission (APEX) trial, bortezomib therapy improved 1-year survival from 66% to 80% compared with dexamethasone alone.22 The combination of lenalidomide and dexamethasone was compared with dexamethasone in 2 large randomized trials in the United States and Europe (MM009, MM010), where the combination was superior to dexamethasone in terms of response rates and median time to progression.23,24
In our study we chose to examine the trends in survival after post-ASCT relapse by using a cutoff of December 2000, contemporaneous with the availability of newer agents. The survival for patients relapsing after December 2000 has doubled compared with those relapsing prior to this date. When post-relapse survival was examined over the time period of the study, there is a steady improvement during the past decade. We further explored the potential reasons for the improvements in survival, focusing on the availability of novel agents. While the ascertainment of this data may be incomplete, being dependent on the follow-up, we demonstrate an improvement in survival among patients who had access to one or more of these drugs. While improvements in supportive care may have contributed to these findings, we believe that introduction of novel drugs played a major role.
Our analysis of the survival trends among patients with newly diagnosed MM, in a large of cohort of patients, demonstrates a significant improvement in survival during the last decade compared with the previous 2 decades. The median survival prior to 1997 was nearly 2.5 years, which has improved to nearly 4 years, a 50% improvement, for patients diagnosed in the last decade. When survival was examined during 6-year periods over the last 36 years, it is clear that there was no trend toward improved survival during the first 4 periods (1971-1994). During 1995-2000 there was a trend toward improved survival compared with previous years. This interval coincides with increased use of high dose therapy (HDT) and likely contributed to this change. The survival improvement seen 1995-2000 is smaller when compared with the average survival improvement of 12 months reported in randomized trials, likely reflecting the proportion of patients who are eligible for stem-cell transplantation.6,7 In the final 6-year period (2000-2006) there was a significant improvement in the survival, independent of the ISS stage, which we believe is due to introduction of novel therapies, both at diagnosis and at the time of subsequent relapse.25,26 Decreased mortality with the newer agents in the early period after diagnosis also likely impacted the survival in this last group. Improvements in supportive care, especially the routine use of bisphosphonates, have improved the care of these patients by reducing the incidence of bony complications, which often contribute to morbidity and, indirectly, to the mortality in these patients.27
These findings are consistent with a recent report from Sweden where clear improvements were seen over a 30-year period among patients with myeloma.28 In this study, the 1-year, 5-year, and 10-year survivals were compared over 1973 to 2003, and continuous improvement was seen during this time period, and as seen in our study the maximum improvement was seen in the last period of study (1994-2003). Similar to our findings, the maximum benefit was seen in the younger patients, particularly in those younger than 60 years of age at diagnosis. The findings from these studies confirm the previous observation regarding the prognostic value of age in patients with myeloma. In addition, survival improvement in the older patients are more likely to be tempered by the increased toxicity seen in this group with novel therapies as well as competing causes of death related to other comorbidities common in this age group.
In our group of patients seen between 1997 and 2006, we saw an improvement in survival of a greater magnitude than that reported in the Swedish study in their last calendar period (1994-2003).28 This is likely due to the larger numbers of patients exposed to the newer drugs in our study. The patients in the Swedish study had access to thalidomide toward the end of the 10-year period, compared with our study, which included patients until 2006, where a larger proportion of patients had access to newer drugs including bortezomib and lenalidomide. Another explanation is the younger age of our cohort, given the greater improvement in survival in the younger patients. The authors in the Swedish study concluded that the improved survival was likely the result of increased use of high-dose therapy in this younger patient group and supported this conclusion by demonstrating the increased numbers of SCT reported to the transplant registries.28 It is also likely that improved life expectancy of the population in general over this time period accounts for some of the results. Unlike the Swedish study, the survival reported here has not been adjusted for the expected mortality rates in this population. Population-based studies done using the Surveillance, Epidemiology, and End Results Program (SEER) database of the United States National Cancer Institute also have demonstrated improvement in the survival of myeloma patients in the past decade. In a study of 40 538 patients with myeloma from SEER from the years 1973-2003, the median survival was 24 months.29 As in the current study, survival was better for females and for younger patients. Early treatment decade (1973-1985) was associated with diminished overall and cause-specific survival compared with the most recent time period studied (1996-2003). Compared with the population-based studies, the current study certainly has the disadvantage of being selected for patients referred to a tertiary care hospital with its attendant caveats. In the Swedish study, patients diagnosed at a nonuniversity center had 12% higher mortality, highlighting the bias introduced by the site of care. Comparison to results from randomized trials should be interpreted with caution, as the spectrum of patients in such studies may not represent the general population, since older patients and those with poor performance status often are excluded.
In conclusion, we demonstrate a clear-cut improvement in the outcome of patients with myeloma in the last decade. In our study we believe that HDT and supportive care were responsible for the trends seen during 1994-2000 and this, along with the novel drugs, contributed to the striking improvement seen since 2000. However, improvement in the supportive care of patients with malignancies, especially better access to growth factor support, decreased incidence of fractures and resultant complication due to widespread use of bisphosphonates, and better management of patients with renal failure all would have contributed to the improving survival. It also highlights the need to target the older patient population for innovative approaches in order to improve outcome in these patients. This finding certainly raises the hope that we are moving closer to making MM a chronic disease, if not a curable one.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Colin Colby and Dirk Larsen, MS, for data management.
This work was supported in part by Hematologic Malignancies Program, Mayo Clinic CR20 program, ASCO Young Investigator Award (S.K.), and grants CA93842 and CA10080 from the National Cancer Institute; National Institutes of Health, and the Department of Health and Human Services.
National Institutes of Health
Authorship
Contribution: S.K. was involved in design of concept, data collection, analysis, and writing the paper; A.D., M.Q.L., S.R.H., S.R.Z., F.K.B., D.D., S.J.R., R.A.K., S.V.R., and M.A.G. were involved in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shaji Kumar, Associate Professor of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kumar.shaji@mayo.edu.