Angiogenesis is the main mechanism of vascular remodeling during late development and, after birth, in wound healing. Perturbations of angiogenesis occur in cancer, diabetes, ischemia, and inflammation. While much progress has been made in identifying factors that control angiogenesis, the understanding of the precise molecular mechanisms involved is incomplete. Here we identify a small GTPase, Rap1b, as a positive regulator of angiogenesis. Rap1b-deficient mice had a decreased level of Matrigel plug and neonatal retinal neovascularization, and aortas isolated from Rap1b-deficient animals had a reduced microvessel sprouting response to 2 major physiological regulators of angiogenesis: vascular endothelial growth factor (VEGF) and basic fibroblasts growth factor (bFGF), indicating an intrinsic defect in endothelial cells. Proliferation of retinal endothelial cells in situ and in vitro migration of lung endothelial cells isolated from Rap1b-deficient mice were inhibited. At the molecular level, activation of 2 MAP kinases, p38 MAPK and p42/44 ERK, important regulators of endothelial migration and proliferation, was decreased in Rap1b-deficient endothelial cells in response to VEGF stimulation. These studies provide evidence that Rap1b is required for normal angiogenesis and reveal a novel role of Rap1 in regulation of proangiogenic signaling in endothelial cells.
Introduction
Angiogenesis, sprouting of new capillaries from existing vascular beds, and vasculogenesis, de novo vessel formation via differentiation of endothelial precursor cells, angioblasts, provide 2 mechanisms through which blood vessels form. While vasculogenesis involves differentiation of embryonic progenitor cells and predominates in the early embryonic development, most vascular remodeling in late development and during physiological processes of organ growth and repair occurs via angiogenesis. This complex process is regulated by a controlled balance of proangiogenic and antiangiogenic factors and, when deregulated, contributes to multiple metastatic, ischemic, inflammatory, and immune disorders.1
Vascular endothelial growth factors (VEGFs), in particular VEGF-A, are involved in the regulation of the processes required for angiogenesis: endothelial cell activation, proliferation, migration, and tubule formation.2 The tyrosine kinase receptor VEGFR2 (flk-1/KDR) is the major receptor responsible for the biochemical effects of VEGF-A on cells and is indispensable for normal vascular development.3 Activation of VEGFR2 leads to recruitment and activation of multiple signaling molecules. Among those are 2 MAP kinases: p42/44 ERK1/2 involved in regulation of endothelial proliferation and p38 MAPK, one of the critical modulators of actin cytoskeleton remodeling required for migration.4 Targeted genetic deletion of either p38α or ERK2 is embryonic lethal, as each has been shown to be required for placental development and angiogenesis.5,–7 Signaling from VEGFR2 is bidirectionally regulated by integrins.8,9
Rap1 is a small GTPase that becomes activated downstream from multiple surface receptors via guanine nucleotide exchange factors (GEFs) and regulates several basic cellular functions: adhesion, migration, polarity, differentiation, and growth.10,11 Rap1 has been shown to regulate integrin activation, and specific molecular links between activation of Rap1 and activation of integrins have been proposed.12 Rap1 has also been shown to activate MAP kinase pathway in several cell types.13 Much of the research on Rap1 in endothelial cells has focused on its role in regulation of cadherin-based cell-cell junctions and vascular permeability.14 Several GEFs have been shown to control Rap1 activity in endothelial cells; increased cellular cAMP activates Epac-induced Rap1 activation promoting endothelial cell barrier formation,11,15,16 while activation of C3G and PDZ-GEF downstream from cell-cell junction molecules, such as cadherins and nectins, induces Rap1 activation upon dissociation of junctions.17
Two closely related isoforms of Rap1 exist: Rap1a and Rap1b, and murine genetic models of both have been described. Rap1a−/− mice have a mild defect in leukocyte adhesion, but normal immune function, fertility, and viability.18 While surviving adult Rap1b-deficient mice have a mild platelet aggregation defect and are protected from thrombosis in vivo, the majority of Rap1b-deficient mice suffer from embryonic bleeding resulting in embryonic and perinatal mortality.19 Because of the severity of the embryonic defect and a smaller size of surviving Rap1b-deficient mice as well as the fact that platelets are not required for embryo hemostasis,20,21 we have considered the possibility that the phenotype of Rap1b-deficient mice results from a defect in vascular development. We assessed angiogenesis in vivo in a postnatal retinal model and in an in vitro model to evaluate the contribution of the defective Rap1b-deficient vessels to the angiogenic phenotype. To establish which endothelial functions were affected in Rap1b-deficient cells, we analyzed cellular proliferation in vivo and migration in vitro. Lastly, to begin to characterize the molecular mechanism underlying the functional defects, we analyzed the ability of vascular growth factors to activate MAP kinases in Rap1b−/− endothelial cells.
Methods
Chemicals were from Sigma-Aldrich (St Louis, MO), unless otherwise indicated.
Animals
All mouse procedures were approved by the Institutional Animal Care and Use Committee. Generation of Rap1b−/− mice was described before.19
Antibodies
PECAM-1–specific rat monoclonal antibody (clone MEC13.3) and ICAM-2–specific rat antimouse monoclonal antibody (clone 3C4) were both from BD Biosciences (San Jose, CA); Alexa Fluor 488–conjugated antibromodeoxyuridine mouse monoclonal antibody was from Invitrogen (Carlsbad, CA). Western blotting was performed with rabbit phospho-p38 MAP kinase (Thr180/Tyr182)–specific antibody (Cell Signaling Technology, Danvers, MA), mouse monoclonal phospho-ERK (Tyr-204)–specific antibody, and goat antiactin polyclonal antibody, followed by species-specific secondary antibodies conjugated to HRP (all from Santa Cruz Biotechnologies, Santa Cruz, CA).
Directed in vivo angiogenesis assay
Directed in vivo angiogenesis assay (DIVAA), a commercially available modification of the standard Matrigel plug assay, was performed according to manufacturer's protocol (Trevigen, Gaithersburg, MD). Briefly, sterile surgical silicone capsules (angioreactors) containing Matrigel were supplemented with angiogenic factors as described and placed subcutaneously in anesthetized wild-type and Rap1b−/− animals into the dorsal flank of mice. Three mice of each genotype were used for each condition, and 2 angioreactors were implanted per mouse. After 14 days, angioreactors were recovered, Matrigel was solubilized, and cells that had invaded the Matrigel were released and stained with calcein-AM. Angiogenesis was measured fluorimetrically using Victor Wallac II fluorescence plate reader (PerkinElmer, Waltham, MA). Excitation wavelength was 485 nm, and emission wavelength was 535 nm.
Analysis of retinal neovascularization
Retinal tissue was prepared from 7- and 14-day-old mouse pups. The animals were anaesthetized by intraperitoneal injection of ketamine (20 mg/kg) and xylazine (6 mg/kg). Paraformaldehyde (PFA) was directly perfused (0.5 mL of 0.5% PFA) into the right ventricle, after which the pups were humanely killed. Both eyes were enucleated and whole eyes were fixed in 2% PFA for 1 hour before being washed in PBS. The retinas were dissected and stained with Texas red–conjugated Griffonia simplicifolia (Bandeiraea) isolectin B4 using a described protocol,22 with the following modifications: mounted retinas were blocked in 5% goat serum/PBS for 30 minutes at room temperature immediately prior to isolectin incubation and labeled sections were mounted in Vectashield (Vector Laboratories, Burlingame, CA). Digital images were captured using inverted fluorescence microscope (Nikon, Melville, NY) and a Spot RT Color digital CCD camera (Diagnostic Instruments, Sterling Heights, MI); total retinal and vascular areas were measured using the freeware program ImageTool, Version 3 (University of Texas, San Antonio, TX).
Determination of plasma VEGF levels
Whole blood was collected via cardiac puncture with a 25-G needle from 6- to 8-day-old wild-type and Rap1b−/− mice anesthetized with 2.5% tribromoethanol into a 1-mL syringe preloaded with 24 μL anticoagulant (HEPES-Tyrode buffer with 150 U/mL heparin). Plasma was collected by centrifugation of anticoagulated blood at 2600g for 10 minutes and stored at −20°C. VEGF levels of each plasma sample in duplicate were measured using Bio-Plex Mouse VEGF detection assay and Bio-Plex 200 instrument powered by Luminex xMAP detection technology on RP1 high target value calibration setting according to the manufacturer's protocol (Bio-Rad, Hercules, CA).
Analysis of bromodeoxyuridine incorporation into dividing retinal endothelial cells
Bromodeoxyuridine (BrdU) solution in sterile Dulbecco Modified Eagle Medium (DMEM) was administered to mice at 0.1 mg/g body weight by intraperitoneal injection. Retinas were prepared as described in “Analysis of retinal neovascularization,” and incorporation of BrdU into endothelial cells was visualized with an anti-BrdU antibody, concurrently with isolectin staining. A quarter of each retina was imaged using a Leica TCS SP2 Laser Scanning Confocal microscope (Leica Micro-systems, Bannockburn, IL) and overlapping images of the imaged area were assembled into a composite using Adobe Photoshop (Adobe Systems, San Jose, CA). BrdU-positive cells were counted in approximately one quarter of each analyzed retina.
Aortic ring sprouting assay and characterization of the neovascular outgrowths
Thin prep aortic ring assay was performed as described before23 using thoracic aortas obtained from 2-month-old Rap1b−/− mice or age-matched wild-type controls. Briefly, 1- to 2-mm aortic rings were embedded in growth factor–depleted Matrigel (BD Biosciences) in 12-well plates in the presence of recombinant human bFGF or VEGF-A at a concentration range from 5 to 100 ng/mL (both from R&D Systems, Minneapolis, MN) in high-glucose DMEM (DMEM; Mediatech, Herndon, VA). As a positive control, rings were stimulated with 5% fetal bovine serum (FBS), which induced an abundant sprouting in rings from both genotypes. The plates were incubated at 37°C, with 5% CO2, for 4 to 6 days, and photographed, and vascular sprouts were scored. The ring assays were performed 6 times for each growth factor.
Murine lung endothelial cell preparation, culture, and analysis
Cells were isolated from lungs of 6- to 9-day-old pups by serial immunoselection with Dynabeads M-450 sheep anti–rat IgG beads (Invitrogen) conjugated to purified rat antimouse CD31 antibody (first sort) and antimouse CD102 antibody, as described before.24 Briefly, lungs were harvested from 2 pups, dissected free of visible connective tissue, and rinsed in high-glucose DMEM containing 20% FBS (Atlanta Biologicals, Atlanta, GA). The tissues were finely minced, and digested in 15 mL collagenase I (180 to 200 U/mL; Worthington Biochemical, Lakewood, NJ) or 10 mL collagenase/dispase (0.05/0.4 U/mL; Roche Diagnostics, Indianapolis, IN) solution in PBS at 37°C for 45 minutes to disperse tissue. Cells were plated on T-25 tissue culture flasks coated with human fibronectin (Invitrogen; at 2.5 μg/mL in sterile filtered PBS) and cultured in high-glucose DMEM containing 20% FCS; 100 μg/mL endothelial cell growth supplement (Biomedical Technologies, Stoughton, MA), 100 μg/mL heparin, nonessential amino acids, 2 mM l-glutamine, sodium pyruvate, 25 mM HEPES, and penicillin/streptomycin as antibiotic and cultured at 37°C in a CO2 incubator. Cells were used within 3 passages following the second sort. The purity of the cultures was assessed by flow cytometric analysis of surface expression of PECAM-1 and VEGFR-2, and the endothelial phenotype was confirmed by positive immunofluorescent staining of confluent cultures for the presence of VE-cadherin in cell-cell junctions.
Analysis of Rap1 mRNA expression
For analysis of mRNA expression, total RNA was isolated from cultured wild-type and Rap1b−/− mouse lung endothelial cells using RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. cDNA was synthesized using RETROscript kit (Ambion, Applied Biosystems, Austin, TX) using random decamers as primers. Quantitative real-time polymerase chain reaction (PCR) was performed using TaqMan gene expression assays for mouse Rap1a, Rap1b, and 18SrRNA as internal control and Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA).
Wound-healing migration assay
Cells were counted at seeding and an equal number of cells was plated for each genotype, 9 × 104 cells per well of a 6-well plate. Migration was assayed within 24 to 48 hours of seeding. Confluent cultures of PECAM-1– and ICAM-2–positive cells were scratched with a 20-μL microtip and placed in serum-free DMEM containing the angiogenic factor, as described. The wound was photographed; the cells were cultured for an additional 12 hours and then photographed again. The distance between the edges of the wound was measured. Each experiment was repeated 4 times with cells obtained from 4 different preparations.
Analysis of signaling in lung endothelial cells
Cells seeded at a subconfluent density (80 000 cells per well of a 6-well tissue culture or 250 000 cells per 60-mm plate) were cultured overnight. To obtain quiescence, cells were placed in serum-free culture medium for 4 to 24 hours and treated with angiogenic cytokines as described. For analysis of Rap1 activity, stimulated cells were lysed in lysis buffer (75 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS in 50 mM Tris-HCl [pH 7.4] with protease and phosphatase inhibitor cocktail), and the GTP-bound fraction of Rap1 was measured using a RalGDS-GST pull-down method as described before.25 An aliquot of each lysate was saved and analyzed for actin content by Western blotting as a measure of protein loading. p38 MAPK and ERK1/2 activation were measured by Western blot analysis using a phospho-p38 MAP kinase and phospho- and actin-specific antibody for protein normalization.
To quantify GTP-bound Rap1, p38, and ERK phosphorylation, intensities of specific bands on Western blots were determined using ImageQuant software analysis (Molecular Dynamics, Sunnyvale, CA) of scanned images. First, normalized values of Rap1 or phospho-specific bands were derived by dividing the value for the phospho-specific signal by the value obtained for actin for the respective condition. Next, fold induction of Rap1-GTP or MAPK phosphorylation was calculated by dividing values normalized for actin content from growth factor–treated samples by values from serum-free control samples. Lastly, the values for fold induction of MAPK phosphorylation of knockout samples were expressed as percentage of wild-type values. Such derived values from individual experiments were averaged and mean fold induction was plotted with SEM as error bars.
Statistical analysis
The hypothesis that Rap1b deficiency leads to a decreased number of BrdU-positive nuclei in the retinas was tested with a one-sided t test. For analysis of neovascularization of DIVAA, values of fluorescence obtained from cells recovered from Matrigel plugs containing 500 ng/mL VEGF or 187.5 ng/mL bFGF and 62.5 ng/mL VEGF were compared between WT and KO mice using a 2-sided t test. P values lower than .05 were considered statistically significant.
Results
In lower organisms and model cellular systems, Rap1 is known to regulate a number of basic phenomena: adhesion, migration, differentiation, and proliferation.26,27 In endothelial cells in vivo these phenomena underlie angiogenesis. We generated Rap1b−/− mice and characterized their platelet phenotype.19 To determine whether angiogenesis, the main mechanism of vascular remodeling during late development and after birth, is affected in Rap1b−/− mice, we used 2 in vivo systems: modification of the Matrigel plug assay and neonatal retinal neovascularization as in vivo models of angiogenesis.
Analysis of neovascularization of implanted Matrigel capsules in vivo
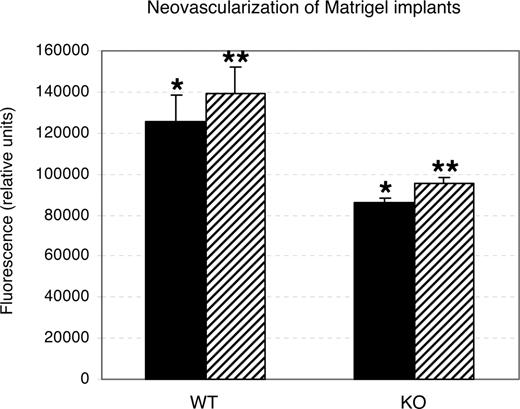
Directed in vivo angiogenesis assay (DIVAA; Trevigen) is a modification of the Matrigel invasion assay that involves subcutaneous implantation of sterile surgical silicone capsules, angioreactors, containing Matrigel supplemented with angiogenic factors. This method has been used to quantitatively assess angiogenesis28,–30 and, compared with standard Matrigel plug assay, may help prevent assay errors due to Matrigel absorption. Angioreactors containing 187.5 ng/mL bFGF + 62.5 ng/mL VEGF or 500 ng/mL VEGF were placed subcutaneously in wild-type and Rap1b−/− animals for 14 days. The number of cells recovered from angioreactors implanted in Rap1b−/− mice was significantly decreased in response to either treatment compared with that in wild-type mice (Figure 1).
Decreased neovascularization of Matrigel implants in Rap1b−/− mice in the directed in vivo angiogenesis assay. Quantitation of calcein-AM fluorescent staining of cells recovered from angioreactors supplemented with 500 ng/mL VEGF (■) or 187.5 ng/mL bFGF and 62.5 ng/mL VEGF (▨) implanted in normal (WT) and Rap1b−/− (KO) mice. Shown are means; error bars are SEM (n = 3). Asterisks indicate the datasets that were compared in a t test for statistical significance. In both instances, there was a statistically significant decrease in neovascularization of Matrigel implants in Rap1b−/− mice (P < .05).
Decreased neovascularization of Matrigel implants in Rap1b−/− mice in the directed in vivo angiogenesis assay. Quantitation of calcein-AM fluorescent staining of cells recovered from angioreactors supplemented with 500 ng/mL VEGF (■) or 187.5 ng/mL bFGF and 62.5 ng/mL VEGF (▨) implanted in normal (WT) and Rap1b−/− (KO) mice. Shown are means; error bars are SEM (n = 3). Asterisks indicate the datasets that were compared in a t test for statistical significance. In both instances, there was a statistically significant decrease in neovascularization of Matrigel implants in Rap1b−/− mice (P < .05).
Analysis of in vivo retinal angiogenesis in rap1b−/− mice
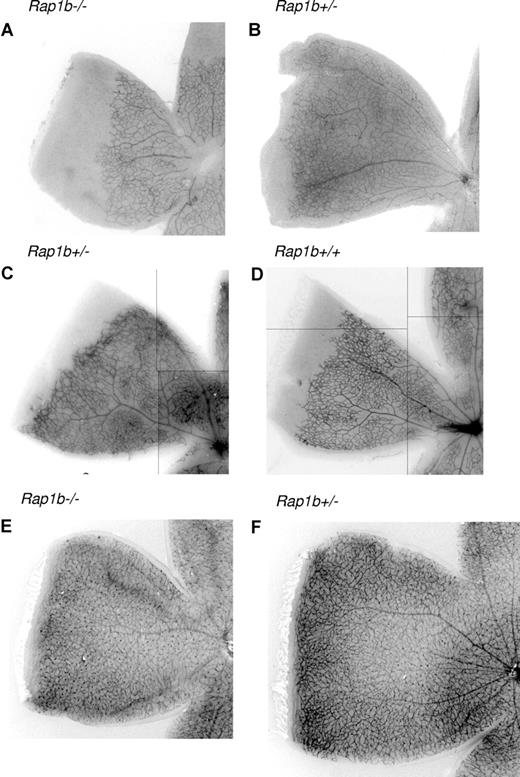
Vascularization of the murine retina commences after birth as the vessels originating at the optic nerve head spread radially over the inner surface of the retina on the pre-existing template of astrocytes, guided by a gradient of VEGF-A to form an initially 2-dimensional vascular plexus.31 Measurement of the fluorescently labeled isolectin-stained vascularized area of the fixed retinas provides a quantitative assessment of angiogenesis.32 Such measurements performed in retinas from 7-day-old (P7) Rap1b−/− mice revealed a significant decrease in the vascular area compared with Rap1b+/− littermates (Figure 2A,B; Table 1). There was no significant reduction in the vascularized area of Rap1b+/− mice compared with wild-type littermates (Figure 2C,D; Table 1). Similar analysis performed at P14 revealed full vascularization of the retinas in Rap1b+/− and littermate Rap1b−/− mice (Figure 2E,F), indicating that the reduced vascularization at P7 is a reflection of a developmental delay rather than complete inhibition of vascularization. Microscopic analysis of retinas immunolabeled for glial fibrillary acidic protein, an astrocyte marker, revealed a normal pattern of astrocyte network in Rap1b-deficient retinas (data not shown), suggesting a vascular rather than a glial defect in Rap1b−/−mice.
Delayed neonatal vascularization in P7 Rap1b−/− retinas. Retinas from P7 littermate Rap1b−/− (A) and Rap1b+/− pups (B); littermate Rap1b+/− (C) and Rap1b+/+ (D) pups; and P14 littermate Rap1b−/− (E) and Rap1b+/− pups (F) were isolated, fixed, mounted, and stained with fluorescent isolectin, as described in “Analysis of retinal neovascularization.” Shown is a representative image of a quarter of each retina. Decrease in vascularization of P7 Rap1b−/− retinas is absent at the P14 stage. Lines have been inserted to indicate composite images.
Delayed neonatal vascularization in P7 Rap1b−/− retinas. Retinas from P7 littermate Rap1b−/− (A) and Rap1b+/− pups (B); littermate Rap1b+/− (C) and Rap1b+/+ (D) pups; and P14 littermate Rap1b−/− (E) and Rap1b+/− pups (F) were isolated, fixed, mounted, and stained with fluorescent isolectin, as described in “Analysis of retinal neovascularization.” Shown is a representative image of a quarter of each retina. Decrease in vascularization of P7 Rap1b−/− retinas is absent at the P14 stage. Lines have been inserted to indicate composite images.
Delayed neonatal vascularization in P7 Rap1b−/− retinas
| . | Vascularized area . | Total area . | |||
|---|---|---|---|---|---|
| Rap1b−/− . | Rap1b+/− . | Rap1b+/+ . | Mutant/control . | Mutant/control . | |
| Rap1b+/− vs Rap1b−/− | 57.3 ± 3.4 | 74.3 ± 2.5 | — | 76.7 ± 3.8 | 89.0 ± 9.9 |
| Rap1b+/− vs Rap1b+/+ | — | 73.7 ± 3.0 | 72.3 ± 3.4 | 102.3 ± 2.9 | 100.2 ± 1.1 |
| . | Vascularized area . | Total area . | |||
|---|---|---|---|---|---|
| Rap1b−/− . | Rap1b+/− . | Rap1b+/+ . | Mutant/control . | Mutant/control . | |
| Rap1b+/− vs Rap1b−/− | 57.3 ± 3.4 | 74.3 ± 2.5 | — | 76.7 ± 3.8 | 89.0 ± 9.9 |
| Rap1b+/− vs Rap1b+/+ | — | 73.7 ± 3.0 | 72.3 ± 3.4 | 102.3 ± 2.9 | 100.2 ± 1.1 |
Data are means plus or minus SEM of percentages of vascularized area to total area or total area relative to littermate controls. Quantitation of the vascularized area of the P7 retinas as a percentage of the total retinal area indicates a reduction in vascularized area of retinas from rap1b−/− mice compared with rap1b+/− littermates (top row), but no difference between rap1b+/− and WT littermates (bottom row; n = 5 for either group). There is no significant difference in the total retinal area between mutant and control mice (far right column).
— indicates not applicable.
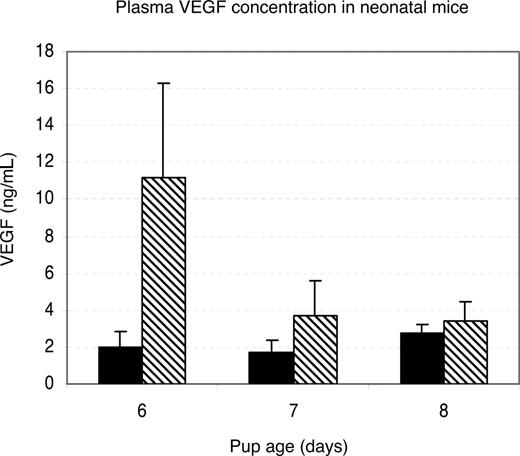
To determine whether lower VEGF concentration could be responsible for the inhibition of neovascularization in Rap1b−/− retinas, we tested VEGF levels in blood plasma collected from 6- to 8-day-old mice using the Bio-Plex Mouse VEGF detection assay. Plasma VEGF was elevated in 6-day-old and normal in 7- and 8-day-old Rap1b−/− mice compared with normal controls (Figure 3). Because Rap1b deficiency leads to an elevated rather than decreased VEGF level, Rap1b is unlikely to regulate angiogenesis through a paracrine mechanism involving up-regulation of VEGF.33
Plasma VEGF levels in neonatal rap1b−/− mice are not decreased. VEGF levels in blood plasma collected from 6- to 8-day-old wild-type (■) and Rap1b−/− mice (▧) were measured fluorescently using the Bio-Plex Mouse VEGF assay. Plotted is the average value; error bars are SEM (n = 3-7 mice per condition, samples assayed in duplicate). There is a significant elevation of VEGF levels in Rap1b−/− mice at day 6 compared with the wild-type mice (P = .09).
Plasma VEGF levels in neonatal rap1b−/− mice are not decreased. VEGF levels in blood plasma collected from 6- to 8-day-old wild-type (■) and Rap1b−/− mice (▧) were measured fluorescently using the Bio-Plex Mouse VEGF assay. Plotted is the average value; error bars are SEM (n = 3-7 mice per condition, samples assayed in duplicate). There is a significant elevation of VEGF levels in Rap1b−/− mice at day 6 compared with the wild-type mice (P = .09).
Decreased in vivo proliferation of Rap1b−/− retinal endothelial cells
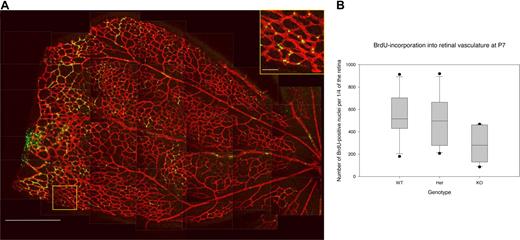
In the developing retina, vessel outgrowth occurs by proliferation of endothelial cells forming the tubes and migration of endothelial tip cells in response to the VEGF gradient released from the underlying astrocytes.31,34 To investigate if Rap1b deficiency affects the proliferation rate of the retinal endothelial cells, we analyzed BrdU incorporation into their nuclei 4 hours after intraperitoneal injection into Rap1b−/−, Rap1b+/−, and normal P7 mice (Figure 4). We found the number of BrdU-positive nuclei significantly lower in Rap1b−/− mice compared with normal mice (284 ± 75 vs 543 ± 101; average ± SEM; P < .05; Figure 4B), while the size of the Rap1b−/− retinas was not significantly different from that of normal retinas (Table 1), indicating a decreased level of endothelial cell proliferation in Rap1b−/− retinas.
Decreased BrdU incorporation into endothelial cells in Rap1b−/−retinas. (A) Low-magnification image (left micrograph) is a composite of confocal images comprising a quarter of the retina isolated from a P7 Rap1b+/− mouse, which had been injected intraperitoneally with BrdU and stained with Texas red–conjugated isolectin (endothelium, red) and FITC-conjugated anti-BrdU antibody (nuclei, green). Bar represents 500 μm. Boxed area is enlarged (insert) to better show the detail of nuclear staining by BrdU. Bar represents 50 μm. Lines have been inserted to indicate sections of the composite image. (B) Quantification of all BrdU-positive nuclei in one quarter of a retina from each analyzed pup. Plotted are data from Rap1b+/+ (WT), Rap1b+/− (Het), and Rap1b−/− (KO) mice. For each dataset, the box shows 25th to 75th percentile range and median. Whiskers extend to the 10th and 90th percentile, and standard error of the mean is plotted. The number of dividing endothelial cells was significantly reduced in Rap1b−/− versus normal retinas (284 ± 75 vs 543 ± 101; n = 6; P < .05).
Decreased BrdU incorporation into endothelial cells in Rap1b−/−retinas. (A) Low-magnification image (left micrograph) is a composite of confocal images comprising a quarter of the retina isolated from a P7 Rap1b+/− mouse, which had been injected intraperitoneally with BrdU and stained with Texas red–conjugated isolectin (endothelium, red) and FITC-conjugated anti-BrdU antibody (nuclei, green). Bar represents 500 μm. Boxed area is enlarged (insert) to better show the detail of nuclear staining by BrdU. Bar represents 50 μm. Lines have been inserted to indicate sections of the composite image. (B) Quantification of all BrdU-positive nuclei in one quarter of a retina from each analyzed pup. Plotted are data from Rap1b+/+ (WT), Rap1b+/− (Het), and Rap1b−/− (KO) mice. For each dataset, the box shows 25th to 75th percentile range and median. Whiskers extend to the 10th and 90th percentile, and standard error of the mean is plotted. The number of dividing endothelial cells was significantly reduced in Rap1b−/− versus normal retinas (284 ± 75 vs 543 ± 101; n = 6; P < .05).
Decreased ex vivo angiogenesis indicates vascular defect in Rap1b−/− mice
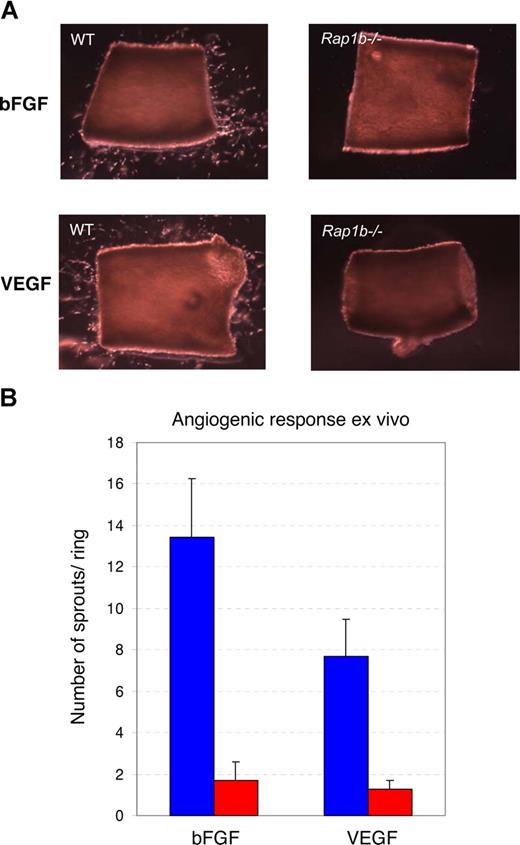
The observed defective angiogenesis in Rap1b−/− retinas could result from an environmental defect other than VEGF expression, such as altered expression of surface receptors on astrocytes providing the template for vascular outgrowth; or it could be a reflection of an intrinsic defect in the retinal endothelial cells leading to a decreased angiogenic response. To test the hypothesis that the angiogenic defect is at least in part due to a defect in vasculature, we examined the ability of VEGF-A, a major physiological regulator of angiogenesis, and of bFGF, another angiogenic factor, to induce microvessel growth from quiescent Rap1b−/− and wild-type aortic explants ex vivo. Wild-type aortas subjected to bFGF and VEGF for 6 days exhibited vessel sprouting, with a maximum number of sprouts occurring at days 4 and 5, after which time the sprouts began to regress (data not shown). At all time points (days 4-6) and all concentrations of growth factor tested, microcapillary outgrowth from Rap1b−/− aortas was reduced compared with that of wild-type aortas and was not overcome by increased concentration of the angiogenic factor or increased length of time in culture (Figure 5 and data not shown). Thus, defective sprouting in the Rap1b−/− aortic cultures is, at least in part, a reflection of the decreased ability of Rap1b−/− vascular cells to become activated by external angiogenic factors. In the absence of exogenous factor, we observed limited sprouting in the wild-type cultures. This spontaneous sprouting, likely due to the release of angiogenic factors from the vessel walls, was reduced in the Rap1b−/− cultures (data not shown). Thus, a paracrine effect may be contributing to the decreased sprouting response in the Rap1b−/− aortas.
Decreased microvessel sprouting from Rap1b−/− aortic rings. (A) Aortic rings prepared from normal (WT) or Rap1b−/− mice were embedded in growth factor–deprived Matrigel and cultured for 4 days in the presence of 50 ng/mL bFGF (top row) or 50 ng/mL VEGF (bottom row). (B) Quantitation of the average number of sprouts per ring from normal (blue bars) or Rap1b−/− (red bars) aortas indicates reduced ex vivo response of Rap1b−/− aortas to both factors. Data from 6 independent experiments; 8 to 14 rings per experiment. Error bars are SEM.
Decreased microvessel sprouting from Rap1b−/− aortic rings. (A) Aortic rings prepared from normal (WT) or Rap1b−/− mice were embedded in growth factor–deprived Matrigel and cultured for 4 days in the presence of 50 ng/mL bFGF (top row) or 50 ng/mL VEGF (bottom row). (B) Quantitation of the average number of sprouts per ring from normal (blue bars) or Rap1b−/− (red bars) aortas indicates reduced ex vivo response of Rap1b−/− aortas to both factors. Data from 6 independent experiments; 8 to 14 rings per experiment. Error bars are SEM.
Decreased migration of Rap1b-deficient lung endothelial cells in vitro
To analyze the effect of Rap1b deficiency on endothelial cell migration, we used primary lung microcapillary endothelial cells isolated from wild-type and Rap1b−/− mice. Fluorescence-activated cell sorting (FACS) analysis of surface expression of PECAM-1 and VEGFR2 revealed homogenous endothelial populations with similar levels of surface receptor expression on wild-type and Rap1b−/− cells, indicating the purity of the endothelial cells (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). The endothelial phenotype was further confirmed by the positive staining for VE-cadherin in adherens junctions of cultured cells (Figure S1B). Because Rap1a is still expected to be present in Rap1b−/− cells, we analyzed total level of Rap1 protein in Rap1b−/− cells to find that it was reduced to 6.4% (± 1.4%) that of wild-type cells (Figure S1C). To assess relative expression levels of the 2 isoforms, we performed quantitative analysis of Rap1a and Rap1b mRNA levels in wild-type and Rap1b−/− cells. We found that Rap1b mRNA was more abundant in wild-type cells than Rap1a mRNA. We did not detect up-regulation of Rap1a mRNA in Rap1b−/− cells.
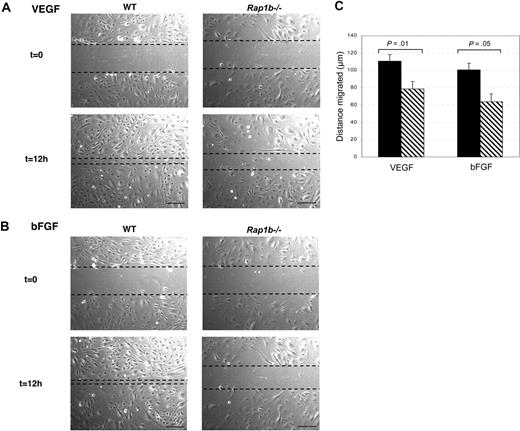
To establish if defective migration might be responsible for the defective angiogenesis in Rap1b−/− mice, we performed a wound healing assay. Confluent monolayer cultures of lung endothelial cells isolated from Rap1b−/− and wild-type mice were wounded with a pipette tip and subjected to VEGF-A (Figure 6A) or bFGF (Figure 6B) stimulation. Cells at the wound edge migrated into the wound, closing it over time. Migration rate expressed as the change in the distance between the edges of the wound was measured over time. Wound healing progress was significantly delayed in Rap1b-deficient cells compared with normal cells in response to either VEGF or bFGF (Figure 6C; P < .05), indicating that Rap1b is required for normal migration in response to angiogenic factor stimulation.
Delayed wound healing by Rap1b-deficient lung endothelial cells. (A) Monolayer cultures of lung endothelial cells isolated from Rap1b−/− and normal (WT) mice were wounded with a pipette tip and subjected to VEGF (A) or bFGF (B) stimulation (t = 0). Cells at the edge of the wound migrated into the wound, closing it over time. After 12 hours, the cells were photographed (t = 12 hours) and migrated distance was measured (C). Bar represents 100 μm. (C) The progress of wound closure, expressed as migrated distance, was significantly delayed in Rap1b−/− cells (▧) compared with wild-type cells (■) in response to either VEGF or bFGF (n = 9, cells isolated from 4 sets of mice).
Delayed wound healing by Rap1b-deficient lung endothelial cells. (A) Monolayer cultures of lung endothelial cells isolated from Rap1b−/− and normal (WT) mice were wounded with a pipette tip and subjected to VEGF (A) or bFGF (B) stimulation (t = 0). Cells at the edge of the wound migrated into the wound, closing it over time. After 12 hours, the cells were photographed (t = 12 hours) and migrated distance was measured (C). Bar represents 100 μm. (C) The progress of wound closure, expressed as migrated distance, was significantly delayed in Rap1b−/− cells (▧) compared with wild-type cells (■) in response to either VEGF or bFGF (n = 9, cells isolated from 4 sets of mice).
Signaling downstream from VEGFR2
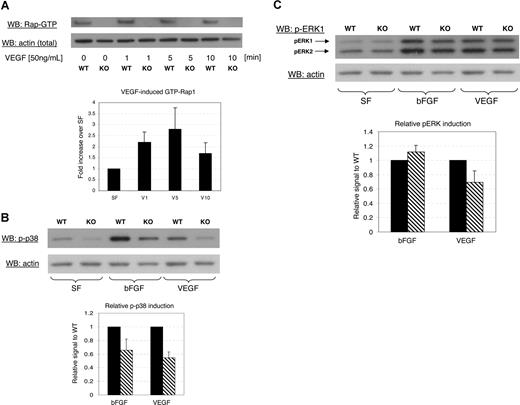
To test if Rap1b is acting downstream from VEGFR2, we assessed Rap1 activation in endothelial cells in response to treatment with 50 ng/mL VEGF-A. We observed a rapid, transient increase in Rap1 activity in wild-type cells with maximum activation at 1 to 5 minutes (Figure 7A). As expected, under these conditions of stimulation, we did not detect Rap1 activity in Rap1b−/− cells (Figure S1C).
Impaired signaling in Rap1b−/− lung endothelial cells. (A) Typical experiment showing time course of Rap1 activation in normal (WT) or Rap1b-deficient (KO) cells in response to treatment with VEGF. GTP-bound Rap1 was pulled down with RalGDS-GST and detected by an anti-Rap1 polyclonal antibody that recognizes both Rap1 isoforms (top blot). The graph depicts quantitation of fold induction of Rap1 activation by VEGF in WT cells in 5 experiments. The values were obtained by normalizing Rap1 signal to actin content in a corresponding lysate sample (bottom blot). Fold induction of Rap1-GTP loading was calculated by dividing values normalized for actin content from VEGF-treated samples by values from nontreated control samples. Fold induction values obtained from 5 experiments were averaged and are expressed as a percentage of nontreated controls; error bars are SEM. (B,C) Decreased MAPK activation in Rap1b-deficient lung endothelial cells. Western blot analysis of the phosphorylation level of p38 MAPK (B) and ERK (C) in normal (WT) or Rap1b-deficient (KO) endothelial cells cultured and serum-starved for 4 hours (SF) or serum-starved and stimulated for 10 minutes with 50 ng/mL of the indicated growth factor (top blots). Shown are representative experiments. The graphs depict quantitation of fold induction of MAPK phosphorylation by the indicated growth factor from 3 separate experiments. To normalize for protein content, the blots were stripped and reprobed with actin-specific antibody (bottom blots). Fold induction of MAPK phosphorylation was calculated by dividing values from growth factor–treated samples normalized for actin content by values from serum-free control samples. The values for fold induction of MAPK phosphorylation of knockout samples (▧) were expressed as percentage of wild-type values (■). Such derived values from individual experiments were averaged and mean fold induction was plotted with SEM as error bars. Bars represent fold induction of MAPK, which was calculated by dividing values from VEGF-treated samples normalized for actin content by values from nontreated control samples.
Impaired signaling in Rap1b−/− lung endothelial cells. (A) Typical experiment showing time course of Rap1 activation in normal (WT) or Rap1b-deficient (KO) cells in response to treatment with VEGF. GTP-bound Rap1 was pulled down with RalGDS-GST and detected by an anti-Rap1 polyclonal antibody that recognizes both Rap1 isoforms (top blot). The graph depicts quantitation of fold induction of Rap1 activation by VEGF in WT cells in 5 experiments. The values were obtained by normalizing Rap1 signal to actin content in a corresponding lysate sample (bottom blot). Fold induction of Rap1-GTP loading was calculated by dividing values normalized for actin content from VEGF-treated samples by values from nontreated control samples. Fold induction values obtained from 5 experiments were averaged and are expressed as a percentage of nontreated controls; error bars are SEM. (B,C) Decreased MAPK activation in Rap1b-deficient lung endothelial cells. Western blot analysis of the phosphorylation level of p38 MAPK (B) and ERK (C) in normal (WT) or Rap1b-deficient (KO) endothelial cells cultured and serum-starved for 4 hours (SF) or serum-starved and stimulated for 10 minutes with 50 ng/mL of the indicated growth factor (top blots). Shown are representative experiments. The graphs depict quantitation of fold induction of MAPK phosphorylation by the indicated growth factor from 3 separate experiments. To normalize for protein content, the blots were stripped and reprobed with actin-specific antibody (bottom blots). Fold induction of MAPK phosphorylation was calculated by dividing values from growth factor–treated samples normalized for actin content by values from serum-free control samples. The values for fold induction of MAPK phosphorylation of knockout samples (▧) were expressed as percentage of wild-type values (■). Such derived values from individual experiments were averaged and mean fold induction was plotted with SEM as error bars. Bars represent fold induction of MAPK, which was calculated by dividing values from VEGF-treated samples normalized for actin content by values from nontreated control samples.
Next, we assessed activation of p38 MAPK, a key regulator of actin cytoskeleton rearrangement required for endothelial cell migration in VEGF-induced angiogenesis.4 We analyzed the ability of both bFGF and VEGF to induce activation-dependent phosphorylation of p38 MAPK and found that treatment of cells with either growth factor induced phosphorylation of p38 MAP kinase, an effect that was significantly decreased in Rap1b−/− cells in response to either factor (Figure 7B). p42/44 ERK is an important regulator of endothelial proliferation. Because we observed a decreased rate of proliferation in the retina (Figure 4), we analyzed the effect of bFGF and VEGF treatment on the activation of p42/44 ERK. We found that activation-specific phosphorylation of p42/44 ERK was decreased in Rap1b−/− cells compared with wild-type cells in response to VEGF, but not bFGF (Figure 7C). This finding is consistent with the decreased proliferation in the retina, where astrocyte-released VEGF is the main angiogenic factor.35
Thus, we conclude that Rap1b deficiency in endothelial cells leads to decreased signaling downstream from VEGF receptor to p38 MAPK and p42/44 ERK. Such decreased signaling via MAPK in endothelial cells may provide at least a partial molecular explanation for the decreased angiogenesis in vivo and ex vivo in Rap1b−/− mice.
Discussion
This study shows that Rap1 is required for normal angiogenesis as Rap1b deficiency leads to decreased neovascularization and endothelial cell proliferation in vivo. Ex vivo, our studies show that the defect is, at least partially, intrinsic in vasculature due to defective endothelial cell activation in response to VEGF-A or bFGF. We identify Rap1b as an important regulator of endothelial cell migration and we show that Rap1b deficiency leads to a decreased ability of either angiogenic factor to activate p38 MAPK, one of the critical biochemical regulators of migration. We also show that activation of p42/44 ERK, a key MAP kinase responsible for proliferative effects of VEGF-A, is decreased in Rap1b−/− cells, a finding consistent with the in vivo findings of decreased Rap1b−/− endothelial cell proliferation in the retina, where VEGF-A is the main angiogenic factor.
Key events involved in angiogenesis are endothelial cell activation, migration, proliferation, and tubule formation. Our studies indicate that Rap1b is involved in the first 3 of those. Both in vivo, in the developing retina, and ex vivo, in aortic rings, Rap1b deficiency leads to decreased vascular proliferation. The lower rate of retinal endothelial cell proliferation is likely to be responsible for the decreased level of vascularization visible at P7, but would still allow for the vessels to spread over the entire retina over time as evidence by fully vascularized primary plexus at P14. The decreased rate of proliferation may still persist at that time, but would be occurring only in deeper levels of the retina, thus not readily amenable to quantitation. The decreased proliferation is not caused by lower levels of VEGF in the plasma, which is actually elevated at day 6 in Rap1b−/− animals. Outside of the retina, where astrocyte-released VEGF is the main angiogenic factor,35 multiple other factors are involved and Rap1b deficiency may affect other aspects of paracrine signaling. One piece of evidence that may support this is the fact that there is lower spontaneous vascular outgrowth from aortic rings in the absence of exogenous growth factor, presumably due to lower release of various angiogenic factors from the vascular wall in Rap1b−/− aortas. Nonetheless, with the addition of exogenous bFGF or VEGF there is an increase in growth in wild-type but not in Rap1b−/− aortas. Previous work has shown that the cells growing out of aortic rings are endothelial cells at the core of the sprout with smooth muscle cells irregularly covering the surface of the sprout.36,37 Reduced vascular sprouting from Rap1b−/− aortas may therefore reflect a defect in both endothelial and smooth muscle cells. While the latter will need to be investigated, endothelial cells form the core of sprouts, and thus the absence of sprouts reflects an endothelial defect. Interestingly, p38 MAPK signaling pathway plays a critical role in mural cell recruitment during neovascularization.38 It will need to be examined if p38 is also decreased in Rap1b−/− smooth muscle cells. In vitro, we find that VEGF-induced activation of ERK is decreased in cells from Rap1b−/− mice. This is consistent with our hypothesis that Rap1b regulates VEGF-induced proliferation by modulating p42/44 ERK activation. In contrast, Rap1b does not appear to be required for bFGF-induced ERK activation. This differential effect is not surprising, as the pathway from VEGF receptor stimulation to ERK activation has been described as atypical, not requiring the standard Grb2-Shc-ras activation upstream from Raf1-MEK-ERK, but rather relying on PKC-dependent activation of Raf1.39 It will be important to determine whether Rap1 is directly involved in regulation of that pathway. Moreover, both the mode and localization of the GEF responsible for activating Rap1 may be critical for the ability of Rap1 to activate downstream signaling and may help explain the differential effects of FGF and VEGF-A on the activation of ERK in Rap1b−/− cells.40 One such site where Rap1 could be interacting with downstream effectors is in the caveolae, where Rap1 localizes.41
Endothelial cell migration is a key step in angiogenesis and a complex process where coordinated signaling from angiogenic cytokine receptors, such as the VEGF receptor, and integrins leads to reorganization of the actin cytoskeleton. Activation of p38 MAP kinase downstream from the VEGF receptor42 is a required step in the process.4 Here, we show the absence of Rap1b in endothelial cells leads to decreased p38 MAPK activation in response to VEGF-A. Rap1 has been previously shown to regulate p38 MAPK in other cellular contexts.43,44 In addition to p38 MAPK activation, Rap1 may regulate other signaling pathways involved in VEGF-induced migration by several mechanisms. Rap1 has a well-documented role in promoting integrin-mediated adhesion in many cell systems,12 including endothelial cells.45 The effect of Rap1 on integrin activation may be direct via one of the several putative effectors of Rap1b described in other cell types,46,–48 or via a cross talk with VEGF downstream signaling49 and alterations in the actin cytoskeleton. Downstream from integrin activation, Rap1 may regulate formation and stability of focal adhesions through its downstream target, the tyrosine kinase Etk/Bmx,50 also shown to affect angiogenesis and arteriogenesis in vivo.51 Rap1 may also be involved in other mechanisms required for directed migration, such as the recruitment of Rac1, promoting both adhesion and lamellipodia formation required for migration.52 Lastly, Rap1 may be regulating migration of endothelial cells by promoting relocalization of its effector capable of interacting with integrins, Nore1b, to the leading edge of migrating cells.52,53
We show that Rap1b becomes rapidly and transiently activated in response to VEGF treatment, but we do not know how this activation occurs. Previous work on Rap1 in endothelial cells has focused on its role in the formation and maintenance of cell-cell junctions14 and indicated that several GEFs are involved in that process. Direct activation of Epac via elevation of cAMP level promotes cell-cell junction formation, while dissociation of cadherin-based junctions leads to activation of Rap1 via PDZ-GEF and C3G and may be a part of a cross-talk mechanism with integrins in epithelial cells.17 C3G, one of the most abundant GEFs expressed in mouse lung endothelial cells (Sribalaji Lakshmikanthan, M.C.-W., unpublished data, June 2007), is a likely candidate for linking VEGFR2 activation and phosphorylation with Rap1 activation. VEGF treatment of human umbilical vein endothelial cells leads to the recruitment of C3G and adapter protein Crk complex to the VEGFR2. Inhibition of Rap1 downstream from C3G in endothelial cells blocks VEGF-induced integrin activation.45 In addition, genetic deletion of C3G leads to an in vivo defect in vascular integrity, hemorrhage, and embryonic lethality of mutant mice, at least in part due to abnormal cell adhesion and migration.54,55 While a recent report has shown that activation of Epac downstream from the A2BAR adenosine receptor leads to activation of ERK1/2 in human umbilical vein endothelial cells, the mechanism in this case did not involve cross talk with the VEGFR2.56 The identity of the activating GEF downstream from the VEGFR2 remains to be established.
Our data show that Rap1b is the predominant Rap1 isoform expressed in endothelial cells, and it is responsible for the majority of Rap1 activity in these cells. This does not exclude the possibility that the existing Rap1a is sufficient for signaling required for blood vessel formation. Alternatively, Rap1b may just be an important positive regulator of angiogenesis, albeit not critically required. Because Rap1a−/−/Rap1b−/− double knockout mice are early embryonic lethal, precluding the analysis of the vessels (M.C.-W., G.C.W., Lawrence Quilliam, unpublished data), a conditional, tissue-specific knockout analysis will have to be used in answering the question of the relative importance of each of the Rap1 isoforms in blood vessel formation.
The in vivo findings from the retinal angiogenesis model, used here as a quantitative model of angiogenesis, may be of a clinical significance in their own right, as deregulated angiogenesis leads to retinopathies, in particular diabetic retinopathy, a leading feature of blinding diseases.57 Further study is required to fully characterize the extent of defects in the Rap1b−/− retinas in order to establish if inhibiting Rap1b activity in the retina could provide a therapeutic strategy in proliferative retinopathies.
In conclusion, these studies show that Rap1b is required for normal angiogenesis and reveal a novel role of Rap1 in regulation of proangiogenic signaling in endothelial cell activation and migration. Thus, Rap1-deficient mice provide an important model to study endothelial cell functions and, in particular, angiogenesis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs M. E. Hartnett and J. McColm and Mr P. Geisen for help with retinal preparation and imaging and Ms L. Botros for technical assistance.
This work was supported by American Heart Association Scientist Development Grant 0235127N (M.C.-W.) and National Institutes of Health grant HL-45100 (G.C.W.).
National Institutes of Health
Authorship
Contribution: M.C.-W. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the paper; A.E.K. performed research, collected and analyzed data, and approved the paper; D.G. performed research, collected and analyzed data, performed statistical analysis, and approved the paper; G.C.W. analyzed data and critically revised the paper; and J.V. performed research, collected and analyzed data, and approved the paper.
Conflict-of-interest disclosure: The authors declare no compet-ing financial interests.
Correspondence: Magdalena Chrzanowska-Wodnicka, Blood Research Institute, BloodCenter of Wisconsin, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: magdalena.wodnicka@bcw.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal