Activation and expansion of T helper (Th) cells followed by regulation of activation are essential to the generation of immune responses while limiting concomitant autoreactivity. In order to characterize T cells reactive towards myeloma-derived monoclonal immunoglobulin (mIg), an autologous coculture assay for single-cell analysis of mIg-responding cells was developed. When cultured with dendritic cells loaded with mIg, CD4+ Th cells from patients with progressing multiple myeloma (MM) showed a proliferative MHC class II–dependent response. CD8+ T-cell reactivity and Th1 activation were consistently low or absent, and Th2 and regulatory cytokines were expressed. The presence of such non-Th1 CD4+ T cells in peripheral blood was independent of treatment status, while the frequencies of responding cells varied between patients and reached the same order of magnitude as those measured for tetanus toxoid–specific Th memory cells. Furthermore, investigations of T-cell subpopulations indicated a possible regulatory role on the mIg responsiveness mediated by suppressive CD25highFOXP3+CD4+ T cells. It is proposed from the present results that a predominant in vivo activation of non-Th1 mIg-reactive CD4+ T cells constitute an Ig-dependent autoregulatory mechanism in human MM, with possible tumor growth supporting or permissive effects.
Introduction
Multiple myeloma (MM), a malignancy of B-lineage origin, is characterized by clonal growth of transformed plasma cells in the bone marrow. The variable regions of the monoclonal immunoglobulin (mIg) are specific for the individual malignant clone, due to the mechanisms of rearrangements and mutations in the corresponding gene.1,2 While the etiology of MM remains largely unknown, interactions involving stromal cells,3 as well as B- and plasma cell differentiation and growth factors such as cytokines released by T-helper type 2 (Th2) cells,4,,–7 are believed to be implicated in the development of MM
Studies of mouse models have provided evidence that T cells are involved in the development of plasma cell tumors.8,9 Whether T cells also play a role in human MM is not yet clear, although a number of T-cell alterations associated with the disease have been described.10,,,,–15 Observations of mIg or “idiotype” reactive Th1 and CD8+ T cells in patients with MM have motivated attempts at immunotherapy based on administrations of mIg in adjuvant-like preparations or loaded in dendritic cells (DCs), mostly with a disappointing outcome.15,16 There is clearly a need for predictive and quantitative individual assessments of pre-existing antigen-specific lymphocytes, in the context of immunotherapy based on administrations of tumor antigens. Such treatment attempts will address possibly long-term antigen experienced and in vivo selected and putatively anergized T cells, a situation immunologically distinct from that encountered when vaccinating with a foreign antigen in order to induce a primary immune response by naive lymphocytes. In addition, patients selected for inclusion in immunotherapy trials have often received prior chemotherapy, which contributes to individual variability in immune status and, thus, to unpredictability of immune responsiveness.
In attempt to identify and describe the population of mIg-reactive lymphocytes in patients with MM, we analyzed responses of T cells upon interaction with autologous antigen-presenting cells consisting of homogenous and functionally synchronized DCs pulsed with the autologous mIg. An obvious prerequisite for reliable determination of autoreactivity is the absence of xeno- or allogeneic antigens in the cultures used. Furthermore, no exogenous growth factors or cytokines were added to the cocultures, and the number of cell selection steps was minimized in attempt to restrain in vitro biased or induced immune responses, and thereby identify an in vivo–selected T-cell population. Short-term cocultures of fresh cells collected repeatedly, together with CFSE labeling in order to quantitate the proliferation of T cells in a mixed cell population, were therefore used. Isolated T-cell populations were further analyzed for autologous regulatory functions.
Methods
Patients and controls
A total of 10 patients diagnosed with MM17 expressing an M-component of the IgG isotype were included (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Five patients were included at the time of diagnosis and peripheral blood samples were collected prior to initiation of therapy. Five of the patients had previously received chemotherapy, one of which remained in complete remission following an autologous stem cell transplantation 4 years prior to the present study. With the latter exception, repeated analyses of M-component concentrations in serum from 9 of the patients included indicated myeloma tumor progression. No laboratory findings or symptoms indicative of infections were identified. White blood cell counts, granulocytes and lymphocytes were within the normal ranges for 9 of the patients at the time of blood collection (Table S1). Healthy age-matched volunteers served as control blood donors. The study was approved by the ethics committee at Karolinska Hospital. Informed consent from all blood donors was obtained in accordance with the Declaration of Helsinki.
Culture and differentiation of DCs
DCs were generated from peripheral blood precursor cells using an adaptation of protocols described previously.18 In brief, peripheral blood mononuclear cells (PBMCs) were isolated from freshly collected heparin-treated blood by density gradient centrifugation. Subsequently, 5 × 106 cells/mL were cultured for adherence for 2 hours, after which nonadherent cells were removed and the adherent cells were cultured in AIM-V medium (Invitrogen, Carlsbad, CA) supplemented with 0.2% autologous serum, 60 ng/mL of human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; Leucomax; Sandoz, Basel, Switzerland) and 50 ng/mL of interleukin 4 (IL-4; Schering-Plough, Kenilworth, NJ). On days 5 and 7 of culture, surface markers on harvested cells characteristic of DCs were analyzed by flow cytometry,18,19 and negativity for other lineage markers (CD3, CD14, CD19, and CD56) was confirmed. Following pulsing of immature DCs with antigen, cells were cultured for an additional 48 hours in the presence of 20 ng/mL of TNF-α (R&D Systems, Minneapolis, MN), and their stage of differentiation was confirmed by analysis of surface marker expression. In vitro matured DCs reached a purity of at least 90%, which was confirmed for each experiment.
Pulsing of DCs with mIg or tetanus toxoid
On the fifth day of culture, immature DCs were incubated for uptake of antigens in serum-free medium. mIgG (M-component) from the sera of patients and polyclonal IgG from healthy donors were purified on a MabTrapG column (Pharmacia, Uppsala, Sweden) as described elsewhere.20 The degree of purity of the mIg fraction was assessed by polyacrylamid gel electrophoresis and the concentration of mIg by nephelometry. The mIg preparations were tested negative for endotoxin. Tetanus toxoid (TT; Statens Serum Institut, Copenhagen, Denmark) was obtained from SBL Vaccine (Stockholm, Sweden). The protocol for loading of immature DCs with antigen was based on pilot experiments in which the uptake of FITC-labeled mIg at varying concentrations, and for distinct periods of incubation, was monitored by flow cytometry and confocal microscopy (Leica; Meyer Instruments, Houston, TX). Different concentrations of TT and mIg (log fold titrations, mIg 1-1000 μg/mL) used to load DCs were also assessed with respect to the kinetics of antigen-presenting functions and T-cell responses in the coculture proliferation assay, and proliferation was also determined by incorporation of thymidine (data not shown). On the basis of these studies, immature DCs were incubated in serum-free RPMI 1640 medium for 2 hours at 37°C with TT or mIg; representative results of 3 and 100 μg/mL of the respective antigen are presented. Subsequently, cells were cultured in serum-free AIM-V medium supplemented with TNF-α (20 ng/mL) for 2 days prior to analysis of DC maturation markers. Flow cytometry was used to verify that mIg did not remain on the cell surface following the pulsing and maturation of DCs.
Coculture of CFSE-labeled PBMCs and autologous DCs
Antigen-pulsed mature DCs were irradiated (25 Gy) and seeded together with carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled (Molecular Probes Europe, Leiden, the Netherlands) PBMCs at a ratio of 1:10 (104 or 105 DCs and 105 or 106 PBMCs) for coculture in AIM-V medium. In order to assess the involvement of MHC class II in antigen presentation, DCs were preincubated with mouse antibodies (5 μg/mL) against human MHC class II (anti–human HLA-DR; BD Pharmingen, San Diego, CA) or with an isotype control, and then washed prior to the coculturing. CFSE-labeled PBMCs from healthy subjects and patients were also cocultured with DCs pulsed with allogeneic mIg purified from patients' sera. Polyclonal IgG purified from sera of 4 healthy blood donors was used for analyses of T-cell responses in cocultures with autologous Ig-pulsed DCs derived from the respective donor. T-cell proliferation in cultures with unpulsed DCs was also analyzed. As a control for activation of T cells, anti-CD3 antibody (1 μg/mL; R&D Systems) was added to cultures of CFSE-labeled PBMCs. When the cell yield was sufficient, the cocultures were restimulated at 1-week intervals by addition of mIg-pulsed mature DCs at a ratio of 1:10 (DC-lymphocyte) in AIM-V medium supplemented with 2% autologous serum and rhIL-2 (20 U/mL; Peprotech, London, United Kingdom). After 1 to 3 such rounds of restimulation, the cells were analyzed for intracellular cytokines and T-cell surface markers by flow cytometry.
Flow cytometry and detection of cytokines
Fluorescence-activated cell sorter (FACS) analysis of cell-surface markers followed standard procedures. In brief, cells were labeled with monoclonal specific antibodies (Ab) or the corresponding isotype control Ab conjugated with FITC, PE, or PerCP (BD Pharmingen; R&D Systems). Supernatants from the various cultures were analyzed for Th1/Th2 cytokines using a cytometric bead array kit (BD Biosciences, San Jose, CA). For detection of intracellular cytokines, harvested cells were incubated with Brefeldin A (Sigma-Aldrich, St Louis, MO) prior to fixation in paraformaldehyde and permeabilization (FACS perm; BD Biosciences), followed by surface staining with fluorochrome-conjugated Ab towards CD4, CD8, and CD3, and intracellular staining with Abs directed against IL-4 and INF-γ. For detection of intracellular FOXP3, fixed and permeabilized cells labeled with Ab toward CD4 and CD25 were incubated with anti-FOXP3 Ab or the respective isotype control Ab (eBioscience, San Diego, CA). Ab-labeled cells and cytometric bead samples were acquired in a FACS Calibur flow cytometer (BD Biosciences) and analyzed by Cell Quest software (BD Biosciences). Concentrations of TGF-β1 were determined by an Elisa procedure according to the manufacturers instructions (OptEIA; BD Biosciences).
T-cell isolation and assessment of CD4+ T-cell inhibititory functions
PBMCs (stored frozen in liquid nitrogen) were stained with Abs towards CD3, CD4, and CD25 or the corresponding isotype controls (BD Biosciences) and subsequently CD25highCD4+ T cells (Tr), CD25−CD4+ T cells, and CD3− cells were isolated using a MoFlo cell sorter (DakoCytomation, Glostrup, Denmark). The threshold of CD25 fluorescence intensity for selection of CD25high Tr was set above the maximum level of fluorescence emitted from CD25-labeled CD8+ T cells (example in Figure 6). Assay of inhibitory functions of the Tr population isolated by cell sorting followed published procedures. In brief, CD25−CD4+ T cells (5-10 × 103 cells/well), and irradiated (25 Gy) CD3− cells (25 × 103 cells/well) in the presence of anti-CD3 Ab, or mIg-pulsed DCs (25 × 103 cells/well), were cultured with different numbers of Tr in 96-well plates. On the fifth day of culture, aliquots of supernatants were collected for assessment of cytokines; [3H]-thymidine was added, and 16 hours later, cells were harvested for determination of thymidine incorporation.
Results
In order to assess T-cell responses towards autologous mIg or alternative antigens, PBMCs collected from the patients with MM were subjected to short-term cultures in 2 steps. First, precursor cells were differentiated in vitro into DCs, which were subsequently “pulsed” with antigen. Second, mature and antigen-loaded DCs were cultured together with freshly collected, CFSE-labeled autologous PBMCs. T-cell proliferation was determined after 7 days of such coculture, and in some cases repeated analyses were performed following restimulation of T cells. The regulatory functions of CD4+ T cells isolated by cell sorting were also examined.
Generation of DCs in vitro
The immature and mature DCs generated in vitro from adherent monocyte precursor cells exhibited phenotypic characteristics typical for their distinct stage of differentiation (“Methods”). After “pulsing” of immature DCs with mIg or alternative antigens on the fifth day of culture, followed by 2 additional days of differentiation in medium supplemented with TNF-α, the mature DCs displayed enhanced expression of MHC classes I and II, CD80, CD83, and CD86; attenuated expression of CD64 and CD32; and no expression of CD14, in agreement with previous reports.18
Proliferation of CD4+ T cells cocultured with autologous mIg-pulsed DCs
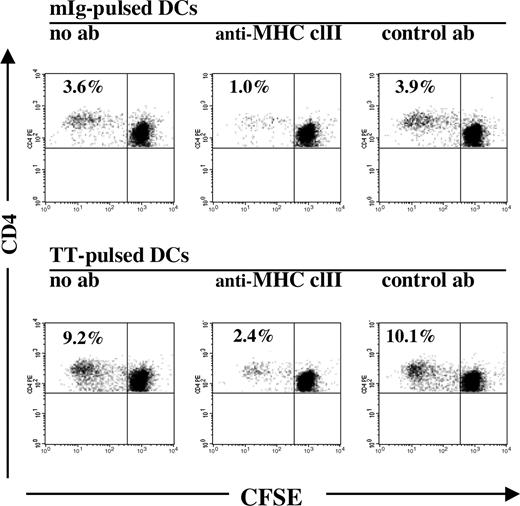
Mature, mIg-pulsed and irradiated DCs were cocultured at a ratio of 1 to 10 with autologous CFSE-labeled PBMCs. Since analyses of PBMCs from 9 of the 10 patients showed T-cell and lymphocyte counts in comparable numbers and within normal ranges at the time of investigation, and additional selection steps of T cells led to some loss of activated cells, we chose to culture PBMCs labeled with CFSE, which allows for a subsequent single-cell analysis of dividing T cells by flow cytometry. Cells were cultured without any addition of exogenous growth factors, cytokines, or serum. The lower limit of detection of T-cell proliferation was 0.1%, that is, reproducible results were obtained after 7 days of coculture if a minimum of 1 of 1000 CFSE-labeled cells had undergone division with a corresponding reduction in the intracellular concentration of CFSE during the culture period. The FACS analysis of CFSE-labeled T cells from patients with MM in progress, cocultured with mIg-pulsed DCs, is exemplified in Figure 1, where the percentage of “CFSE-low” cells thus denotes the cumulative fraction of T cells that had divided during the culture period.
Proliferation of CD4+ T cells cocultured with autologous DCs pulsed with mIg or TT. The percentages of proliferating cells among the total CD4+ T-cell population analyzed after 7 days of coculture are indicated, as deduced from the relative numbers of CD4+ T cells with reduced intracellular concentrations of CFSE (CFSE-low). Middle graphs depict a decrease in proliferation of CD4+ T cells when antigen-pulsed DCs were preincubated with anti–MHC class II antibodies (ab), in comparison with preincubation with isotype control (right graphs) or without antibody (left graphs).
Proliferation of CD4+ T cells cocultured with autologous DCs pulsed with mIg or TT. The percentages of proliferating cells among the total CD4+ T-cell population analyzed after 7 days of coculture are indicated, as deduced from the relative numbers of CD4+ T cells with reduced intracellular concentrations of CFSE (CFSE-low). Middle graphs depict a decrease in proliferation of CD4+ T cells when antigen-pulsed DCs were preincubated with anti–MHC class II antibodies (ab), in comparison with preincubation with isotype control (right graphs) or without antibody (left graphs).
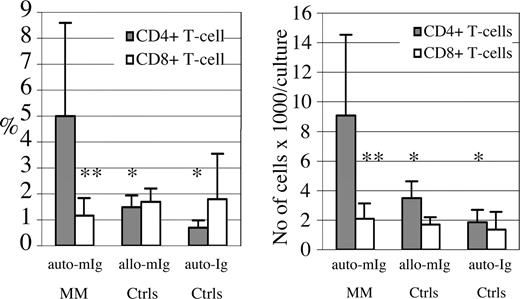
Analyses of T-cell proliferation in such cocultures showed that between 0.7% and 10.9% (with a mean ± SD of 5.0% ± 3.6%) of the CD4+ T cells had divided during the 7 days of coculture with autologous mIg-pulsed DCs (Figures 1,2; Table S1). In contrast, the CD8+ T cells demonstrated a significantly lower degree or absence of proliferation (Figure 2). The dominance of CD4+ versus CD8+ T-cell proliferation was independent of individual variability in the ratio of CD4+ to CD8+ T cells (Table S1), and this pattern was also confirmed by calculating the total numbers of responding CD4+ and CD8+ T cells per culture (Figure 2). In the single patient with MM in complete remission, no significant proliferation of either the CD4+ or CD8+ T-cell population was observed. Controls for Fc-mediated or nonspecific effects included pulsing of DCs derived from healthy blood donors with the mIg preparations from patients with MM, or purified polyclonal IgG for analyses of T-cell proliferation in cultures with Ig-pulsed DCs. A significantly lower or background degree of CD4+ T-cell proliferation was measured after such cultures (Figure 2). An occasional limited proliferation of CD4+ T cells was observed (< 2%) when the mIg preparations were assessed in cultures of nonautologous cells (Figure 2), possibly related to alloreactivity towards allogeneic Ig determinants. The results indicated that the increased responses of CD4+ T cells from patients with MM towards autologous mIg-pulsed DCs were not due to nonspecific effects of Ig, a conclusion which was substantiated by MHC-blocking experiments.
Proliferation of T cells cocultured with Ig-pulsed autologous DCs. Immature DCs were pulsed with autologous mIg from sera of patients with progressing MM (auto-mIg MM; 9 patients). DCs were subsequently subjected to differentiation in the presence of TNF-α for 2 days and irradiated prior to coculture with autologous PBMCs labeled with CFSE at a ratio of 1:10 (DC/PBMC). Cells were harvested after 7 days of culture for FACS analysis of surface T-cell markers and intracellular concentration of CFSE (Figure 1). mIg preparations from patients were also used for pulsing of DCs derived from healthy subjects prior to cultures with CFSE-labeled PBMCs (allo-mIg Ctrls; mean values of 8 different mIgs are shown). Alternatively, autologous polyclonal Ig (auto-Ig Ctrls; 4 control donors) were used for pulsing of DCs derived from the respective healthy subject prior to cultures with autologous CFSE-labeled PBMCs. The mean percentages plus or minus SD (left graph) and mean total cell numbers/culture plus or minus SD (right graph) of CD4+ and CD8+ T cells that had proliferated during culture (CFSE-low) of representative experiments are depicted. Significant differences (**P < .01; *P < .05; t test) versus the CD4+ T-cell response to auto-mIg pulsed DCs of patients with MM (auto-mIg MM) are indicated.
Proliferation of T cells cocultured with Ig-pulsed autologous DCs. Immature DCs were pulsed with autologous mIg from sera of patients with progressing MM (auto-mIg MM; 9 patients). DCs were subsequently subjected to differentiation in the presence of TNF-α for 2 days and irradiated prior to coculture with autologous PBMCs labeled with CFSE at a ratio of 1:10 (DC/PBMC). Cells were harvested after 7 days of culture for FACS analysis of surface T-cell markers and intracellular concentration of CFSE (Figure 1). mIg preparations from patients were also used for pulsing of DCs derived from healthy subjects prior to cultures with CFSE-labeled PBMCs (allo-mIg Ctrls; mean values of 8 different mIgs are shown). Alternatively, autologous polyclonal Ig (auto-Ig Ctrls; 4 control donors) were used for pulsing of DCs derived from the respective healthy subject prior to cultures with autologous CFSE-labeled PBMCs. The mean percentages plus or minus SD (left graph) and mean total cell numbers/culture plus or minus SD (right graph) of CD4+ and CD8+ T cells that had proliferated during culture (CFSE-low) of representative experiments are depicted. Significant differences (**P < .01; *P < .05; t test) versus the CD4+ T-cell response to auto-mIg pulsed DCs of patients with MM (auto-mIg MM) are indicated.
MHC class II restriction of the mIg-associated proliferative response
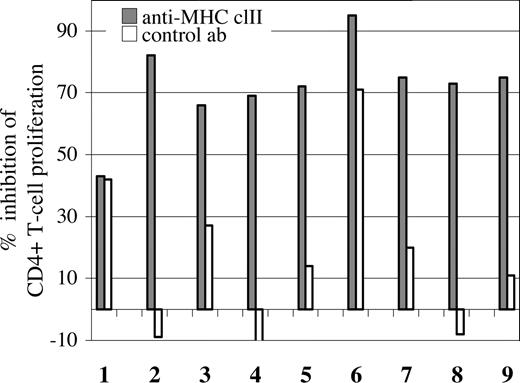
In order to examine the possible involvement of MHC class II in the observed response of CD4+ T cells, mIg-pulsed DCs were preincubated with blocking antibodies directed against MHC class II prior to coculturing, which led to a pronounced (> 65%) decrease in the proliferation of CD4+ T cells from 8 of the9 patients with a proliferative response (Figures 1,3). In one patient, preincubation with the isotype control antibody also interfered with the proliferation of CD4+ T cells, although to a lesser extent than the anti–MHC class II antibodies (71% vs 95% inhibition, respectively; Figure 3; patient no. 6). Together, these results indicate that for at least 7 of 9 patients, MHC class II was involved in antigen presentation to CD4+ T cells specific for mIg-derived peptides.
Decreased CD4+ T-cell proliferation by antibody blocking of MHC class II. mIg-loaded DCs were preincubated with anti–HLA-DR or isotype control antibodies prior to coculturing with CFSE-labeled PBMCs, as in Figure 2. For each patient (n = 9), the percentage inhibition of the CD4+ T-cell proliferation by blocking (▩) or isotype control antibodies (□) are presented (example of FACS analysis in Figure 1). In the case of patient nos. 2, 4, and 8, the control antibody increased the percentage of proliferating cells marginally, resulting in negative values.
Decreased CD4+ T-cell proliferation by antibody blocking of MHC class II. mIg-loaded DCs were preincubated with anti–HLA-DR or isotype control antibodies prior to coculturing with CFSE-labeled PBMCs, as in Figure 2. For each patient (n = 9), the percentage inhibition of the CD4+ T-cell proliferation by blocking (▩) or isotype control antibodies (□) are presented (example of FACS analysis in Figure 1). In the case of patient nos. 2, 4, and 8, the control antibody increased the percentage of proliferating cells marginally, resulting in negative values.
CD4+ and CD8+ T-cell responses to DCs pulsed with TT
TT was used as an alternative antigen in order to evaluate the efficiency of the present coculture model with respect to presentation of exogenous antigens to T cells. This approach might also provide an indication of memory responses of T cells from patients with MM to alternative antigens. Therefore, the proliferation of and cytokine production by T cells from 6 of the patients who had previously been vaccinated with TT as well as from 4 vaccinated healthy volunteer blood donors, in coculture with DCs pulsed with TT were examined.
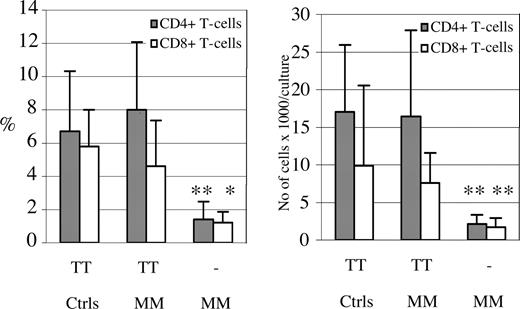
In contrast to what was observed in cultures with DCs pulsed with mIg, both the CD4+ and CD8+ T cells from all 6 patients responded in culture with TT-pulsed DCs with significant increases in proliferation (with mean values of 8.0% and 4.3% and ranges of 1.8%-15.1% and 1.0%-9.4% for CD4+ and CD8+ T cells, respectively), which were comparable with the responses of T cells from healthy vaccinated blood donors (Figure 4). Preincubation of TT-pulsed DCs with anti–MHC class II Ab prior to coculturing inhibited the proliferative response of CD4+ T cells from 5 of the 6 patients by more than 70% (exemplified in Figure 1). There was no correlation found between the degree of CD4+ T-cell responses towards TT and mIg.
CD4+ and CD8+ T-cell proliferation in cultures with autologous DCs pulsed with TT. Immature DCs pulsed with TT or unpulsed (−), were subjected to differentiation in the presence of TNF-α and subsequently irradiated prior to coculture, as in the case of mIg. The proliferative response of T cells from 6 patients with MM (nos. 1, 2, 5-8) and 4 healthy blood donors (Ctrls), all of whom had been vaccinated against TT, were analyzed after 7 days of coculture with TT-pulsed DCs. The mean percentages plus SD (left) and total numbers/culture plus SD (right) of dividing “CFSE-low” CD4+ and CD8+ T cells are depicted. There was no significantly different T-cell response towards TT between the patients with MM and control subjects. Significant differences comparing the CD4+ and CD8+ T-cell proliferation in cultures with TT-pulsed versus unpulsed DCs are indicated (**P < .01; *P < .05; t test).
CD4+ and CD8+ T-cell proliferation in cultures with autologous DCs pulsed with TT. Immature DCs pulsed with TT or unpulsed (−), were subjected to differentiation in the presence of TNF-α and subsequently irradiated prior to coculture, as in the case of mIg. The proliferative response of T cells from 6 patients with MM (nos. 1, 2, 5-8) and 4 healthy blood donors (Ctrls), all of whom had been vaccinated against TT, were analyzed after 7 days of coculture with TT-pulsed DCs. The mean percentages plus SD (left) and total numbers/culture plus SD (right) of dividing “CFSE-low” CD4+ and CD8+ T cells are depicted. There was no significantly different T-cell response towards TT between the patients with MM and control subjects. Significant differences comparing the CD4+ and CD8+ T-cell proliferation in cultures with TT-pulsed versus unpulsed DCs are indicated (**P < .01; *P < .05; t test).
The pattern of Th cytokine production
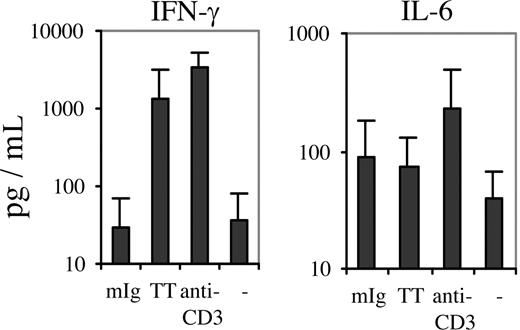
Since analyses of T cells cultured with mIg-pulsed DCs showed a predominant Th cell response, the levels of Th1 and Th2 cytokines in the culture supernatants were determined in order to identify a putative polarity in Th activity. The concentrations of IFN-γ in the supernatants were consistently low or at background values (Figure 5). This finding did not reflect an inability of the patients' T cells to respond to T-cell receptor (TCR) stimulation by producing IFN-γ (Figure 5), since anti-CD3 ligation induced a greater than 100-fold mean increase in IFN-γ production by the same cell population (3280 pg/mL), a response comparable with that of anti-CD3–stimulated T cells from healthy control subjects (2770 pg/mL). Furthermore, the mean concentrations of IFN-γ in cocultures of patients' T cells with autologous DCs loaded with TT were increased (1360 pg/mL; control T cells, 2305 pg/mL).
Concentrations of IFN-γ and IL-6 in cocultures with mIg- or TT-pulsed autologous DCs. Supernatants from cocultures with mIg-, TT- and unpulsed DCs, as well as from anti-CD3–stimulated PBMCs, were collected for analysis of cytokine concentrations by cytometric bead array. IFN-γ (left graph) and IL-6 (right graph) were measured in such cultures for 8 of the patients with a proliferative response towards mIg-pulsed DCs (Figure 2) and 5 of the 6 patients that had received a TT vaccination (Figure 4). The mean concentrations (+SD) are shown (y-axis in log scale).
Concentrations of IFN-γ and IL-6 in cocultures with mIg- or TT-pulsed autologous DCs. Supernatants from cocultures with mIg-, TT- and unpulsed DCs, as well as from anti-CD3–stimulated PBMCs, were collected for analysis of cytokine concentrations by cytometric bead array. IFN-γ (left graph) and IL-6 (right graph) were measured in such cultures for 8 of the patients with a proliferative response towards mIg-pulsed DCs (Figure 2) and 5 of the 6 patients that had received a TT vaccination (Figure 4). The mean concentrations (+SD) are shown (y-axis in log scale).
Preincubation of TT-pulsed DCs with antibodies blocking MHC class II inhibited this increase by approximately 40%, indicating that MHC class II–restricted Th1 cells were participating in the response to TT. The concentrations of IL-6 in cocultures with mIg-pulsed DCs (Figure 5) were comparable with those obtained with TT (control T cells, 149 pg/mL).
The yield of cells obtained from some blood samples allowed additional cytokine analyses. Thus, reanalyses of the culture supernatants and/or detection of intracellular IL-4 and IFN-γ were performed following restimulation of lymphocytes from 4 of the patients by repeated additions of autologous mIg-pulsed DCs at 1-week intervals. The cytokine pattern identified was similar to that of short-term cultures since the level of IFN-γ was consistently low or below the limit of detection. Intracellular IL-4 was detected in 9.7% to 25.6% of the restimulated CD4+ T cells, while the corresponding values for intracellular IFN-γ was 1.3% to 2.8% (data not shown). IL-6 and IL-10 concentrations increased in 2 of the restimulated cell cultures, while IFN-γ remained below detection limit upon restimulation (data not shown). The results were thus consistent with a non-Th1 cytokine pattern upon restimulation with mIg-pulsed DCs. Furthermore, TGF-β was detected in cocultures containing mIg-pulsed DCs.
Regulation of the activation of Th cells
Since the fraction of autoreactive Th cells that responded to autologous mIg varied among the patients (Figure 2; Table S1), and the patterns of cytokines were characteristic of Th2 effector cells and compatible with the presence of regulatory T cells, we examined whether variations in the functions and/or numbers of regulatory T cells correlated to the degree of Th response for each individual patient. For this purpose, Tr were isolated from PBMCs by cell sorting, FOXP3 expression was verified, and sorted cells were cocultured with autologous CD25−CD4+ T cells for assessment of suppressive activity. T cells from 7 healthy donors provided a measure of normal suppressive functions of Tr.
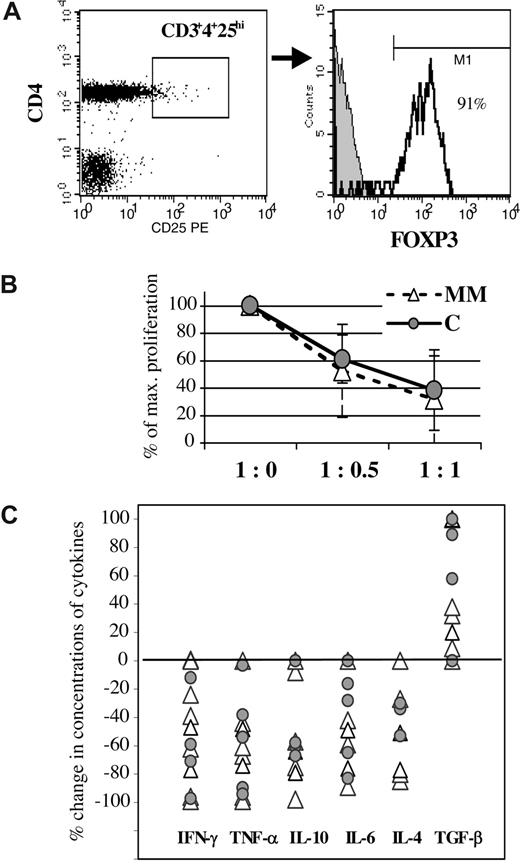
For all the cell samples examined from patients with MM, more than 90% (range, 91%-97%) of the population of CD25highCD4+ T cells stained positively for intracellular expression of FOXP3 (Figure 6A), thus confirming crucial markers of regulatory T cells.21 Moreover, most Tr expressed TGF-β on the cell surface (data not shown). These Tr were functionally active, as reflected in their ability to inhibit proliferation (Figure 6B) and suppress both Th1- and Th2-type cytokines produced by CD25−CD4+ T cells (Figure 6C), with the exception of Tr from a single patient, which exhibited no suppression of cytokine production. In most cases, the Tr-dependent reductions in the levels of Th cytokines were paralleled by an elevation in the concentration of TGF-β (Figure 6C). In summary, the phenotype, inhibitory functions, and a certain individual variability in the degree of suppressive effects demonstrated by Tr isolated from peripheral blood of patients with MM were comparable with those observed from control subjects (Figure 6).
The phenotype and functions of CD25highCD4+ T cells. (A) Analysis of Tr for intracellular expression of FOXP3. The threshold of CD25 fluorescence intensity for the analysis gate of Tr was set above the maximum level of fluorescence emitted from CD25-labeled CD8+ T cells. For all the patient samples, more than 90% of the CD25highCD4+ T cells were FOXP3+ (histogram). Gray-shaded histogram depicts the background fluorescence intensity of the isotype control antibody. (B) Suppression of anti-CD3–induced proliferation of CD25−CD4+CD3+ T cells by different relative numbers of Tr. The 100% value on the y-axis indicates the maximal anti-CD3–induced proliferation (cpm) of CD25−CD4+CD3+ T cells without Tr present (1:0). The mean percentages (± SD) of the maximal response assessed at different ratios of these 2 CD4+ T-cell subpopulations are depicted for patients (MM; n = 9) and control subjects (C; n = 5). The degree of Tr-dependent inhibition of proliferation was not significantly different between the groups. (C) The Tr-mediated suppression of proliferation was accompanied by decreased concentrations of Th cytokines in the culture supernatants and, in most cases, elevated levels of TGF-β. The concentrations of cytokines in cultures with Tr present (1:1) are expressed in percentage of the concentrations obtained in cultures without Tr (1:0), which is plotted for each patient (△) and control subjects ( ) for the respective cytokine. (Occasional increases in the concentration of TGF-β of more than 100% are plotted as 100% for graphics reasons.)
) for the respective cytokine. (Occasional increases in the concentration of TGF-β of more than 100% are plotted as 100% for graphics reasons.)
The phenotype and functions of CD25highCD4+ T cells. (A) Analysis of Tr for intracellular expression of FOXP3. The threshold of CD25 fluorescence intensity for the analysis gate of Tr was set above the maximum level of fluorescence emitted from CD25-labeled CD8+ T cells. For all the patient samples, more than 90% of the CD25highCD4+ T cells were FOXP3+ (histogram). Gray-shaded histogram depicts the background fluorescence intensity of the isotype control antibody. (B) Suppression of anti-CD3–induced proliferation of CD25−CD4+CD3+ T cells by different relative numbers of Tr. The 100% value on the y-axis indicates the maximal anti-CD3–induced proliferation (cpm) of CD25−CD4+CD3+ T cells without Tr present (1:0). The mean percentages (± SD) of the maximal response assessed at different ratios of these 2 CD4+ T-cell subpopulations are depicted for patients (MM; n = 9) and control subjects (C; n = 5). The degree of Tr-dependent inhibition of proliferation was not significantly different between the groups. (C) The Tr-mediated suppression of proliferation was accompanied by decreased concentrations of Th cytokines in the culture supernatants and, in most cases, elevated levels of TGF-β. The concentrations of cytokines in cultures with Tr present (1:1) are expressed in percentage of the concentrations obtained in cultures without Tr (1:0), which is plotted for each patient (△) and control subjects ( ) for the respective cytokine. (Occasional increases in the concentration of TGF-β of more than 100% are plotted as 100% for graphics reasons.)
) for the respective cytokine. (Occasional increases in the concentration of TGF-β of more than 100% are plotted as 100% for graphics reasons.)
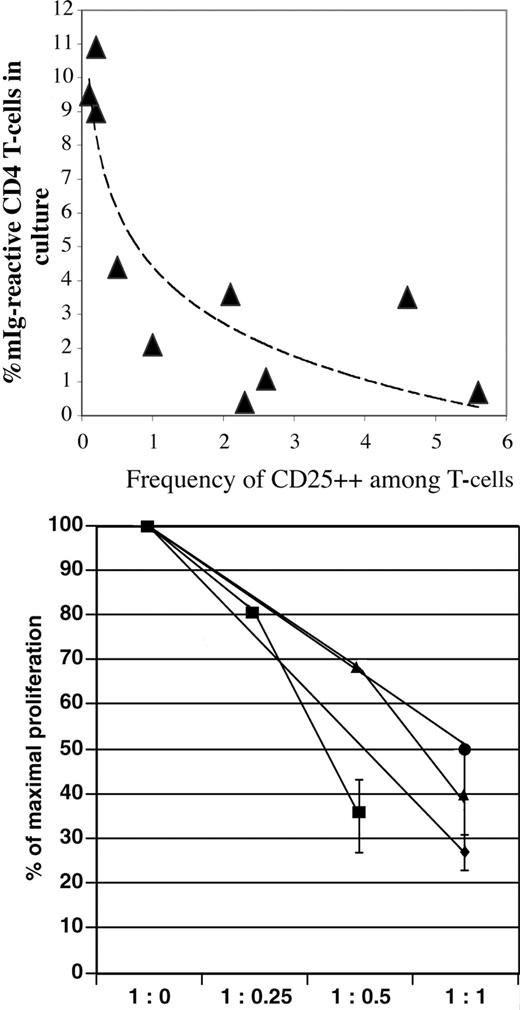
However, the frequency of such functional CD25high Tr within the population of CD4+ T cells or expressed in relationship to the total CD3+ T-cell population (the latter is chosen for data presentation due to the individual variability in the CD4/CD8 ratio) showed a pronounced variability among individual patients with MM. Furthermore, the frequency of functional Tr among peripheral T cells tended to be inversely correlated to the degree of the proliferative response to mIg-pulsed DCs. Thus, patients' T cells that exhibited pronounced proliferative Th responses at day 7 of coculture with mIg-pulsed DCs contained significantly decreased frequencies of Tr (< 1% of CD3+ T cells) as compared with control T-cell populations, and conversely, a relatively lower proliferative response to mIg-pulsed DCs was associated with normal to increased frequency of Tr (Figure 7; top panel).
Inverse correlation of CD25highFOXP3+CD4+ Tr frequency and CD4+ T-cell proliferative response to mIg-pulsed DCs. (Top) Decreased frequencies of CD25highFOXP3+CD4+ Tr among peripheral CD3+ T-lymphocytes (x-axis) correlated with increased proliferative response to mIg-pulsed DCs for the individual MM patient (x- and y-values for patient nos. 1-10 and a trendline are plotted). The mean percentage of dividing CD4+ T cells after 7 days of coculture with mIg-pulsed DCs is shown (y-axis). Corresponding mean Tr frequency of 7 control donors was 2.1% (SD, 0.9%). (Bottom) Suppression of CD4+ T-cell proliferative response to mIg-pulsed DCs during coculture in the presence of autologous Tr. As in Figure 6, the 100% value on the y-axis depicts the proliferation of CD4+ T cells without Tr present (1:0). Proliferation assessed at increasing concentration of Tr (1:0.25, 1:0.5, 1:1) is expressed as a percentage (± SD, triplicate cultures) of maximal response. The results of DC cocultures with sorted CD4+ T-cell populations from 4 patients are shown. Due to limited cell sorting yield of Tr, it was not possible in all experiments to assess proliferation at every ratio indicated (x-axis).
Inverse correlation of CD25highFOXP3+CD4+ Tr frequency and CD4+ T-cell proliferative response to mIg-pulsed DCs. (Top) Decreased frequencies of CD25highFOXP3+CD4+ Tr among peripheral CD3+ T-lymphocytes (x-axis) correlated with increased proliferative response to mIg-pulsed DCs for the individual MM patient (x- and y-values for patient nos. 1-10 and a trendline are plotted). The mean percentage of dividing CD4+ T cells after 7 days of coculture with mIg-pulsed DCs is shown (y-axis). Corresponding mean Tr frequency of 7 control donors was 2.1% (SD, 0.9%). (Bottom) Suppression of CD4+ T-cell proliferative response to mIg-pulsed DCs during coculture in the presence of autologous Tr. As in Figure 6, the 100% value on the y-axis depicts the proliferation of CD4+ T cells without Tr present (1:0). Proliferation assessed at increasing concentration of Tr (1:0.25, 1:0.5, 1:1) is expressed as a percentage (± SD, triplicate cultures) of maximal response. The results of DC cocultures with sorted CD4+ T-cell populations from 4 patients are shown. Due to limited cell sorting yield of Tr, it was not possible in all experiments to assess proliferation at every ratio indicated (x-axis).
Such correlation resembled the observed dependency on the ratio of Tr to responder CD4+ T cells for inhibition of proliferation as shown in Figure 6. To address whether the autologous Tr mediated inhibitory functions could target the mIg-responding Th cells, experiments were repeated for 4 patients with a sufficient cell yield after the sorting procedure. mIg-pulsed DCs were cultured with autologous CD4+ T cells with increasing concentrations of Tr, as described for Figure 6. The presence of autologous Tr decreased the proliferation of responding CD4+ T cells (Figure 7; bottom panel), thus indicating a Tr-dependent regulation of the T-cell response.
Discussion
The present study reveals a predominance of mIg-autoreactive CD4+ Th cells exhibiting a non-Th1 phenotype in the peripheral blood of patients with progressing MM. Analysis of the differential content of CFSE in proliferating T cells, most of which had divided more than 5 times during 7 days of culture with antigen-presenting cells, indicated that the frequency of mIg-responding cells in the pool of peripheral CD4+ T cells ranged between 1/104 and 1/103. Such values are orders of magnitude above frequencies expected to be associated with the response of naive T cells to foreign antigens and thus indicate an in vivo activation and expansion of specific cells, similar to what was observed for the anti-TT responses of T cells from individuals who had been vaccinated against TT. This mIg-related T-cell response also shared several characteristics with the response of memory cells to TT (Figure 1), including the kinetics of cell division, the range of response frequencies, and expression of memory markers (eg, CD45RO) by responding cells.
However, there were also major differences between the autoreactive response to mIg and the anti-TT response, including the degree of involvement of CD8+ T cells and Th1 effector cells, as might be expected given the distinct origin and molecular nature of the antigens. The general predominance of CD4+ over CD8+ T cells in responding to mIg-loaded DCs is consistent with the notions of a preferential MHC class II–dependent pathway for exogenously loaded antigens in antigen-presenting cells, whereas antigens expressed intracellularly would mainly access the MHC class I presentation pathway. The degree of such compartmentalization may vary for different antigens as suggested previously, and exogenous antigens internalized into DCs may also gain access to the MHC class I pathway.22
The subpopulation of peripheral T cells from vaccinated patients and healthy control subjects that responded to TT included CD8+ T cells in addition to CD4+ T cells, an observation compatible with reports on cross-presentation of this antigen.23 Furthermore, both Th1 and Th2 effector T cells were activated towards TT, as indicated by the pattern of the cytokines produced. In contrast, the levels of IFN-γ, the hallmark of Th1 function, in culture supernatants and expressed intracellularly in T cells cultured with mIg-pulsed DCs were consistently low or below detection level. At the same time, one or several B-lineage helper cytokines, including those identified as risk factors in the development of MM,4,,–7 were expressed. The pattern of expression of TNF-α was similar to that observed for IFN-γ (ie, measured at low or background levels; data not shown).
Together, these results indicate that DCs derived from blood precursor cells of patients with MM evoke “composite” T-memory responses to non–self-exogenous antigens, in contrast to the response to mIg. The observed differences are interpreted as reflecting the nature of the antigens and the processing/presentation pathways of antigen-presenting cells, as well as a prior in vivo selection of the responding T-cell population, rather than any major limitation associated with the cell culture model used (including possible antigen-presenting cell dysfunction) or a generally deficient or skewed responsiveness of patients' T cells. The capacity of the CD4+ and CD8+ T-cell populations to respond with production of IFN-γ, as well as with proliferation, was further confirmed by TCR ligation experiments.
Studies of murine models have demonstrated the importance of T cells either promoting the development of plasma cell tumors9 or mediating resistance (eg, by idiotype-specific CD4+ T cells in a transgenic model of myeloma).8 Regulatory implications of idiotype associated Th1 or CD8+ T cells in human MM have also been proposed.15 Our results, obtained by a culture method allowing for the identification of dividing cells on a single-cell level in autologous conditions with limited additions of supplemental growth factors, point to a predominant non-Th1 CD4+ T-cell population reactive with mIg-pulsed DCs. The present findings suggest that in human MM, mIg-associated CD4+ T cells activated in vivo may not be, at least not in a population-predominant fashion, committed to an “antitumor” phenotype, but may include B-lineage–supporting helper cells. Consistently, our previous study of lymphocytes cultured with mIg-pulsed DCs indicated an absence of anti–mIg-, in addition to myeloma cell–associated cytotoxic T cells (CD4+ and CD8+) in peripheral blood of patients with MM.19 Possible growth-promoting effects of mIg-specific Th2 cells on clonal myeloma cells could either be indirect (eg, by providing Th2 cytokines in the circulation and/or in the local tumor environment) or via direct interactions of specific Th cells, the latter of which would be dependent on presentation of endogenous Ig peptides in the context of MHC class II expressed on the myeloma or clonotypic precursor B cells.24,–26 Autologous MHC class II–dependent antigen presentation of peptides derived from endogenous Ig to T cells has been described previously.27,–29 Furthermore, T cells autoreactive against epitopes in the IgVH region have been proposed to regulate the production of the corresponding antibodies30,–32 in addition to mediate tolerance in autoimmune disease to peptides derived from IgG.30,33
Since T cells cultured with DCs disclosed an individually variable expansion of an mIg-reactive subpopulation of Th cells (Figure 2), and the cultured T-cell population included regulatory T cells, we examined whether variations in the functional status and/or frequencies of Tr correlated to the Th response towards mIg for the individual patients. The autologous inhibitory functions of CD4+CD25highFOXP3+ Tr from the peripheral blood of patients were similar to those of control subjects when assessed at defined ratios (Figure 6). The production of TGF-β in cultures with Tr present and the expression of TGF-β on the cell surface of Tr indicate that TGF-β was a potential mediator of suppression.34
However, pronounced variations in the frequency of such functional Tr were observed among patients with MM (Figure 7; top panel), and there was an inverse relationship between the numbers (relative and absolute) of functional CD25highFOXP3+ Tr present in the total T-cell population and responsiveness to mIg-pulsed DCs. Thus, T cells from patients containing a normal to high frequency of Tr exhibited a relatively attenuated proliferative response in culture, consistent with the observed concentration-dependent inhibitory activity demonstrated by Tr (Figure 6), and conversely, the proliferative T-cell responses to mIg-pulsed DCs were enhanced when Tr frequencies were reduced (Figure 7 top panel). Further evidence for such Tr-dependent suppressive functions was obtained from coculture experiments of mIg-pulsed DCs and isolated CD4+ T-cell populations from 4 patients (Figure 7 bottom panel), indicating a Tr-dependent regulation of the mIg-autoreactive T-cell response. No such correlation between the proliferative response to TT and frequency of Tr could be found.
Since the description of CD25+ regulatory T cells by Sakaguchi et al,35 Tr have been detected in association with a variety of human diseases, including cancer. Tr in peripheral blood from patients with MM were recently described as either dysfunctional36 or present in elevated frequencies and active in assays of allogeneic suppression.37 The present results are technically not comparable with those reports, since we used sorted T-cell populations instead of bulk cultures for analyses of T-regulatory functions, which were measured towards autologous and not allogeneic target CD4+ T cells, in order to analyze the regulation of autoreactive CD4+ T cells in association with MM.
Collectively, our results point to (1) a predominance among peripheral T cells of non-Th1 CD4+ T cells reactive with mIg-presenting cells; and (2) a negative regulation of CD4+ T-cell activation, including mIg-autoreactive Th cells, mediated by suppressive Tr in MM.
mIg-reactive T cells of alternative phenotypes might obviously coexist among tumor antigen–associated immune reactivities, and the resulting effector functions upon activation could be modulated by regulatory T cells, analogous to mechanisms involved in certain autoimmune disorders.
A tumor antigen–associated Th2 activation, possibly insufficiently regulated by Tr, could be a risk factor in MM, a hypothesis that is compatible with the results from our studies of MM-associated IL-10 and CTLA-4 polymorphisms.38,39 Whether increased frequencies of mIg- or alternative tumor antigen–reactive non-Th1 cells are associated with tumor progression, and whether the Th populations include cells of a putative pathologic importance, remain to be investigated in extended studies. If such a “tumor feedback” mechanism as proposed here exists, it could have implications for the development of immunotherapies in MM and other B-lineage–derived tumors. mIg-directed therapies (eg, administration of DCs loaded with mIg) might be associated with a risk for a predominant activation of pre-exisiting circulating mIg-specific Th2 cells. Administration of DCs may in addition reinforce tolerance mechanisms, as indicated by observations of Tr expansions in peripheral blood of MM patients after injections of DCs.40 It was also reported that protein mixtures derived from myeloma cells and internalized in DCs induce primarily regulatory T cells,41 which further emphasizes the conclusion that an understanding of the complex relationships between B-lineage tumor cells and the immune system is a prerequisite for the development of effective immunotherapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The discussions with Prof G. Holm are gratefully acknowledged.
This study was supported by the Torsten and Ragnar Söderberg foundation, Sweden and the Karolinska Institutet, Sweden.
Authorship
Contribution: M.O. and M.A. performed research and analyzed data; A.G. collected and analyzed data and reviewed the paper; and A.S. designed and performed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Sundblad, Centre for Molecular Medicine, L8:00, Karolinska University Hospital, S-171 76 Stockholm, Sweden; e-mail: anne.sundblad@ki.se.
References
Author notes
M.O. and M.A. are equal contributors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal