Activin-A is a transforming growth factor-β (TGF-β) superfamily member that plays a pivotal role in many developmental and reproductive processes. It is also involved in neuroprotection, apoptosis of tumor and some immune cells, wound healing, and cancer. Its role as an immune-regulating protein has not previously been described. Here we demonstrate for the first time that activin-A has potent autocrine effects on the capacity of human dendritic cells (DCs) to stimulate immune responses. Human monocyte-derived DCs (MoDCs) and the CD1c+ and CD123+ peripheral blood DC populations express both activin-A and the type I and II activin receptors. Furthermore, MoDCs and CD1c+ myeloid DCs rapidly secrete high levels of activin-A after exposure to bacteria, specific toll-like receptor (TLR) ligands, or CD40 ligand (CD40L). Blocking autocrine activin-A signaling in DCs using its antagonist, follistatin, enhanced DC cytokine (IL-6, IL-10, IL-12p70, and tumor necrosis factor-α [TNF-α]) and chemokine (IL-8, IP-10, RANTES, and MCP-1) production during CD40L stimulation, but not TLR-4 ligation. Moreover, antagonizing DC-derived activin-A resulted in significantly enhanced expansion of viral antigen-specific effector CD8+ T cells. These findings establish an immune-regulatory role for activin-A in DCs, highlighting the potential of antagonizing activin-A signaling in vivo to enhance vaccine immunogenicity.
Introduction
Dendritic cells (DCs) form sentinel networks within the body sampling the microenvironment for pathogens, tissue injury, and inflammation via an array of pattern recognition receptors.1,–3 Pathogen encounter induces DC maturation, resulting in profound alterations in DC function. Antigen uptake is reduced, antigen processing is enhanced, and proinflammatory mediators are released.4,,,–8 The class, magnitude, and timing of cytokine or chemokine release are under exquisite control through both autocrine and paracrine signals as well as the signal strength and magnitude of the initiating stimulus.8 DC cytokine and chemokine production can be induced by specific classes of stimuli. These include CD40 ligand (CD40L) and pathogen signals, such as toll-like receptor (TLR) agonists (eg, lipopolysaccharide [LPS] or intact bacteria). Appropriate release of cytokines, chemokines, and other soluble mediators by DCs and neighboring cells induces and moderates inflammation, recruits innate effectors, and regulates T-cell functions.9,,–12 Many cytokines/chemokines produced by DCs at the epicenter of infection and inflammation, such as interleukin-6 (IL-6),13,14 IL-8,4,15,16 IL-1017,18 as well as the potent T helper 1 (Th-1) cytokine, IL-12p70,19,20 have pleiotropic effects ranging from enhancing to inhibitory depending on the context and target cell type. However, uncontrolled cytokine/chemokine release within this microenvironment can also result in inappropriate T- and B-cell responses and subsequent immunopathology.21,–23 In this regard, the immune system has evolved to coordinately express mediators that attenuate exaggerated or inappropriate responses so as to minimize tissue damage and immunopathology (eg, prostaglandin E2 (PGE2), adenosine triphosphate (ATP), transforming growth factor-β [TGF-β]).24
Activin-A is a homodimer of activin-βA subunits and was first described as a reproductive factor that accentuates the release of follicle-stimulating hormone.25 It is a member of the TGF-β superfamily of cytokines and intimately shares with TGF-β the Smad intracellular signaling proteins.26 The signaling, however, occurs through separate and distinct serine threonine kinase receptor subunits, and its release into the circulation during acute systemic inflammation differs from TGF-β.27 Activin-A signals through heteromeric receptor complexes consisting of both type I (ALK 2, 4, or 7) and type II (ActRIIA and ActRIIB) receptors. In addition, it is known to be pivotal in developmental and reproductive processes, inhibition of tumor cell proliferation via apoptosis,28 protection against ischemic brain injury,29 induction of wound healing,30 pancreatic fibrosis,31 exacerbation of rheumatoid arthritis,32 embryonic stem-cell renewal and pluripotency,33,34 and differentiation of erythroid lineage cells.35 Furthermore, activin-Smad signaling pathways can be activated at distinct maturation stages of thymopoiesis in mice36 and can either inhibit or stimulate rat thymocyte growth and differentiation,37 suggesting a complex regulatory role in T-cell development.
The biologic activity of activin-A is controlled at many levels, including through its interaction with the high-affinity binding protein follistatin.38,,–41 Two follistatin molecules bind one activin molecule and neutralize the ligand by burying one-third of activin residues, resulting in antagonism of both type I and II activin receptors.42 The role of follistatin in inflammatory processes appears to be part of a short feedback loop that attenuates the effects of activin-A.43 Although activin-A is the highest affinity ligand for follistatin, this interaction is not exclusive, and follistatin can also bind certain bone morphogenic proteins (BMPs)44 and myostatin.45
The pleiotropic role of activin-A and follistatin in diverse biologic systems, particularly their role in inflammation, led us to investigate their potential effects on DC function. Because DCs play a pivotal role in linking innate and adaptive immune responses, we examined whether activin-A and follistatin regulate such DC functions. Our studies show that several types of human DCs produce and respond to activin-A and that autocrine activin-A production by DCs potently attenuates their pro-inflammatory potential as well as their T-cell stimulatory capacity, including the expansion of antigen specific CD8+ T cells. These potent autocrine and paracrine immune-regulatory effects of activin-A have important implications for our understanding of immune regulation and for the development of strategies to manipulate immune function in vivo.
Methods
Access to and manipulation of peripheral blood mononuclear cells from healthy human volunteers for use in the present study was approved by the Institutional Human Ethics committee at Austin Hospital. Informed consent was obtained in accordance with the Declaration of Helsinki.
Cell culture
Peripheral blood mononuclear cells from buffy coats of healthy donors (Red Cross Blood Bank, Melbourne, Australia) were prepared by Ficoll-Paque density gradient centrifugation (GE Healthcare, Little Chalfont, United Kingdom). Monocytes, CD1c+ peripheral blood DCs (PBDCs), CD123+ plasmacytoid DCs (PDCs), B cells, CD4+, or CD8+ T cells were isolated by positive selection using magnetic beads specific for CD14, CD1c, BDCA-4, CD19, CD4, or CD8, respectively. Natural killer (NK) cells were isolated using a human NK-cell isolation kit (Miltenyi Biotec, Auburn, CA). Cell purity was greater than 96% as determined by flow cytometry.
Human monocyte-derived DCs (MoDCs) were generated by culturing CD14+ cells with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 for 6 to 7 days. Cell cultures were maintained in RPMI 1640 supplemented with 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 60 mg/L penicillin, 12.5 mg/L streptomycin, 2 mM l-glutamine, 1% nonessential amino acids, and 10% heat-inactivated fetal calf serum (culture media). MoDCs or freshly isolated CD1c+ or PDCs were plated at 5 × 105 cells/mL in fresh culture media, or serum-free media (AIM-V, Invitrogen, Carlsbad, CA), supplemented with GM-CSF plus IL-4, GM-CSF, or IL-3, respectively, and stimulated with the following conditions: 2 μg/mL CD40L-trimer (kind gift from Immunex, Seattle, WA), live Escherichia coli (5 × 106/mL), 100 ng/mL E coli–derived LPS (Sigma-Aldrich, St Louis, MO), 1 μg/mL R-848, 1 μg/mL Pam3Cys, and 10 μg/mL poly I:C, which bind TLR 4, 7/8, 2/6, and 3, respectively (all from InvivoGen, San Diego, CA), 250 μM ATP, or 350 ng/mL PGE2 (Sigma-Aldrich).
RT and quantitative real-time PCR
RNA was isolated from MoDCs, myeloid CD1c+ or PDCs using the RNeasy Mini Kit (QIAGEN, Hilden, Germany), and cDNA was synthesized as previously described46 ; 1 μL cDNA was used as template for quantitative reverse-transcribed polymerase chain reaction (RT-PCR). Gene expression levels were quantified using ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). Primers were designed for detection of activin receptors, activin-βA, BMP 4 or 7, myostatin, and nodal mRNA and used in combination with probes from a Universal ProbeLibrary (Roche Applied Science, Indianapolis, IN). Predeveloped assay reagents for IL-6, IL-10, and IL-12p35 were obtained from Applied Biosystems and used in multiplex reactions with 18S rRNA predeveloped assay reagents for normalization. PCR reactions were set up in 96-well plates and analyzed using SDS program, version 1.9. Relative expression was calculated using the ΔCt method and was expressed relative to a calibrator, in this case MoDCs cultured in the presence of GM-CSF and IL-4.
ΔCt = Ctgene − Ct18S
ΔΔCtsample = ΔCtsample − ΔCtGM/IL-4
Relative expression = 2−ΔΔCtsample
Activin RT-PCR primers:
Activin-βA (F) ggagggcagaaatgaatgaa (R) ctgctggagacagggaagac.
ALK-2 (F) ctggccaagctgtggagtgctgccaa (R) gtactggcgtgtctagaggtcatgt.
ALK-4 (F) ggaaagcttatggcgcgagtcggccgga (R) gatcgtagacgagctcctggagcgt.
ActRIIA (F) cgacgacattgttttgctacc (R) ccccgcaattaacataagtgg.
ActRIIB (F) gagaattcggaacatgacggcgccctg (R) cctcgagccttgatctccag.
quantitative RT-PCR primers:
Activin-βA (F) ctcggagatcatcacgtttg (R) ccttggaaatctcgaagtgc.
ALK-2 (F) tcgccctcatgaatagctg (R) tttggcagtgtgacgcttac.
ALK-4 (F) gacgctccaggatcttgtct (R) gtgcgctggacaaagagg.
ActRIIA (F) gactttgggttggccttaaa (R) gtacctccgggtaccaacct.
ActRIIB (F) tgtcaagatcttcccactcca (R) catgccaggtgtgctgaa.
BMP-4 (F) tccacagcactggtcttgag (R) ctgggatgttctccagatgtt.
BMP-7 (F) tcagcgtttatcaggtgctc (R) ccagagggtacggctgtc.
Myostatin (F) tggtcatgatcttgctgtaacc (R) cttgacctctaaaaacggattca.
Nodal (F) agacatcatccgcagccta (R) caaaagcaaacgtccagttct.
Western immunoblot analysis
MoDCs were plated in fresh culture media supplemented with GM-CSF plus IL-4 and stimulated for 4 hours with 2 μg/mL CD40L or 100 ng/mL LPS. Smad 2/3, phospho-Smad 2, and β-actin were measured in samples using specific antibodies according to the manufacturer's instructions (Cell Signaling Technology, Danvers, MA).
Cytokine ELISA and antibody arrays
Cytokine enzyme-linked immunosorbent assay (ELISA) kits (BD OptEIA) were used to quantify IL-6, 10, 12 (p70), and tumor necrosis factor-α (TNF-α, BD Biosciences, San Jose, CA). Activin-A was measured in media samples using a specific ELISA47 according to the manufacturer's instructions (Oxford Bio-Innovations, Oxford, United Kingdom) with some modifications for media samples as described previously.48 The average intraplate percent coefficient of variation was 8.3% (n = 15 plates), the interplate percent coefficient of variation was 6.6%, and the limit of detection was 0.01 ng/mL. Chemokines (IP-10, MCP-1, IL-8, and RANTES) were measured using the human Chemokine Kit I Cytometric Bead Array system (BD Biosciences) according to the manufacturer's instructions, using a FACSCalibur flow cytometer (BD Biosciences).
Effects of binding activin-A in vitro
To assess effects of blocking activin-A signaling, 20 to 400 ng/mL recombinant human follistatin (R&D Systems, Minneapolis, MN) was added to cell cultures. Recombinant human activin-A was also purchased from R&D Systems.
Activin-A knockdown in MoDCs
Complexes of Hiperfect and activin-βA chain targeted siRNA were formed according to the manufacturer's instructions (QIAGEN, Valencia, CA). Targeted or Allstar negative control complexes, at a final concentration of5 nM siRNA, were added to 2 × 104 MoDCs cultured in fresh GM-CSF and IL-4 in 96-well flat-bottomed plates. After 24 hours of culture, MoDCs were stimulated with 2 μg/mL CD40L for a further 24 hours and supernatants collected and analyzed for cytokine production.
Effects of binding DC-derived activin-A on flu matrix specific CD8+ T-cell expansion
To facilitate cross-presentation, immature HLA-A2 restricted MoDCs were pulsed with chemically inactivated whole flu particles and ISCOMATRIX adjuvant (both from CSL, Melbourne, Australia) for 6 hours. After washing, 2 × 104 MoDCs were cultured with 2 × 105 autologous CD8+ T cells in media containing 10 U/mL IL-2 and 20 ng/mL GM-CSF. After 9 days of culture, cells were counted and cocultured for 4 hours with HLA-A2 restricted T2 cells pulsed with Flu matrix peptide in the presence of brefeldin-A and anti-CD107a Ab (BD Biosciences). Intracellular interferon-γ (IFN-γ) was determined using a standard intracellular cytokine staining assay.
Statistics
Unless indicated, results are expressed as the mean plus or minus 1 SD of 3 or more donors with P less than or equal to .05 considered significant.
Results
Gene regulation of activin-A receptors, activin-A, BMP 4 or 7, and myostatin in human monocyte-derived DCs and blood DC populations
To assess the relevance of the activin system to human DC populations, we assessed mRNA expression patterns of type I and II human activin receptors and activin-βA subunit by RT-PCR in MoDCs or DCs purified directly from blood. Initial analyses demonstrated that MoDCs expressed activin type I and II receptors and activin-βA subunit (data not shown). To investigate the kinetics of gene expression, we stimulated MoDCs with either trimeric CD40L or LPS and performed quantitative RT-PCR.
First, ALK-2 mRNA was detected at very low levels in all MoDC culture conditions (not shown). CD40L or LPS stimulation resulted in early down-regulation of ALK-4 mRNA (Figure 1A), but longer periods of stimulation resulted in significantly increased expression of ALK-4 mRNA above controls by 24 hours (Figure 1A). These same CD40L- or LPS-stimulated MoDCs significantly up-regulated activin RIIA mRNA after 2 hours, but this decreased to background levels by 6 hours (Figure 1B). Expression of activin RIIB mRNA differed markedly, with early down-regulation in response to CD40L or LPS (Figure 1C). CD40L stimulation increased activin RIIB mRNA over time, but LPS continued to be suppressive (Figure 1C). Activin-βA subunit mRNA was substantially increased within 2 hours of either CD40L or LPS stimulation (Figure 1D). These levels peaked at 4 to 6 hours but decreased by 24 hours (Figure 1D). Strikingly, these same MoDCs did not express mRNA of BMP 4 or 7 or myostatin (Figure 1E,F), suggesting that under these stimulatory conditions MoDCs probably do not produce these proteins.
Activin-A receptor and protein gene expression by DCs. MoDCs were cultured in either GM-CSF + IL-4 or with CD40L (2 μg/mL) or LPS (100 ng/mL) for 2 to 24 hours before being harvested. DCs were then lysed and mRNA extracted and quantitative RT-PCR performed for detection of activin type I and II receptors (A-C), activin βA subunit (D), BMP 4 or 7 (E), myostatin (F), or β-actin. Data represent the mean plus or minus 1 SD of 3 separate donors. *P ≤ .01, **P ≤ .05 versus medium alone; CD123+ plasmacytoid (G) or CD1c+ blood DCs (H) were lysed immediately after purification or cultured in medium or stimulated with CD40L and RT-PCR performed for detection of activin type I and II receptors, activin βA subunit, or β-actin. Data shown are one representative experiment from 3 separate donors.
Activin-A receptor and protein gene expression by DCs. MoDCs were cultured in either GM-CSF + IL-4 or with CD40L (2 μg/mL) or LPS (100 ng/mL) for 2 to 24 hours before being harvested. DCs were then lysed and mRNA extracted and quantitative RT-PCR performed for detection of activin type I and II receptors (A-C), activin βA subunit (D), BMP 4 or 7 (E), myostatin (F), or β-actin. Data represent the mean plus or minus 1 SD of 3 separate donors. *P ≤ .01, **P ≤ .05 versus medium alone; CD123+ plasmacytoid (G) or CD1c+ blood DCs (H) were lysed immediately after purification or cultured in medium or stimulated with CD40L and RT-PCR performed for detection of activin type I and II receptors, activin βA subunit, or β-actin. Data shown are one representative experiment from 3 separate donors.
CD123+ and CD1c+ PBDCs expressed low levels of ALK2 and ALK4 type I receptors, whereas the constitutively expressed activin RIIA receptor was more prominently expressed (Figure 1G,H). Interestingly, culture alone induced activin-βA subunit mRNA in the CD123+ and CD1c+ PBDCs (Figure 1G,H). Together, these results suggest a complex level of activin receptor and activin-A gene regulation in response to either CD40L or LPS by MoDCs and that the activin system may also be important in the blood DC populations.
Activated MoDCs secrete large amounts of activin-A
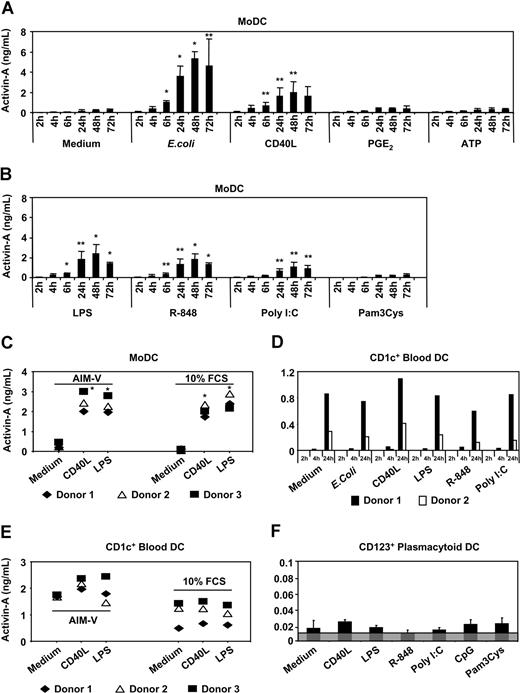
To test whether up-regulation of activin-βA subunit mRNA translated into increased secretion of activin-A protein, we cocultured human MoDCs in medium containing fresh GM-CSF and IL-4 in the presence of either intact E coli, or CD40L, or various TLR ligands (eg, LPS, R-848, poly I:C, or Pam3Cys) or the inflammatory mediators PGE2 or ATP. Consistent with the quantitative RT-PCR data, intact E coli induced the highest secretion of activin-A protein (Figure 2A). Under these conditions, significant levels of activin-A protein were detected by 6 hours, reaching 5 ng/mL by 48 hours. As with E coli stimulation, signaling through CD40 (Figure 2A) or TLR 4 (Figure 2B) produced very similar kinetics and magnitude of activin-A release by MoDCs, with significant levels detected at 6 hours, peaking by 48 hours to 2 ng/mL (Figure 2A,B). Although MoDCs express receptors for the inflammatory mediators PGE249 and ATP,46 addition of either did not stimulate activin-A release above levels detected in unstimulated MoDC cultures (Figure 2A). Both R-848 and polyI:C stimulated MoDCs to produce significant amounts of activin-A after 6 hours of stimulation, whereas Pam3Cys was ineffective (Figure 2B). To exclude the possibility that serum factors were influencing activin-A production by MoDCs, we cultured immature MoDCs in either serum-free media (AIM-V) or standard fetal calf serum-containing medium supplemented with GM-CSF and IL-4. Figure 2C shows that MoDCs secreted equivalent levels of activin-A whether stimulated in serum-containing or serum-free conditions.
Activin-A production by DCs. Immature MoDCs (A-C) or purified CD1c+ DC (D-E) or CD123+ PDCs (F) were cultured in 96-well plates for 2 to 72 hours (MoDCs) or 2 to 24 hours (CD1c+ DCs) or 24 hours (CD123+ PDCs) in either serum-containing or serum-free culture medium alone or with the indicated stimuli. Supernatants were collected and assayed for activin-A. Data represent the mean plus or minus 1 SD of 3 separate donors or individual donors are shown. Gray shaded area in panel F indicates the assays level of sensitivity. *P ≤ .01, **P ≤ .05 versus medium alone.
Activin-A production by DCs. Immature MoDCs (A-C) or purified CD1c+ DC (D-E) or CD123+ PDCs (F) were cultured in 96-well plates for 2 to 72 hours (MoDCs) or 2 to 24 hours (CD1c+ DCs) or 24 hours (CD123+ PDCs) in either serum-containing or serum-free culture medium alone or with the indicated stimuli. Supernatants were collected and assayed for activin-A. Data represent the mean plus or minus 1 SD of 3 separate donors or individual donors are shown. Gray shaded area in panel F indicates the assays level of sensitivity. *P ≤ .01, **P ≤ .05 versus medium alone.
Blood DC subsets, but not other immune cells, in human blood spontaneously secrete activin-A protein after isolation
With MoDCs secreting large amounts of activin-A in response to the various classes of stimuli tested, we next investigated whether the CD1c+ or CD123+ PBDC populations were also major producers of activin-A protein. CD1c+ PBDCs were stimulated for 2, 4, or 24 hours with either intact E coli or CD40L or TLR ligands (LPS, R-848 or poly I:C). Low levels of activin-A were detected by 4 hours after stimulation reaching by 24 hours to between 0.4 and 1 ng/mL (Figure 2D). Consistent with the fact that these cells spontaneously mature on in vitro culture50 (and our unpublished data), it was found that unstimulated CD1c+ PBDCs produced equivalent levels of activin-A to those detected after stimulation with the various classes of stimuli tested (Figure 2D). Furthermore, CD1c+ PBDCs produced similar levels of activin-A (with or without stimuli) irrespective of culture in serum-containing or serum-free media (Figure 2E). Although CD123+ PDCs also spontaneously up-regulate activin-βA subunit mRNA on in vitro isolation, they produced very low levels of activin-A protein irrespective of CD40L or TLR stimulation (Figure 2F).
To examine whether other immune cells produce activin-A, purified T cells, B cells, and NK cells were stimulated with appropriate activation-inducing stimuli (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Unlike DCs, neither activated human CD4+ or CD8+ T cells, nor B cells or NK cells secreted activin-A after 24 hours of stimulation (Figure S1). This is in contrast to activated murine splenic CD4+ T cells.51
Activin-A specifically regulates CD40L-mediated release of IL-6, IL-12p70, TNF-α, and IL-10
Stimuli such as CD40L, LPS, R-848 or poly I:C are potent inducers of MoDC maturation and cytokine secretion.8,49 A novel finding of the present study is that these stimuli rapidly induce MoDCs to secrete nanogram amounts of activin-A. To test if activin-A produced by MoDCs results in autocrine/paracrine signaling, we measured Smad 2/3 and phospho-Smad 2 levels after 4 hours of stimulation. We found increased Smad 2/3 and phospho-Smad 2 levels in stimulated MoDC populations (Figure 3A).
Smad signaling, nodal expression, TGF-β production, and effects of follistatin on CD40L-mediated activin-A production by MoDCs. Immature MoDCs were cultured in 24-well plates for 4 hours with either GM-CSF + IL-4 alone or with CD40L (2 μg/mL), LPS (100 ng/mL), R-848 (1 μg/mL), or poly I:C (10 μg/mL). (A) DCs were then lysed, protein extracted, and Western blotting performed for detection of Smad 2/3, phospho-Smad 2, or β-actin. Data shown are one representative experiment from 3 separate donors. Nodal mRNA expression was assessed by quantitative RT-PCR in the same MoDC donors represented in Figure 1A-F. (B) Immature MoDCs were cultured in 96-well plates either with GM-CSF + IL-4 alone or with 2 μg/mL CD40L or 100 ng/mL LPS for 24 hours (C) or cultured in 96-well plates with 2 μg/mL CD40L together with increasing concentrations of follistatin (D). Supernatants were collected after 24 hours or at the times indicated and assayed for TGF-β (C) and/or activin-A (C,D) protein by ELISA. Data represent the mean plus or minus 1 SD of 3 separate donors. *P ≤ .01 versus CD40L only.
Smad signaling, nodal expression, TGF-β production, and effects of follistatin on CD40L-mediated activin-A production by MoDCs. Immature MoDCs were cultured in 24-well plates for 4 hours with either GM-CSF + IL-4 alone or with CD40L (2 μg/mL), LPS (100 ng/mL), R-848 (1 μg/mL), or poly I:C (10 μg/mL). (A) DCs were then lysed, protein extracted, and Western blotting performed for detection of Smad 2/3, phospho-Smad 2, or β-actin. Data shown are one representative experiment from 3 separate donors. Nodal mRNA expression was assessed by quantitative RT-PCR in the same MoDC donors represented in Figure 1A-F. (B) Immature MoDCs were cultured in 96-well plates either with GM-CSF + IL-4 alone or with 2 μg/mL CD40L or 100 ng/mL LPS for 24 hours (C) or cultured in 96-well plates with 2 μg/mL CD40L together with increasing concentrations of follistatin (D). Supernatants were collected after 24 hours or at the times indicated and assayed for TGF-β (C) and/or activin-A (C,D) protein by ELISA. Data represent the mean plus or minus 1 SD of 3 separate donors. *P ≤ .01 versus CD40L only.
Nodal and TGF-β also signal through Smad 2/3. To investigate the potential contribution of either to the enhanced Smad 2/3 signaling, we tested the same MoDC donors used in the analysis shown in Figure 1 for nodal mRNA expression by quantitative RT-PCR as well as measure TGF-β production in culture supernatants. The same MoDCs, which expressed high levels of activin-βA mRNA (Figure 1), were negative for nodal mRNA compared with the positive control, skeletal muscle (Figure 3B). Similarly, although CD40L or LPS stimulated MoDCs secreted large amounts of activin-A, they did not secrete TGF-β (Figure 3C).
These results suggest that the combined effects of increased expression of activin receptors on stimulated MoDCs (Figure 1A-C) and increased activin-A (but not nodal or TGF-β) production by these same MoDCs (Figure 2A-C) would probably result in enhanced autocrine/paracrine activin signaling.
To investigate whether early autocrine/paracrine release of activin-A by MoDCs was a potentiating feedback loop to further activin-A production, we blocked activin-A signaling using the high affinity activin-A binding protein, follistatin.38,39,41 Here, MoDCs were activated with CD40L in the presence of increasing concentrations of follistatin and supernatants tested for activin-A production. Figure 3D shows that addition of increasing amounts of follistatin within the first 2 to 4 hours of CD40L stimulation resulted in a dose-dependent decrease in the amount of activin-A produced by MoDCs. However, addition of even 400 ng/mL of follistatin 6 hours after CD40L stimulation was unable to abrogate the levels of activin-A produced by MoDCs. This suggests that either the levels of activin-A produced by 6 hours were no longer neutralizable by follistatin or that the signaling cascade initiated in activin receptor-expressing MoDCs by 6 hours could not be reversed by subsequent activin-A neutralization with follistatin (Figure 3D).
In an in vivo LPS challenge model, activin-A is rapidly released into the circulation and is detectable before IL-6 and just preceding TNF-α release.52 The present study demonstrated that live E coli, LPS, or CD40L induces MoDCs to secrete large amounts of activin-A, and we hypothesized that activin-A release by MoDCs may influence their subsequent IL-6 production. To test this hypothesis, we cultured MoDCs together with either LPS or CD40L in the presence of increasing concentrations of follistatin. After 4, 24, or 48 hours, we collected culture supernatants and assayed for IL-6. By 24 hours, LPS stimulated the production of large amounts of IL-6, but neutralization of activin-A by addition of biologically effective levels of follistatin did not alter the levels of either IL-6 (Figure 4A), IL-8, or IL-12p70 (data not shown) produced by MoDCs. In stark contrast, CD40 stimulation in the presence of increasing concentrations of follistatin resulted in a dose-dependent increase in IL-6 production by the same MoDCs to levels achieved with either LPS (Figure 4A) or E coli (data not shown). The levels of IL-6 were significantly higher than those induced by CD40L alone at 4 hours, peaked by 24 hours, and remained elevated even at 48 hours (Figure 4A). Importantly, the addition of follistatin alone did not induce MoDCs cytokine secretion (Figure S2A,D).
The effects of blocking endogenous activin-A with follistatin on LPS or CD40L-mediated IL-6 production by MoDCs. Immature MoDCs were cultured in 96-well plates for 4 to 24 hours with either GM-CSF and IL-4 alone or with LPS or CD40L in the absence or presence of increasing concentrations of follistatin (A-C). Supernatants were collected at the various times indicated (A) or after 24 hours (B,C) and assayed for IL-6 production by ELISA; 2 × 104 immature MoDCs were cultured in 96-well flat-bottomed plates in media supplemented with GM-CSF and IL-4 and transfected with Alexa Fluor-labeled siRNA or control nontargeted siRNA or targeted activin βA chain siRNA. Efficiency of transfection was measured by flow cytometry (D). Transfected MoDCs were stimulated with 2 μg/mL CD40L for 24 hours, and supernatants were assayed for IL-6 production (E). Data represent the mean plus or minus 1 SD of 3 separate donors, except in panel D where one representative donor is shown and panel E where individual donors are shown. *P ≤ .01, **P ≤ .05 versus CD40L only; †P ≤ .01 versus CD40L stimulated nontargeted siRNA transfected MoDCs.
The effects of blocking endogenous activin-A with follistatin on LPS or CD40L-mediated IL-6 production by MoDCs. Immature MoDCs were cultured in 96-well plates for 4 to 24 hours with either GM-CSF and IL-4 alone or with LPS or CD40L in the absence or presence of increasing concentrations of follistatin (A-C). Supernatants were collected at the various times indicated (A) or after 24 hours (B,C) and assayed for IL-6 production by ELISA; 2 × 104 immature MoDCs were cultured in 96-well flat-bottomed plates in media supplemented with GM-CSF and IL-4 and transfected with Alexa Fluor-labeled siRNA or control nontargeted siRNA or targeted activin βA chain siRNA. Efficiency of transfection was measured by flow cytometry (D). Transfected MoDCs were stimulated with 2 μg/mL CD40L for 24 hours, and supernatants were assayed for IL-6 production (E). Data represent the mean plus or minus 1 SD of 3 separate donors, except in panel D where one representative donor is shown and panel E where individual donors are shown. *P ≤ .01, **P ≤ .05 versus CD40L only; †P ≤ .01 versus CD40L stimulated nontargeted siRNA transfected MoDCs.
To investigate this effect further, we repeated this experiment using either CD40L or a range of LPS concentrations (1-150 ng/mL) together with an even higher dose of follistatin (400 ng/mL). Once again, antagonizing activin-A signaling with follistatin dramatically enhanced CD40L-mediated IL-6 production by MoDCs (Figure 4B) but did not enhance LPS-mediated IL-6 production at any of the LPS doses tested (Figure 4C). Furthermore, quantitative RT-PCR analysis indicated a 15-fold increase in IL-6 mRNA at 2 hours after MoDC stimulation with CD40L and the highest dose of follistatin (Figure S2B), peaking to 30-fold at 4 hours and decreasing by 6 to 24 hours after stimulation (Figure S2B). To confirm this effect as activin-A specific, we used targeted siRNA to knock down the activin-A βA chain gene in MoDCs. We found, even with modest transfection efficiency (Figure 4D), that silencing of activin-A genes resulted in a significant increase in IL-6 production after CD40L stimulation compared with nontargeted siRNA controls (Figure 4E).
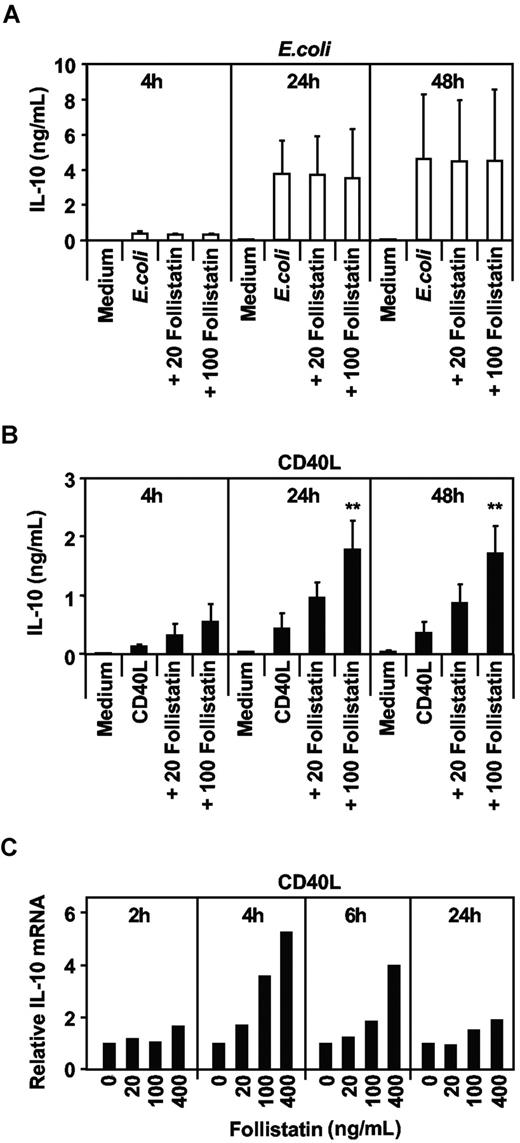
To more fully investigate the effects of activin-A on MoDC cytokine secretion, we screened supernatants for a larger array of cytokines and chemokines, including IL-10. Side-by-side comparisons of the same MoDC donors showed that antagonizing activin-A with follistatin did not affect E coli–mediated IL-10 (Figure 5A) but specifically enhanced CD40L-mediated IL-10 secretion (Figure 5B). Addition of follistatin enhanced IL-10 secretion by MoDCs in a dose-dependent manner, correlating with increased IL-10 mRNA, which peaked at 4 hours and waned by 24 hours (Figure 5C).
The effects of blocking endogenous activin-A with follistatin on E coli or CD40L-mediated IL-10 production by MoDCs. Immature MoDCs were cultured in 96-well plates for 24 hours with either GM-CSF and IL-4 alone or with E coli (A) or CD40L (B) in the absence or presence of increasing concentrations of follistatin. Supernatants were collected at the various times indicated and assayed for IL-10 production by ELISA. Data represent the mean plus or minus 1 SD of 3 separate donors (**P ≤ .02 vs CD40L only). MoDCs stimulated with CD40L in the presence of increasing concentrations of follistatin were lysed, mRNA extracted at times indicated, and IL-10 qRT-PRC performed (C). One of 2 donors is shown.
The effects of blocking endogenous activin-A with follistatin on E coli or CD40L-mediated IL-10 production by MoDCs. Immature MoDCs were cultured in 96-well plates for 24 hours with either GM-CSF and IL-4 alone or with E coli (A) or CD40L (B) in the absence or presence of increasing concentrations of follistatin. Supernatants were collected at the various times indicated and assayed for IL-10 production by ELISA. Data represent the mean plus or minus 1 SD of 3 separate donors (**P ≤ .02 vs CD40L only). MoDCs stimulated with CD40L in the presence of increasing concentrations of follistatin were lysed, mRNA extracted at times indicated, and IL-10 qRT-PRC performed (C). One of 2 donors is shown.
We extended these studies to investigate the effects of activin-A on the Th-1-inducing cytokine, IL-12p70, as well as pro-inflammatory TNF-α. Although the kinetics of IL-12p35 mRNA expression (the rate-limiting subunit in the formation of bioactive IL-12p70) differed markedly from that of IL-6, blockade of activin-A by follistatin resulted in 15-fold greater levels of IL-12p35 mRNA by 24 hours compared with MoDCs stimulated with CD40L alone (Figure S2C). This translated into a dose-dependent enhancement in MoDC IL-12p70 secretion of approximately 8 ng/mL (Figure 6A). As with IL-6 production, follistatin alone in the absence of any other stimulus did not induce IL-12p70 secretion by MoDCs (Figure S2D). TNF-α secretion mirrored IL-12p70 production with a dose-dependent enhancement, peaking at 2 ng/mL after 24 hours (Figure 6B). Furthermore, blockade of the major type I activin receptor (ALK4) using the chemical inhibitor SB431542 had similar enhancing effects in CD40L-mediated MoDC cytokine production to that seen with the natural antagonist, follistatin (data not shown).
The effects of blocking endogenous activin-A with follistatin on CD40L-mediated IL-12, TNF-α, and chemokine production by MoDCs. Immature MoDCs were cultured in 96-well plates for 24 hours with either GM-CSF and IL-4 alone or with CD40L in the absence or presence of increasing concentrations of follistatin, and supernatants were collected after 24 hours and assayed by ELISA for IL-12p70 (A) or TNF-α (B) or assayed for IL-8 (C), IP-10 (D), RANTES (E), and MCP-1 (F) production using a Cytometric Bead Array system. Data represent the mean plus or minus 1 SD of 3 separate donors. *P ≤ .01, **P ≤ .05 versus CD40L only.
The effects of blocking endogenous activin-A with follistatin on CD40L-mediated IL-12, TNF-α, and chemokine production by MoDCs. Immature MoDCs were cultured in 96-well plates for 24 hours with either GM-CSF and IL-4 alone or with CD40L in the absence or presence of increasing concentrations of follistatin, and supernatants were collected after 24 hours and assayed by ELISA for IL-12p70 (A) or TNF-α (B) or assayed for IL-8 (C), IP-10 (D), RANTES (E), and MCP-1 (F) production using a Cytometric Bead Array system. Data represent the mean plus or minus 1 SD of 3 separate donors. *P ≤ .01, **P ≤ .05 versus CD40L only.
Thus, although CD40L, LPS, and E coli induce large amounts of activin-A, blocking activin-A signaling with follistatin appears to specifically modulate MoDCs production of IL-6, IL-10, or IL-12p70 in response to CD40L but not to LPS or E coli.
Activin-A specifically regulates CD40L-mediated release of IL-8, IP-10, RANTES, and MCP-1
As with cytokines, chemokines play an integral part in recruiting leukocyte effectors and shaping immune responses. We hypothesized that DC derived activin-A may also play a regulatory role in modulating CD40L-induced chemokine secretion. MoDCs culture supernatants were screened using a multiplex bead array system for multiple chemokines within the same culture sample. Figure 6C-F shows that antagonizing CD40L-induced activin-A production with follistatin substantially enhanced MoDC production of IL-8, IP-10, RANTES, and MCP-1. Taken together, these results reveal a previously undescribed regulatory role of activin-A in CD40L-mediated cytokine and chemokine secretion by DCs.
Activin-A specifically regulates Ag-specific CD8+ T-cell expansion
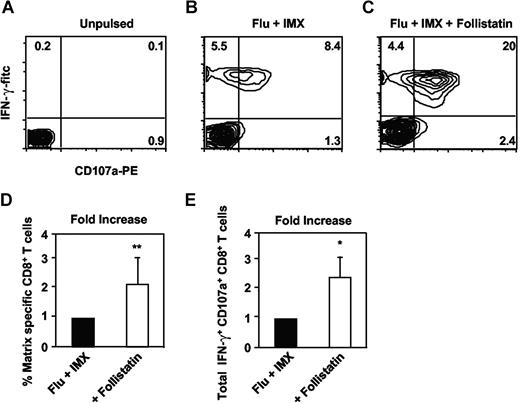
Considering DCs' prominent role in regulating cellular immune responses, the ability of activin-A to regulate a DC-mediated T-cell response was next examined. Examination of purified CD8+ T cells indicated that type I and II activin receptors were up-regulated in response to anti-CD3/CD28 treatment (data not shown). Purified CD8+ T cells were cocultured with autologous MoDCs pulsed with chemically inactivated influenza virus and the saponin-based adjuvant, ISCOMATRIX. This adjuvant was used to efficiently target antigens into the class I major histocompatibility complex MHC processing pathway in DCs for class I MHC cross-presentation.7,53 Blockade of DC-derived activin-A, using follistatin, significantly increased both the percentage and total number of influenza matrix-specific CD8+ T cells displaying effector function (ie, Ag-specific IFN-γ secretion and lytic granule exocytosis based on CD107a expression). Figure 7A-C shows a representative donor, whereas Figure 7D,E shows combined results. Thus, activin-A not only regulates DC cytokine output via autocrine/paracrine mechanisms but also limits the capacity of these DCs to expand antigen-specific CD8+ T-cell effectors. Importantly, these regulatory effects of DC-derived activin-A could be antagonized by follistatin, revealing a greater T-cell stimulatory potential of Ag-loaded DCs.
The effects of blocking endogenous activin-A with follistatin on influenza matrix specific CD8+ T-cell expansion. Immature MoDCs were pulsed with inactivated influenza particles together with ISCOMATRIX adjuvant for 6 hours before washing and coculture with purified autologous CD8+ T cells with or without addition of 400 ng/mL follistatin. After 9 days, cultures were counted and restimulated with influenza matrix peptide pulsed T2 cells together with anti-CD107a antibody in the presence of brefeldin-A. (A-C) Typical analysis. (D) Percentage of IFN-γ+ CD8+ T cells. (E) Total IFN-γ+ CD107a+ CD8+ T cells, calculated from 4 individual donors. *P ≤ .02, **P ≤ .05 versus no follistatin.
The effects of blocking endogenous activin-A with follistatin on influenza matrix specific CD8+ T-cell expansion. Immature MoDCs were pulsed with inactivated influenza particles together with ISCOMATRIX adjuvant for 6 hours before washing and coculture with purified autologous CD8+ T cells with or without addition of 400 ng/mL follistatin. After 9 days, cultures were counted and restimulated with influenza matrix peptide pulsed T2 cells together with anti-CD107a antibody in the presence of brefeldin-A. (A-C) Typical analysis. (D) Percentage of IFN-γ+ CD8+ T cells. (E) Total IFN-γ+ CD107a+ CD8+ T cells, calculated from 4 individual donors. *P ≤ .02, **P ≤ .05 versus no follistatin.
To test if activin-A directly regulated in vitro CD8+ T-cell activation and expansion in the above conditions or acted primarily via the DC, carboxyfluorescein diacetate succinimidyl ester-labeled CD8+ T cells were cultured with or without anti-CD3/CD28 stimulation with increasing doses of recombinant activin-A. Even doses of activin-A 10-fold higher than those produced by MoDCs could not directly suppress CD8+ T-cell division or IFN-γ production (Figure S3A-C), suggesting that activin-A, under these conditions, does not directly regulate T-cell functions but rather primarily acts by regulating DC function.
Discussion
The actions of activin-A span multiple facets of human biology. Most studied with regard to reproductive and developmental biology,54 recent reports indicate an involvement in stem cell differentiation, local and systemic inflammation, as well as disease states, such as cancer, rheumatoid arthritis, and diabetes.32,52,55,,–58 Although DCs are often at the center of many of these disease states, reports implicating activin-A in the regulation of DC function are rare. To date, one report has demonstrated costaining of activin-A with DC-SIGN+ DCs in human skin59 and another using transgenic mice overexpressing follistatin has shown reduced Langerhans cell numbers in the epidermis.60 In another study, activin-A has been implicated in the regulation of inflammation by demonstrating rapid systemic release of activin-A within 30 minutes after intravenous injection of LPS, this preceding the release of systemic TNF-α, IL-1β, and IL-6.52 In addition, up-regulation of activin-βA subunit mRNA and activin-A protein has been reported in diseased and/or inflamed tissues.30,32,59,61 These are all environments where DCs are either rapidly recruited to or present in large numbers. Consequently, we sought to identify a novel relationship between activin-A and regulation of DC function.
Our initial findings indicated that both in vitro-generated MoDCs and in vivo–derived blood DC populations express mRNAs for the activin-βA subunit and activin receptors. This provided the first indication that human DCs are both producers of, and are receptive to, activin-A. This was confirmed when activin-βA subunit mRNA was found to be up-regulated in response to LPS or CD40L and that MoDCs produced extremely high levels of dimeric activin-A protein in response to various stimuli. An earlier report has also demonstrated LPS and 1,25-dihydroxyvitamin D3 induced transcription of activin-βA subunit mRNA in human monocytes,62 an observation likely consistent with our findings in human MoDCs. Serum factors did not play a major role in the final activin-A output by DCs because similar levels of activin-A were produced by MoDCs or blood DCs when stimulated in serum-free media.
We found that early production of activin-A by CD40L-stimulated DCs potentiates further activin-A release. This effect can be attenuated by follistatin only when added within the first 4 hours of CD40L stimulation but not after 6 hours, presumably when comparatively low, neutralizable amounts of activin-A were present (Figure 3). Alternatively, the signaling cascade initiated in activin receptor-expressing MoDCs by 6 hours could not be reversed by subsequent activin-A neutralization with follistatin. Given that the ELISA can detect both free and follistatin-bound activin-A, the reduction in activin-A detected in MoDC cultures after addition of follistatin was not trivially due to masking of the activin-A epitopes recognized by the ELISA antibodies. Although follistatin can block several ligands (eg, activin-A, BMP 4, BMP 7, and myostatin),44,45 neither resting nor stimulated MoDCs expressed mRNA for these other follistatin targets. This suggests that the effects of follistatin on CD40L-stimulated DCs were predominantly via activin-A blockade. Furthermore, neither resting nor stimulated MoDCs expressed mRNA for nodal nor did they produce TGF-β, suggesting that autocrine activin-A signaling is most likely responsible for the enhanced Smad 2/3 signaling shown in Figure 3.
These data led to the hypothesis that MoDC-derived activin-A production may affect their cytokine production in an autocrine/paracrine manner. Our analyses also revealed a putative functional dichotomy in how autocrine/paracrine activin-A regulates MoDCs functions induced by either CD40/CD40L or TLR/MyD88/TRIF signaling pathways. Neutralization of MoDC-derived activin-A (either by follistatin or activin-A siRNA) during CD40 stimulation profoundly enhanced MoDCs production of cytokines and chemokines, whereas it did not alter cytokine/chemokine production induced by TLR stimuli (eg, LPS or E coli). These findings suggest that MoDC-derived activin-A attenuates CD40-mediated cytokine/chemokine production, and its neutralization reveals the cytokine/chemokine output of an unrestrained DC.
In addition, these findings also suggest a putative regulatory link between activin/Smad pathway signaling and the regulation of the CD40/TRAF/NF-κB signaling cascade but a less direct one between activin/Smad with the TLR/MyD88/TRIF pathways in MoDCs. Several studies have now linked Smad and TRAF/NF-κB signaling pathways. Smad signaling prevents proinflammatory cytokine production through inhibition of p38 mitogen-activated protein kinase and NF-κB in macrophages.63 Functional cooperativity between NF-κB and Smads has also been demonstrated in that κB sites alone are sufficient to mediate transcriptional activation by another Smad pathway ligand, TGF-β, and that this requires an intact NF-κB pathway.64 These putative signaling pathway differences between DC types are an area of active research within our group.
Interestingly, activin-A secretion is induced in monocytes and bone marrow stromal fibroblasts via activated CD4+ T cells.65 This is consistent with the regulatory effects of activin-A in the present study on MoDCs but was not observed when CD1c+ blood DCs were cultured with CD40L and follistatin (data not shown), suggesting differences in these DC types.
A regulatory role for CD40L-mediated activin-A release by DCs within the lymph node environment is easily reconciled. Here, unrestrained cytokine release by DCs (such as IL-6, IL-8, IL-10, and IL-12p70 as well as chemokines) may result in chronic overstimulation and recruitment of immune effectors within the local microenvironment with the potential, if left unchecked, to induce immunopathology and malignant transformation.66,–68
In addition, CD40L can also be expressed by non-T cells within inflamed tissues (eg, activated platelets, macrophages, neutrophils, and endothelial cells).69,70 Interestingly, DCs are relatively abundant within inflamed tissues, sites where large amounts of follistatin are also detected.43 Thus, it is possible that inflamed tissue-derived CD40L at peripheral sites will activate DCs in the presence of follistatin, resulting in proinflammatory-type DCs that release multiple pro-inflammatory cytokines and chemokines forming epicenters of inflammation. Such environments may very well aid recruitment of both innate and adaptive immune cell effectors to sequester and eradicate invading pathogens.
Lastly, we demonstrated that activin-A not only has an autocrine role in regulating DC cytokine/chemokine production but also regulates their induction of Ag-specific CD8+ T cells. This is an important finding considering many current vaccine strategies aim to target DCs via intramuscular or subcutaneous administration. Penetration of skin and subsequent tissue damage by the vaccine needle represents a putative “danger” signal to cutaneous DCs likely accompanied by local activin-A release. It is conceivable that coadministration of follistatin to antagonize local activin-A release may enhance vaccine efficacy. Alternatively, activating antigen loaded DCs ex vivo with CD40L in the presence of follistatin before reinfusion into patients may also enhance their immunostimulatory capacity in vivo.
Together, these results provide novel insights into the regulation of DC cytokine and chemokine production, highlighting the intricate interplay between activin-A and follistatin in regulating the final cytokine/chemokine output of DCs in response to specific physiologic stimuli. These mediators should now be explored as targets for manipulation so as to enhance the efficacy of therapeutic vaccine-based immunotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Suzanne Robson, Oliver Klein, Lisa Ebert, and Ian Davis for helpful discussion; Marina Zuber, Anne O'Connor, and Susan Hayward for additional technical assistance; and Amgen for provision of soluble CD40L trimer.
This work was supported by a Program Grant from the Australian National Health and Medical Research Council (NH&MRC) and the Ludwig Institute for Cancer Research (N.C.R.) and by a Program Grant (Regkey 334011) from the NH&MRC (D.J.P.). E.M. is an employee of CSL Limited and an Honorary Senior Research Fellow of the Ludwig Institute for Cancer Research.
Authorship
Contribution: N.C.R. designed and performed research, collected and analyzed data, and wrote the paper; D.J.P. contributed vital reagents, performed research, collected and analyzed data, and wrote the paper; T.M., A.S., S.S., and T.T. performed research and collected and analyzed data; V.P. designed and performed research and collected and analyzed data; N.K. and D.Z. performed research and collected and analyzed data; K.W. contributed vital reagents and wrote the paper; I.H. and H.W. performed research and collected and analyzed data; W.C. wrote the paper; J.C. analyzed data and wrote the paper; and E.M. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: One of the authors (E.M.) is employed by a company (CSL Limited), but no reagent or potential product was studied in this paper, nor has this author any financial interest in this work. All other authors declare no competing financial interests.
Correspondence: Neil Robson, Ludwig Institute for Cancer Research, Melbourne Centre for Clinical Sciences, Heidelberg, Victoria 3084, Australia; e-mail: neil.robson@ludwig.edu.au.
References
Author notes
J.C. and E.M. contributed equally to this work.