Gastrointestinal graft-versus-host disease (GVHD) is a common and potentially life-threatening complication after allogeneic hematopoietic stem-cell transplantation (HSCT). Noninvasive tests for assessment of GVHD activity are desirable but lacking. In the present study, we were able to visualize intestinal GVHD-associated inflammation in an allogeneic murine transplantation model by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) in vivo. A predominant localization of intestinal GVHD to the colon was verified by histology and fluorescence reflectance imaging of enhanced green fluorescent protein (EGFP)–expressing donor cells. Colonic infiltration by EGFP+ donor lymphocytes matched increased FDG uptake in PET examinations. These preclinical data were prospectively translated into 30 patients with suspected intestinal GVHD beyond 20 days after transplantation. A total of 14 of 17 patients with a diagnostic histology showed significant FDG uptake of the gut, again predominantly in the colon. No increased FDG uptake was detected in 13 patients without histologic evidence of intestinal GVHD. Our findings indicate that FDG-PET is a sensitive and specific noninvasive imaging technique to assess intestinal GVHD, map its localization, and predict and monitor treatment responsiveness. Novel targeted tracers for PET may provide new insights into the pathophysiology of GVHD and bear the potential to further improve GVHD diagnosis.
Introduction
Improving results of allogeneic hematopoietic stem-cell transplantation (HSCT) have led to growing acceptance of this treatment as a potentially curative therapy for various malignant and nonmalignant diseases.1,,,,,–7 While the donor graft is enriched for hematopoietic progenitor and stem cells, it also contains T lymphocytes, which assist hematopoietic engraftment, restore T cell–dependent immunity, and are crucial to immunologic tumor control, usually referred to as graft-versus-tumor effect.3,8 Besides these beneficial attributes, donor T cells are also responsible for graft-versus-host disease (GVHD), a leading cause of morbidity and lethality in patients who have undergone HSCT.9,10 Depending on the source of the hematopoietic stem cell graft, the conditioning regimen, and the immunosuppressive treatment, the frequencies of acute GVHD vary from 20% to 50% in patients with an HLA-identical sibling donor,11,–13 and may reach 60% to 80% in patients with an HLA-compatible unrelated donor.14 One remarkable feature of GVHD is its predilection for certain organ systems. Among them in descending frequency are the epithelial surfaces of the skin and mucous membranes, the crypts of the gastrointestinal tract, and the biliary ducts of the liver. About 80% of patients with acute GVHD have skin involvement, and more than 50% have gastrointestinal GVHD.9,15
Intestinal GVHD is a progressive process that can affect all sections of the alimentary tract, albeit the terminal ileum and the colon are predominant sites. Symptoms are nonspecific and may include anorexia, nausea, vomiting, watery diarrhea, intestinal bleeding, abdominal pain, and ileus16 that may also develop secondary to numerous other causes, such as infections, conditioning, and drug toxicity.17,,–20
Although clinical investigation, laboratory tests, and histology usually lead to an early diagnosis of skin or liver GVHD, gastrointestinal GVHD is less readily diagnosed.21 This is of particular concern, as GVHD remains one of the most detrimental complications after allogeneic transplantation, and the presence of gut involvement has been related to increased lethality and poor response to treatment.22,–24 Therefore, early recognition of intestinal GVHD is important, as prompt therapeutic intervention may prevent progression to higher-grade disease and improve outcome.24,,,,–29
The current reference standard for diagnosis and staging of intestinal GVHD combines assessment of clinical symptoms with histologic analyses of mucosal biopsy specimens obtained by endoscopy of the upper and lower intestinal tract.9 This approach is unsatisfactory for several reasons. First, invasive diagnostic procedures, such as endoscopic biopsies, are associated with health hazards in patients who are frequently thrombocytopenic and have a poor performance status.30 Second, the histology of early intestinal GVHD is characterized by rather nonspecific features, such as apoptotic bodies and cellular infiltrates, and can only be interpreted in context with the course of symptoms.27 Third, the correlation of the extent of intestinal GVHD and endoscopic appearance is not definitive,31 and affected areas may occasionally be missed.
Encouraged by observations in an experimental murine transplantation model, we systematically tested the potential of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) for diagnosis and monitoring of intestinal GVHD in patients after allogeneic HSCT. We show for the first time that FDG-PET is a sensitive and specific diagnostic imaging technique suited to noninvasively map the activity and distribution of intestinal GVHD and predict and monitor treatment response.
Methods
Mice and bone marrow transplantation
BALB/c and C57BL/6 mice were purchased from Charles River Laboratories (Bad Sulzfeld, Germany), and CB6F1 mice were bred at the animal facility of the University of Muenster. C57BL/6-Tg(ACTB-EGFP)1Osb/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Female mice between 12 to 18 weeks of age were used for bone marrow transplantation (BMT) experiments as donors and recipients. All mice were kept in laminar flow racks under pathogen-free conditions. Transplant recipients received sterilized food and sterilized water supplemented with cotrimoxazole (200 mg/L). Mice received transplants according to a standard protocol as previously described.32,33 Briefly, bone marrow cells were harvested by flushing tibia and femurs of donor mice. For GVHD induction, CB6F1 (H2b × d) recipients were lethally irradiated with 9 Gy total body irradiation (TBI) from a 60Co source at a dose rate of 128 cGy/minute and reconstituted with either syngeneic bone marrow cells (BMCs) and additional splenocytes (2 × 107 BMCs and 1 × 107 splenocytes) from CB6F1 donors (syngeneic control) or with a GVHD-inducing inoculum of both haploidentical allogeneic BMCs (2 × 107 cells) and additional splenocytes (107 cells) from BALB/c (H2d) donors (allogeneic GVHD group). A second control group of CB6F1 mice received BMC grafts without splenocytes from allogeneic BALB/c donors (allogeneic control without GVHD). All animal care and procedures were in accordance with European regulations and were approved by the regional governmental review board.
Systemic and histopathologic analysis of GVHD in mice
GvHD was monitored by calculating the loss in total body weight. Body weights were measured before transplantation and 3 times a week after transplantation. Clinical GVHD intensity was scored by assessing weight loss, posture, activity, fur texture, and skin integrity.34 Histopathologic analyses of the bowel were performed in a blinded fashion on hematoxylin and eosin (H&E)–stained tissue. Microscopic analyses were performed with a BX51 light microscope (Olympus, Hamburg, Germany) equipped with a 40×/0.75 NA objective lens and a DP70 camera (Olympus) using Cell A Analysis software (Olympus Software Imaging Solutions 1986-2007, Muenster, Germany).
PET imaging of mice in vivo
Imaging was performed on a 32-module quadHIDAC small-animal PET scanner (Oxford Positron Systems, Oxford, United Kingdom). The scanner has an effective and uniform spatial resolution of less than 1 mm (full width at half maximum [FWHM]).35 Animals had unrestricted access to water and their normal food before scanning. Mice were anesthetized with isoflurane and placed on a heating pad to maintain a body temperature within the normal range. FDG (10 MBq in 100 μL 0.9% saline) was injected intravenously 1 hour prior to the PET scan. List-mode data were acquired for 15 minutes and subsequently reconstructed into a single image volume with a voxel size of 0.4 × 0.4 × 0.4 mm3. Intestinal glucose uptake was analyzed by abdominal regions of interest (ROIs). Based on the ROIs, uptake was calculated as percentage of the injected dose of radioactivity (% ID).
Combined FDG-PET and fluorescence reflectance imaging in mice
CB6F1 mice received transplants from allogeneic C57BL/6-Tg(ACTB-EGFP) donors as described. On day 21 after transplantation mice, were killed 1 hour after intravenous application of 50 MBq FDG, and the whole bowel was removed for further analysis. The bowel was placed on the bed of the quadHIDAC scanner for ex vivo PET imaging. Directly afterward, the bowel was measured on an In-Vivo FX Imaging System (Kodak Molecular Imaging Systems, New Haven, CT) for detection of enhanced green fluorescent protein–positive (EGFP+) donor cells by fluorescence reflectance imaging (FRI). Fluorescence was read-out by excitation with a filtered white light source and a dedicated band-pass emission filter. For GFP excitation, 465 nm was used; for emission, 535 nm was used. Image acquisition times were 30 seconds per sample at maximum photon flux. ROIs were selected and analyzed with the ImageJ software (W. S. Rasbrand, National Institutes of Health, Bethesda, MD). In order to detect CD4+ or CD8+ donor cells in the colon, acetone-fixed cryosections were incubated with unlabeled primary antibody (GK1.5 or 53-6.7; BD Biosciences, Heidelberg, Germany), followed by biotin-conjugated anti–rat IgG (Jackson ImmunoResearch, West Grove, PA), horseradish peroxidase (HRP)–conjugated streptavidin and Alexa Fluor546 Tyramide (Molecular Probes, Eugene, OR). EGFP was detected directly in frozen sections. Nuclei were stained with DAPI (Molecular Probes). Fluorescence microscopic analyses were performed with an Axioskop2plus (Carl Zeiss MicroImaging, Goettingen, Germany) equipped with an A-Plan 10×/0.25Ph1 NA objective and an AxioCam HRc camera (Carl Zeiss MicroImaging), using Axiovision software 4.5 SP1 (Carl Zeiss MicroImaging).
Patients
Between November 2004 and August 2007, patients after allogeneic HSCT with suspected intestinal GVHD were prospectively enrolled into an exploratory study comparing FDG-PET findings with endoscopic, histologic, and clinical criteria for the diagnosis of intestinal GVHD. Patients with a prior history of intestinal GVHD were excluded, as well as those who had developed diarrhea within 20 days after HSCT, since in these patients conditioning therapy might have caused histologic features that mimic GVHD. In total, 10 women and 20 men (median age, 47 years; range, 24-68 years) who underwent transplantation for different malignant hematologic diseases (24 patients with acute leukemia, 4 with lymphoma, 1 with myeloma, and 1 with myelofibrosis) from matched related (n = 14) or unrelated donors (n = 16) were examined by FDG-PET. Conditioning regimens, including TBI, were used in 24 patients (4 Gy in 4 patients, 8 Gy in 9 patients, and 12 Gy in 11 patients). Endoscopic evaluations and histopathologic analyses of mucosal samples were performed in 28 patients within less than 1 week from FDG-PET examination and before any additional immunosuppressants were administered. In 2 patients, diarrhea ceased spontaneously before endoscopic evaluation, and no other symptoms of GVHD were observed. Thus, these 2 patients were rated as GVHD−. All patients were monitored weekly for cytomegalovirus. Stool samples were screened for viral, bacterial, or fungal gastrointestinal infections. Daily stool volume was quantified for staging and grading of GVHD and in order to monitor treatment response.26 Written informed consent was obtained from each patient in accordance with the Declaration of Helsinki before PET scan and endoscopic examinations. All study procedures were reviewed and approved by the Ethikkommission der Aerztekammer Westfalen-Lippe and Medizinischen Fakultaet der Westfaelische Wilhelms University Muenster.
FDG-PET imaging of patients
FDG-PET was performed as a standard procedure. All patients were studied after 6 hours of fasting. Normal blood glucose concentration (< 6.7 mM) was confirmed in all patients. Whole-body PET (2 minutes of transmission, 6 minutes of emission per bed position) was performed 60 minutes after intravenous administration of 5 MBq/kg body weight of FDG (ECAT EXACT 921/47; CTI/Siemens, Knoxville, TN). Immediately before scanning, patients were asked to void their bladder. PET images were analyzed visually by 3 experienced nuclear medicine physicians (consensus reading) blinded to the diagnosis and symptoms of the patients. FDG uptake was graded (0 = normal, 1 = mildly pathologic, 2 = severely pathologic) for individual bowel segments (small intestine: duodenum, jejunum, ileum; large intestine: ascending colon, transverse colon, descending colon, sigmoid colon, and rectum). Based on the segmental analyses and the overall impression, scans were grouped into those with physiologic or abnormal intestinal FDG uptake. Regional FDG uptake was quantitatively assessed as maximum standardized uptake value (SUVmax = maximum concentration of activity in the tissue ROI [kBq/mL] divided by injected activity [MBq]/body weight [g]).
Endoscopic and histologic evaluation in patients
The rectum, sigmoid, and, where feasible, the cecum and terminal ileum were explored endoscopically, and biopsy specimens were taken. The performing physician was blinded to the PET results but was aware of the clinical symptoms and laboratory tests. Histopathologic analyses of the mucosal specimens were assessed by a pathologist experienced in diagnosing GVHD in a blinded fashion on H&E-stained tissues.
Statistics
Data are presented as means plus or minus standard deviation (SD) or plus or minus standard error of the mean (SEM) as indicated. Box plots were used to illustrate the association of PET findings with treatment response and prognosis of intestinal GVHD. The 2-tailed Mann-Whitney U test was used for the statistical analysis of in vivo data. P less than .05 was considered statistically significant. All statistical analyses were performed using SPSS 13.0 software (Chicago, IL). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for FDG-PET results as categorized by blinded consensus reading. Sensitivity was calculated as the number of patients identified as PET positive divided by the total number of patients with intestinal GVHD according to clinical and histopathologic evaluation. Specificity was defined as the number of patients with negative PET results divided by the total number of patients without intestinal GVHD according to clinical and histopathologic evaluation. PPV was calculated as the number of PET-positive patients with intestinal GVHD divided by the total number of patients with (true and false) positive PET results. NPV was calculated as the number of PET-negative patients without intestinal GVHD divided by the total number of patients with (true and false) negative PET results.
Results
In a murine allogeneic HSCT model, FDG-PET visualizes tissue inflammation associated with histologically confirmed intestinal GVHD
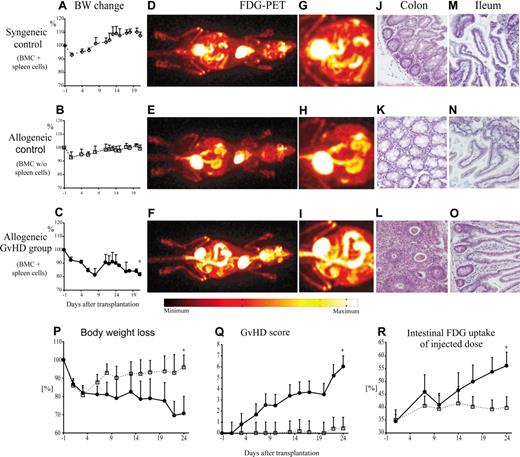
CB6F1 recipients were lethally irradiated and reconstituted with either syngeneic bone marrow and additional splenocytes from CB6F1 donors (syngeneic control) or with a GVHD-inducing inoculum of both haploidentical allogeneic bone marrow and additional splenocytes from parental BALB/c donors (allogeneic GVHD group). A second control group received bone marrow grafts without splenocytes from allogeneic BALB/c donors (allogeneic control without GVHD). After transplantation, mice of all groups lost weight during the first week, probably related to TBI toxicity. Animals of the syngeneic and allogeneic control groups later recovered their weight (Figure 1A,B) and showed no clinical evidence of disease. In recipients of transplants of allogeneic grafts containing additional splenocytes, this recovery was transient, and mice started losing weight progressively 12 days after transplantation (Figure 1C) and developed typical signs of GVHD. PET imaging on day 21 after transplantation showed a pronounced increase of FDG uptake throughout the colon in recipients with clinical GVHD as compared with both control groups (Figure 1D-I). With the exception of Peyer patches, increased FDG uptake was not detected in the small bowel of any mice from the control or GVHD groups. In line with the findings from the PET scans, histopathologic analyses showed no signs of GVHD in the colon of control mice (Figure 1J,K). In contrast, histopathologic examination revealed GVHD of the colon with distinct cell infiltrations and single-cell apoptoses in the allogeneic GVHD group (Figure 1L). There was no histologic evidence of GVHD in the small gut in either control or GVHD groups (Figure 1M-O).
Intestinal GVHD in mice after allogeneic HSCT was associated with increased local FDG uptake mainly localized to the colon. Lethally irradiated CB6F1 mice received 2.0 × 107 BMCs alone (allogeneic control; □) or together with 1.0 × 107 splenocytes (allogeneic GvHD group; ●) from parental BALB/c donors. In the syngeneic control group, CB6F1 recipients were given transplanted of grafts from CB6F1 mice containing 2.0 × 107 BMCs and 1.0 × 107 splenocytes (◇). Body weight loss was used as a measure of GVHD in recipient mice after syngeneic (A) or allogeneic (B,C,P) HSCT. In vivo imaging after application of 10 MBq FDG with a small-animal PET scanner 21 days after transplantation (D-I) demonstrated a marked increase of FDG uptake in the colons of animals with GVHD (F,I) compared with physiologic FDG uptake in the gut of both control groups (panels D,G and E,H). Histopathology of the colon revealed GVHD with tissue infiltration by lymphocytes and mucosa cell apoptosis in the GVHD group (L) and no evidence of GVHD in control animals (J,K). In the small intestine of recipient mice with or without GVHD, only low levels of FDG uptake were observed. Histopathologic analyses of the small intestine showed normal mucosa in all groups (M-O). In an independent set of experiments, intensity and progression of GVHD were correlated with FDG-PET results. GVHD severity was monitored using a clinical score (Q), as detailed in “Methods,” and by body weight loss (P). At each time point, 4 to 6 transplant recipients per group were examined in vivo by PET scan 1 hour after application of 10 MBq FDG. FDG uptake associated with gastrointestinal inflammation was quantified by calculation of FDG uptake in the gut as percentage of the dose injected (R). The results are representative of at least 3 independent experiments. Original magnification for histopathology was 100-fold. Error bars indicate positive standard deviations for each time point. *Statistically significant differences versus both control groups (P < .05).
Intestinal GVHD in mice after allogeneic HSCT was associated with increased local FDG uptake mainly localized to the colon. Lethally irradiated CB6F1 mice received 2.0 × 107 BMCs alone (allogeneic control; □) or together with 1.0 × 107 splenocytes (allogeneic GvHD group; ●) from parental BALB/c donors. In the syngeneic control group, CB6F1 recipients were given transplanted of grafts from CB6F1 mice containing 2.0 × 107 BMCs and 1.0 × 107 splenocytes (◇). Body weight loss was used as a measure of GVHD in recipient mice after syngeneic (A) or allogeneic (B,C,P) HSCT. In vivo imaging after application of 10 MBq FDG with a small-animal PET scanner 21 days after transplantation (D-I) demonstrated a marked increase of FDG uptake in the colons of animals with GVHD (F,I) compared with physiologic FDG uptake in the gut of both control groups (panels D,G and E,H). Histopathology of the colon revealed GVHD with tissue infiltration by lymphocytes and mucosa cell apoptosis in the GVHD group (L) and no evidence of GVHD in control animals (J,K). In the small intestine of recipient mice with or without GVHD, only low levels of FDG uptake were observed. Histopathologic analyses of the small intestine showed normal mucosa in all groups (M-O). In an independent set of experiments, intensity and progression of GVHD were correlated with FDG-PET results. GVHD severity was monitored using a clinical score (Q), as detailed in “Methods,” and by body weight loss (P). At each time point, 4 to 6 transplant recipients per group were examined in vivo by PET scan 1 hour after application of 10 MBq FDG. FDG uptake associated with gastrointestinal inflammation was quantified by calculation of FDG uptake in the gut as percentage of the dose injected (R). The results are representative of at least 3 independent experiments. Original magnification for histopathology was 100-fold. Error bars indicate positive standard deviations for each time point. *Statistically significant differences versus both control groups (P < .05).
GVHD activity parallels FDG uptake of the colon
To determine the dependence of FDG uptake of the colon on GVHD activity over time, we performed serial PET scans of recipients of transplants of BMCs (allogeneic control group) or BMCs and additional splenocytes (GVHD group). Again, initial weight loss was followed by almost complete recovery in control mice after 2 weeks, while recipients of the GVHD group progressively lost weight (Figure 1P). This GVHD-related weight loss was mirrored by rising GVHD scores (Figure 1Q). FDG-PET scans were performed on days 1, 7, 10, 14, 17, 21, and 24 after allogeneic transplantation. Beyond day 7 after transplantation, control animals showed a stable uptake of the administered FDG dosage of approximately 40%, while in recipients of the GVHD group, the FDG uptake in the colon increased to more than 55% over time, indicating aggravation of GVHD-associated tissue inflammation (Figure 1R).
Increased FDG uptake correlates with migration of donor cells into colon tissue
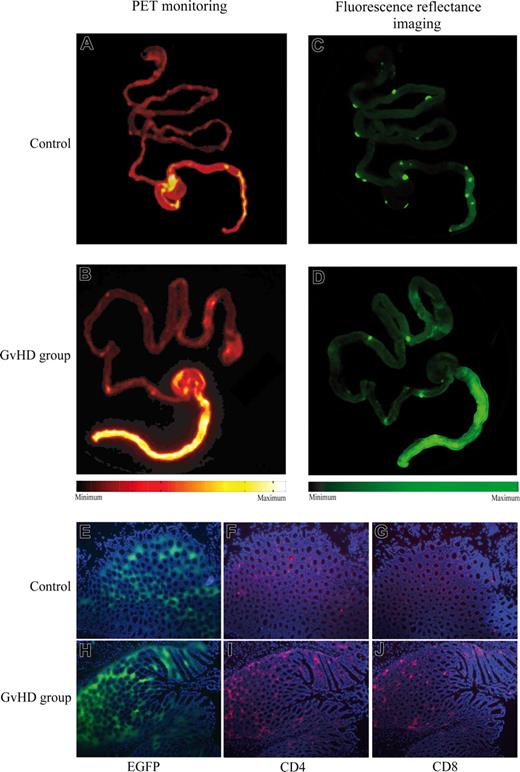
PET imaging and histologic analyses revealed that in the murine transplantation models, GVHD-associated inflammation of the gut was mainly localized to the colon. In order to determine whether the GVHD-associated increase in FDG uptake correlated with organ-specific tissue infiltration by donor cells, we used EGFP-expressing allogeneic donor mice. On day 21 after transplantation, CB6F1 recipients were killed 1 hour after intravenous application of FDG, and the whole gut was removed for PET scanning. As before, recipients of transplants of allogeneic BMCs and splenocytes showed marked FDG uptake preferentially in the colon (Figure 2B) compared with basal FDG uptake in the colon of GVHD-negative mice that received transplants of BMCs alone (Figure 2A). Following PET scans, fluorescence reflectance imaging was performed in order to detect infiltration of intestinal tissue by EGFP+ donor cells. EGFP+ donor cells were predominantly located to Peyer patches and the colon (Figure 3C,D). Matching the significantly increased PET signals, massive infiltration of colonic tissue by EGFP+ cells was observed in mice with intestinal GVHD (Figure 2D). Additional analyses of these colon specimens by fluorescence microscopy demonstrated donor cell infiltration of the mucosa and submucosa (Figure 2E,H). Immunofluorescence staining of the same samples further identified the infiltrating EGFP+ cells predominantly as CD4+ and CD8+ lymphocytes (Figure 2F,G,I,J).
Intestinal FDG-PET signals correlated with tissue infiltration by EGFP+ donor lymphocytes. Lethally irradiated CB6F1 mice received transplants of BMCs alone (A,C) or with bone marrow and additional splenocytes (B,D) from C57BL/6-Tg(ACTB-EGFP) donors. At 1 hour after application of 50 MBq FDG on day 21 after transplantation, the whole gut was removed and scanned by PET (A,B). Subsequently, fluorescence reflectance imaging was performed to detect EGFP+ donor cells (C,D). Colocalized with intestinal FDG uptake, fluorescence imaging demonstrated an accumulation of EGFP+ donor cells in Peyer plaques and in the colon of mice with intestinal GVHD. Analyses of these colon specimens by fluorescence microscopy showed tissue infiltration by EGFP+ donor cells (E,H). Immunofluorescene staining revealed predominant CD4+ (F,I) and CD8+ (G,J) lymphocyte infiltration in EGFP+ areas. For simplification, spleen images were removed from the original PET and fluorescence images, as they were not relevant for intestinal GVHD. The results are representative of at least 3 independent experiments.
Intestinal FDG-PET signals correlated with tissue infiltration by EGFP+ donor lymphocytes. Lethally irradiated CB6F1 mice received transplants of BMCs alone (A,C) or with bone marrow and additional splenocytes (B,D) from C57BL/6-Tg(ACTB-EGFP) donors. At 1 hour after application of 50 MBq FDG on day 21 after transplantation, the whole gut was removed and scanned by PET (A,B). Subsequently, fluorescence reflectance imaging was performed to detect EGFP+ donor cells (C,D). Colocalized with intestinal FDG uptake, fluorescence imaging demonstrated an accumulation of EGFP+ donor cells in Peyer plaques and in the colon of mice with intestinal GVHD. Analyses of these colon specimens by fluorescence microscopy showed tissue infiltration by EGFP+ donor cells (E,H). Immunofluorescene staining revealed predominant CD4+ (F,I) and CD8+ (G,J) lymphocyte infiltration in EGFP+ areas. For simplification, spleen images were removed from the original PET and fluorescence images, as they were not relevant for intestinal GVHD. The results are representative of at least 3 independent experiments.
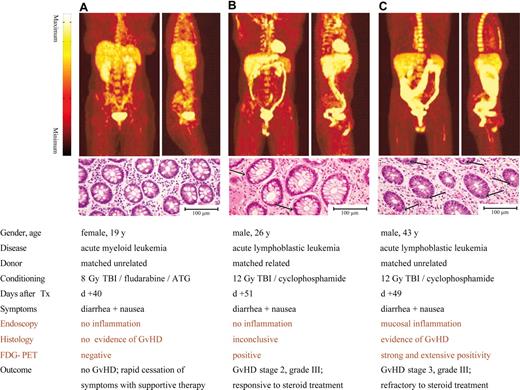
Comparison of FDG-PET images with endoscopic and histopathologic findings in relation to clinical presentation and outcome in patients with suspected intestinal GVHD after allogeneic HSCT. A significant FDG uptake was detected in the colons of patients with clinical signs of GVHD and diagnostic or inconclusive histopathologic findings (B,C). The results are representative of 30 different patients with suspected gastrointestinal GVHD. Arrows indicate apoptotic mucosa cells, compared with physiological findings in a patient with no intestinal GVHD (A).
Comparison of FDG-PET images with endoscopic and histopathologic findings in relation to clinical presentation and outcome in patients with suspected intestinal GVHD after allogeneic HSCT. A significant FDG uptake was detected in the colons of patients with clinical signs of GVHD and diagnostic or inconclusive histopathologic findings (B,C). The results are representative of 30 different patients with suspected gastrointestinal GVHD. Arrows indicate apoptotic mucosa cells, compared with physiological findings in a patient with no intestinal GVHD (A).
FDG uptake maps intestinal GVHD in patients
The preclinical data suggested that FDG-PET could be applied to patients suspected of having intestinal GVHD for visual mapping and assessment of GVHD-associated bowel inflammation. In order to test this hypothesis, 30 patients with clinical signs of intestinal GVHD were enrolled into a prospective cohort study comparing FDG-PET with clinical, endoscopic, and histopathologic evaluation (Table 1). Patients within 20 days of transplantation were excluded to avoid false positive endoscopic or histopathologic results caused by treatment toxicity that might mimic GVHD. As expected from the preclinical mouse studies, an increased intestinal accumulation of FDG was typically observed in patients suffering from clinical GVHD. Figure 3 displays characteristic findings of FDG-PET in the clinical scenarios of no evident GVHD with no to physiologic FDG uptake in the bowel (Figure 3A; Video S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), increased intestinal FDG uptake in GVHD stage II (Figure 3B; Video S2), and massively increased FDG uptake throughout the entire large bowel in GVHD stage III (Figure 3C; Video S3). Visual consensus reading revealed abnormal FDG uptake in 14 of 17 patients with GVHD (FDG-positive, 82%), whereas no single instance of abnormal FDG uptake was found in the 13 patients without GVHD (FDG-negative, 0%). Overall, increased intestinal FDG uptake was found to be a sensitive and very specific noninvasive biomarker of GVHD (sensitivity, 82%; specificity, 100%; NPV, 81%; and PPV, 100%; Table 1.). Patients with increased FDG uptake did not differ from patients with physiologic FDG uptake with respect to clinical characteristics such as symptoms or microbiological findings that potentially influence intestinal FDG uptake (Table 1).
Patient characteristics
| . | FDG-PET negative (n=16 patients) . | FDG-PET positive (n=14 patients) . |
|---|---|---|
| Intestinal GVHD positive, no. patients | 3 | 14 |
| Intestinal GVHD negative, no. patients | 13 | 0 |
| Symptoms, no. patients | ||
| Nausea/vomiting | 6 | 6 |
| Diarrhea > 500 mL/d | 11 | 7 |
| Diarrhea > 1000 mL/d | 5 | 7 |
| Positive microbiological findings | 9 | 6 |
| No. patients* | ||
| Clostridium difficile toxin | 4 | 1 |
| VRE | 1 | 4 |
| CMV | 3 | 1 |
| HHV 6 | 1 | 0 |
| Hepatitis B virus | 1 | 1 |
| Candida spp. | 3 | 0 |
| GVHD of the skin | 8 | 10 |
| Suspected GVHD of the liver | 3 | 2 |
| Clinical grading of GVHD at PET examination, no. patients | ||
| II | 10 | 7 |
| III | 6 | 7 |
| . | FDG-PET negative (n=16 patients) . | FDG-PET positive (n=14 patients) . |
|---|---|---|
| Intestinal GVHD positive, no. patients | 3 | 14 |
| Intestinal GVHD negative, no. patients | 13 | 0 |
| Symptoms, no. patients | ||
| Nausea/vomiting | 6 | 6 |
| Diarrhea > 500 mL/d | 11 | 7 |
| Diarrhea > 1000 mL/d | 5 | 7 |
| Positive microbiological findings | 9 | 6 |
| No. patients* | ||
| Clostridium difficile toxin | 4 | 1 |
| VRE | 1 | 4 |
| CMV | 3 | 1 |
| HHV 6 | 1 | 0 |
| Hepatitis B virus | 1 | 1 |
| Candida spp. | 3 | 0 |
| GVHD of the skin | 8 | 10 |
| Suspected GVHD of the liver | 3 | 2 |
| Clinical grading of GVHD at PET examination, no. patients | ||
| II | 10 | 7 |
| III | 6 | 7 |
FDG-PET results were blinded to treating physicians; the diagnosis of intestinal GVHD was made on the basis of clinical symptoms and signs (eg, stool volume quantification) and histopathologic results.
VRE indicates vancomycin-resistant Enterococcus; CMV, cytomegalovirus; and HHV6, human herpesvirus 6.
Microbiological results from stool analyses within 2 weeks of PET scan. CMV reactivation was diagnosed from blood samples; in none of the patients, histology showed evidence for intestinal CMV infection.
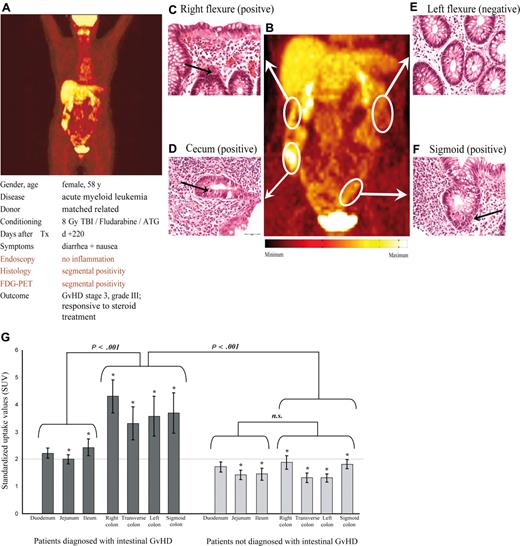
When analyzing regional distribution of the FDG signal throughout the bowel, a characteristic pattern was found. In most FDG-positive patients, enhanced FDG uptake was present in the large bowel, with up to 13 of 14 patients showing intense FDG accumulation in the ascending colon, whereas only 3 FDG-positive patients showed abnormal FDG uptake in the duodenum or jejunum and 6 patients showed abnormal FDG uptake in the ileum. In representative patients, the regional distribution of the FDG signal was correlated to multiple endoscopic biopsies. Figure 4 shows a typical example where the regional FDG uptake is clearly correlated with histologic findings of GVHD in the corresponding biopsies.
FDG-PET identifies areas of inflammation in a case of segmental manifestations of intestinal GVHD and allows noninvasive monitoring of treatment response. The top panels (A-F) show findings in a representative patient diagnosed with intestinal GVHD. PET images showed segmental inflammation of the right colon (SUV, 5.1) and sigmoid colon (SUV, 2.2), and normal FDG uptake in the transverse (SUV, 1.0) and left (SUV, 1.6) colon segments (A,B). Histopathology of serial biopsies of the whole colon verified GVHD in those segments matching with increased local glucose uptake (C,D,F). Histopathology of colon segments with normal PET findings showed no evidence of GVHD (E). Depicted histologies are representative of at least 3 independent mucosal samples of each colon segment. Arrows indicate examples of apoptotic mucosa cells. (G) SUVs of FDG in 30 patients with (left side) or without (right side) clinical proven intestinal GVHD showed significant differences. Analyses of different intestinal segments in patients without intestinal GVHD showed a mean SUV of less than 2. In contrast, colon segments of patients with intestinal GVHD showed significant higher SUV compared with respective segments of the small gut as well as in comparison with colon segments of patients without intestinal GVHD. Error bars indicate SEM for each segment. *Statistically significant differences for each bowel segment of patients with GVHD compared corresponding segments of patients without GVHD (P < .05).
FDG-PET identifies areas of inflammation in a case of segmental manifestations of intestinal GVHD and allows noninvasive monitoring of treatment response. The top panels (A-F) show findings in a representative patient diagnosed with intestinal GVHD. PET images showed segmental inflammation of the right colon (SUV, 5.1) and sigmoid colon (SUV, 2.2), and normal FDG uptake in the transverse (SUV, 1.0) and left (SUV, 1.6) colon segments (A,B). Histopathology of serial biopsies of the whole colon verified GVHD in those segments matching with increased local glucose uptake (C,D,F). Histopathology of colon segments with normal PET findings showed no evidence of GVHD (E). Depicted histologies are representative of at least 3 independent mucosal samples of each colon segment. Arrows indicate examples of apoptotic mucosa cells. (G) SUVs of FDG in 30 patients with (left side) or without (right side) clinical proven intestinal GVHD showed significant differences. Analyses of different intestinal segments in patients without intestinal GVHD showed a mean SUV of less than 2. In contrast, colon segments of patients with intestinal GVHD showed significant higher SUV compared with respective segments of the small gut as well as in comparison with colon segments of patients without intestinal GVHD. Error bars indicate SEM for each segment. *Statistically significant differences for each bowel segment of patients with GVHD compared corresponding segments of patients without GVHD (P < .05).
In a further step, regional intestinal FDG uptake was quantified by analyses of maximum SUVs of ROIs placed on distinct bowel segments (duodenum, jejunum, ileum, right colon, transverse colon, left colon, and sigmoid colon). Figure 4G shows the distribution of FDG uptake assessed by SUV over the intestinal segments. Again, quantitative FDG uptake was found to be significantly higher in all GVHD-positive large bowel segments (increase in FDG uptake ranging from 89% to 129% in GVHD-positive against GVHD-negative) and, to a lesser degree, in 2 of 3 small bowel segments (increase in FDG uptake ranging from 27% to 56% in GVHD-positive against GVHD-negative) as shown in Figure 4G.
Monitoring of treatment response and prognostic significance
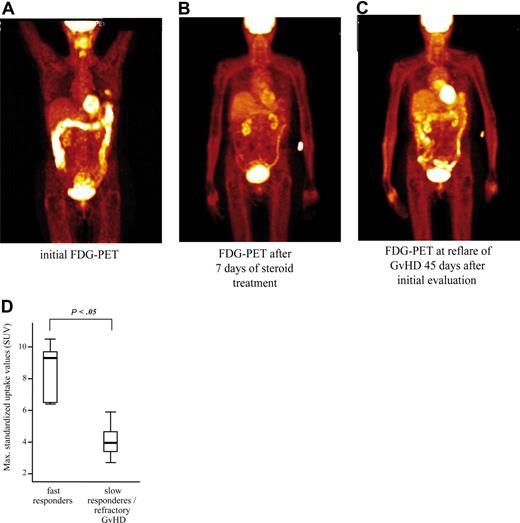
After initially showing increased FDG uptake by PET, 8 patients with recurring or persisting symptoms of intestinal GVHD received a second FDG-PET scan 7 to 20 days after initial PET examination and intensification of immunosuppressive treatment. Of these patients, 4 had responded to GVHD treatment and showed a significant decrease in mean FDG uptake of the colon in all segments from an initial mean SUV plus or minus SEM of 6.9 (± 0.5) to 2.6 (± 0.3; representative example shown in Figure 5A,B). Two of the responders subsequently relapsed with intestinal GVHD. In one of those patients, a third FDG-PET scan was performed at the time of relapse and had turned positive again (Figure 5C). The other relapsing patient died of refractory GVHD before receiving another PET evaluation. In the 4 patients with persisting symptoms, FDG uptake of the colon in the initial PET scan was only moderately increased and remained unchanged on follow-up FDG-PET examinations (initial, maximum SUV ± SEM of 2.6 ± 0.4; follow-up, 2.8 ± 0.3). A total of 3 of these patients died from refractory GVHD, and in a fourth patient, GVHD responded only slowly after prolonged immunosuppressive treatment. These findings gave rise to the hypothesis that patients with rapidly responsive intestinal GVHD have higher SUV at initial examination, indicative of a more active inflammatory component, than nonresponders or slow responders. The assumption of a prognostic significance of the initial PET scan was supported when treatment response of all patients with PET-confirmed GVHD was related to initial FDG uptake (Figure 5D).
Prognostic assessment and monitoring of therapy response of intestinal GVHD with sequential FDG-PET scansin a patient with late onset acute intestinal GVHD. (A) The initial scan showed a distinct inflammation of the whole colon. At that time, histopathology of mucosal specimens obtained from different colon segments revealed typical features of GVHD. (B) After 7 days of corticosteroid treatment, re-evaluation by FDG-PET showed nearly normal glucose uptake of the colon in line with almost complete clinical response of the GVHD. (C) After 45 days, clinical relapse of intestinal GVHD was associated with reappearance of increased glucose uptake of the initially affected sites of the colon. These individual results are representative of 4 different patients with repeated FDG-PET examinations after treatment of gastrointestinal GVHD. (D) Comparison of maximum initial FDG uptake in segmental analysis of the bowel showed that patients with fast response to immunosuppressive treatment within 1 week (5 patients) had higher maximal FDG uptake than those 9 patients with slowly responding (4 patients) or refractory (5 patients) GVHD (presentation of median, minimum, and maximum SUV with quartiles of each group). ATG indicates antithymocyte globulin.
Prognostic assessment and monitoring of therapy response of intestinal GVHD with sequential FDG-PET scansin a patient with late onset acute intestinal GVHD. (A) The initial scan showed a distinct inflammation of the whole colon. At that time, histopathology of mucosal specimens obtained from different colon segments revealed typical features of GVHD. (B) After 7 days of corticosteroid treatment, re-evaluation by FDG-PET showed nearly normal glucose uptake of the colon in line with almost complete clinical response of the GVHD. (C) After 45 days, clinical relapse of intestinal GVHD was associated with reappearance of increased glucose uptake of the initially affected sites of the colon. These individual results are representative of 4 different patients with repeated FDG-PET examinations after treatment of gastrointestinal GVHD. (D) Comparison of maximum initial FDG uptake in segmental analysis of the bowel showed that patients with fast response to immunosuppressive treatment within 1 week (5 patients) had higher maximal FDG uptake than those 9 patients with slowly responding (4 patients) or refractory (5 patients) GVHD (presentation of median, minimum, and maximum SUV with quartiles of each group). ATG indicates antithymocyte globulin.
Discussion
Our observations in mice receiving allogeneic marrow grafts and additional splenocytes for the induction of GVHD suggested that inflammatory activity associated with intestinal GVHD can be assessed by FDG-PET. In view of previous evidence localizing the priming phase of intestinal GVHD to the small intestine and Peyer patches,36,37 our experiments also strongly point to a difference between initiation and the effector phase of intestinal GVHD (ie, the ensuing inflammatory reaction mainly involving the large bowel).
Reasons why other investigators have localized intestinal GVHD to the small gut in addition to the large bowel may reside in apparent differences of the experimental models used, and the timing of assessment (eg, before and after engraftment).38 While some authors have described a predominant inflammation of the colon in their experimental models,39 others have reported inflammation of both the large and small bowel,40 or mainly of the small bowel.41 Differences were not only related to the experimental models used, they also varied between different investigators using the same donor and recipient strains. These differences imply that epigenetic factors such as housing conditions and in consequence differences in microbial colonization could have an influence on GVHD manifestation.
In addition, different aspects of intestinal GVHD visualized by others and our group account for obvious differences in topical assignments. It is important to stress that we did not assess intestinal GVHD directly, but rather visualized GVHD-associated inflammation resulting in enhanced FDG uptake. Therefore, the relatively higher FDG uptake in the colon when compared with the small gut might be caused by a stronger inflammatory response in the colon, where lipopolysaccharides and other microbial proinflammatory stimuli predominantly reside. A weak inflammatory response in the small gut might result in a low FDG uptake and might not be distinguishable from the physiologic FDG background activity in the small gut. These potential limitations of PET sensitivity in detecting inflammatory reactions in different intestinal segments need to be addressed in further studies. Nonetheless, we were able to show that donor T cells were the main driving force of the observed inflammation of colonic tissue. In addition, the progression of overall GVHD activity in the mouse model paralleled increasing inflammation of the gut over time. In these respects, our murine model reflects the essential features of intestinal GVHD after allografting (ie, donor T cell–mediated tissue inflammation with apoptoses, progression over time without treatment, and extensive inflammatory involvement of the large bowel) that readily explains the impressive clinical symptoms.
In patients, manifestations of gastrointestinal GVHD include diarrhea, bleeding, vomiting, and abdominal pain.21 Though nonspecific, these symptoms are the basis for clinical diagnosis and staging. Confirmation of the diagnosis requires the demonstration of apoptosis on mucosal biopsy.9 However, although the diagnosis of gastrointestinal GVHD is unwarranted in its absence, apoptosis is not specific for GVHD.27,42 Furthermore, discordances between biopsy findings from the upper and lower gastrointestinal tract are as common as 28%.43,44 Hence, the segment of gut to target for optimal yield of diagnostic biopsies has remained a matter of controversy.16,27 While some centers favor gastric and, in particular, small bowel biopsies,17,45 others found the distal colon to be the biopsy site with the highest diagnostic yield of up to 82%.46 Thus, it is at least arguable whether gastrointestinal GVHD is a panintestinal process.9 Finally, endoscopic examination with mucosal biopsies in these ill patients who are frequently thrombocytopenic and neutropenic is associated with an increased risk of bleeding and infection. Indeed, endoscopic procedural lethality in HSCT patients has been reported as high as 1.8%.30
Lately, considerable progress has been made in devising novel, noninvasive, radiographic imaging techniques to diagnose inflammatory bowel diseases such as ulcerative colitis and Crohn disease.47 Although still experimental, FDG-PET has been found to be particularly useful in the detection of inflamed areas throughout the entire intestinal tract in Crohn disease, with strikingly high sensitivity and specificity. Particularly in the early phase of intestinal GVHD, other noninvasive methods such as computed or magnetic resonance tomographic imaging and, possibly, high-resolution transabdominal ultrasound and color Doppler are probably less suited to detect acute intestinal GVHD. In addition, they have not been validated for monitoring of treatment response of intestinal GVHD, but are suitable to detect pathological features of advanced intestinal GVHD such as thickening and edema of the intestinal wall.48,49 Thus, they may be useful complementary diagnostic methods providing additional information particularly in advanced or treatment refractory stages of intestinal GVHD.43,50,51
Our clinical results demonstrate that FDG-PET imaging can advance noninvasive diagnostic mapping and monitoring of intestinal GVHD and possibly allow early prognostic assessment of GVHD responsiveness to therapy. We show that FDG-PET detects intestinal inflammation secondary to GVHD early after onset of clinical symptoms with a particularly high specificity and positive predictive value. As demonstrated in 8 of our 30 patients, FDG-PET imaging showed segmental inflammation associated with intestinal GVHD that could be confirmed by targeted biopsies, while biopsies from PET-negative segments were uninformative. In all 17 patients with intestinal GVHD, the diagnosis was confirmed by biopsies from large bowel segments. Serial biopsies from the ileum down to the colon were performed in 6 patients. In only 2 of them, biopsies from the ileum, besides those from the large bowel segments, were positive for GVHD. Therefore, extended prospective studies should include a larger number of patients with serial biopsies from the ileum down to the large bowel.
We were also able to show that PET imaging readily detects treatment responses as well as flare-ups during tapering of immune-suppressive therapy. The relationship between only moderately increased FDG uptake, indicating less GVHD-associated inflammatory activity, and delayed treatment response or treatment resistance may be a prognostic finding of paramount clinical importance. However, these findings have to be interpreted with caution and warrant independent confirmation, as our study cohort comprised only 30 patients. In addition, patients with suspected early intestinal GVHD within 20 days after transplantation were not examined. Since TBI-associated intestinal toxicity and early intestinal GVHD are closely related and most difficult to differentiate, it will be of particular interest to evaluate early intestinal GVHD in future prospective studies of this PET approach.
In 3 patients with a histologic diagnosis of intestinal GVHD, FDG-PET scans were judged normal on visual analyses. One of these 3 patients had new onset of hepatitis B virus reinfection. A second patient judged PET negative on visual analysis had increased FDG uptake of the colon revealed by quantitative analysis (SUV up to 2.8). The third patient had both liver and intestinal GVHD. The calculation of SUV in this patient showed markedly increased FDG uptake of the liver and of the intestine, suggesting that visual analyses of FDG uptake of the gut may be false negative in cases of accompanying GVHD in the liver, a major visual reference organ. Further studies will have to verify whether such patients can indeed be regarded as false negative in FDG-PET. With this patient cohort, we were able to reproduce our preclinical findings and to demonstrate the diagnostic specificity in distinguishing GVHD-associated inflammation from similar clinical presentations secondary to preconditioning and other drug toxicities or nonspecific viral or bacterial bowel infections. On the other hand, in this cohort of sequential patients we had only limited opportunities to perform FDG-PET in patients with proven specific infections of the gut, since early pre-emptive or prophylactic antiviral or antibacterial treatment have made infections caused by, for example, cytomegalovirus or Clostridium difficile rare events.
Thus, FDG-PET imaging is useful as a noninvasive method in diagnosing, visual mapping, and monitoring of the inflammatory component of intestinal GVHD. It also bears the potential for early prognostic GVHD assessment that may lead to better guidance of immunosuppressive treatment strategies. Additional prospects for future clinical applications as well as experimental studies on the mechanisms causing and controlling intestinal GVHD arise from the potential of applying novel tracers designed for imaging of more specific targets such as cellular subpopulations, inflammatory mediators, or signaling molecules.52,–54
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Christine Bätza, Anne Kanzog, and Uta Schnöckel for excellent technical support in the PET studies, and we are grateful to our patients and clinical staff members who participated in and worked on the study.
This work was supported in part by grants from the Wilhelm Sander Foundation (2004.110.1), the German Josè Carreras Leukemia Foundation (DJCLS R 05/35), the Medical Faculty/University of Muenster (IMF-AL120407, IZKF-ZPG 4b), and the Deutsche Forschungsgemeinschaft grant SFB-656 A4.
Authorship
Author contributions: M. Stelljes, S.H., J.A., M. Schäfers, O.S., W.E.B., and J.K. designed and performed experiments, interpreted and analyzed data, generated figures, and wrote the manuscript. V.S., S.V., C.O., C.P., and C.B. performed in vitro and in vivo mouse experiments. G.K., M.L., C.F., T.K. and G.S. performed and analyzed the clinical examinations.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joachim Kienast, Department of Medicine/Hematology and Oncology, University of Muenster, Albert-Schweitzer-Str 33, D-48129 Muenster, Germany; e-mail: kienast@uni-muenster.de; or Michael Schäfers, Department of Nuclear Medicine, University of Muenster, Albert-Schweitzer-Str 33, 48129 Muenster, Germany; e-mail: schafmi@uni-muenster.de.
References
Author notes
M. Stelljes, S.H., and J.A. contributed equally to this study.