Abstract
Since the breakthroughs in combination chemotherapy of patients with Hodgkin disease (HD) starting in the 1960s, prognosis of patients has been rising steadily. Trends in long-term survival of patients with HD on the population level should therefore be monitored in an as timely as possible manner. We assessed trends in age specific 5- and 10-year relative survival of patients with HD in the United States from 1980-1984 to 2000-2004 from the 1973-2004 database of the Surveillance, Epidemiology, and End Results (SEER) Program. Period analysis was used to disclose recent developments with minimum delay. Overall, 5-year relative survival steadily increased from 73.5% to 85.2% (+11.7 percentage units), and 10-year relative survival increased from 62.1% to 80.1% (+18.0 percentage units) between 1980-1984 and 2000-2004, according to period analysis. The increase was particularly pronounced for patients aged 45 to 59 years and 60 years and older (increases in 10-year relative survival by 24.8 and 23.3 percentage points, respectively). Nevertheless, a strong age gradient persisted, with 10-year relative survival of 92.7%, 88.7%, 84.9%, 76.2%, and 44.9% in patients aged 15 to 24 years, 25 to 34 years, 35 to 44 years, 45 to 54 years, and 60 years and older, respectively, in 2000-2004. Our period analysis discloses ongoing, major improvement in long-term survival of patients with HD in recent years, particularly among older patients.
Introduction
Before breakthroughs and introduction of combination chemotherapy in the late 1960s and 1970s, Hodgkin disease (HD) was a highly lethal malignancy, with 5-year survival well below 10%.1 With the introduction of combination chemotherapy in the 1970s, prospects for complete remission and long-term survival increased sharply, but long-term adverse effects of therapy, in particular risk of second malignancy, remained a major concern.2 Subsequent studies have attempted to decrease the side effects of chemotherapy regimens while maintaining or improving the therapeutic efficacy.3,4 Pertinent progress in long-term prognosis should be monitored in a manner as timely as possible, but is only disclosed with substantial delay by conventional methods of survival analysis. We aimed for timely disclosure of trends from 1980-1984 to 2000-2004 of long-term survival of patients with HD by novel techniques of period survival analysis.5,6 Due to the differential application, efficacy, and tolerance of novel therapies according to age, we were specifically interested in age-specific trends of prognosis.
Methods
All data presented in this paper are derived from the 1973-2004 limited-use database of the Surveillance, Epidemiology, and End Results (SEER) Program of the United States National Cancer Institute issued in April 2007.7 Data included in the 1973-2004 SEER database are from population-based cancer registries in Connecticut, New Mexico, Utah, Iowa, Hawaii, Atlanta, Detroit, Seattle–Puget Sound, and San Francisco–Oakland, which together cover a population of about 30 million people. Geographic areas were selected for inclusion in the SEER Program based on their ability to operate and maintain a high-quality population-based cancer reporting system and for their epidemiologically significant population subgroups. The SEER population is comparable with the general United States population with regard to measures of poverty and education, though it tends to be more urban and has a higher proportion of foreign-born persons than the latter.
The SEER database included 16 402 patients aged 15 years or older with a first diagnosis of HD (and no previous cancer diagnosis) between 1980-2004, who have been followed for vital status until the end of 2004. After exclusion of 42 patients (0.26%) who were reported by autopsy only and 46 patients (0.28%) who were reported by death certificate only, there remained 16 314 patients (99.46%) for the analysis.
Survival (5- and 10-year) was calculated for the calendar periods 1980-1984, 1985-1989, 1990-1994, 1995-1999, and 2000-2004 using the period analysis methodology.5 Furthermore, we tested for statistical significance of trends in 5- and 10-year year survival between 1980-1984 and 2000-2004 by a recently described modeling approach.6 All analyses were carried out separately for the following 5 major age groups: 15 to 24 years, 25 to 34 years, 35 to 44 years, 45 to 59 years, and 60 years or older.
With period analysis, first proposed by Brenner and Gefeller in 1996,8 only survival experience during the period of interest is included in the analysis. This is achieved by left truncation of observations at the beginning of the period in addition to right censoring at its end. It has been shown by extensive empirical evaluation that period analysis provides more up-to-date long-term survival figures than traditional “cohort-based” survival analysis, and quite closely predicts long-term survival expectations of cancer patients diagnosed within the period of interest. Nevertheless, even period analysis may still remain somewhat too pessimistic in cases of ongoing major improvement in survival.9,10 With follow-up data available by the end of 2004, 5-year survival could meanwhile be calculated retrospectively for cohorts of patients diagnosed in 1980-1984, 1985-1989, 1990-1994, and 1995-1999, and 10-year survival estimates could meanwhile be calculated for cohorts of patients diagnosed in 1980-1984, 1985-1989, and 1990-1994. In our study, we present such estimates for the cohort of patients diagnosed in 1980-1984. Otherwise, we consistently provide results from period analysis for all time windows, however, to ensure comparability with the 1995-1999 and 2000-2004 results, which are available by period analysis only, and to allow for valid assessment of trends over time.
In addition to 5-year survival from diagnosis, we also calculated 5-year survival in the subsequent 5 years among patients who have already survived 1, 2, 3, 4, or 5 years since diagnosis. This way, trends and recent achievements in late survival can be analyzed specifically. They are of particular interest, given the concern about potential late adverse treatment effects and ongoing attempts to minimize them. For the same reason, and given the relatively young age of most patients with HD, timely monitoring of very-long-term survival is also important. We therefore provide a period analysis for survival over 25 years following diagnosis by age group based on data from the 2000-2004 period. This analysis was restricted to the 4 younger age groups, because 25-year survival is less relevant and estimates are statistically too imprecise for the oldest age group.
According to standard practice in population-based cancer survival analysis, relative rather than absolute survival was calculated. Relative survival reflects survival of patients with cancer compared with survival of the general population. It is calculated as the ratio of absolute survival of patients with cancer divided by the expected survival of a group of persons of the corresponding sex, age, and race in the general population.11,12 Estimates of expected survival were derived according to the so-called Ederer II method13 using US sex-, age-, and race-specific life tables.14 For analyses of 25-year relative survival, expected survival was derived according to Hakulinen's method.15
Results
Numbers of patients by age group and calendar period are shown in Table 1. Overall numbers gradually increased by approximately 10% between 1980-1984 and 2000-2004. However, this overall modest increase was entirely due to a strong increase in case numbers in patients aged 45 to 59 years (+45%) and especially in patients aged 35 to 44 years (+82%), whereas patient numbers slightly declined in the other age groups. Nevertheless, patients aged 25 to 34 years were the largest group in all time periods (overall, 27.6%), despite a decline by more than 10% in the 2 most recent periods. The number of patients exceeded 380 for each combination of age group and calendar period.
Patients with HD by age group and calendar period
| Age . | Calendar period, no. patients . | Total no. patients . | ||||
|---|---|---|---|---|---|---|
| 1980-1984 . | 1985-1989 . | 1990-1994 . | 1995-1999 . | 2000-2004 . | ||
| All | 3082 | 3243 | 3294 | 3312 | 3383 | 16 314 |
| 15 to 24 y | 814 | 801 | 720 | 659 | 695 | 3 689 |
| 25 to 34 y | 881 | 935 | 957 | 902 | 832 | 4 507 |
| 35 to 44 y | 386 | 530 | 582 | 652 | 704 | 2 854 |
| 45 to 59 y | 402 | 426 | 453 | 540 | 581 | 2 402 |
| 60 y and over | 599 | 551 | 582 | 559 | 571 | 2 862 |
| Age . | Calendar period, no. patients . | Total no. patients . | ||||
|---|---|---|---|---|---|---|
| 1980-1984 . | 1985-1989 . | 1990-1994 . | 1995-1999 . | 2000-2004 . | ||
| All | 3082 | 3243 | 3294 | 3312 | 3383 | 16 314 |
| 15 to 24 y | 814 | 801 | 720 | 659 | 695 | 3 689 |
| 25 to 34 y | 881 | 935 | 957 | 902 | 832 | 4 507 |
| 35 to 44 y | 386 | 530 | 582 | 652 | 704 | 2 854 |
| 45 to 59 y | 402 | 426 | 453 | 540 | 581 | 2 402 |
| 60 y and over | 599 | 551 | 582 | 559 | 571 | 2 862 |
The 1980-1984 period analysis would have predicted 5-year relative survival of the cohort of patients diagnosed in 1980-1984 almost perfectly, whereas the 10-year survival period survival analysis would have remained somewhat too conservative (pessimistic; Table 2). According to period analysis, 5-year relative survival increased from 73.5% in 1980 to 1984 to 85.2% in 2000-2004 for all ages combined, an increase of 11.7 percentage points (P value for trend is less than .001). An even stronger increase by 18.0 percentage points, from 62.1% to 80.1%, was seen for 10-year relative survival. In 1980-1984, there was a very strong age gradient in survival, with 5-year relative survival ranging from 86.9% in patients aged 15 to 24 years to 34.8% in patients aged 60 years and older, and 10-year relative survival ranging from 79.8% to 21.6%. A major and significant increase in survival over time was observed in all age groups, but the increase was particularly large for patients aged 45 to 59 years and 60 years and older. In this way, the age gradient was reduced in recent years, although a substantial age gradient still persists. In the 2000-2004 period analysis, age-specific 5-year relative survival ranged from 94.1% to 58.8%, and age-specific 10-year relative survival ranged from 92.7% to 44.9%.
Relative survival in percentage of patients with HD by age groups, calendar period, and type of analysis*
| Relative survival . | Calendar period (type of analysis) . | Increase, %† . | |||||
|---|---|---|---|---|---|---|---|
| 1980-1984 (cohort) . | 1980-1984 (period) . | 2000-2004 (period) . | |||||
| PE, % . | SE . | PE, % . | SE . | PE, % . | SE . | ||
| 5-y | |||||||
| All | 74.5 | 0.8 | 73.5 | 0.9 | 85.2 | 0.7 | 11.7 |
| 15-24 y | 86.7 | 1.2 | 86.9 | 1.2 | 94.1 | 0.9 | 7.2 |
| 25-34 y | 86.3 | 1.2 | 85.2 | 1.3 | 92.6 | 0.9 | 7.4 |
| 35-44 y | 81.9 | 2.0 | 81.2 | 2.3 | 89.3 | 1.2 | 8.1 |
| 45-59 y | 68.5 | 2.5 | 69.0 | 2.5 | 83.3 | 1.8 | 14.3 |
| 60+ y | 37.7 | 2.3 | 34.8 | 2.3 | 58.8 | 2.6 | 24.0 |
| 10-y | |||||||
| All | 66.4 | 0.9 | 62.1 | 1.1 | 80.1 | 0.8 | 18.0 |
| 15-24 y | 80.4 | 1.4 | 79.8 | 1.6 | 92.7 | 1.1 | 12.9 |
| 25-34 y | 78.8 | 1.4 | 74.0 | 1.9 | 88.7 | 1.1 | 14.7 |
| 35-44 y | 75.7 | 2.3 | 68.2 | 3.4 | 84.9 | 1.5 | 16.7 |
| 45-59 y | 55.5 | 2.8 | 51.4 | 3.3 | 76.2 | 2.2 | 24.8 |
| 60+ y | 25.2 | 2.4 | 21.6 | 2.5 | 44.9 | 3.1 | 23.3 |
| Relative survival . | Calendar period (type of analysis) . | Increase, %† . | |||||
|---|---|---|---|---|---|---|---|
| 1980-1984 (cohort) . | 1980-1984 (period) . | 2000-2004 (period) . | |||||
| PE, % . | SE . | PE, % . | SE . | PE, % . | SE . | ||
| 5-y | |||||||
| All | 74.5 | 0.8 | 73.5 | 0.9 | 85.2 | 0.7 | 11.7 |
| 15-24 y | 86.7 | 1.2 | 86.9 | 1.2 | 94.1 | 0.9 | 7.2 |
| 25-34 y | 86.3 | 1.2 | 85.2 | 1.3 | 92.6 | 0.9 | 7.4 |
| 35-44 y | 81.9 | 2.0 | 81.2 | 2.3 | 89.3 | 1.2 | 8.1 |
| 45-59 y | 68.5 | 2.5 | 69.0 | 2.5 | 83.3 | 1.8 | 14.3 |
| 60+ y | 37.7 | 2.3 | 34.8 | 2.3 | 58.8 | 2.6 | 24.0 |
| 10-y | |||||||
| All | 66.4 | 0.9 | 62.1 | 1.1 | 80.1 | 0.8 | 18.0 |
| 15-24 y | 80.4 | 1.4 | 79.8 | 1.6 | 92.7 | 1.1 | 12.9 |
| 25-34 y | 78.8 | 1.4 | 74.0 | 1.9 | 88.7 | 1.1 | 14.7 |
| 35-44 y | 75.7 | 2.3 | 68.2 | 3.4 | 84.9 | 1.5 | 16.7 |
| 45-59 y | 55.5 | 2.8 | 51.4 | 3.3 | 76.2 | 2.2 | 24.8 |
| 60+ y | 25.2 | 2.4 | 21.6 | 2.5 | 44.9 | 3.1 | 23.3 |
PE indicates point estimate; and SE, standard error.
P value for trend from 1980-1984 to 2000-2004 (period analysis) less than .001 for all entries.
Increase from 1980-1984 to 2000-2004 in percentage points (period analysis).
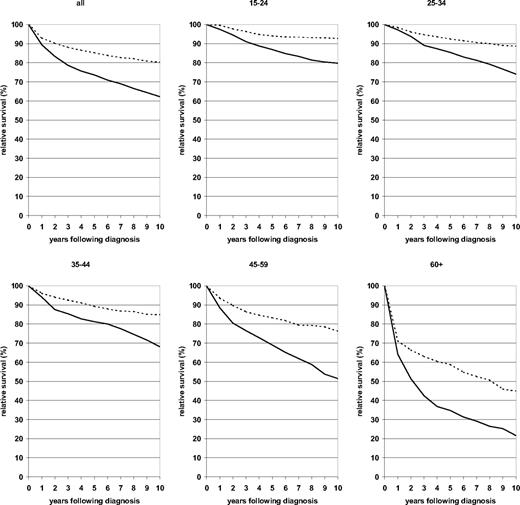
A more comprehensive illustration of the development of the survival curves over 10 years following diagnosis from the earliest to the most recent period is given in Figure 1. In contrast to many other malignancies, the relative survival curves for patients with HD do not flatten out within 10 years following diagnosis. This pattern reflects a substantial proportion of late deaths, especially among elderly patients. However, with the exception of the oldest age group, the decline of the survival curves between 5 and 10 years following diagnosis was strongly reduced over time. For the youngest age group, the relative survival curve hardly declined any longer beyond 5 years after diagnosis in the 2000-2004 period analysis.
Ten-year relative survival curves of patients with HD by major age groups. Period analysis for 1980-1984 (—) and 2000-2004 ( ).
).
Ten-year relative survival curves of patients with HD by major age groups. Period analysis for 1980-1984 (—) and 2000-2004 ( ).
).
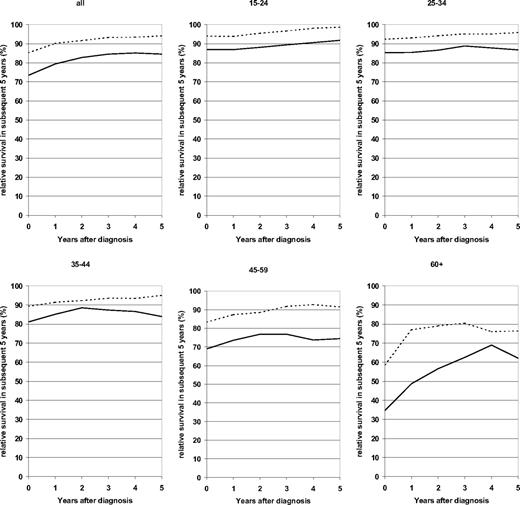
Furthermore, for each of the 3 younger age groups, 5-year relative survival in the subsequent 5 years steadily increased with each year from diagnosis, starting from approximately at or above 90% at the time of diagnosis and exceeding 95% after 5 years following diagnosis in the 2000-2004 period analysis (Figure 2). Conditional survival within the subsequent 5 years exceeded 90% from the third year on in patients aged 45 to 59 years, and it stabilized at levels around 80% among patients aged 60 years or older after surviving the first year following diagnosis in the 2000-2004 period analysis.
Relative survival in subsequent 5 years at various times after diagnosis. Patients with HD by age group. Period analysis for 1980-1984 (—) and 2000-2004 ( ).
).
Relative survival in subsequent 5 years at various times after diagnosis. Patients with HD by age group. Period analysis for 1980-1984 (—) and 2000-2004 ( ).
).
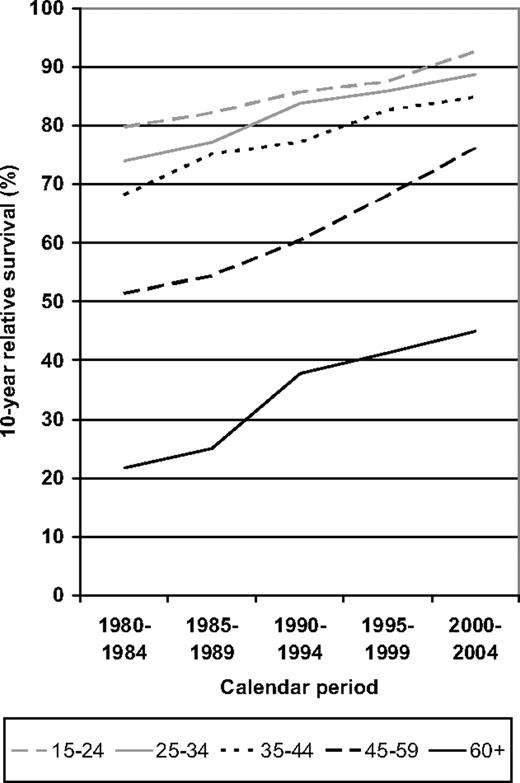
To address the question of when the strongest increase in survival was achieved for the various age groups, 10-year relative survival is shown for each of the 5 calendar periods under investigation in Figure 3. Steady improvement over time was seen for all age groups. The relatively small age gradient between the 3 younger age groups further decreased over time. In the 2 older age groups, there was only moderate improvement from the 1980-1984 to the 1985-1989 period. However, improvement was particularly pronounced in the more recent periods.
Period analysis of 10-year relative survival of patients with HD by major age groups in defined calendar periods from 1980-1984 to 2000-2004.
Period analysis of 10-year relative survival of patients with HD by major age groups in defined calendar periods from 1980-1984 to 2000-2004.
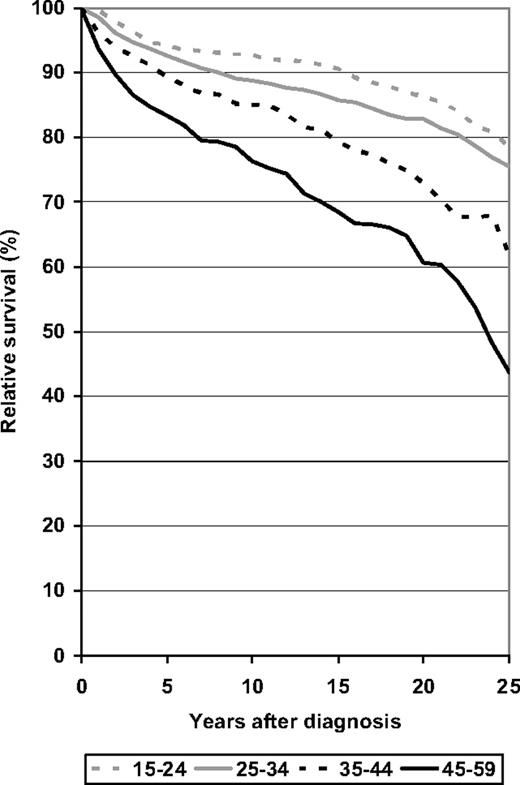
Finally, relative survival over 25 years following diagnosis as derived from the 2000-2004 period analysis is shown for the 4 younger age groups. In all age groups, the decline of the relative survival curve seems to accelerate rather than level off after 10 to 15 years following diagnosis, reaching levels of 79%, 76%, 62%, and 44% after 25 years among patients aged 15 to 24 years, 25 to 34 years, 35 to 44 years, and 45 to 59 years, respectively.
Relative survival over 25 years following diagnosis of patients with HD by major age groups. Period analysis for 2000-2004.
Relative survival over 25 years following diagnosis of patients with HD by major age groups. Period analysis for 2000-2004.
Discussion
This application of period analysis to age specific long-term survival of patients with HD discloses continuing improvement at all ages between 1980-1984 and 2000-2004. From 1985-1989 on, improvement was particularly strong in patients aged 45 to 59 years and 60 years and older. In this way, the strong age gradient in prognosis was reduced to some extent. With the exception of the oldest age group, improvement in survival was particularly strong in the longer run (ie, when patients had already survived the first few years after diagnosis). In patients aged 15 to 24 years, 25 to 34 years, and 35 to 44 years, conditional 5-year relative survival in the subsequent 5 years exceeded 95% among patients who have survived 5 years from diagnosis. However, substantial late mortality persisted even in 2000-2004 among patients who had survived 10 to 15 years since diagnosis. To the best of our knowledge, this is the first in-depth population based analysis of the long-term survival of HD patients by age using the period analysis methodology. Using this approach, we could demonstrate that previously reported progress in outcomes of HD patients in the 1970s, 1980s, and 1990s16,17 continued at an unbroken pace in the early 21st century. These results provide encouraging news in the long-standing and ongoing battle for enhanced long-term life prospects of patients with HD.
HD was first treated with combination chemotherapy, using mechlorethamine, vincristine, procarbazine, and prednisone (MOPP) or a similar regimen with cyclophosphamide substituting for mechlorethamine (COPP) in the 1960s.18,19 These and similar combinations initially produced remissions in 80% or more of patients with HD and was curative in many cases. A second combination regimen, doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), was introduced in the late 1970s.20 Its use was initially restricted to second-line therapy, but in 1987 it was demonstrated to produce results that were as good as or better than those produced by MOPP or COPP, with less toxicity, after which it became the standard first-line therapy of choice.21 In particular, fewer cases of secondary myelodysplastic syndrome (MDS) and acute lymphoblastic leukemia (AML) were observed after the administration of ABVD as compared with MOPP, making it a better choice in a disease that occurs frequently in younger patients. Further attempts to improve chemotherapy in HD were largely unsuccessful until the German Hodgkin Lymphoma Study Group (GHLSG) reported on the results of HD9, a study conducted between 1993-1998. In this study, they compared COPP/ABVD, a hybrid regimen alternating COPP with ABVD, with a new regimen consisting of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and predisone (BEACOPP) in standard or increased doses in advanced HD. They report better complete response rates and 5-year overall survival rates with the increased-dose BEACOPP regimen.22 However, the BEACOPP regimen, like the MOPP regimen, has a relatively high risk of secondary AML. Efforts to find a way to decrease the toxicity of this regimen while keeping its efficacy are under way.
Radiation therapy (XRT) has played a role in the treatment of HD since the 1950s. XRT alone can be curative in early-stage HD. However, a GHLSG study of XRT alone versus 2 courses of chemotherapy with COPP/ABVD followed by XRT in stage I or II HD demonstrated that the addition of 2 courses of chemotherapy improves survival even in early-stage patients.3,23 Therefore, only patients with true stage Ia disease with no adverse prognostic indicators are treated with XRT alone. In general, the movement in recent years has been toward less XRT for HD, as studies have shown smaller fields and smaller doses of XRT to be as effective as larger fields and higher doses but with lower toxicities.3,23 In addition, XRT may lead to secondary malignancies and cardiovascular problems later in life.2,24 Therefore, efforts to find the minimum effective dose and extent of XRT may have benefited some patients in terms of limiting their risk of long-term toxic effects.
Patients with HD were among the first to be treated with stem-cell transplantation (SCT) in the early 1970s.25 However, SCT did not become a part of the routine treatment for recurring HD until the late 1980s to early 1990s, when trials of autologous SCT demonstrated that it was superior to conventional chemotherapy in relapsed or refractory HD, with cure rates of 40% to 70%.26,27 Autologous SCT is the current standard of care for eligible patients with refractory or relapsed HD, and improvements in SCT such as the use of peripheral blood stem-cell collection as well as the extension of the use of SCT to older patients has improved the outlook for patients with refractory and relapsed HD. In general, allogenic SCT has been shown to be more toxic and no more effective than autologous SCT in HD,28 and therefore has not been used much outside the setting of clinical trials.
Survival in older patients with HD has improved steadily and to a greater extent than has survival in younger patients, so that the gap in survival between older and younger patients is decreasing. However, patients aged 60 years and older still have a much worse prognosis than younger patients. Patients aged 45 to 59 years also do worse than younger patients, although not to quite the same extent. The grounds for this difference may be multifactorial. Some researchers have suggested that HD may have a different biology in older adults, with more aggressive behavior29,30 ; however, others have found no major differences in known prognostic factors in older versus younger patients.31 In addition, older adults are more likely to have comorbid problems and less likely to tolerate chemotherapy or SCT well and may not benefit from more aggressive regimens as do younger patients.32,33 Undertreatment may also play a role.29,31 The improvement in survival in older patients seen between the 1980s and early 21st century may reflect more aggressive treatment of this patient population, including increased use of SCT, particularly in patients aged 45 to 59 years, as well as improvements in supportive care that allow the more aggressive treatment to be carried out reasonably safely in older patients. Efforts to find the ideal balance between efficacy and toxicity in older patients with HD are ongoing.34
Multiple studies have demonstrated late complications of HD therapy. These include secondary cancers,2,24,35,36 heart disease,37 stroke,38 pulmonary disease,39 and secondary amenorrhea.40 An excess risk of death due to both hematologic and solid malignancies after successful treatment for HD has been documented.2,24,35,41 The risk of hematologic malignancies, particularly secondary MDS and AML, was highest in the first 10 years after treatment and is decreased in patients diagnosed from 1985-2001 compared with patients diagnosed from 1970-1984 and in patients diagnosed under age 35 years, although there is still a risk above baseline for patients younger than 35 years diagnosed from 1985-2001.35 The risk of solid tumors continues to be increased at least 20 years after diagnosis. A higher risk of breast cancer and supradiaphragmatic tumors has been noted in patients who received XRT versus those who did not, but there is still an increase in patients treated with chemotherapy alone as compared with the general population, and the risk of infradiaphragmatic tumors is no different in patients who received chemotherapy only and those who received XRT.24 In contrast to hematologic malignancies, the increase in risk of secondary solid tumors appears to be less in patients who were diagnosed at older ages.24 An increased risk of death due to cardiac disease after treatment for HD, particularly if XRT or anthracyclines were involved in the treatment, has been observed as well. The greatest increased risk is for patients diagnosed under the age of 35 years, and there may be an increased risk for patients diagnosed before 1980 compared with those diagnosed in 1980 or later.37 Although the risk of cardiac disease and at least some secondary malignancies may be reduced by the use of current protocols, the risk of these and other chronic medical problems remains increased in survivors of HD as compared with their age cohort. Close monitoring of long-term survivors of HD for possible cardiovascular disease and secondary malignancies as well as aggressive treatment of treatable risk factors may help reduce the risk of these secondary causes of death.
In conclusion, our population-based period survival analysis discloses the steady ongoing improvement in 5- and 10-year survival of patients with HD. Timely disclosure of this progress to researchers, clinicians, and patients, enabled by period analysis, should encourage the ongoing efforts in the fight against this formerly deadly disease. Nevertheless, the substantial late mortality even in 2000-2004 among patients who have survived 10 to 15 years remains of concern. It has to be considered, however, that even the 2000-2004 period analysis relies on patients who had their diagnosis and primary treatment in the 1980s or early 1990s for survival beyond 10 to 15 years after diagnosis. To the extent that treatment has become both more efficient and less toxic since then, the very-long-term survival expectations of patients diagnosed from 2000-2004 are expected to be higher than suggested by our 2000-2004 period analysis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
D.P. was supported by a Faculty Research Visit Grant from the German Academic Exchange Service (DAAD) and a Merit Review Grant from the US Department of Veterans Affairs.
Authorship
Contribution: H.B. designed the work and carried out the analysis; H.B. and D.P. wrote the paper; and A.G. critically reviewed and contributed to finalizing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hermann Brenner, Division of Clinical Epidemiology & Aging Research, German Cancer Research Center, Bergheimer Str. 20, D-69115 Heidelberg, Germany; e-mail: h.brenner@dkfz-heidelberg.de.