Abstract
Approximately 10% of the neonates with Down syndrome (DS) exhibit a unique transient leukemia (TL). Though TL resolves spontaneously in most patients, early death and development of myeloid leukemia (ML-DS) may occur. Prognostic factors as well as treatment indication are currently uncertain. To resolve that issue, we prospectively collected clinical, biologic, and treatment data of 146 patients with TL. The 5-year overall survival (OS) and event-free survival (EFS) were 85% plus or minus 3% and 63% plus or minus 4%, respectively. Multivariate analysis revealed a correlation between high white blood cell (WBC) count, ascites, preterm delivery, bleeding diatheses, failure of spontaneous remission, and the occurrence of early death. Treatment with cytarabine (0.5-1.5 mg/kg) was administered to 28 patients with high WBC count, thrombocytopenia, or liver dysfunction. The therapy had a beneficial effect on the outcome of those children with risk factors for early death (5-year EFS, 52% ± 12% vs 28% ± 11% [no treatment]; P = .02). Multivariate analysis demonstrated its favorable prognostic impact. A total of 29 (23%) patients with TL subsequently developed ML-DS. Patients with ML-DS with a history of TL had a significantly better 5-year EFS (91% ± 5%) than those without documented TL (70% ± 4%), primarily due to a lower relapse rate. A history of TL may therefore define a lower-risk ML-DS subgroup. This study was registered at www.clinicaltrials.gov as no. NCT 00111345.
Introduction
Infants with Down syndrome (DS) are at a high risk of developing a transient myeloproliferative disorder (TMD) or transient leukemia (TL).1 It has been estimated that 5% to 10% of all infants with DS exhibit this syndrome.2 TL is unique to children with DS (or trisomy 21 mosaicism) and is characterized by the clonal proliferation of myeloid blasts with megakaryoblastic or erythroblastic features.3 In most patients, TL is self limiting and disappears during the first months of life. The majority of the affected individuals are asymptomatic, and the diagnosis is confirmed by routine complete blood cell (CBC) analysis. A subset of children presents with severe symptoms and potentially lethal peri- and postnatal complications. Previous reports suggested a risk of 11% to 17% for early death.4,5
Hematologic abnormalities, such as polycythemia, anemia, thrombocytopenia, thrombocytosis, or increased erythrocyte mean corpuscular volume, are common findings in patients with DS.6 In addition, 13% to 33% of the patients with TL develop acute myeloid leukemia (AML) within the first 4 years of life.5,7,8 The unique type of AML in DS is referred to as myeloid leukemia of DS (ML-DS),8 which is characterized by the frequent occurrence of acute megakaryoblastic leukemia (AMKL or FAB M7–like), a low diagnostic white blood cell (WBC) count, and young age. Although TL and ML-DS show similar morphologic, immunologic, and genetic features, the course of disease is different. All children with ML-DS require intensive cytostatic chemotherapy to achieve complete remission (CR). In several protocols, relatively high toxic death rates during induction have been observed, due to a high susceptibility for infections and drug-related toxicity.9 Previous reports suggested an increased drug sensitivity, and excellent results have been obtained if attenuated chemotherapy protocols were applied (event-free survival [EFS], approximately 80%).9-12
Recently, acquired mutations in exon 2 of the gene encoding for the transcription factor GATA1 (localized on chromosome X) have been identified in leukemic blasts from virtually all patients with ML-DS and with TL, leading to the exclusive expression of a truncated GATA1 protein (GATA1s).13,14 Two studies reported that GATA1s is insufficient to induce leukemia in the absence of trisomy 21 in either humans15 or mice.16 However, it remains unknown which factors on chromosome 21 cooperate with the oncogenic GATA1s and which factors drive this transition from preleukemia to ML-DS in only a part of these children.17-20
In the past, most of the knowledge about the natural history and biology of TL was gathered from case reports and retrospective reviews.5,8,21 Recently, Massey et al published the first prospective survey of 48 patients with TL (POG 9481).4 Due to the size of the study, their ability to evaluate the correlation between covariates and outcome was limited. In some previous studies, cytostatic therapy for children with TL was recommended, but proven evidence of efficacy is lacking.22,23 To date, clear prognostic factors, as well as treatment indications, remain elusive.
To address this issue, we prospectively collected the clinical, biological, cytogenetic, and therapeutic data of the largest TL cohort reported to date (n = 146).
Methods
Patients
Between January 1, 1993, and December 31, 2006, 146 children with trisomy 21 and morphologic evidence of myeloid blasts in the peripheral blood or bone marrow aspirate (more than 5%) within the first 6 months of life were registered in the trials of the AML-BFM study group. After confirmation of diagnosis, parental informed consent for data registration and follow-up was obtained in all patients according to local laws and regulations. All investigations have been approved by the Institutional Review Board and Ethics Committees. Approval was obtained from the Ethical Committee of the rztekammer Westfalen-Lippe (3VCreutzig3) and confirmed by the Ethical Committee of the Hannover Medical School (no. 4378), the institutional review boards for these studies.
Clinical and laboratory data
The following clinical data were collected: sex, birth mode, gestational age, birth weight and length, congenital malformations, time of diagnosis, symptoms at diagnosis, clinical presentation and general condition at diagnosis, and presence of organomegaly or pericardial/pleural/ascitic effusions. The following laboratory data were obtained: CBC and WBC counts, coagulation parameters, percentage of blasts in the peripheral blood and in the bone marrow, liver enzymes (alanine aminotransferase [ALT], aspartate aminotransferase [AST], and alkaline phosphatase), direct and indirect bilirubin, and renal function. Pathologic coagulation was diagnosed in accordance to the National Cancer Institute Common Toxicity Criteria (NCI CTC) version 2.0 as grade 2 or higher (disseminated intravascular coagulation; international normalized ratio or partial thromboplastin time more than 1.5-fold higher than upper limits of normal). Whereas data on CBC counts, WBC count and percentage of blasts were documented in 128 children, data of the various clinical symptoms were available in 78 to 146 patients, depending on the parameter. Follow-up data were documented in all children.
Diagnostic data
In all children, central reference morphology, cytochemistry (ie, peroxidase, periodic acid Schiff, and esterase), and immunophenotyping were performed at the AML-BFM reference laboratory (Medical School Hannover [from 2005 on], University Children's Hospital Muenster [1993-2005], and reviewed in an annual expert meeting by external hematologists.
Immunophenotyping was performed as described previously.3 Status of remission of TL or response to low-dose cytarabine therapy was analyzed at the age of 6 months.
Genetic data
Constitutional trisomy 21 was confirmed by the participating hospitals. Molecular genetics and cytogenetics of the megakaryoblasts were performed with standard methods at the AML-BFM reference laboratories.24 Results of the cytogenetic analysis of the megakaryoblasts were available in 80 (55%) children. Lack of data concerned mostly children of the early study phase or failures. Single and miscellaneous chromosomal aberrations, which have not been reported in association with leukemia, were considered as unspecific aberrations. More than 3 chromosomal aberrations were considered as a complex karyotype (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
After August 2003, we screened for GATA1 mutations in the blasts of 25 children. DNA was extracted from peripheral blood, bone marrow, or smears using the NucleoSpin Blood Kit (MACHEREY-NAGEL, Düren, Germany). Amplification of exons 2 and 3 and bidirectional sequencing on an ABI 3700 sequencer (Applera, Foster City, CA) were performed as described previously.17,25 Bidirectional sequence traces were assembled and analyzed for mutations using ClustalW.26
Therapy
Low-dose cytarabine (0.5 to 1.5 mg/kg) treatment for 3 to 12 days was recommended in case of clinical impairment due to high WBC count (> 50 000 leukocytes/μL), thrombocytopenia (platelet count < 100 × 109/L [100 000/μL]), or signs of cholestasis or liver dysfunction. Cholestasis was defined as increased conjugated bilirubin (15 mg/mL). Liver dysfunction was considered in case of cholestasis, severe increase of transaminases (more than 2 times the standard deviation [SD] of the normal values), or hepatomegaly (more than 3 cm above the age adjusted norm confirmed by ultrasound). Children with ML-DS were recommended to be treated according to the AML-BFM protocols,9 which provided separate treatment guidelines for children with ML-DS (AML-BFM 93, 98 and 04). For comparison, all patients with ML-DS (n = 142; acute megakaryoblastic leukemia, younger than 5 years) without documented TL treated according to the same AML-BFM protocols and registered during the same time period were used.9
Statistical analysis
EFS was defined as the time from diagnosis to the date of last follow-up or first event. Events were development of AML or death from any cause. Survival was defined as the time of diagnosis to death from any cause. The Kaplan-Meier method was used to estimate survival rates,27 differences were compared with the 2-sided log-rank test.28 Cumulative incidence (CI) functions for competing events were constructed by the method of Kalbfleisch and Prentice,29 and were compared with the Gray test.30 Differences in the distribution of individual parameters among patient subsets were analyzed using the chi-squared test for categorized variables and the Mann-Whitney U test for continuous variables. The Cox proportional hazards model has been used to obtain the estimates and the 95% confidence interval of the relative risk for prognostic factors.31 We performed Cox multivariate analysis in 2 steps. First, we only included all findings at diagnosis, regardless of their significance in the univariate analysis. In a second step, we also included those variables that occurred during the course of the disease. The limited power of subgroup analysis due to small numbers has to be considered.
Results
Patient characteristics
Between 1993 and 2006, 146 children with TL were registered in the AML-BFM studies. The ratio of males (n = 80) to females (n = 66) was 1.2:1.0. The median age at diagnosis was 3 days, ranging from 0 to 65 days. The percentage of children born by preterm labor was 47% (n = 37); the earliest delivery was at 30 weeks of gestation. Prenatal diagnosis of TL was made in one child of our series at week 35 of gestation by umbilical blood sampling. The median birth weight of 2850 g corrected for gestational age corresponded to the 25th percentile, ranging from the third to the 85th (Table 1).
Clinical and laboratory findings at diagnosis and during the course of the disease
| . | Total (n = 146) . | Early death (n = 22) . | AML (n = 29) . | Cytarabine (n = 28) . |
|---|---|---|---|---|
| Median birth weight, kg (range)* | 2.9 (1.3-5.6) | 2.6 (1.3-4.2) | 2.8 (2-3.8) | 2.8 (1.3-5.6) |
| Median birth length, cm (range)† | 48.0 (34-66) | 46.5 (36-53) | 49.0 (41-66) | 48.5 (36-56) |
| Median gestational age, wk (range)‡ | 37.0 (30-41) | 35.0 (30-40) | 38.0 (34-40) | 36.0 (30-40) |
| Median WBC count, × 109/L (range)§ | 40.3 (3.9-556) | 74 (6-410) | 42.1 (5.1-131) | 62.4 (5.5-556) |
| Median platelets, × 109/L§ | 119 (4-1047) | 149 (30-717) | 81 (22-1047) | 135 (20-1047) |
| Median hemoglobin, g/L (range)§ | 14.5 (4.8-25.7) | 13.8 (9.2-18.7) | 14.3 (8.4-20.4) | 13.7 (6.7-21.7) |
| Median blasts PB, % (range)§ | 39 (2-95) | 37 (3-95) | 32.5 (9-81) | 38 (4-95) |
| Median blasts BM, % (range)* | 32 (5-93) | 18 (5-45) | 15 (10-67) | 17 (265-54) |
| Elevated AST, % (no.)‖ | 28 (27) | 29 (5) | 27 (4) | 48 (12) |
| Elevated ALT, % (no.)‖ | 24 (23) | 35 (6) | 27 (4) | 52 (13) |
| Pathologic coagulation, % (no.)* | 22 (23) | 45 (9) | 18 (3) | 42 (11) |
| Hydrops fetalis, % (no.)¶ | 5 (7) | 23 (5) | 3 (1) | 18 (5) |
| Pleural effusion, % (no.) ¶ | 16 (24) | 23 (5) | 31 (9) | 29 (8) |
| Pericardial effusion, % (no.) ¶ | 12 (17) | 14 (3) | 21 (6) | 21 (6) |
| Ascites, % (no.) ¶ | 8 (12) | 32 (7) | 10 (3) | 14 (4) |
| Splenomegaly, % (no.)* | 42 (44) | 53 (10) | 53 (9) | 62 (16) |
| Hepatomegaly, % (no.)* | 60 (62) | 68 (13) | 65 (11) | 73 (19) |
| Cholestasis, % (no.)* | 15 (16) | 37 (7) | 12 (2) | 23 (6) |
| Liver fibrosis, % (no.)* | 7 (7) | 35 (7) | 0 (0) | 12 (3) |
| Liver dysfunction, % (no.) ‡ | 41 (32) | 65 (11) | 54 (7) | 63 (10) |
| Renal failure, % (no.)* | 6 (6) | 32 (6) | 0 (0) | 8 (2) |
| Bleeding diatheses, % (no.)* | 9 (9) | 32 (6) | 0 (0) | 23 (6) |
| Intensive care, % (no.) ¶ | 25 (37) | 68 (15) | 21 (6) | 46 (13) |
| Ventilation required, % (no.)* | 29 (30) | 74 (14) | 24 (4) | 42 (11) |
| Therapy applied, % (no.) ¶ | 19 (28) | 32 (7) | 21 (6) | 100 (28) |
| Cardiac defects, % (no.) ¶ | 47 (68) | 59 (13) | 45 (13) | 50 (14) |
| Intestinal stenosis, % (no.)* | 1 (1) | 5 (1) | 0 (0) | 4 (1) |
| Free trisomy, % (no.)* | 66 (69) | 80 (16) | 59 (10) | 77 (20) |
| Mosaicism, % (no.)* | 7 (7) | 5 (1) | 6 (1) | 0 (0) |
| Translocation, % (no.)* | 2 (2) | 0 (0) | 6 (1) | 0 (0) |
| . | Total (n = 146) . | Early death (n = 22) . | AML (n = 29) . | Cytarabine (n = 28) . |
|---|---|---|---|---|
| Median birth weight, kg (range)* | 2.9 (1.3-5.6) | 2.6 (1.3-4.2) | 2.8 (2-3.8) | 2.8 (1.3-5.6) |
| Median birth length, cm (range)† | 48.0 (34-66) | 46.5 (36-53) | 49.0 (41-66) | 48.5 (36-56) |
| Median gestational age, wk (range)‡ | 37.0 (30-41) | 35.0 (30-40) | 38.0 (34-40) | 36.0 (30-40) |
| Median WBC count, × 109/L (range)§ | 40.3 (3.9-556) | 74 (6-410) | 42.1 (5.1-131) | 62.4 (5.5-556) |
| Median platelets, × 109/L§ | 119 (4-1047) | 149 (30-717) | 81 (22-1047) | 135 (20-1047) |
| Median hemoglobin, g/L (range)§ | 14.5 (4.8-25.7) | 13.8 (9.2-18.7) | 14.3 (8.4-20.4) | 13.7 (6.7-21.7) |
| Median blasts PB, % (range)§ | 39 (2-95) | 37 (3-95) | 32.5 (9-81) | 38 (4-95) |
| Median blasts BM, % (range)* | 32 (5-93) | 18 (5-45) | 15 (10-67) | 17 (265-54) |
| Elevated AST, % (no.)‖ | 28 (27) | 29 (5) | 27 (4) | 48 (12) |
| Elevated ALT, % (no.)‖ | 24 (23) | 35 (6) | 27 (4) | 52 (13) |
| Pathologic coagulation, % (no.)* | 22 (23) | 45 (9) | 18 (3) | 42 (11) |
| Hydrops fetalis, % (no.)¶ | 5 (7) | 23 (5) | 3 (1) | 18 (5) |
| Pleural effusion, % (no.) ¶ | 16 (24) | 23 (5) | 31 (9) | 29 (8) |
| Pericardial effusion, % (no.) ¶ | 12 (17) | 14 (3) | 21 (6) | 21 (6) |
| Ascites, % (no.) ¶ | 8 (12) | 32 (7) | 10 (3) | 14 (4) |
| Splenomegaly, % (no.)* | 42 (44) | 53 (10) | 53 (9) | 62 (16) |
| Hepatomegaly, % (no.)* | 60 (62) | 68 (13) | 65 (11) | 73 (19) |
| Cholestasis, % (no.)* | 15 (16) | 37 (7) | 12 (2) | 23 (6) |
| Liver fibrosis, % (no.)* | 7 (7) | 35 (7) | 0 (0) | 12 (3) |
| Liver dysfunction, % (no.) ‡ | 41 (32) | 65 (11) | 54 (7) | 63 (10) |
| Renal failure, % (no.)* | 6 (6) | 32 (6) | 0 (0) | 8 (2) |
| Bleeding diatheses, % (no.)* | 9 (9) | 32 (6) | 0 (0) | 23 (6) |
| Intensive care, % (no.) ¶ | 25 (37) | 68 (15) | 21 (6) | 46 (13) |
| Ventilation required, % (no.)* | 29 (30) | 74 (14) | 24 (4) | 42 (11) |
| Therapy applied, % (no.) ¶ | 19 (28) | 32 (7) | 21 (6) | 100 (28) |
| Cardiac defects, % (no.) ¶ | 47 (68) | 59 (13) | 45 (13) | 50 (14) |
| Intestinal stenosis, % (no.)* | 1 (1) | 5 (1) | 0 (0) | 4 (1) |
| Free trisomy, % (no.)* | 66 (69) | 80 (16) | 59 (10) | 77 (20) |
| Mosaicism, % (no.)* | 7 (7) | 5 (1) | 6 (1) | 0 (0) |
| Translocation, % (no.)* | 2 (2) | 0 (0) | 6 (1) | 0 (0) |
Comparison between different subgroups and the total group of TL.
n = 104.
n = 46.
n = 78.
n = 128.
n = 96.
Data were available in all children (n=146).
Presentation at diagnosis
Most of the patients presented with hepatomegaly (60%), splenomegaly (42%), pleural/pericardial effusions (23%), or ascites (12%; Table 1). Only 14 (10.2%) children did not have relevant clinical symptoms (World Health Organization [WHO] grade 1) at diagnosis. A total of 7 children with TL diagnosed after birth suffered from hydrops fetalis. In 66 (45%) children cardiac defects were diagnosed.
Median WBC count at diagnosis was 40.3 × 109/L (range, 3.9-556 × 109/L), median platelet count was 119 × 109/L (119 000/μL; range, 4-1047 × 109/L [4000-1 047 000/μL), and median hemoglobin was 145 g/L (14.5 g/dL; range, 48-257 g/L [4.8-25.7 g/dL]; Table 1). The percentage of children with high WBC count (> 100 ×109/L [100 000/μL]) and thrombocytopenia (platelet count < 100 × 109/L [100 000/μL]) were 20% and 41%, respectively.
A total of 37 (25%) children were referred to an intensive care unit for respiratory failure (n = 11), prematurity (n = 3), abnormal coagulation (n = 7), hydrops fetalis (n = 7), or cardiac defects (n = 21).
Immunophenotyping
Blasts from children with TL expressed a unique immunophenotype with stem cell antigens (CD34/CD117; 93%), myeloid antigens (CD33/CD13; 100%), megakaryocytic antigens (CD41, CD42b; 62%), thrombospondin receptor (CD36; 100%), natural killer cell antigen CD56 (79%), and T-cell antigen CD7 (100%). The blast cells of all patients with TL were positive for thrombopoietin receptor (TPO-R) and IL-3 receptor alpha (IL-3Rα), whereas erythropoietin receptor (EPO-R) and IL-6 receptor alpha (IL-6Rα) were absent.
Taken together, the immunophenotype of blast cells from children with TL is characterized by the expression of CD33+/CD13+/−/CD38+/CD117+/CD34+/CD7+/CD56+/−/CD36+/CD71+/ CD42b+/CD4dim+/TPO-R+/EPO-R−/IL-3Rα+/IL-6Rα-, as described previously.3
Genetics
Most of the patients showed free constitutional trisomy 21 (Table 1). A total of 7 children had trisomy 21 mosaicism and another 2 children carried translocations of chromosome 21. In addition to the constitutional trisomy 21, cytogenetic aberrations were found in 17 (21%) of 80 children, and mostly involved unspecific aberrations (23%) or complex karyotypes (29%). MLL gene rearrangements, as diagnosed by fluorescence in situ hybridization, were the most frequent single abnormality (n = 3; 17%). The remainder consisting of “other aberrations” summed to 29% (n = 5). Interestingly, all children with TL and MLL gene rearrangements achieved spontaneous remission, although one child progressed to ML-DS later on. Another TL patient who had the translocation t(12;21) died due to ascites and respiratory failure despite rapid clearance of the blasts.
Most patients (n = 63; 79%) had trisomy 21c as the only cytogenetic aberration. Additional cytogenetic aberrations did not turn out to be a prognostic marker. Overall survival (OS), EFS, and CI of death were similar in the patients with and without additional cytogenetic aberrations (5-year OS, 82% ± 9% vs 76% ± 5%, Plog rank = .6; and 5-year EFS, 62% ± 12% vs 60% ± 6%, Plog rank = .71; CI death, 18% ± 13% vs 20% ± 5%, PGray = .75).
Children who later developed ML-DS did not differ in cytogenetics when compared with children who stayed disease free. In 3 children, no aberrations in addition to constitutional trisomy 21 were shown in paired TL and ML-DS samples. In another 5 children who developed ML-DS after TL, clonal evolution with additional acquired AML associated aberrations could be detected (del(1q), der(3q), trisomy 19, trisomy 11; Table S1); however, a loss of additional aberration was also found.
GATA1 mutations were identified in all 25 children analyzed. All mutations laid within exon 2, leading either to the introduction of a premature stop codon, a frameshift, or an altered splicing, which results in the expression of truncated GATA1s. Sequencing data of 4 children from whom material was available for both TL and ML-DS revealed the same GATA1 mutation in the paired cases, suggesting clonal stability.
Outcomes
CR was achieved in 122 (84%) children with TL. Most of the patients (n = 97; 66%) showed spontaneous remission (Table 1).
In total, 24 children died, including 22 within the first 6 months of life. Two children older than 1 year died secondary to ML-DS. Causes of early death were cardiac defects (18%; n = 4), multiorgan failure associated with high WBC count (27%; n = 6), liver fibrosis (32%; n = 7), and other congenital abnormalities (23%; n = 5). Excluding death from cardiac defects, congenital abnormalities, or ML-DS, in 13 children (9%), death was caused by mainly TL-related complications. A total of 32 (21%) children developed symptoms of liver dysfunction with conjugated hyperbilirubinemia or elevated liver enzymes. As 24 of those had cardiac defects as well, it is difficult to determine the etiology of the liver dysfunction.
Liver fibrosis was diagnosed in 7 children, and all of them died. In 4 children, liver biopsies were performed and extramedullary hematopoiesis, infiltration with megakaryoblasts, fibrosis, and cholestasis were proven in 2 of them after death.
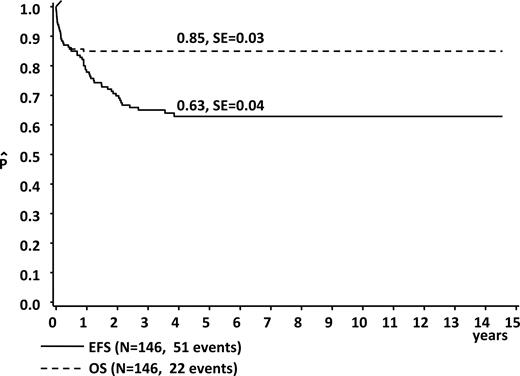
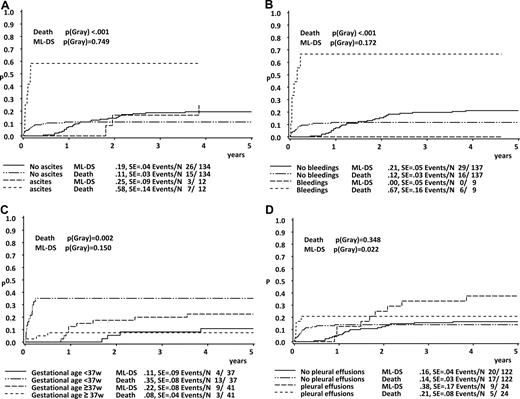
The estimated 5-year OS and EFS for the whole group were 85% plus or minus 3% and 63% plus or minus 4%, respectively (Figure 1). Frequent findings at diagnosis in the children who went on to early death in comparison to those who did not were hydrops fetalis (23%; n = 5 vs 2%, n = 2; Pχ2 < .001), ascites (32%; n = 7 vs 11%, n = 9; Pχ2 < .001), pathologic coagulation parameters (45%; n = 6 vs 16%, n = 14; Pχ2 < .001), and higher median WBC count (74 × 109/L [74 000/μL] vs 34.6 × 109/L [34 600/μL];PU test = .005; Table 1). Children with ascites (n = 12) at diagnosis invariably had a fatal outcome, with a 5-year EFS of 0% (vs 68% ± 4% without ascites, n = 134; Plog rank < .001).
Event-free and overall survival in children with Down syndrome and transient leukemia.
Event-free and overall survival in children with Down syndrome and transient leukemia.
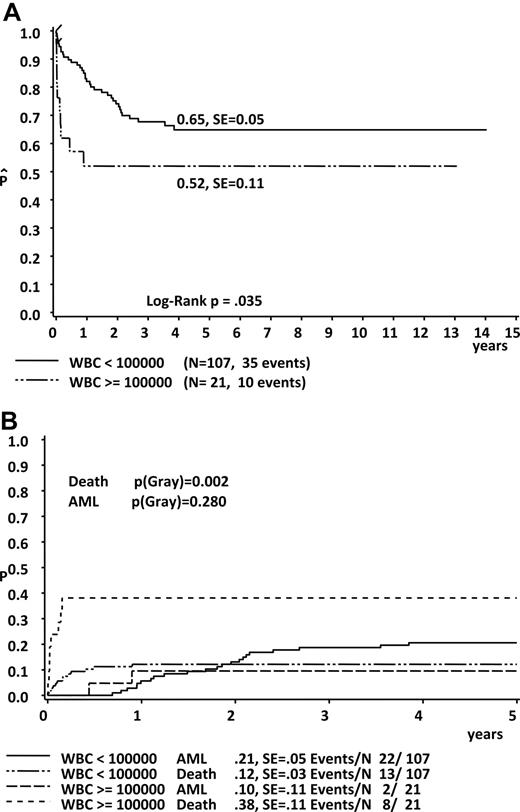
Using univariate analysis (Gray test), we could determine high WBC count (WBC > 100 × 109/L), hydrops fetalis, ascites, effusions (pleural, pericardial, ascites or hydrops), coagulopathy, bleeding diatheses, platelet levels higher than 100 × 109/L, preterm delivery, and low birth weight (< 3 kg) as findings at diagnosis to be significantly associated with the incidence of early death (Table 2; Figures 2B,3A-D). During the course of the disease the occurrence of cholestasis, liver fibrosis, liver dysfunction, renal failure, and failure of spontaneous remission appeared to correlate with the probability of early death (Table 2). Covariates associated with a poor EFS (log-rank test) were high WBC count, effusions, hydrops fetalis, pleural effusions, liver dysfunction, renal failure, coagulopathy, and bleeding diatheses (Table 3; Figure 2A). There was no significant difference in the 5-year OS between boys and girls (5-year EFS, 64% ± 5% vs 60% ± 7%; Plog rank = .72) and between patients, who were asymptomatic or symptomatic at diagnosis (5-year EFS, 76% ± 12% vs 64% ± 4%; Plog rank = .25).
Findings at diagnosis and during the course of TL significantly associated with a higher cumulative incidence of death (CID)
| Variable . | CID at 5 y, % . | PGray . | |
|---|---|---|---|
| Yes . | No . | ||
| At diagnosis | |||
| High WBC count (> 100 ×109/L) | 38 ± 11 | 12 ± 3 | .002 |
| Hydrops fetalis | 71 ± 17 | 12 ± 3 | < .001 |
| Ascites | 58 ± 14 | 11 ± 3 | < .001 |
| Effusions (pleural, pericardial, ascites, or hydrops) | 30 ± 8 | 11 ± 3 | .008 |
| Coagulopathy | 39 ± 10 | 11 ± 3 | < .001 |
| Bleeding diatheses | 67 ± 16 | 12 ± 3 | < .001 |
| Platelet levels > 100 × 109/L | 21 ± 5 | 6 ± 3 | .023 |
| Preterm delivery | 35 ± 8 | 8 ± 4 | .002 |
| Birth weight less than 3 kg | 33 ± 9 | 14 ± 4 | .021 |
| During course of disease | |||
| Cholestasis | 38 ± 12 | 12 ± 3 | .007 |
| Liver fibrosis | 100 ± 13 | 12 ± 3 | < .001 |
| Liver dysfunction | 31 ± 8 | 11 ± 3 | .003 |
| Renal failure | 100 | 11 ± 3 | < .001 |
| Variable . | CID at 5 y, % . | PGray . | |
|---|---|---|---|
| Yes . | No . | ||
| At diagnosis | |||
| High WBC count (> 100 ×109/L) | 38 ± 11 | 12 ± 3 | .002 |
| Hydrops fetalis | 71 ± 17 | 12 ± 3 | < .001 |
| Ascites | 58 ± 14 | 11 ± 3 | < .001 |
| Effusions (pleural, pericardial, ascites, or hydrops) | 30 ± 8 | 11 ± 3 | .008 |
| Coagulopathy | 39 ± 10 | 11 ± 3 | < .001 |
| Bleeding diatheses | 67 ± 16 | 12 ± 3 | < .001 |
| Platelet levels > 100 × 109/L | 21 ± 5 | 6 ± 3 | .023 |
| Preterm delivery | 35 ± 8 | 8 ± 4 | .002 |
| Birth weight less than 3 kg | 33 ± 9 | 14 ± 4 | .021 |
| During course of disease | |||
| Cholestasis | 38 ± 12 | 12 ± 3 | .007 |
| Liver fibrosis | 100 ± 13 | 12 ± 3 | < .001 |
| Liver dysfunction | 31 ± 8 | 11 ± 3 | .003 |
| Renal failure | 100 | 11 ± 3 | < .001 |
Significantly impaired prognosis in children with high WBC count at diagnosis. (A) EFS. (B) Cumulative incidence of death and ML-DS.
Significantly impaired prognosis in children with high WBC count at diagnosis. (A) EFS. (B) Cumulative incidence of death and ML-DS.
Cumulative incidence of death and of ML-DS in children with TL. Patients grouped according to the appearance of (A) ascites, (B) bleeding diatheses, (C) preterm delivery, and (D) pleural effusions.
Cumulative incidence of death and of ML-DS in children with TL. Patients grouped according to the appearance of (A) ascites, (B) bleeding diatheses, (C) preterm delivery, and (D) pleural effusions.
Findings at diagnosis and during the course of TL significantly associated with a poor EFS
| Variable . | 5-year EFS, % . | Plog rank . | |
|---|---|---|---|
| Yes . | No . | ||
| At diagnosis | |||
| High WBC count (>100 ×109/L) | 52 ± 11 | 65 ± 5 | .035 |
| Effusions | 31 ± 9 | 71 ± 4 | < .001 |
| Hydrops fetalis | 14 ± 13 | 66 ± 4 | < .001 |
| Pleural effusions | 36 ± 11 | 68 ± 4 | .0071 |
| Coagulopathy | 48 ± 10 | 68 ± 6 | .013 |
| Bleeding diatheses | 33 ± 16 | 67 ± 5 | .001 |
| During course of disease | |||
| Liver dysfunction | 47 ± 9 | 73 ± 7 | .013 |
| Renal failure | 0 | 68 ± 5 | < .001 |
| Variable . | 5-year EFS, % . | Plog rank . | |
|---|---|---|---|
| Yes . | No . | ||
| At diagnosis | |||
| High WBC count (>100 ×109/L) | 52 ± 11 | 65 ± 5 | .035 |
| Effusions | 31 ± 9 | 71 ± 4 | < .001 |
| Hydrops fetalis | 14 ± 13 | 66 ± 4 | < .001 |
| Pleural effusions | 36 ± 11 | 68 ± 4 | .0071 |
| Coagulopathy | 48 ± 10 | 68 ± 6 | .013 |
| Bleeding diatheses | 33 ± 16 | 67 ± 5 | .001 |
| During course of disease | |||
| Liver dysfunction | 47 ± 9 | 73 ± 7 | .013 |
| Renal failure | 0 | 68 ± 5 | < .001 |
Multivariate analysis confirmed a strong correlation between high WBC count, preterm delivery, and ascites (findings at diagnosis), as well as failure of spontaneous clearing of peripheral blasts (during the course of TL), and both the incidence of early death and a poor EFS (Table 4). Furthermore, bleeding diatheses were predictive for early death, and the presence of renal failure was predictive for a poor EFS using multivariate analysis.
Multivariate model of prognostic factors for early death
| Variable . | Hazard ratio . | Confidence interval, 95% . | P . |
|---|---|---|---|
| High-risk | |||
| Preterm delivery (less than 37 wk) | 4.10 | 1.13-14.93 | .032 |
| Ascites | 4.56 | 1.54-13.46 | .006 |
| High WBC count (> 100 ×109/L) | 5.01 | 1.74-14.39 | .003 |
| Bleeding diatheses | 11.01 | 3.56-34.02 | < .001 |
| Low-risk | |||
| Spontaneous remission | 0.01 | 0.00-0.06 | < .001 |
| Cytarabine treatment | 0.11 | 0.04-0.31 | < .001 |
| Variable . | Hazard ratio . | Confidence interval, 95% . | P . |
|---|---|---|---|
| High-risk | |||
| Preterm delivery (less than 37 wk) | 4.10 | 1.13-14.93 | .032 |
| Ascites | 4.56 | 1.54-13.46 | .006 |
| High WBC count (> 100 ×109/L) | 5.01 | 1.74-14.39 | .003 |
| Bleeding diatheses | 11.01 | 3.56-34.02 | < .001 |
| Low-risk | |||
| Spontaneous remission | 0.01 | 0.00-0.06 | < .001 |
| Cytarabine treatment | 0.11 | 0.04-0.31 | < .001 |
Among the 124 children who survived the first 6 months of life, 29 (23.4%) subsequently developed ML-DS. A total of 27 children progressed to ML-DS after clearing the peripheral blood (PB) from TL blasts, and 2 children had persistent evidence of TL and did not achieve CR (n = 2; ages 8 and 12 months). The median age at diagnosis of ML-DS was 1.5 years (range, 0.5-3 years), which is not significantly different in comparison to those ML-DS patients without a history of TL (1.8 years; range, 0.5-3.8 years; Pt test = .12).
The patients who presented with pleural effusions (PGray = .022) and thrombocytopenia (platelets < 100 × 109/L; PGray = .033) at diagnosis of TL, had a higher risk of developing subsequent ML-DS (Figure 3D). The correlation between pleural effusions and the progression to ML-DS appeared also significant in the multivariate analysis. There was no difference in the WBC count in the group of TL patients who developed ML-DS in comparison to those who did not.
We identified 7 infants with trisomy 21 mosaicism; 4 boys and 3 girls. One boy died due to multiorgan failure, and 1 boy developed ML-DS. He was treated according to the AML-BFM 2004 protocol and continues to remain in remission, as do the other 5 children with trisomy 21 mosaicism.
Treatment results
A total of 28 children received a short course of cytarabine treatment. Children treated with low-dose cytarabine had more severe symptoms at the beginning of treatment than untreated children (Table 1). Among these 28 patients, 13 (46%) had to be referred to an intensive care unit, whereas only 24 (20%; Pχ2 < .001) among the untreated patients required intensive care. A total of 11 children (39%; Pχ2 < .01) had pathologic coagulation parameters, and 6 showed bleeding diatheses (21% vs 4%; Pχ2 < .001). Among 7 diagnosed patients with liver fibrosis, 3 were in the treatment group (10% vs 3%; Pχ2 < .001).
Interestingly, despite these profound differences in the composition of the groups, EFS and OS did not differ significantly in the treated versus the untreated group (5-year EFS, 51% ± 11% vs 66% ± 5%; Plog rank = .24; 5-year OS 78% ± 8% vs 85% ± 3%; Plog rank = .44). A total of 6 (21%) children went on to early death despite therapy. Causes of death were severe cardiac defects (n = 2), multiorgan failure because of cardiac defects in combination with sepsis (n = 1), multiorgan failure after severe intracerebral bleeding diatheses and deep vein thrombosis (n = 1), and liver fibrosis (n = 3).
Considering only those patients with either significant risk factors (identified by multivariate analysis: high WBC count, ascites, preterm delivery, and bleeding) or without spontaneous remission, the cumulative incidence of death (CID) was significantly lower in the treatment group compared with the rest of the group (CID, 24% ± 9%, n = 26 vs 72% ± 11%, n = 18; PGray = .001; Figure 4). The above-mentioned multivariate analysis performed to identify factors associated with early death, clearly demonstrated a favorable impact of cytarabine treatment on the prevention of early death (relative risk [RR] 0.11; 95% CI, 0.04-0.31; P < .001; Table 4).
Outcome of patients with TL with and without treatment with low-dose cytarabine. (A) EFS of those patients with TL with high WBC count, ascites, preterm delivery, and bleeding diatheses and without spontaneous remission. (B) Cumulative incidence of death.
Outcome of patients with TL with and without treatment with low-dose cytarabine. (A) EFS of those patients with TL with high WBC count, ascites, preterm delivery, and bleeding diatheses and without spontaneous remission. (B) Cumulative incidence of death.
A total of 6 (27%) of 22 treated children who achieved CR subsequently developed ML-DS. The frequency of ML-DS was similar in the treated and the untreated group (27% vs 23%; P = .46).
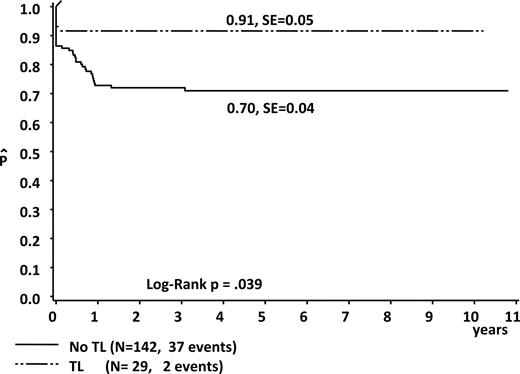
In total, 27 of 29 children with ML-DS were treated according to the intensity-adapted AML-BFM 93, 98, or 2004 protocols, and all children survived without event (5-year EFS, 100%). One child died prior to the start of therapy due to a very severe untreatable cardiac defect. One other child has not been treated because of a bad general condition after severe intracerebral bleedings. However, the 5-year EFS after diagnosis of ML-DS for all 29 patients was 91% plus or minus 5%, which is significantly higher than the 5-year EFS of those ML-DS patients without documented TL diagnosed in the same period of time (70% ± 4%; Plog rank = .039; n = 142; Figure 5). Patients with ML-DS with a history of TL had a lower cumulative relapse rate in comparison to those ML-DS without TL (0% vs 9% ± 3%). Considering only those children who received therapy for ML-DS (n = 27 vs n = 139); 5-year EFS was 100% versus 74% plus or minus 4% (Plog rank = .011). The outcome of the patients with non-DS AMKL (n = 106) in the same period of time was significantly lower (5-year EFS, 47% ± 5%, Plog rank < .001; 5-year OS, 55% ± 5%, Plog rank < .001).32
EFS in children with ML-DS and a history of TL compared with those with ML-DS and no history of TL (enrolled between 1993 and 2006).
EFS in children with ML-DS and a history of TL compared with those with ML-DS and no history of TL (enrolled between 1993 and 2006).
Discussion
DS is the most common chromosomal disorder, with an occurrence of 1 in every 700 live births. Approximately 10% of the infants with DS present with TL, but the true incidence of TL could only be estimated so far.33,34 No population-based study among patients with DS has yet been carried out to establish the true incidence of this disorder. Between 1993 and 2006, 146 children with TL were enrolled in our nationwide AML-BFM registry, a lower frequency than expected. Between 2001 and 2006, approximately 1.3% of all infants with DS were registered with TL per year.35 This high discrepancy is most likely caused by a failure to diagnose TL in children with DS without or with only mild symptoms. Population-based prospective studies are ongoing in Germany (lower Saxony) and within the Dutch Childhood Oncology Group (DCOG; M. Zwaan, personal communication, January 2007).
In the past, most of the knowledge about the natural history and biology of TL was gathered from case reports and retrospective reviews.5,8,21 Recently, Massey et al published the first prospective survey of 48 patients with TL (POG 9481).4 In our study, we prospectively collected the clinical, biological, and cytogenetic data of the largest cohort of patients with TL (n = 146) reported to date. Altogether, the 5-year OS and the EFS of all children with TL were 85% plus or minus 3% and 63 plus or minus 4%, respectively, which is in accordance to previous results.4 The unfavorable survival rates emphasize that TL should be regarded as a serious disease, and therapy should be considered early (see below in this section). As a result, we propose to include TL or a screening for the presence of GATA1 mutations in the diagnostic survey of newborns with DS to identify individuals where severe complications may occur.
Because of the large number of patients with TL enrolled in this study, we could examine covariates that predict EFS, early death, and progression to ML-DS, and could define findings associated with early death and a poor prognosis (Tables 2,3). A total of 5 covariates (high WBC count, ascites, preterm delivery, bleeding diatheses, and failure of spontaneous remission) also reached significance using multivariate analysis, underlining their true impact as independent risk factors for early death (Table 4). As high WBC count, ascites, and preterm delivery are features at presentation, they can be used as early predictors for the outcome. Using univariate proportional hazard models, the Children's Oncology Group suggested a correlation between higher WBC count, elevated transaminases, failure to clear peripheral blasts, respectively, and early death.4,26 Due to the limited sample size in their study (n = 48) and a loss of significance from multiple hypothesis testing, their results were tentative. Examination of this large population confirms several of the factors of the POG-9481 study and adds new prognostic indicators to that list.
The appearance of thrombocytopenia (platelets < 100 × 109/L) and pleural effusions predicted the progress to ML-DS. Pleural effusions as a variable reached significance in the multivariate analysis. The splanchopleura is a site of early embryonic hematopoiesis.36 The pleural tissue derived from this embryonic structure could serve as a niche for the leukemic cells from fetal origin as well as for extramedullary hematopoiesis. The resulting pleural effusion may be secondary.37 Thrombocytopenia may reflect the infiltration of the blasts in the bone marrow and the suppression of the normal hematopoiesis.
In this study, we prospectively investigated if the reduction of the proliferative activity of blasts by a treatment with low-dose cytarabine might improve prognosis. Al Kasim et al26 and Dormann et al27 recommended therapy in case of clinical symptoms of liver dysfunction. In the AML-BFM studies, treatment was recommended in all children with clinical impairment associated with TL such as high WBC count, severe thrombocytopenia, or liver dysfunction. Using both univariate and multivariate analysis, we could demonstrate a beneficial effect of the treatment with cytarabine. This is the first report that suggests a benefit for low-dose cytarabine therapy for a group of patients with TL who present with one of the previously defined risk factors (see “Results”). The fact that 3 children died due to liver fibrosis suggests that late therapy is insufficient to revert an ongoing fibrotic process. The paradox that the 5-year EFS and 5-year OS was similar in the treatment and nontreatment group while multivariate analysis revealed a beneficial effect of the treatment can be explained by the nature of this analysis, which corrects for confounding prognostic factors. As a result, we propose an early consideration of treatment, especially if findings are apparent that we and others showed to be associated with early death. This may help to improve the unfavorable outcome for all children with TL.
The fact that the rate for the development of ML-DS was similar in the treated and the untreated TL group suggests an insufficiency of the applied low-dose cytarabine treatment to prevent the progression from the preleukemic phase to leukemia in all children. Of note, the power to detect differences among the groups may be limited. However, the objective of the cytarabine treatment in this study was to reduce the bulk of leukemic blasts to improve the prognosis of children with a severe occurrence of the disease. The “ML-DS prevention trial” (EudraCT no. 2006-002962-20) is currently ongoing within the AML-BFM group to assess if the progression from TL to ML-DS may be blocked by true eradication of the GATA1s+ clone using low-dose cytarabine treatment and minimal residual disease (MRD) diagnostics.
In total, 29 (23%) children subsequently developed ML-DS. These data are consistent with other studies, which report a 20% to 30% risk of leukemia.1,4,38 The favorable outcome of all 27 treated children is significantly better than the outcome for those children with ML-DS without documented TL (Figure 5).9,10 Therefore, this report might have identified a subgroup which is characterized by almost 100% leukemia free-survival (LFS), in which therapy can potentially be reduced. To date, no prognostic subgroups have been detected within ML-DS that would allow a risk-stratified approach, with the aim to select those patients in whom further therapy reduction would be possible without compromising prognosis. The responsible factors for the better treatment outcome for this subgroup remain elusive. We could rule out a difference in the age at diagnosis. It is unclear whether ML-DS is preceded by TL in all patients and whether the variable presentation at diagnosis reflects the size of the TL clone. The size of the TL clone itself might depend on the time point when the GATA1 mutation is acquired and on additional mutations further accelerating proliferation. An increased sensitivity of these cells against cytostatic therapy might be a consequence of a higher proliferation index. If ML-DS could arise de novo without preceding TL, the TL status might define 2 different biologic subgroups. A prospective nationwide study screening all children with DS for TL may help to address these issues.
In conclusion, our results have important implications for the treatment of both TL and ML-DS. The definition of clear treatment indication may help to improve the outcome for all children with TL. By identifying a ML-DS subgroup with 100% LFS, this report makes a big step toward risk-stratified therapy in children with ML-DS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr C. M. Zwaan and V. G. Sankaran for critical reading; C. Augsburg, T. Reinke, and M. Wackerhahn for technical assistance; J.-E. Müller for data management; and U. Bernsmann for assistance with the management of the TL studies.
This work was supported by Deutsche Krebshilfe 50-2728, Madeleine Schickedanz Foundation, and the José Carreras Leukemia Foundation.
National Institutes of Health
Authorship
J.H.K. analyzed and interpreted the data and wrote the manuscript, with contribution from D.R. D.R. and U.C. designed the study. M.Z. performed statistical analysis. C.L. performed immunophenotyping and K.R. performed the mutation analysis. M.D., A.P., M.J., and C.L. collected data.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Dirk Reinhardt, Department of Pediatric Hematology/Oncology, Medical School Hannover, Carl Neuberg Str 1, 30625 Hannover, Germany; e-mail: reinhardt.dirk@mh-hannover.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal