Abstract
Dasatinib is a multitargeted drug that blocks several tyrosine kinases. Apart from its well-known antileukemic activity, the drug has attracted attention because of potential immunosuppressive and anti-inflammatory effects. We report that dasatinib at 1 μM completely blocks anti-IgE–induced histamine release in blood basophils in healthy donors, and allergen-induced release of histamine in sensitized individuals. In addition, dasatinib inhibited FcϵRI-mediated release of IL-4 and IgE-mediated up-regulation of CD13, CD63, CD164, and CD203c in basophils. The effects of dasatinib were dose-dependent (IC50: 50-500 nM) and specific for FcϵRI activation in that the drug failed to inhibit C5a-induced or Ca-ionophore–induced histamine release. Interestingly, at lower concentrations, dasatinib even promoted FcϵRI-dependent histamine release in basophils in allergic subjects. In consecutive studies, dasatinib was found to interact with and block several FcϵRI downstream targets in basophils, in-cluding Btk. Correspondingly, FcϵRI-mediated histamine secretion in basophils was markedly reduced in Btk knockout mice and in a patient with Btk deficiency. However, the remaining “low-level” mediator secretion in Btk-deficient cells was fully blocked down again by 1 μM dasatinib. Together, these data suggest that dasatinib inhibits FcϵRI-mediated activation of basophils through multiple signaling molecules including Btk. Dasatinib may be an interesting agent for immunologic disorders involving Btk-dependent responses or/and FcϵRI activation of basophils.
Introduction
Basophils are circulating effector cells of allergic reactions.1-4 These cells express the high-affinity receptor for immunoglobulin E (IgE), FcϵRI, and synthesize numerous proinflammatory and vasoactive mediators such as histamine, leukotrienes, and cytokines.2-6 Most of these mediators including histamine, are stored within the metachromatic granules of basophils. Cross-linking of FcϵRI on basophils by an allergen or by other compounds is followed by rapid release of mediators and cytokines into the extracellular space with consecutive edema formation and tissue inflammation that may lead to the clinical picture of an anaphylactic reaction.1-6
Activation of the FcϵRI is associated with activation of several signal transduction pathways.3-9 Tyrosine phosphorylation of several kinases is an early event in FcϵRI-dependent signaling.6-17 Tyrosine kinases (TKs) that undergo rapid activation upon IgE receptor cross-linking include Src family kinases such as Lyn,10-12 spleen tyrosine kinase (Syk),13-15 and Bruton tyrosine kinase (Btk).16,17 In particular, it has been reported that FcϵRI cross-linking is followed by rapid phosphorylation of these kinases in basophils and mast cells.10-20 It has also been described that natural deficiency of relevant signaling molecules, their enforced knockout, or their pharmacologic deactivation is associated with inhibition of FcϵRI-dependent mediator secretion.21-25 Therefore, these kinases represent interesting targets of therapy.26-28 However, unfortunately, most kinase inhibitors under investigation have not been developed far enough to reach clinical application or are experimental compounds that cannot be applied in clinical trials because of their unfavorable toxicity profiles.
Dasatinib, previously referred to as BMS-354825, is a novel targeted drug that inhibits a number of TKs including PDGFR, KIT, BCR/ABL, and Src kinases.29-32 Based on its effects on BCR/ABL, dasatinib is used to treat patients with imatinib-resistant chronic myeloid leukemia (CML).33 More recently, we and others have shown that dasatinib also inhibits the growth of neoplastic mast cells, an effect that is considered to be mediated by dasatinib-induced inhibition of KIT.34-36
However, despite its strong impressive action on KIT and Src kinases, the effects of dasatinib on activation of human basophils or mast cells have not been described yet. We here show that dasatinib acts as a potent inhibitor of FcϵRI-dependent activation of human basophils, and that this drug effect is mediated at least in part through inhibition of Btk.
Methods
Monoclonal antibodies and other reagents
The anti-IgE monoclonal antibody (mAb) E124.2.8 (Dϵ2), the FITC-labeled mAb CLB-gran12 (CD63), and PE-conjugated mAb 97A6 (CD203c) were purchased from Immunotech (Marseille, France); the PE-labeled mAb R3-242 (CD13), PE-labeled mAb N6B6 (CD164), and isotype-matched control antibodies (FITC-IgG1, PE-IgG1, PE-IgG2a), from Becton Dickinson Biosciences (San Jose, CA); and the APC-labeled mAb AC145 (CD123), from Miltenyi Biotech (Bergisch Gladbach, Germany). A specification of antibodies is shown in Table 1. Dasatinib (BMS-354825) was produced at Bristol-Myers Squibb (New Brunswick, NJ). Stock solutions of dasatinib were prepared by dissolving in dimethyl sulfoxide (DMSO; Merck, Darmstadt, Germany). RPMI 1640 medium and fetal calf serum (FCS) were purchased from PAA laboratories (Pasching, Austria); l-glutamine and Iscoves modified Dulbecco medium (IMDM), from Gibco Life Technologies (Gaithersburg, MD); and calcium ionophore A23187 and recombinant (r) C5a, from Sigma-Aldrich (St Louis, MO). The recombinant allergens rBet v 1 and rPhl p 5 were obtained from Biomay (Vienna, Austria), and natural (n) Der p 1 was prepared by affinity chromatography using the monoclonal antibody 4C1 as described.37
Specification of antibodies
| CD . | Clone . | Ig class . | Fluorochrome . | Source . |
|---|---|---|---|---|
| CD13 | R3-242 | IgG1 | PE | BD Biosciences |
| CD63 | CLB-gran12 | IgG1 | FITC | Immunotech |
| CD123 | AC145 | IgG1 | APC | Miltenyi Biotech |
| CD164 | N6B6 | IgG2a | PE | BD Biosciences |
| CD203c | 97A6 | IgG1 | PE | Immunotech |
| Anti-IgE (Dϵ2) | E124.2.8 | IgG1 | — | Immunotech |
| Isotype control | MOPC-21 | IgG1 | FITC | BD Biosciences |
| Isotype control | MOPC-21 | IgG1 | PE | BD Biosciences |
| Isotype control | G155-178 | IgG2a | PE | BD Biosciences |
| CD . | Clone . | Ig class . | Fluorochrome . | Source . |
|---|---|---|---|---|
| CD13 | R3-242 | IgG1 | PE | BD Biosciences |
| CD63 | CLB-gran12 | IgG1 | FITC | Immunotech |
| CD123 | AC145 | IgG1 | APC | Miltenyi Biotech |
| CD164 | N6B6 | IgG2a | PE | BD Biosciences |
| CD203c | 97A6 | IgG1 | PE | Immunotech |
| Anti-IgE (Dϵ2) | E124.2.8 | IgG1 | — | Immunotech |
| Isotype control | MOPC-21 | IgG1 | FITC | BD Biosciences |
| Isotype control | MOPC-21 | IgG1 | PE | BD Biosciences |
| Isotype control | G155-178 | IgG2a | PE | BD Biosciences |
PE indicates phycoerythrin; BD, Becton Dickinson; FITC, fluorescein isothiocyanate; APC, allophycocyanin; and —, not labeled.
Enrichment of human blood basophils
Peripheral blood was obtained from 16 healthy individuals, 1 patient with chronic myeloid leukemia (CML), 1 patient with acute myeloid leukemia (AML) presenting with basophilia, 1 patient with Bruton disease (Btk deficiency), and 18 patients allergic to one or more of the following allergens: Bet v 1, Phl p 5, Der p 1. Informed consent was obtained in each case. The patients' characteristics (allergic donors) are shown in Table 2. The study was approved by the institutional review board (Medical University of Vienna) and informed consent was obtained in accordance with the Declaration of Helsinki. Peripheral blood was collected in heparin- or EDTA-containing tubes. Basophils were enriched by dextran sedimentation as described.38 For Western blot experiments, blood was layered over Ficoll to isolate “mononuclear” cells (MNCs). The percentage of basophils in MNC preparations ranged from 5% to 40%. Cell viability was always more than 90% as assessed by trypan blue exclusion test.
Patient characteristics (allergic donors)
| Patient no. . | Age, y . | f/m . | Symptoms . | Therapy . | Allergen-specific IgE – CLA/RAST class . | SPT . | ||
|---|---|---|---|---|---|---|---|---|
| HDM . | BP . | TGP . | ||||||
| 1 | 26 | m | RC, U | AH* | 4(c)/3(r) | 4(c)/5(r) | 4(c)/4(r) | nt |
| 2 | 28 | m | RC | AH* | 1(c)/nt | 1(c)/nt | 4(c)/4(r) | + |
| 3 | 27 | f | RC, D | AH* | nt/nt | 4(c)/nt | 4(c)/nt | + |
| 4 | 24 | m | RC, D | AH* | 3(c)/nt | 4(c)/nt | 4(c)/nt | nt |
| 5 | 45 | m | RC | No | nt/nt | 4(c)/nt | nt/nt | nt |
| 6 | 26 | m | RC | AH* | 4(c)/nt | 4(c)/nt | 4(c)/nt | nt |
| 7 | 27 | f | RC | AH* | nt/6(r) | nt/6(c) | nt/6(r) | nt |
| 8 | 34 | f | RC | AH* | nt/nt | nt/nt | nt/3(r) | + |
| 9 | 25 | m | RC, D, P | AH*, CS* | 1(c)/nt | 4(c)/nt | 4(c)/nt | nt |
| 10 | 62 | m | RC, AA, U | CS | nt/3(r) | nt/4(r) | nt/3(r) | + |
| 11 | 24 | f | RC | No | 4(c)/nt | nt/nt | 1(c)/nt | nt |
| 12 | 27 | m | RC | No | 4(c)/nt | nt/nt | nt/nt | + |
| 13 | 22 | f | RC | No | nt/3(r) | nt/3(r) | nt/nt | + |
| 14 | 23 | f | RC, D | AH* | nt/4(r) | nt/6(r) | nt/nt | + |
| 15 | 17 | f | RC, D | CS | 4(c)/nt | nt/nt | nt/nt | + |
| 16 | 24 | f | RC | No | 4(c)/nt | nt/nt | nt/nt | nt |
| 17 | 30 | f | RC, D | AH* | 4(c)/nt | 2(c)/nt | 4(c)/nt | + |
| 18 | 32 | m | RC, AA | CS | 4(c)/nt | 4(c)/nt | 4(c)/nt | + |
| Patient no. . | Age, y . | f/m . | Symptoms . | Therapy . | Allergen-specific IgE – CLA/RAST class . | SPT . | ||
|---|---|---|---|---|---|---|---|---|
| HDM . | BP . | TGP . | ||||||
| 1 | 26 | m | RC, U | AH* | 4(c)/3(r) | 4(c)/5(r) | 4(c)/4(r) | nt |
| 2 | 28 | m | RC | AH* | 1(c)/nt | 1(c)/nt | 4(c)/4(r) | + |
| 3 | 27 | f | RC, D | AH* | nt/nt | 4(c)/nt | 4(c)/nt | + |
| 4 | 24 | m | RC, D | AH* | 3(c)/nt | 4(c)/nt | 4(c)/nt | nt |
| 5 | 45 | m | RC | No | nt/nt | 4(c)/nt | nt/nt | nt |
| 6 | 26 | m | RC | AH* | 4(c)/nt | 4(c)/nt | 4(c)/nt | nt |
| 7 | 27 | f | RC | AH* | nt/6(r) | nt/6(c) | nt/6(r) | nt |
| 8 | 34 | f | RC | AH* | nt/nt | nt/nt | nt/3(r) | + |
| 9 | 25 | m | RC, D, P | AH*, CS* | 1(c)/nt | 4(c)/nt | 4(c)/nt | nt |
| 10 | 62 | m | RC, AA, U | CS | nt/3(r) | nt/4(r) | nt/3(r) | + |
| 11 | 24 | f | RC | No | 4(c)/nt | nt/nt | 1(c)/nt | nt |
| 12 | 27 | m | RC | No | 4(c)/nt | nt/nt | nt/nt | + |
| 13 | 22 | f | RC | No | nt/3(r) | nt/3(r) | nt/nt | + |
| 14 | 23 | f | RC, D | AH* | nt/4(r) | nt/6(r) | nt/nt | + |
| 15 | 17 | f | RC, D | CS | 4(c)/nt | nt/nt | nt/nt | + |
| 16 | 24 | f | RC | No | 4(c)/nt | nt/nt | nt/nt | nt |
| 17 | 30 | f | RC, D | AH* | 4(c)/nt | 2(c)/nt | 4(c)/nt | + |
| 18 | 32 | m | RC, AA | CS | 4(c)/nt | 4(c)/nt | 4(c)/nt | + |
The CLA class was defined by the following score: 0, 0-26 luminescence units (LU); 1, 27-65 LU; 2, 66-142 LU; 3, 143-242 LU; and 4, more than 242 LU. The RAST class was defined by the following score: 0, 0-0.34 kUA/L; 1, 0.35-0.69 kUA/L; 2, 0.70-3.49 kUA/L; 3, 3.50-17.49 kUA/L; 4, 17.50-49.99 kUA/L; 5, 50.00-99.99 kUA/L; and 6, 100 kUA/L or more.
f indicates female; m, male; CLA, chemiluminescence (c); RAST, radioallergosorbent test (r); SPT, skin prick test; HDM, house dust mite; BP, birch pollen; TGP, timothy grass pollen; RC, rhinoconjunctivitis; U, urticaria; AH, antihistamines; nt, not tested; D, dyspnea; P, pruritus; CS, corticosteroids; and AA, allergic asthma.
These drugs were taken only on demand by patients.
Histamine release assay with human basophils
The histamine release assay was performed on dextran-enriched basophils (healthy donors, n = 14; allergic donors, n = 11; CML donors, n = 3) essentially as described.38 In typical experiments, cells (1.5 × 106/mL) were incubated in RPMI 1640 medium containing 10% FCS in the presence or absence of dasatinib (1 μM) for 30 minutes at 37°C, then washed, and thereafter incubated with various concentrations of anti-IgE antibody E124.2.8 (0.001-10 μg/mL), or various concentrations of allergens (0.001-1 μg/mL each allergen) at 37°C for another 30 minutes. In priming experiments, normal basophils were incubated with the anti-IgE antibody in the presence or absence of IL-3 (100 ng/mL). After exposure to anti-IgE or allergens, cells were centrifuged at 4°C, and the cell-free supernatants recovered and analyzed for histamine content by radioimmunoassay (RIA; Immunotech). In time course experiments, dasatinib (1 μM) was applied for various time periods (1-90 minutes). To define the IC50, basophils were preincubated with various concentrations of dasatinib (0.001-1 μM) before being exposed to anti-IgE (1 μg/mL) or allergen (1 μg/mL). In selected experiments, the calcium ionophore A23187 (0.01-10 μg/mL) or recombinant C5a (0.1-100 nM) was applied as basophil-activating reagents. All experiments were performed in triplicates.
Culture of mouse basophils and release experiments
Mouse basophils were cultured from bone marrow progenitor cells using interleukin-3 (IL-3) essentially as described.39,40 Bone marrow cells were obtained from the tibia and femurs of killed wild-type mice or Btk−/− mice41 (mixed background: C57BL/6 × 129 Sv; 4 to 6 weeks of age, purchased from Jackson Laboratory, Bar Harbor, ME), and were resuspended in RPMI 1640 medium containing 10% FCS, antibiotics, glutamine (0.2 mM), and β-mercaptoethanol (50 μM) (all from Sigma). At day 0, bone marrow cells were seeded at a density of 0.5 × 106 cells/mL in RPMI 1640 medium containing 10% FCS and 5 ng/mL recombinant murine IL-3 (Peprotech, London, United Kingdom). On day 7, basophils were harvested. In a separate set of experiments (n = 3), cultured mouse basophils were enriched by flow cytometry and cell sorting on a FACSAria (Becton Dickinson Biosciences). In these experiments, basophils were defined as KIT-negative cells expressing IgE receptors, and by their characteristic side scatter properties. The PE-labeled anti–mouse KIT/CD117 mAb 2B8 was purchased from BD Pharmingen (Heidelberg, Germany). For flow cytometric detection of the IgE receptor, basophils were preincubated with murine (m)IgE (C38-2; BD Pharmingen), washed, and then incubated with the FITC-labeled anti-IgE mAb R35-72 (BD Pharmingen). The percentage of basophils before sorting ranged between 10% and 14%. After sorting, the purity of basophils amounted to 55% to 75%, as determined by Wright-Giemsa staining and flow cytometry. For release experiments, cells (impure cells: 1-2.5 × 106 cells/mL; enriched basophils: 0.5 × 106 cells/mL) were cultured in RPMI 1640 medium with freshly added IL-3 and 1 μg/mL mIgE overnight. Thereafter, cells were washed in phosphate-buffered saline (PBS) and then were incubated with dasatinib (1 μM). In histamine release experiments, basophils were incubated with dasatinib for 30 minutes at 37°C, washed, and then were incubated with various concentrations (10-1000 ng/mL) of trinitrophenol (TNP). Then, cells were centrifuged at 4°C, and cell-free supernatants and cell lysates were examined for the content of histamine by RIA. In IL-4 secretion experiments, IgE-exposed basophils were transferred to TNP-BSA–coated plates (Biosearch Technologies, Novato, CA) in the presence or absence of dasatinib (1 μM) at 37°C for 24 hours. Thereafter, supernatants were harvested and stored at −80°C. IL-4 was measured by a commercial enzyme-linked immunosorbent assay (ELISA; specific for murine IL-4) according to the instructions of the manufacturer (BD Pharmingen). Experiments were performed in triplicates throughout the study. All studies in mice were conducted according to protocols approved by the Austrian Federal Ministry for Education, Science, and Art.
Western blotting
Western blotting and immunoprecipitation (IP) were performed essentially as described36,42 using lysates of KU812 cells, primary CML cells, or normal blood MNCs. The FcϵRI-expressing human basophil cell line KU81243 was kindly provided by Dr Kenji Kishi (Niigata University, Niigata, Japan). Primary cells were analyzed after exposure to control medium or anti-IgE antibody (1 μg/mL) at 37°C for 30 minutes. For IP, protein G sepharose beads (GE Healthcare, Little Chalfont, United Kingdom) and an anti-Lyn antibody (Cell Signaling Technology, Beverly, MA) were applied. The following antibodies were used for Western blot analysis: anti-Btk (Santa Cruz Biotechnology, Santa Cruz, CA), anti–phospho-Btk (Cell Signaling Technology), anti-Lyn (Cell Signaling Technology), anti–phospho-Lyn (Cell Signaling Technology), anti–phospho-Hck (Santa Cruz Biotechnology), and antiphosphotyrosine mAb 4G10 (Upstate Biotechnology, Lake Placid, NY). All antibodies were applied at a work dilution of 1:1000 at 4°C overnight. Antibody reactivity was made visible by sheep anti–mouse or donkey anti–rabbit antibodies, Lumingen PS-3 detection reagent (all from GE Healthcare), and CL-Xposure film (Pierce Biotechnology, Rockford, IL).
Drug-affinity purification and mass spectrometry
For drug-affinity binding and mass spectrometry (MS) experiments, the basophil cell line KU81243 and c-dasatinib, synthesized by WuXi PharmaTech (Shanghai, China), were used. Drug pulldown experiments were performed using KU812 total cell lysates (TCL, input amount loaded: 120 μg), and tryptically digested proteins were analyzed by nanoLC tandem MS as described.44 The acquired data were searched against the human IPI database with the MASCOT search engine (MatrixScience, London, United Kingdom), and the identified proteins were validated and clustered into protein groups using EpiCenter (Proxeon, Odense, Denmark).
Antibody staining and flow cytometry
To examine the effects of dasatinib on expression of activation-linked antigens, flow cytometric experiments were performed using KU812 and primary blood basophils. KU812 cells were incubated with dasatinib (1 or 3 μM) for 24 hours, before being stained. Primary basophils in whole blood samples were incubated with dasatinib (0.01-1 μM) for 15 minutes at 37°C. In time course experiments, dasatinib (1 μM) was applied for various time periods (1-90 minutes). After incubation, basophils were washed and incubated with anti-IgE mAb E124.2.8 (1 μg/mL) at 37°C for 15 minutes. Thereafter, cells were washed in PBS supplemented with EDTA (20 mM), subjected to erythrocyte lysis with FACS-lysing solution (Becton Dickinson Biosciences), and then analyzed by multicolor flow cytometry using combinations of fluorochrome-conjugated antibodies as described.45,46 For basophil detection, the CD203c mAb 97A6 or the CD123 mAb AC145 was used. Additional mAbs used were R3-242 (CD13), CLB-gran12 (CD63), and N6B6 (CD164). KU812 cells were examined for CD antigen expression by single-color flow cytometry. Expression of surface antigens was analyzed on a FACSCalibur (Becton Dickinson Biosciences) or a FACScan (Becton Dickinson Biosciences).
Results
Dasatinib blocks IgE-dependent histamine release in normal blood basophils
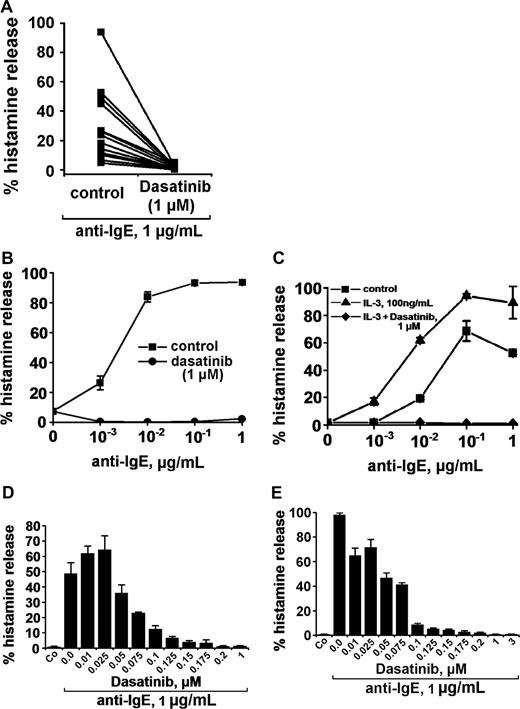
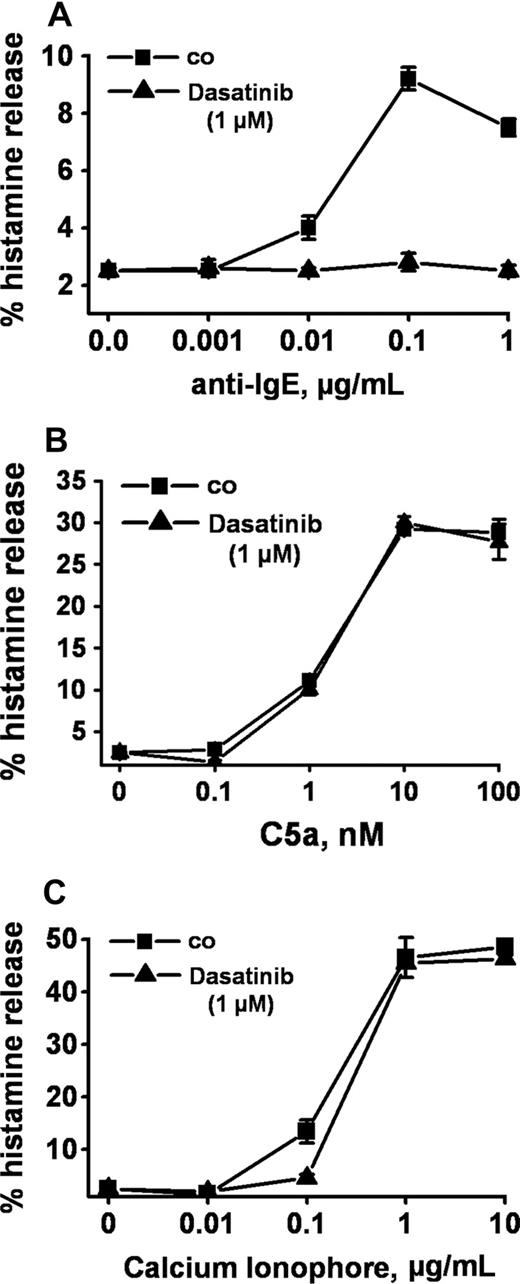
Anti-IgE, as expected, induced release of histamine from human basophils in all 14 healthy donors examined (percentage release with anti-IgE, mean ± SD: 28.0% ± 24.5% [range: 4.8%-93.6%] versus spontaneous histamine release: 1.0% ± 0.8% [range: 0.0%-3.2%], P < .05). At 1 μM, dasatinib was found to completely inhibit the anti-IgE–induced release of histamine in all donors examined (Figure 1A) and at all concentrations of anti-IgE tested (Figure 1B). Complete inhibition of release by dasatinib (1 μM) was also seen when basophils were primed with IL-3 and then were exposed to anti-IgE (Figure 1C). In addition, we found that dasatinib down-regulates histamine release in CML basophils, in which BCR/ABL may act as a cotrigger of signal transduction (similar to IL-3 priming). In time course experiments, the maximum effect of dasatinib on histamine release was observed within 1 minute of incubation (not shown). The effects of dasatinib on IgE-mediated histamine release were dose-dependent with IC50 values ranging between 50 and 100 nM, without a difference seen between normal basophils (Figure 1D) and CML basophils (Figure 1E). An interesting phenomenon was that at lower concentrations, dasatinib was found to slightly up-regulate histamine secretion in normal basophils (Figure 1D), but not in CML basophils (Figure 1E). Whereas dasatinib blocked the IgE-dependent release of histamine in all donors examined, no inhibition was seen when histamine release was provoked by rhC5a or the calcium ionophore A23187 (Figure 2). Dasatinib did not reduce viability of basophils under the experimental conditions applied (not shown).
Effects of dasatinib on histamine release in human basophils. (A) Basophils from 14 healthy donors were incubated with control medium (control) or dasatinib (1 μM) at 37°C for 30 minutes. Then, cells were washed and exposed to an anti-IgE antibody (1 μg/mL) for 30 minutes. Thereafter, cells were centrifuged and the cell-free supernatants and lysates examined for histamine content by RIA. Results show the percentage of histamine release. The inhibitory effect of dasatinib on histamine release was significant (P < .05). (B) Basophils were preincubated in control medium (■-■) or dasatinib, 1 μM (●-●) for 30 minutes. Thereafter, basophils were washed and incubated in various concentrations of anti-IgE (37°C, 5% CO2) for 30 minutes. Then, cells were centrifuged at 4°C and the cell-free supernatants and lysates examined for the amounts of histamine. Results show the percentage of released histamine and represent the mean (± SD) from triplicates. (C) Normal basophils were incubated in control buffer (■-■), or in buffer with IL-3 (100 ng/mL) in the absence (▲-▲) or presence (♦-♦) of dasatinib (1 μM) for 30 minutes (37°C). Thereafter, cells were washed and incubated in various concentrations of anti-IgE (37°C, 30 minutes). Then, cells were centrifuged and histamine levels measured in cell-free supernatants and cell lysates. Results show the percentage of released histamine and represent mean (± SD) values from triplicates in one donor. (D-E) Dose-dependent effects of dasatinib on anti-IgE–induced release of histamine from normal basophils (D) and CML basophils (E). Basophils were preincubated in control medium or in various concentrations of dasatinib (as indicated) for 30 minutes at 37°C. Then, cells were washed and incubated with anti-IgE (1 μg/mL) for 30 minutes. After centrifugation at 4°C, cell-free supernatants and lysates were analyzed for the con-tent of histamine. Results show the percentages of released histamine and represent the means (± SD) from triplicates.
Effects of dasatinib on histamine release in human basophils. (A) Basophils from 14 healthy donors were incubated with control medium (control) or dasatinib (1 μM) at 37°C for 30 minutes. Then, cells were washed and exposed to an anti-IgE antibody (1 μg/mL) for 30 minutes. Thereafter, cells were centrifuged and the cell-free supernatants and lysates examined for histamine content by RIA. Results show the percentage of histamine release. The inhibitory effect of dasatinib on histamine release was significant (P < .05). (B) Basophils were preincubated in control medium (■-■) or dasatinib, 1 μM (●-●) for 30 minutes. Thereafter, basophils were washed and incubated in various concentrations of anti-IgE (37°C, 5% CO2) for 30 minutes. Then, cells were centrifuged at 4°C and the cell-free supernatants and lysates examined for the amounts of histamine. Results show the percentage of released histamine and represent the mean (± SD) from triplicates. (C) Normal basophils were incubated in control buffer (■-■), or in buffer with IL-3 (100 ng/mL) in the absence (▲-▲) or presence (♦-♦) of dasatinib (1 μM) for 30 minutes (37°C). Thereafter, cells were washed and incubated in various concentrations of anti-IgE (37°C, 30 minutes). Then, cells were centrifuged and histamine levels measured in cell-free supernatants and cell lysates. Results show the percentage of released histamine and represent mean (± SD) values from triplicates in one donor. (D-E) Dose-dependent effects of dasatinib on anti-IgE–induced release of histamine from normal basophils (D) and CML basophils (E). Basophils were preincubated in control medium or in various concentrations of dasatinib (as indicated) for 30 minutes at 37°C. Then, cells were washed and incubated with anti-IgE (1 μg/mL) for 30 minutes. After centrifugation at 4°C, cell-free supernatants and lysates were analyzed for the con-tent of histamine. Results show the percentages of released histamine and represent the means (± SD) from triplicates.
Selective inhibition of IgE-dependent histamine release in basophils by dasatinib. Dextran-enriched basophils from a healthy donor were preincubated with dasatinib (1 μM; ▲) or control medium (■) for 30 minutes. Then, cells were exposed to control buffer (co) or to various concentrations of anti-IgE (0.001-1 μg/mL, A), recombinant (r) C5a (0.1-100 nM) (B), or Ca-ionophore A23187 (0.01-10 μg/mL, C) for 30 minutes. After centrifugation, histamine was measured in supernatants and cell lysates by RIA. Results show the percentages of released histamine (relative to total histamine content) and represent the means (± SD) from triplicates in one donor. Identical results were obtained in a second donor.
Selective inhibition of IgE-dependent histamine release in basophils by dasatinib. Dextran-enriched basophils from a healthy donor were preincubated with dasatinib (1 μM; ▲) or control medium (■) for 30 minutes. Then, cells were exposed to control buffer (co) or to various concentrations of anti-IgE (0.001-1 μg/mL, A), recombinant (r) C5a (0.1-100 nM) (B), or Ca-ionophore A23187 (0.01-10 μg/mL, C) for 30 minutes. After centrifugation, histamine was measured in supernatants and cell lysates by RIA. Results show the percentages of released histamine (relative to total histamine content) and represent the means (± SD) from triplicates in one donor. Identical results were obtained in a second donor.
Dasatinib inhibits allergen-induced histamine release in human basophils
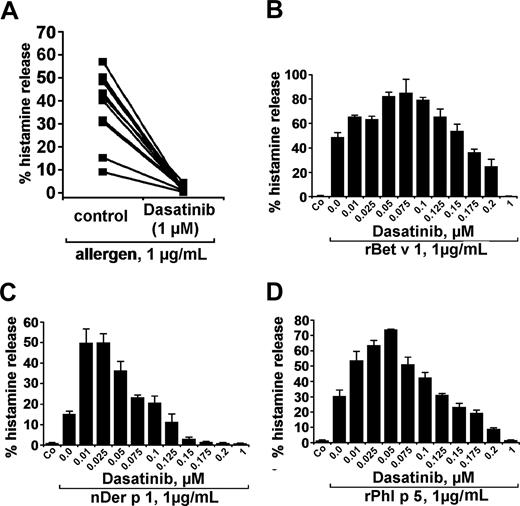
In a next step, we examined the effects of dasatinib on basophil histamine release induced by recombinant or natural allergens in patients allergic to the birch pollen allergen Bet v 1, grass pollen allergen Phl p 5, or/and house dust mite allergen Der p 1. The respective 3 allergens were found to induce histamine release in basophils with comparable potencies (percentage histamine release). At 1 μg/mL, the allergens induced 37.8% (± 14.9%) histamine release (range: 9.1%-56.9%) compared with a spontaneous release of 2.1% (± 1.5%; range: 0.6%-5.0%; P < .05). As visible in Figure 3A, dasatinib was found to inhibit histamine secretion from basophils induced by allergens in all sensitized individuals tested. Again, the effects of dasatinib on allergen-induced histamine release were found to be dose-dependent with IC50 values ranging between 100 and 200 nM. Typical examples for dose-dependent effects of the allergens are shown in Figure 3B-D. As in normal blood basophils, lower concentrations of dasatinib were found to even promote allergen-induced histamine secretion in basophils. Interestingly, this unexpected augmenting effect of low-dose dasatinib on histamine release was even more pronounced in allergic patients (allergen-dependent reactions) compared with healthy individuals (normal basophils exposed to anti-IgE). These data suggest that low-dose dasatinib may even promote basophil activation under certain conditions.
Effects of dasatinib on histamine release in blood basophils of allergic donors. (A) Basophils of allergic donors (n = 11) were incubated with control medium or dasatinib (1 μM) at 37°C in 5% CO2 for 30 minutes. Then, cells were washed and exposed to allergens (1 μg/mL) for 30 minutes. Thereafter, basophils were centrifuged and the cell-free supernatants and lysates examined for the content of histamine by RIA. The inhibitory effect of dasatinib on histamine release was found to be significant (P < .05). (B-D) Dose-dependent effects of dasatinib on histamine release provoked by the allergens rBet v 1 (birch pollen), nDer p 1 (house dust mite), and rPhl p5 (timothy grass). Basophils from allergic donors were preincubated in control medium or various concentrations of dasatinib (0.01-1 μM) for 30 minutes (37°C). Then, cells were washed and exposed to rBet v 1, nDer p 1, or rPhl p 5 (each 1 μg/mL) for 30 minutes as indicated. After centrifugation at 4°C, cell-free supernatants and cell lysates were analyzed for histamine content by RIA. Results show the percentages of released histamine and represent mean (± SD) values from triplicates.
Effects of dasatinib on histamine release in blood basophils of allergic donors. (A) Basophils of allergic donors (n = 11) were incubated with control medium or dasatinib (1 μM) at 37°C in 5% CO2 for 30 minutes. Then, cells were washed and exposed to allergens (1 μg/mL) for 30 minutes. Thereafter, basophils were centrifuged and the cell-free supernatants and lysates examined for the content of histamine by RIA. The inhibitory effect of dasatinib on histamine release was found to be significant (P < .05). (B-D) Dose-dependent effects of dasatinib on histamine release provoked by the allergens rBet v 1 (birch pollen), nDer p 1 (house dust mite), and rPhl p5 (timothy grass). Basophils from allergic donors were preincubated in control medium or various concentrations of dasatinib (0.01-1 μM) for 30 minutes (37°C). Then, cells were washed and exposed to rBet v 1, nDer p 1, or rPhl p 5 (each 1 μg/mL) for 30 minutes as indicated. After centrifugation at 4°C, cell-free supernatants and cell lysates were analyzed for histamine content by RIA. Results show the percentages of released histamine and represent mean (± SD) values from triplicates.
Dasatinib inhibits IgE-dependent up-regulation of CD13, CD63, CD164, and CD203c on basophils
Basophil activation is typically associated with up-regulation of certain activation-linked cell-surface antigens. The most important of these antigens appear to be CD13, CD63, CD164, and CD203c.45-47 As expected, exposure of normal blood basophils to anti-IgE (1 μg/mL) was followed by a substantial increase in expression of these surface antigens. As visible in Figure 4A, dasatinib was found to inhibit anti-IgE–induced up-regulation of CD13, CD63, CD164, and CD203c on basophils in all healthy subjects examined. The effects of dasatinib on CD antigen expression on basophils were dose dependent with an IC50 ranging between 250 and 500 nM (Figure 4B). Corresponding effects were observed in allergic individuals. In these donors, the allergens were found to augment the expression of all activation-linked CD antigens tested in all donors examined, without major differences when comparing potencies of the 3 allergens or the responses of individual donors in each group. As visible in Figure 4C, dasatinib inhibited allergen-induced up-regulation of CD13, CD63, CD164, and CD203c on basophils in all patients examined. The effects of dasatinib were found to be dose-dependent (IC50 ranging between 500 and 750 nM) without major differences among the allergen species applied (Figure 4D).
Dasatinib inhibits the IgE-dependent up-regulation of activation-linked cell-surface antigens on blood basophils. (A) Peripheral blood cells of healthy donors were preincubated with dasatinib (1 μM) or control buffer for 15 minutes, washed, and then were exposed to control buffer (co) or an anti-IgE mAb (1 μg/mL) for 15 minutes (37°C). Expression of surface antigens on blood basophils was assessed by multicolor flow cytometry as described in the text. Anti-IgE-induced up-regulation of CD antigens was calculated from mean fluorescence intensities (MFIs) obtained with stimulated (MFIstim) and unstimulated (MFIco) cells and was expressed as stimulation index (MFIstim:MFIco). (B) Dose-dependent inhibition of anti-IgE–induced expression of cell surface antigens by dasatinib. Blood basophils were incubated with dasatinib (0.01-1.0 μM) or control buffer for 15 minutes, washed, and then were exposed to control buffer (co) or anti-IgE (1 μg/mL) for 15 minutes (37°C). Expression of CD antigens on basophils was assessed by multicolor flow cytometry. Anti-IgE–induced up-regulation of CD antigens was expressed as stimulation index (MFIstim:MFIco). Results represent the mean (± SD) values of 5 independent experiments (5 donors). Significances in surface marker expression after exposure to anti-IgE are indicated by asterisk (*). (C) Peripheral blood cells of 15 allergic patients were incubated with dasatinib (1.0 μM) or control buffer for 15 minutes, washed, and then exposed to control buffer (control) or to allergens rBet v 1, nDer p 1, or rPhl p 5 (each 1 μg/mL) for 15 minutes (37°C). Expression of CD surface antigens on blood basophils was assessed by multicolor flow cytometry. Allergen-induced up-regulation of CD antigens was expressed as stimulation index (MFIstim:MFIco). (D) Dose-dependent inhibition of allergen-induced expression of cell surface antigens by dasatinib. Basophils were incubated with dasatinib (0.01-1.0 μM) or control buffer for 15 minutes, washed, and then were exposed to control buffer (co) or to various allergens (each 1 μg/mL) for 15 minutes (37°C) as indicated. Allergen-induced up-regulation of CD antigens on basophils was expressed as stimulation index. Results represent the mean (± SD) values of 5 independent experiments (5 donors) for each allergen. Significances in changes in surface marker expression after exposure to allergens are indicated by asterisk (*).
Dasatinib inhibits the IgE-dependent up-regulation of activation-linked cell-surface antigens on blood basophils. (A) Peripheral blood cells of healthy donors were preincubated with dasatinib (1 μM) or control buffer for 15 minutes, washed, and then were exposed to control buffer (co) or an anti-IgE mAb (1 μg/mL) for 15 minutes (37°C). Expression of surface antigens on blood basophils was assessed by multicolor flow cytometry as described in the text. Anti-IgE-induced up-regulation of CD antigens was calculated from mean fluorescence intensities (MFIs) obtained with stimulated (MFIstim) and unstimulated (MFIco) cells and was expressed as stimulation index (MFIstim:MFIco). (B) Dose-dependent inhibition of anti-IgE–induced expression of cell surface antigens by dasatinib. Blood basophils were incubated with dasatinib (0.01-1.0 μM) or control buffer for 15 minutes, washed, and then were exposed to control buffer (co) or anti-IgE (1 μg/mL) for 15 minutes (37°C). Expression of CD antigens on basophils was assessed by multicolor flow cytometry. Anti-IgE–induced up-regulation of CD antigens was expressed as stimulation index (MFIstim:MFIco). Results represent the mean (± SD) values of 5 independent experiments (5 donors). Significances in surface marker expression after exposure to anti-IgE are indicated by asterisk (*). (C) Peripheral blood cells of 15 allergic patients were incubated with dasatinib (1.0 μM) or control buffer for 15 minutes, washed, and then exposed to control buffer (control) or to allergens rBet v 1, nDer p 1, or rPhl p 5 (each 1 μg/mL) for 15 minutes (37°C). Expression of CD surface antigens on blood basophils was assessed by multicolor flow cytometry. Allergen-induced up-regulation of CD antigens was expressed as stimulation index (MFIstim:MFIco). (D) Dose-dependent inhibition of allergen-induced expression of cell surface antigens by dasatinib. Basophils were incubated with dasatinib (0.01-1.0 μM) or control buffer for 15 minutes, washed, and then were exposed to control buffer (co) or to various allergens (each 1 μg/mL) for 15 minutes (37°C) as indicated. Allergen-induced up-regulation of CD antigens on basophils was expressed as stimulation index. Results represent the mean (± SD) values of 5 independent experiments (5 donors) for each allergen. Significances in changes in surface marker expression after exposure to allergens are indicated by asterisk (*).
Dasatinib inhibits IgE-dependent production and secretion of IL-4 in basophils
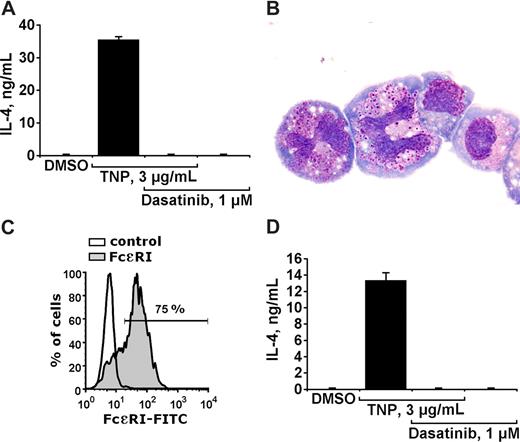
A number of previous and more recent data suggest that activation of basophils is not only followed by histamine secretion, but is also associated with production and secretion of various cytokines such as IL-4.3-5 In the present study, we examined the effects of dasatinib on IgE-dependent production and secretion of IL-4 in cultured mouse basophils. As visible in Figure 5, dasatinib at 1 μM completely blocked the TNP-induced secretion of IL-4 into the supernatants of cultured mouse basophils. Figure 5A shows the effect of dasatinib in impure cells. To exclude an effect of dasatinib on mast cells (mast-cell progenitors), enriched sorted mouse basophils (IgE-receptor positive, KIT negative) were used (Figure 5B,C). Dasatinib was found to block IL-4 production/secretion in enriched mouse basophils in the same way as in impure cells (Figure 5D). Dasatinib (1 μM) did not affect viability of mouse basophils (not shown). All in all, these data suggest that dasatinib blocks not only histamine secretion in basophils but also the IgE-dependent production/secretion of IL-4.
Dasatinib inhibits IgE-dependent production/secretion of IL-4 in mouse basophils. (A) Cultured mouse basophils preincubated with TNP-specific IgE, were exposed to control medium (containing DMSO) or to TNP (3 μg/mL) in the presence or absence of dasatinib (1 μM) as indicated, for 24 hours. Then, cells were centrifuged and the cell-free supernatants recovered and examined for the content of IL-4 by ELISA. Panel A shows a typical experiment performed with impure cells. Results representing the means (± SD) from triplicates. (B) Enriched sorted basophils stained with Wright-Giemsa stain. Acquisition of figures was performed by an Olympus DP11 camera connected to an Olympus BX50F4 microscope equipped with 100×/1.35 UPlan-Apo objective lenses (Olympus, Hamburg, Germany). Images were processed with Adobe Photoshop software version 7.0 (Adobe Systems, San Jose, CA). Magnification ×100. (C) Flow cytometric detection of the IgE receptor on enriched basophils using IgE and an anti-IgE antibody. As visible, the purity of IgE receptor–positive basophils amounted to more than 70%. (D) Effects of dasatinib on IL-4 production/secretion in sorted mouse basophils (conditions as in A). Results represent the means (± SD) from triplicates.
Dasatinib inhibits IgE-dependent production/secretion of IL-4 in mouse basophils. (A) Cultured mouse basophils preincubated with TNP-specific IgE, were exposed to control medium (containing DMSO) or to TNP (3 μg/mL) in the presence or absence of dasatinib (1 μM) as indicated, for 24 hours. Then, cells were centrifuged and the cell-free supernatants recovered and examined for the content of IL-4 by ELISA. Panel A shows a typical experiment performed with impure cells. Results representing the means (± SD) from triplicates. (B) Enriched sorted basophils stained with Wright-Giemsa stain. Acquisition of figures was performed by an Olympus DP11 camera connected to an Olympus BX50F4 microscope equipped with 100×/1.35 UPlan-Apo objective lenses (Olympus, Hamburg, Germany). Images were processed with Adobe Photoshop software version 7.0 (Adobe Systems, San Jose, CA). Magnification ×100. (C) Flow cytometric detection of the IgE receptor on enriched basophils using IgE and an anti-IgE antibody. As visible, the purity of IgE receptor–positive basophils amounted to more than 70%. (D) Effects of dasatinib on IL-4 production/secretion in sorted mouse basophils (conditions as in A). Results represent the means (± SD) from triplicates.
Dasatinib binds to Btk and blocks Btk activation in basophils
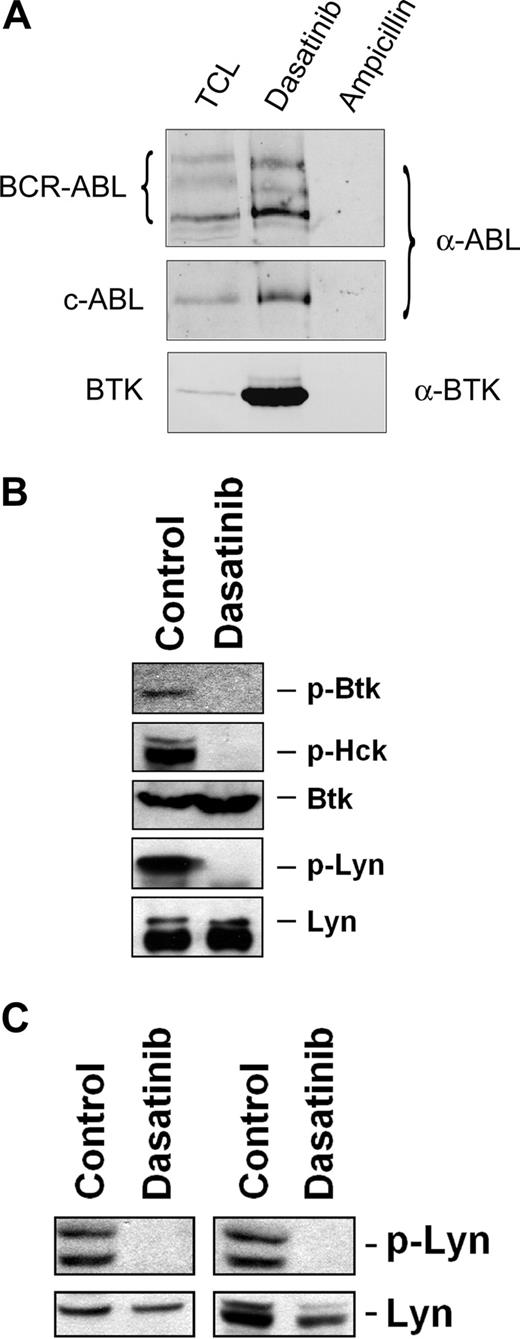
Recent data suggest that FcϵRI-dependent histamine release is mediated through a pathway involving Btk, and that dasatinib binds to and deactivates Btk in CML cells.44 Therefore, we were interested to learn whether dasatinib can also bind to Btk in human basophils. As assessed by drug-affinity chromatography followed by MS, we were able to show that dasatinib binds to several different key target kinases, including Btk, in the human basophil cell line KU812 (Table 3). Western blotting confirmed that dasatinib binds to Btk in KU812 basophils (Figure 6A). In addition, we were able to show that dasatinib blocks the activation (phosphorylation) of Btk and of several other target kinases, such as Hck or Lyn, in KU812 cells (Figure 6B). As exemplified for Lyn in Figure 6C, the same effect of dasatinib was seen when examining anti-IgE–exposed primary blood basophils obtained from patients with myeloid leukemias including one with CML (where BCR/ABL may promote signaling) and one with AML with basophilia (BCR/ABL negative). In addition, as determined by immunoblotting using antiphosphotyrosine antibody 4G10, dasatinib was found to block phosphorylation of all detectable kinases in IgE receptor cross-linked blood basophils (not shown).
Proteins from KU812 total cell lysate purified on dasatinib-matrix
| Entrez name . | Description . | MW, kDa . | Protein score . | Unique peptides . | SC, % . |
|---|---|---|---|---|---|
| BTK | Bruton agammaglobulinemia tyrosine kinase | 76.2 | 1818 | 31 | 49.2 |
| LYN | Tyrosine-protein kinase LYN | 58.6 | 1432 | 18 | 36.9 |
| CSK | c-Src tyrosine kinase | 50.7 | 1113 | 17 | 42.4 |
| EPHB4 | EPH receptor B4 | 108.3 | 432 | 13 | 16.3 |
| MAPK14 | Mitogen-activated protein kinase 14 (p38α) | 41.3 | 275 | 12 | 33.1 |
| YES1 | Proto-oncogene tyrosine-protein kinase YES | 60.8 | 564 | 9 | 17.3 |
| TEC | TEC protein tyrosine kinase | 73.6 | 272 | 9 | 15.8 |
| ABL1 | Proto-oncogene tyrosine-protein kinase ABL1 | 122.9 | 636 | 9 | 11.7 |
| SRC | Tyrosine-protein kinase SRC-1 | 59.8 | 556 | 8 | 15.7 |
| KIT | v-KIT Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | 109.9 | 184 | 6 | 5.6 |
| BCR | Breakpoint cluster region | 137.7 | 414 | 6 | 5.8 |
| FYN | Protein-tyrosine kinase FYN | 54.5 | 237 | 5 | 9.5 |
| EPHA5 | EPH receptor A5 | 114.8 | 105 | 5 | 5.5 |
| PRO3078 | — | 7.7 | 308 | 4 | 60.3 |
| ZAK | Sterile alpha motif and leucine zipper containing kinase AZK | 51.6 | 82 | 3 | 5.9 |
| HSPA8 | Heat shock 70kDa protein 8 | 70.9 | 88 | 3 | 5.7 |
| FRK | FYN-related kinase | 58.3 | 90 | 3 | 5.3 |
| ABL2 | v-ABL Abelson murine leukemia viral oncogene homolog 2 (Abelson-related gene) | 124.6 | 122 | 3 | 2.5 |
| PPAT | Phosphoribosyl pyrophosphate amidotransferase | 57.4 | 81 | 2 | 4.3 |
| FGR | Gardner-Rasheed feline sarcoma viral (v-FGR) oncogene homolog | 59.5 | 69 | 2 | 3.4 |
| Entrez name . | Description . | MW, kDa . | Protein score . | Unique peptides . | SC, % . |
|---|---|---|---|---|---|
| BTK | Bruton agammaglobulinemia tyrosine kinase | 76.2 | 1818 | 31 | 49.2 |
| LYN | Tyrosine-protein kinase LYN | 58.6 | 1432 | 18 | 36.9 |
| CSK | c-Src tyrosine kinase | 50.7 | 1113 | 17 | 42.4 |
| EPHB4 | EPH receptor B4 | 108.3 | 432 | 13 | 16.3 |
| MAPK14 | Mitogen-activated protein kinase 14 (p38α) | 41.3 | 275 | 12 | 33.1 |
| YES1 | Proto-oncogene tyrosine-protein kinase YES | 60.8 | 564 | 9 | 17.3 |
| TEC | TEC protein tyrosine kinase | 73.6 | 272 | 9 | 15.8 |
| ABL1 | Proto-oncogene tyrosine-protein kinase ABL1 | 122.9 | 636 | 9 | 11.7 |
| SRC | Tyrosine-protein kinase SRC-1 | 59.8 | 556 | 8 | 15.7 |
| KIT | v-KIT Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | 109.9 | 184 | 6 | 5.6 |
| BCR | Breakpoint cluster region | 137.7 | 414 | 6 | 5.8 |
| FYN | Protein-tyrosine kinase FYN | 54.5 | 237 | 5 | 9.5 |
| EPHA5 | EPH receptor A5 | 114.8 | 105 | 5 | 5.5 |
| PRO3078 | — | 7.7 | 308 | 4 | 60.3 |
| ZAK | Sterile alpha motif and leucine zipper containing kinase AZK | 51.6 | 82 | 3 | 5.9 |
| HSPA8 | Heat shock 70kDa protein 8 | 70.9 | 88 | 3 | 5.7 |
| FRK | FYN-related kinase | 58.3 | 90 | 3 | 5.3 |
| ABL2 | v-ABL Abelson murine leukemia viral oncogene homolog 2 (Abelson-related gene) | 124.6 | 122 | 3 | 2.5 |
| PPAT | Phosphoribosyl pyrophosphate amidotransferase | 57.4 | 81 | 2 | 4.3 |
| FGR | Gardner-Rasheed feline sarcoma viral (v-FGR) oncogene homolog | 59.5 | 69 | 2 | 3.4 |
KU812 proteins were purified on dasatinib-matrix, cleaned up only for keratin. Proteins are sorted by the number of their unique peptides identified. The BCR-ABL fusion protein, which as a whole is not represented in the IPI database, is split into its 2 components, BCR and ABL1. Protein scores and sequence coverage (SC) are based on unique peptides. Protein scores are Mascot standard scores.
MW indicates molecular weight in kDa as defined by the particular IPI database entry; and —, no description available in IPI database.
Dasatinib binds to and blocks phosphorylation of Btk in human basophils. (A) Anti-ABL and anti-BTK immunoblots of KU812 pulldowns with dasatinib and ampicillin (negative control). The samples were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 7% gel. Regions corresponding to BCR-ABL (∼ 210 kDa), c-ABL (∼ 145 kDa), and BTK (∼ 75 kDa) are displayed. TCL indicates total cell lysate (120 μg TCL protein loaded). (B) Immunoblot of lysates derived from KU812 cells after cells had been exposed to control medium or dasatinib (1 μM) at 37°C for 4 hours. Antibodies against phosphorylated Btk (p-Btk), p-Hck, total Btk, and total Lyn were applied as indicated. For detection of p-Lyn, immunoprecipitation was performed using total Lyn antibody and antiphosphotyrosine 4G10 antibody. (C) Basophils obtained from 2 leukemia patients with basophilia (AML, left panel; CML, right panel) were preincubated with control medium or dasatinib (3 μM) at 37°C for 15 minutes, and then were exposed to an anti-IgE antibody (1 μg/mL) at 37°C for 10 minutes. After incubation, cells were recovered and subjected to Western blotting using antibodies against p-Lyn and total Lyn protein. Western blotting was performed as described in the text.
Dasatinib binds to and blocks phosphorylation of Btk in human basophils. (A) Anti-ABL and anti-BTK immunoblots of KU812 pulldowns with dasatinib and ampicillin (negative control). The samples were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 7% gel. Regions corresponding to BCR-ABL (∼ 210 kDa), c-ABL (∼ 145 kDa), and BTK (∼ 75 kDa) are displayed. TCL indicates total cell lysate (120 μg TCL protein loaded). (B) Immunoblot of lysates derived from KU812 cells after cells had been exposed to control medium or dasatinib (1 μM) at 37°C for 4 hours. Antibodies against phosphorylated Btk (p-Btk), p-Hck, total Btk, and total Lyn were applied as indicated. For detection of p-Lyn, immunoprecipitation was performed using total Lyn antibody and antiphosphotyrosine 4G10 antibody. (C) Basophils obtained from 2 leukemia patients with basophilia (AML, left panel; CML, right panel) were preincubated with control medium or dasatinib (3 μM) at 37°C for 15 minutes, and then were exposed to an anti-IgE antibody (1 μg/mL) at 37°C for 10 minutes. After incubation, cells were recovered and subjected to Western blotting using antibodies against p-Lyn and total Lyn protein. Western blotting was performed as described in the text.
All in all, these data suggest that basophils display multiple molecular targets for dasatinib including Btk. Our data also show that dasatinib binds to several of these key signaling kinases that play a role in basophil histamine release (Table 3). The inhibitory effect of dasatinib on the activity of these kinases confirms previous observations made with CML cells.29-31,44
Contribution of Btk to histamine release in basophils and role of additional dasatinib targets
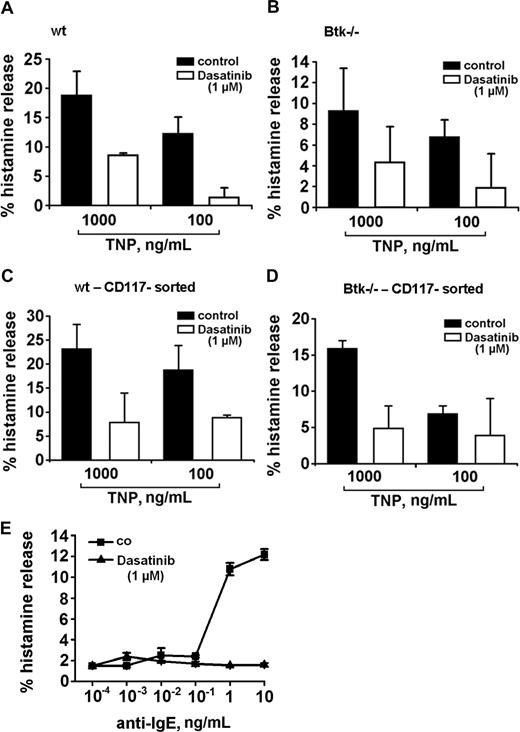
To define the functional role of Btk and of the other targets in the secretory response of basophils, we took advantage of 2 Btk knockout models. In a first step, Btk knockout mice were examined. Basophils grown from the bone marrow of these mice were found to display a functionally active FcϵRI. However, when compared with wt mice, cultured basophils of Btk knockout mice were found to release lower amounts of histamine when challenged with IgE + TNP (Figure 7). Interestingly, dasatinib was able to inhibit the residual histamine release in the same way as in basophils obtained from wt mice (Figure 7A-D), suggesting that dasatinib interacts with additional relevant targets apart from Btk in mouse basophils. In a next step, we examined cells from a patient with Btk deficiency (Bruton disease). Similar to data obtained in Btk knockout mice, the anti-IgE–dependent release of histamine was relatively weak (12%), but dasatinib was still able to block the residual anti-IgE–induced histamine release down to baseline levels (Figure 7E). We also asked whether Btk plays a role in IL-4 secretion in basophils. However, unlike histamine release, the TNP-induced secretion of IL-4 in mouse basophils did not differ quantitatively (was not significantly reduced) when comparing wt mice with Btk knockout mice (data not shown).
Dasatinib blocks histamine release in Btk-deficient basophils. (A,B) Cultured mouse basophils obtained from wild-type (wt) mice (A,C) or Btk-deficient mice (B,D) were preincubated with TNP-specific IgE. Basophils were examined as impure cells (A,B) or as sorted enriched basophils (C,D). Preloaded basophils were washed and incubated in control medium or in medium containing dasatinib (1 μM). Thereafter, cells were exposed to control medium or to TNP (0.1 or 1 μg/mL) at 37°C for 30 minutes. Then, cells were centrifuged at 4°C and lysates and cell-free supernatants examined for the content of histamine by RIA. Results show the percentages of released histamine and represent the means (± SD) from triplicates in one typical experiment each. Analyzing all values in all experiments, the effects of dasatinib on histamine release were found to be significant in wt mice (TNP, 1μg/mL: 28.3% ± 15.1% vs TNP + dasatinib, 1 μM: 5.8% ± 9.8%; P < .05) as well as in Btk-deficient animals (TNP, 1 μg/mL: 16.5% ± 7.3% vs TNP + dasatinib: 3.5% ± 2.1%; P < .05). (E) Basophils from a patient with Btk deficiency were preincubated in control medium (co, ■-■) or dasatinib, 1 μM (▲-▲) for 30 minutes. Thereafter, basophils were washed and incubated in various concentrations of anti-IgE (37°C, 5% CO2) for 30 minutes. Then, cells were centrifuged at 4°C and the cell-free supernatants and lysates examined for histamine content by RIA. Results show the percentages of released histamine and represent the means (± SD) from triplicates.
Dasatinib blocks histamine release in Btk-deficient basophils. (A,B) Cultured mouse basophils obtained from wild-type (wt) mice (A,C) or Btk-deficient mice (B,D) were preincubated with TNP-specific IgE. Basophils were examined as impure cells (A,B) or as sorted enriched basophils (C,D). Preloaded basophils were washed and incubated in control medium or in medium containing dasatinib (1 μM). Thereafter, cells were exposed to control medium or to TNP (0.1 or 1 μg/mL) at 37°C for 30 minutes. Then, cells were centrifuged at 4°C and lysates and cell-free supernatants examined for the content of histamine by RIA. Results show the percentages of released histamine and represent the means (± SD) from triplicates in one typical experiment each. Analyzing all values in all experiments, the effects of dasatinib on histamine release were found to be significant in wt mice (TNP, 1μg/mL: 28.3% ± 15.1% vs TNP + dasatinib, 1 μM: 5.8% ± 9.8%; P < .05) as well as in Btk-deficient animals (TNP, 1 μg/mL: 16.5% ± 7.3% vs TNP + dasatinib: 3.5% ± 2.1%; P < .05). (E) Basophils from a patient with Btk deficiency were preincubated in control medium (co, ■-■) or dasatinib, 1 μM (▲-▲) for 30 minutes. Thereafter, basophils were washed and incubated in various concentrations of anti-IgE (37°C, 5% CO2) for 30 minutes. Then, cells were centrifuged at 4°C and the cell-free supernatants and lysates examined for histamine content by RIA. Results show the percentages of released histamine and represent the means (± SD) from triplicates.
Discussion
Dasatinib is a novel multikinase inhibitor that is successfully used to treat patients with imatinib-resistant CML.33,48-50 In addition to its growth-inhibitory activity on leukemic cells, dasatinib may also exhibit anti-inflammatory and immunosuppressive effects.30,36,51 We here show that dasatinib is a most potent inhibitor of basophil activation, an issue that may reveal novel interesting indications and concepts for this drug. In particular, dasatinib was found to block multiple key functions in basophils including IgE-mediated histamine release and IL-4 secretion as well as IgE-dependent expression of activation-linked cell surface antigens. Another most interesting observation was that the effects of dasatinib on basophils are specific for FcϵRI-dependent activation, but are not seen with C5a-activated basophils.
The effects of dasatinib on basophils occurred at “pharmacologic” drug concentrations. In fact, in most experiments, the IC50 was clearly lower than 1 μM. Moreover, at 1 μM, dasatinib completely blocked histamine secretion to baseline levels. To test whether this impressive effect may also be seen in basophils exposed to a “priming” cytokine reflecting more the situation in physiologic tissues, we repeated histamine release experiments with basophils preincubated (primed) with IL-3. Priming of basophils with IL-3 is usually associated with an enhanced response to IgE-dependent stimuli.38 However, even in IL-3–primed basophils, dasatinib at 1 μM produced complete inhibition of basophil activation and mediator secretion. In addition, we were also able to show that dasatinib at 1 μM blocks IgE-dependent histamine release in CML basophils, in which BCR/ABL is considered to act as an additional signaling molecule similar to IL-3–primed basophils. Together, these observations provide evidence that dasatinib is a novel potent inhibitor of basophil activation.
Apart from IgE-dependent triggers, a number of other factors and molecules may contribute to basophil activation in an inflammatory reaction.2-4,53 We were therefore interested to learn whether the inhibitory (anti-inflammatory) effect of dasatinib on basophils is restricted to IgE-dependent activation or is also seen when basophils are activated by IgE-independent compounds. To address this question, basophils were incubated with the Ca-ionophore A23187 or with recombinant C5a, both of which are known to induce histamine release. However, unlike IgE-dependent release, neither the Ca-ionophore–induced nor the C5a-induced release of histamine from basophils was found to be inhibitable by dasatinib. This observation suggests that the dasatinib-induced inhibition of basophil activation is specific for FcϵRI activation.
FcϵRI cross-linkage on basophils is associated with activation of several signal transduction pathways, expression and up-regulation of various surface molecules, and production and/or release of several mediators and cytokines.6-20,45-47,54,55 In this study, dasatinib was found to block not only the IgE-mediated release of histamine, but also the anti-IgE–induced up-regulation of several activation-linked cell surface molecules on basophils, including CD13, CD63, CD164, and CD203c. In addition, dasatinib was found to block the IgE-mediated production/secretion of IL-4 in cultured (impure and enriched) mouse basophils. These data suggest that dasatinib blocks IgE-dependent basophil activation in general, not only histamine release.
A number of different signaling molecules have been implicated in the regulation of basophil activation provoked by FcϵRI cross-linkage.6-20 Among these molecules are tyrosine kinases such as Src family kinases, spleen tyrosine kinase, Syk, and the Bruton tyrosine kinase, Btk.10-20 The results of our study show that dasatinib binds to and blocks several of these kinases including Btk in basophils, which confirms our recent observations in CML cells.44,56 The observation that Btk can serve as a target of dasatinib was also supported by the observation that basophils in Btk knockout mice exhibit a reduced capacity to release histamine upon IgE-dependent activation of basophils compared with wild-type mice. On the other hand, dasatinib was still found to completely decrease the “residual” histamine secretion in Btk-deficient basophils down to baseline levels, and the same result was obtained with basophils derived from a patient with Btk deficiency. These data suggest that dasatinib blocks mediator secretion in basophils through multiple targets, not only Btk, which is consistent with the observation that dasatinib bound to multiple kinases in our drug-affinity purification and MS approach.
An important question was whether the inhibitory effects of dasatinib on basophils would also be seen in allergic patients. We therefore incubated basophils obtained from allergic donors with dasatinib, and found that dasatinib inhibits the allergen-induced histamine release in almost the same way as the anti-IgE–induced release of histamine in normal basophils. However, the concentrations of dasatinib required to block histamine release to baseline levels were found to be slightly higher in allergic basophils compared with normal basophils, and the same was found to hold true for IgE-dependent up-regulation of CD molecules. Nevertheless, dasatinib blocked histamine secretion and CD antigen expression in all allergic donors examined.
From our data, dasatinib may be regarded an interesting new agent for the treatment of immunologic (allergic) disorders associated with IgE-dependent activation of basophils. This notion was supported by the observation that the effects of dasatinib occurred at pharmacologically reachable drug concentrations.57 However, it remains unknown whether the same effects can be obtained in vivo using dasatinib in allergic patients. Moreover, dasatinib also has significant side effects including edema formation, pleural effusions, cytopenia, and exanthema.33,48,50,58 Therefore, it remains unknown whether dasatinib would indeed succeed as an antiallergic drug. In this regard, it is also noteworthy that we found that lower concentrations of dasatinib even promote the release of histamine from human basophils, especially in allergic subjects. Although the mechanism of this effect remains unknown, the observation predicts that dasatinib may even cause edema formation or effusions under certain conditions or in distinct anatomic sites where the drug can less well accumulate. An interesting observation was that this release-promoting effect of dasatinib at lower drug concentrations was not observed in CML basophils. As mentioned above, the biochemical basis of the paradoxical increase in histamine release in sensitized basophils observed at low drug concentrations remains at present unknown. One possibility may be that some of the dasatinib targets even block IgE-dependent histamine release. In this regard, it is of interest that one of the key targets of dasatinib, Lyn, has recently been described as a potential negative regulator of IgE-dependent secretion of histamine in mouse basophils.59,60
In summary, our data show that dasatinib inhibits IgE-mediated basophil activation and histamine secretion in vitro. This drug effect may contribute to antiallergic and immunosuppressive effects of dasatinib. Whether this effect can be exploited for patients with IgE-dependent allergies or other immunologic disorders involving basophils, Btk, and IgE remains to be determined in forthcoming studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Heike Kaltenegger for skillful technical assistance, Jacques Colinge for expert analysis of mass spectrometry data, and Oliver Hantschel for helpful discussions.
This work was supported by the Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich (FWF), grants nos. 01801, 01803, 01809, 01815, and Y-163; and the Austrian Federal Ministry for Science and Research (bm.wf), GEN-AU program, grant GZ 200.142/1-IV/1/2006. U.S. was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) Schm 2128/1-1.
Authorship
Contribution: M.K. performed key laboratory experiments on basophil activation, analyzed the data, and contributed to the drafting of the article; U.S., A.S., and W.E. contributed experiments on Btk-deficient mice and mouse bone marrow–derived cultured basophils; A.V. and H.H. contributed histamine release experiments and measurements of histamine and IL-4; C.B. contributed flow cytometry experiments; K.V.G. contributed Western blot and IP experiments; M.W., C.L., W.R.T., S.V., and R.V. contributed recombinant allergens as well as allergic patients; K.L.B. performed mass spectrometry analysis; U.R. and G.S.-F. contributed drug-binding studies and logistic and budget support; F.Y.L. contributed essential reagents; and P.V. contributed the study design, logistics, and budget support, and drafted and approved the final version of the article.
Conflict-of-interest disclosure: One coauthor (F.Y.L.) is employed by a company (Bristol-Myers Squibb, Princeton, NJ) whose potential product (targeted drug) was studied in the present work. All other authors declare no competing financial interests.
Correspondence: Peter Valent, Department of Internal Medicine I, Division of Hematology and Hemostaseology, Medical University of Vienna, Austria; e-mail: peter.valent@meduniwien.ac.at.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal