Abstract
Thyroid hormone and its cognate receptor (TR) have been implicated in the production of red blood cells. Here, we show mice deficient for TRα have compromised fetal and adult erythropoiesis. Erythroid progenitor numbers were significantly reduced in TRα−/− fetal livers, and transit through the final stages of maturation was impeded. In addition, immortalized TRα−/− erythroblasts displayed increased apoptosis and reduced capacity for proliferation and differentiation. Adult TRα−/− mice had lower hematocrit levels, elevated glucocorticoid levels, and an altered stress erythropoiesis response to hemolytic anemia. Most TRα−/− animals contained markedly altered progenitor numbers in their spleens. Strikingly, 20% of TRα−/− mice failed to elicit a stress erythropoiesis response and recovered very poorly from hemolytic anemia. We conclude that an underlying erythroid defect exists in TRα−/− mice, demon-strating the importance of TRα to the erythroid compartment.
Introduction
Binding of erythropoietin (Epo) to its cell-surface receptor activates signaling cascades initiated by JAK2.1 Knockout studies have demonstrated the importance of Epo, the Epo receptor, JAK2, ras, raf, and STAT5 to erythropoiesis.2-6 We have shown that the Src kinase Lyn acts as an important secondary kinase for Epo signaling, both in vitro7 and in vivo.8 A yeast 2-hybrid screen identified several known signaling molecules that form part of the Lyn pathway, including HS1 and Cbp.9,10 Trip1 (thyroid hormone receptor interacting protein) was also isolated as a Lyn-interacting protein, providing an unexpected link between the Epo and thyroid hormone signaling pathways.11 Our in vitro analyses showed that thyroid hormone (T3) stimulated proliferation of erythroid cells, but inhibited Epo-induced differentiation.11 Significantly, T3 has been implicated in the regulation of erythropoiesis, with both hypothyroid and hyperthyroid patients displaying red cell abnormalities.12,13
The thyroid hormone receptor (TR) is a member of the nuclear receptor superfamily,14 and the TRα isoform is expressed exclusively in the erythroid compartment.15 Altering the TRα content of chicken erythroblasts affects the balance between proliferation and differentiation,16 and a mutated version of this receptor (v-erbA) causes avian erythroleukemias.17,18 While TRα−/− mice have defects in several organs,19-21 neonatal mice also display deficiencies in splenic erythropoiesis.22 In this study, we examined the impact of TRα deletion on fetal and adult erythropoiesis.
Methods
Fetal liver cells from TRα1−/− or TRβ−/− mice,21 on a 129sv background, were isolated from day-12.5 embryos and then plated in methylcellulose for analysis of erythroid progenitors.8 Fetuses were photographed using a Canon EOS 400D camera (Canon, Ohta-ku, Japan) and images processed using Adobe Photoshop CS2 (v9.02; Adobe Systems, San Jose, CA). Adult (12 weeks old) mice were treated with phenylhydrazine to induce anemia.8 Cytocentrifuged splenocytes were stained with neutral benzidine (Sigma-Aldrich, St Louis, MO) to detect hemoglobin counterstained with Giemsa (BDH, Kilsyth, Australia), while blood smears were stained with methylene blue (Sigma-Aldrich) to identify reticulocytes.8 Cytocentrifuge preparations were mounted in DePex Mounting Medium (Biolabs, Victoria, Australia). Micrographs were acquired using an Olympus BX51 microscope fitted with a 40×/0.75 UPlanFl objective, connected to an Olympus DP12 digital camera. Image-Pro Plus (v5.0; Media Cybernetics, Bethesda, MD) was used to process acquired images. Proliferation was measured by CellTitre 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI), while cortisol and Epo were determined using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN), according to the manufacturers' instructions. Cells were stained for flow cytometry with CD-71-FITC, TER-119-PE (BD Pharmingen, San Diego, CA), or annexin V.8 Immortalized cell lines from knockout mice were generated by exposing fetal liver progenitors to the J2 retrovirus as described previously.23,24 Erythroid burst-forming units (BFU-Es) and erythroid colony-forming units (CFU-Es) were determined using methylcellulose cultures, as described elsewhere.11
Results and discussion
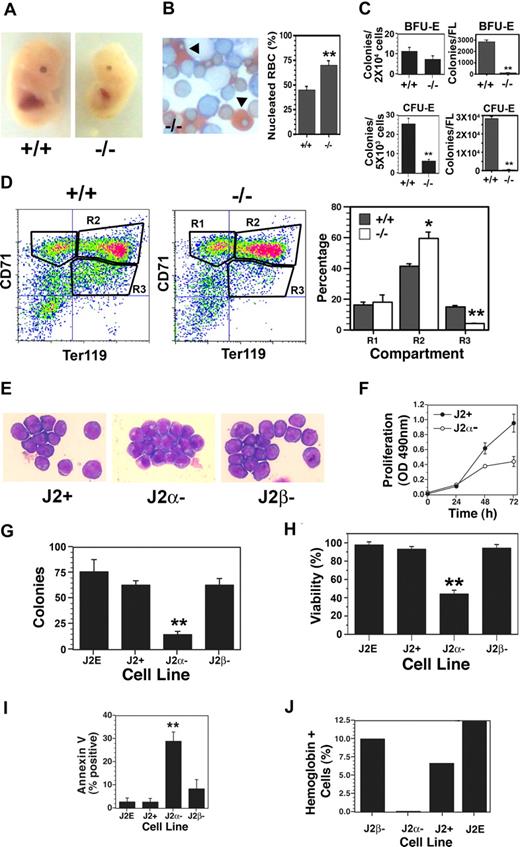
As our previous studies with T3 were conducted with fetal liver progenitors11 and the fetal liver-derived J2E cell line,23 we examined the impact of TRα deletion on fetal erythropoiesis in vivo. Interestingly, TRα heterozygotes produced 31 +/+, 50 +/−, and 12 −/− fetuses; this varied significantly (P < .01) from the expected Mendelian ratio of 1:2:1, suggesting potential embryonic lethality of −/− fetuses. TRα−/− fetuses generally had smaller livers (Figure 1A) and a higher percentage of nucleated (primitive) erythrocytes compared with enucleate (definitive) red blood cells (Figure 1B). Progenitor levels were significantly lower in the knockout mice (Figure 1C) when expressed as percentage of cells plated; strikingly, with the reduced fetal liver size, total BFU-E and CFU-E levels were decreased dramatically (Figure 1C). Flow cytometric analyses revealed a marked accumulation of cells in the differentiating R2 compartment (CD71high/Ter119high) of TRα−/− mice (Figure 1D); conversely, a significant reduction in the mature R3 compartment (CD71low/Ter119high) was observed. Thus, transit through terminal differentiation was altered substantially in erythroblasts isolated from TRα−/− fetal livers. Together, these data indicate abnormalities in TRα−/− erythropoiesis, which could affect fetal viability. In contrast, erythropoiesis in TRα+/− and TRβ−/− fetal livers was indistinguishable from wild-type animals (data not shown).
Altered fetal erythropoiesis in TRα−/− mice. (A) Day-12.5 fetuses of TRα+/+ (+/+) and TRα−/− (−/−) mice. (B) Neutral benzidine/Giemsa-stained fetal liver cells from day-12.5 TRα+/+ and TRα−/− embryos, with percentage of nucleated red blood cells (RBCs, arrowheads) enumerated (n = 3). (C) Erythroid colony assays (BFU-Es and CFU-Es) of fetal liver cells from day-12.5 TRα+/+ and TRα−/− embryos (n = 3). (D) Flow cytometric analysis of fetal liver cells from day-12.5 TRα+/+ and TRα−/− embryos, stained with CD71 and TER119. Erythroid populations corresponding to different stages of maturation (R1, R2, R3) were enumerated (n = 5). (E) Morphology of Giemsa-stained immortalized erythroblasts from day-12.5 TRα+/+ (J2+), TRα−/− (Jα−), and TRβ−/− (Jβ−) fetal livers. (F) Proliferation of J2+ and J2α− cells was measured by a colorimetric (OD: 490 nm) MTT-based assay (n = 6). (G) Clonogenicity of immortalized erythroid cells was determined using methylcellulose cultures (n = 3). (H) Viability of immortalized erythroid cells was determined by eosin dye exclusion (n = 6). (I) Apoptosis was assessed by annexin V staining of immortalized erythroid cells (n = 4). (J) Hemoglobin production was identified by benzidine staining in response to Epo treatment (48 hours). Means (± SD) are shown. Statistically significant (2-way ANOVA) differences are indicated (*P ≤ .05; **P ≤ .01).
Altered fetal erythropoiesis in TRα−/− mice. (A) Day-12.5 fetuses of TRα+/+ (+/+) and TRα−/− (−/−) mice. (B) Neutral benzidine/Giemsa-stained fetal liver cells from day-12.5 TRα+/+ and TRα−/− embryos, with percentage of nucleated red blood cells (RBCs, arrowheads) enumerated (n = 3). (C) Erythroid colony assays (BFU-Es and CFU-Es) of fetal liver cells from day-12.5 TRα+/+ and TRα−/− embryos (n = 3). (D) Flow cytometric analysis of fetal liver cells from day-12.5 TRα+/+ and TRα−/− embryos, stained with CD71 and TER119. Erythroid populations corresponding to different stages of maturation (R1, R2, R3) were enumerated (n = 5). (E) Morphology of Giemsa-stained immortalized erythroblasts from day-12.5 TRα+/+ (J2+), TRα−/− (Jα−), and TRβ−/− (Jβ−) fetal livers. (F) Proliferation of J2+ and J2α− cells was measured by a colorimetric (OD: 490 nm) MTT-based assay (n = 6). (G) Clonogenicity of immortalized erythroid cells was determined using methylcellulose cultures (n = 3). (H) Viability of immortalized erythroid cells was determined by eosin dye exclusion (n = 6). (I) Apoptosis was assessed by annexin V staining of immortalized erythroid cells (n = 4). (J) Hemoglobin production was identified by benzidine staining in response to Epo treatment (48 hours). Means (± SD) are shown. Statistically significant (2-way ANOVA) differences are indicated (*P ≤ .05; **P ≤ .01).
Immortalized cell lines were then generated to examine erythroblasts with different genotypes in greater detail and compared with the wild-type J2E cell line. Each of the cell lines derived from wild-type (J2+), TRα−/− (J2α−), and TRβ−/− (J2β−) fetal livers displayed morphologic features of immature erythroid cells (Figure 1E), as well as cell-surface markers of erythroid progenitors (CD71high/Ter119low). Significantly, the TRα−/− lines grew poorly compared with wild-type clones (Figure 1F), and had markedly reduced clonogenicity (Figure 1G). The decreased growth rate and clonogenicity of the TRα−/− cells could be attributed to substantially reduced viability (Figure 1H), caused by increased apoptosis (Figure 1I). In addition, the TRα−/− lines were incapable of synthesizing hemoglobin in the presence of Epo (Figure 1J). These data indicate that the absence of TRα resulted in increased cell death, and severely compromised the capacity for proliferation and differentiation.
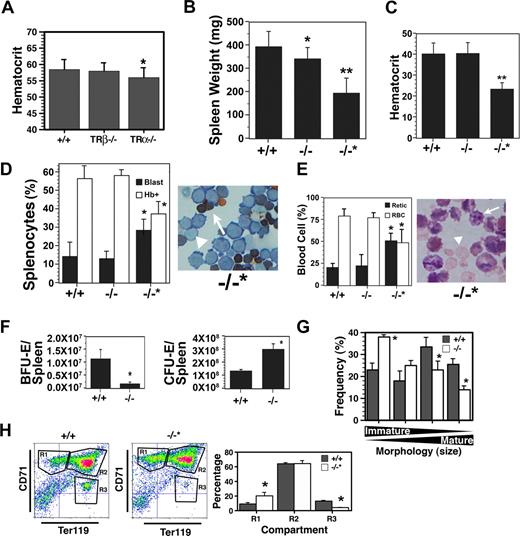
The erythroid compartment of adult mice was examined next, and a modest but statistically significant decrease in hematocrit level was detected in TRα−/−, but not TRα+/− (data not shown) or TRβ−/−, animals (Figure 2A). No differences in circulating Epo were observed in the different strains, however, in TRα−/− animals glucocorticoid (cortisol) levels were elevated 10-fold (20 ng/mL) compared with TRβ−/− or wild-type controls (< 2 ng/mL). Since glucocorticoids have been shown to be involved in stress erythropoiesis,25 this marked rise in cortisol indicates activation of a compensatory mechanism to maintain red cell production.
Altered adult erythropoiesis in TRα−/− mice. (A) Hematocrit level of 10-week-old TRα+/+ (n = 32), TRβ−/− (n = 28), and TRα−/− (n = 38) mice. (B) Spleen weight of TRα+/+ (+/+), TRα−/− (−/−), and TRα−/− “poor responder” (−/−*) mice 3 days after phenylhydrazine treatment (n = 6). Spleen weights before treatment were 100 to 120 mg. (C) Hematocrit level of mice taken 3 days after phenylhydrazine treatment (n = 6). (D) Enumeration of hemoglobin-positive (Hb+, arrow) and proerythroblasts (blast, arrowhead) in the spleens of phenylhydrazine-treated mice (n = 3). (E) Percentage of circulating reticulocytes (arrow) and erythrocytes (arrowhead) from mice 3 days after phenylhydrazine treatment (n = 3). (F) Total erythroid progenitors (BFU-Es and CFU-Es) in the spleens of TRα+/+ (+/+) and TRα−/− (−/−) mice 3 days after phenylhydrazine treatment (n = 3). (G) Morphology of erythroblasts from TRα+/+ (+/+) and TRα−/− (−/−) cultured for 3 days ex vivo (n = 3). (H) Flow cytometric analysis of spleen cells from TRα+/+ (+/+) and TRα−/−“poor responders” (−/−*) stained with CD71 and Ter119 (n = 4). Means (± SD) are shown. Statistically significant (2-way ANOVA) differences are indicated (*P ≤ .05; **P ≤ .01).
Altered adult erythropoiesis in TRα−/− mice. (A) Hematocrit level of 10-week-old TRα+/+ (n = 32), TRβ−/− (n = 28), and TRα−/− (n = 38) mice. (B) Spleen weight of TRα+/+ (+/+), TRα−/− (−/−), and TRα−/− “poor responder” (−/−*) mice 3 days after phenylhydrazine treatment (n = 6). Spleen weights before treatment were 100 to 120 mg. (C) Hematocrit level of mice taken 3 days after phenylhydrazine treatment (n = 6). (D) Enumeration of hemoglobin-positive (Hb+, arrow) and proerythroblasts (blast, arrowhead) in the spleens of phenylhydrazine-treated mice (n = 3). (E) Percentage of circulating reticulocytes (arrow) and erythrocytes (arrowhead) from mice 3 days after phenylhydrazine treatment (n = 3). (F) Total erythroid progenitors (BFU-Es and CFU-Es) in the spleens of TRα+/+ (+/+) and TRα−/− (−/−) mice 3 days after phenylhydrazine treatment (n = 3). (G) Morphology of erythroblasts from TRα+/+ (+/+) and TRα−/− (−/−) cultured for 3 days ex vivo (n = 3). (H) Flow cytometric analysis of spleen cells from TRα+/+ (+/+) and TRα−/−“poor responders” (−/−*) stained with CD71 and Ter119 (n = 4). Means (± SD) are shown. Statistically significant (2-way ANOVA) differences are indicated (*P ≤ .05; **P ≤ .01).
To examine the response to stress erythropoiesis, mice were subjected to hemolytic anemia induced by phenylhydrazine treatment. Prior to exposure to phenylhydrazine, no differences were observed in red cell indices, reticulocytes, spleen weights, or cell numbers (Figure 2A and data not shown). The majority of TRα−/− animals underwent characteristic splenomegaly due to stress erythropoiesis, although the spleens were slightly smaller (Figure 2B). The hematocrit level of most TRα−/− mice recovered to the same extent as wild-type animals (Figure 2C), and the levels of proerythroblasts and hemoglobin-synthesizing cells were comparable (Figure 2D); moreover, the reticulocyte-erythrocyte ratio in peripheral blood was similar (Figure 2E). However, the erythroid progenitor content in the TRα−/− spleens was altered markedly—total BFU-Es in TRα−/− spleens were approximately 10% of wild-type spleens, whereas total CFU-Es were 200% to 300% higher in TRα−/− spleens (Figure 2F). These data strongly suggest that compensatory mechanisms were operating in TRα−/− mice to elicit a stress erythropoiesis response. In addition, ex vivo cultures revealed TRα−/− spleens contained more immature erythroblasts, and fewer hemoglobin-producing cells (Figure 2G), reflecting inefficient transit through terminal differentiation. Collectively, these results indicate that the erythroid compartment of adult TRα−/− mice was compromised, especially with emergency erythropoiesis. No differences were observed among wild-type, TRα+/−, and TRβ−/− mice.
Unexpectedly, a small group of animals (∼ 20%) failed to undergo splenomegaly during hemolytic anemia (Figure 2B). While no differences were observed in various parameters (eg, hematocrit level, spleen weight [100-120 mg], red cell indices) prior to phenylhydrazine treatment (data not shown), these animals became severely anemic and recovered poorly from hemolytic anemia (Figure 2C); and their spleens contained fewer hemoglobin-positive erythroblasts (Figure 2D). The elevated reticulocyte count in peripheral blood also indicated defective red cell maturation (Figure 2E). Flow cytometric analyses revealed a significant accumulation of immature R1 compartment cells, combined with a marked reduction in more mature erythroblasts of the R3 compartment (Figure 2H). Thus, multiple defects in the erythroid differentiation pathway were observed in this cohort of TRα−/− animals. Studies are currently under way to identify possible modifier genes present in these mice that promote the poor stress erythropoietic response.
Data presented in this paper demonstrate that erythroid defects are evident during fetal and adult life of TRα−/− mice, extending the red cell abnormalities beyond postnatal splenic erythropoiesis.22 A major consequence of TRα−/− deletion was impaired capacity of immature erythroid cells to traverse terminal differentiation. This study adds to the mounting evidence that members of the nuclear receptor superfamily (including the glucocorticoid receptor25 ) play a significant role in red blood cell production. Thus, the interplay between cytokine and nuclear receptor signaling pathways is vital for the production of functional erythrocytes.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Jacques Samarut and Dr Karine Gauthier, Laboratoire de Biologie Moléculaire et Cellulaire, Lyon, France, for their generous provision of the TRα−/− and TRβ−/− mice.
This work was supported by grants from the National Health and Medical Research Council of Australia (303101 and 403987), the Medical Research Foundation of Royal Perth Hospital, the University of Western Australia, and the Center for Food and Genomic Medicine.
Authorship
Contribution: T.S.K. designed and supported the research, designed, supported and performed experiments, analyzed data, and contributed to writing the paper; C.J.P., M.R.E., and J.R.S. performed experiments; P.J.L. supported the research; S.P.K. supported the research, designed experiments, analyzed results, and contributed to writing the paper; E.I. designed and supported the research, designed and undertook experiments, analyzed data, and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: S. Peter Klinken, WAIMR, Level 6 MRF Building, Rear 50 Murray Street, Perth, WA 6000, Australia; e-mail: pklinken@waimr.uwa.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal