Abstract
Young adult Hodgkin lymphoma (YAHL) is associated clinically with altered immunity, including a systemic defect in cell-mediated responses. There is strong evidence of a genetic contribution to risk, so we hypothesized that heritable alterations in cytokine production associated with Th1 function may contribute to susceptibility. We identified twin pairs in whom at least one member had YAHL and measured interleukin-2 (IL-2), interleukin-12 (IL-12), and interferon-γ (IFN-γ) levels in PHA-stimulated peripheral blood mononuclear cell supernatant in 90 case-twins, 84 of their disease-free twins (unaffected cotwins), and 90 matched controls. Mean difference and mean percentage difference in cytokine levels between case-twins and controls, and unaffected cotwins and controls were determined using analysis of covariance. YAHL case-twins and their unaffected cotwins had IL-12 levels that were 60.6% (P = .002) and 49% (P = .04) lower than those of their matched controls, respectively. IL-2 levels were significantly higher in case-twins (P = .049), but not unaffected cotwins (P = .57), compared with controls. Differences in IFN-γ levels were not statistically significant in either comparison. An IL-12 polymorphism known to regulate expression was associated with a 2.8-fold (P = .03) increase in YAHL risk. Thus, both case-twins and their unaffected cotwins had a decreased ability to produce IL-12, which may contribute to YAHL susceptibility.
Introduction
Hodgkin lymphoma is characterized by 3 distinctive age-specific incidence peaks associated with different demographic factors, probably representing distinct etiologies.1 Young adult Hodgkin lymphoma (YAHL), diagnosed approximately from ages 13 to 45 years, consists mainly of an Epstein-Barr virus (EBV)–negative, nodular sclerosis subtype, and has been historically strongly (positively) correlated with higher socioeconomic status and low sibship size.2 These factors suggest an association between early childhood isolation from infection and risk, consistent with delayed exposure to a common childhood infection (“polio model”).3 Although Epstein-Barr virus infection would be consistent with this set of factors, the best study to date found no increased risk of EBV-negative Hodgkin lymphoma, the most common type of YAHL, among 40 000 persons with laboratory-documented infectious mononucleosis.4 (The same study did demonstrate a 20-fold risk of EBV-positive Hodgkin lymphoma within 5 years of the infectious mononucleosis diagnosis, but EBV-positive Hodgkin lymphoma accounts for only 10% to 25% of the disease in young adults.)
Genetic factors also contribute to YAHL susceptibility. Excess risk has been reported in siblings5 and family members6 of cases, and we found a greater excess risk in identical (monozygotic, MZ) cotwins compared with fraternal (dizygotic, DZ) cotwins of cases.7 An immune response phenotype associated with altered cytokine levels constitutes a likely candidate for a heritable risk factor since the pathological and clinical hallmark of Hodgkin lymphoma is immune dysregulation.8 Untreated HL patients have a defect in cell-mediated immunity as evidenced by delayed hypersensitivity to new and recall antigens, depressed T-cell proliferation in response to mitogens, and natural killer cell activity, consistent with lower T-helper lymphocyte 1 (Th1) cytokine activity.8 There is also evidence of an exaggerated T-helper lymphocyte 2 (Th2) cytokine response, for example, elevated IgE levels, eosinophilic infiltrate in the tumor, and extensive fibrosis in the EBV-negative nodular sclerosis tumors, probably due to interleukin-13 production by the Hodgkin Reed-Sternberg (H-RS) cells.8
Single nucleotide polymorphisms (SNPs) have been identified in promoter regions of many cytokine genes and in genes encoding cytokine receptors, often without impact on cytokine secretion or function, thus measurement of SNPs is not a substitute for measurement of cytokine levels. On the other hand, since cytokine measurement may be altered following cytotoxic therapy, or as a result of the disease process itself, patients may not make good subjects for these studies. Unaffected cotwins of cases may serve as more reliable subjects to define predisposing factors because they share 50% (DZ) or 100% (MZ) of the genes in common with their YAHL case-twin but are not affected by the disease. The usefulness of the unaffected cotwins as surrogate cases depends on the extent to which cytokine responses are under genetic control. Several studies have demonstrated that cytokine levels are at least partially heritable, with heritability estimates ranging from 37% for interleukin (IL)-49 to 85% for interferon (IFN)-γ.10
We previously reported significantly higher IL-6 levels in unaffected MZ cotwins of YAHL case-twins compared with matched controls and also found that an IL-6 promoter SNP, consistently associated with lower levels of IL-6, was protective.11 Here we describe comparisons of levels of cytokines associated with induction of cytotoxic T-cell and natural killer cell activity among prevalent YAHL cases long in remission, their unaffected cotwins, and matched controls. Of note, the main purpose of this study was not to evaluate immune function associated with clinical disease, but instead to infer predisposition to disease via the unaffected cotwins.
Methods
The study was approved by the Institutional Review Board of the Keck School of Medicine of the University of Southern California, and written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki.
Twin pairs
We identified MZ twin pairs in whom at least one member (case-twin) had been diagnosed with Hodgkin lymphoma between ages 13 and 45 years from the rosters of the International Twin Study12 and California Twin Program.13 Of 115 available pairs, 4 were ineligible, 18 refused, one twin was deceased in 21 pairs, and in 14, neither twin could be located, leaving 58 MZ pairs who participated in our study. Of these, 53 of the cotwins of cases remained disease free (unaffected cotwin) and 5 had developed YAHL (affected cotwin).
A sample of DZ twin pairs discordant for young adult YAHL identified from the same twin registries were also contacted. Of the 56 pairs identified, the case was deceased in 12 pairs, one pair was ineligible, we were unable to locate either member of 4 pairs, and at least one member of 7 pairs refused. No DZ cotwin had developed YAHL. Blood samples were obtained from both members of the 32 remaining DZ pairs. In summary, samples were obtained from both members of 90 twin pairs in whom at least one twin was diagnosed with YAHL.
Pathology reports and histopathologic slides were obtained for 96% of the YAHL cases and were reviewed by a single hematopathologist (B.N.N.) with the following histopathologic distribution: 57 (66%) nodular sclerosis, 15 (17%) mixed cellularity, 8 (9%) lymphocyte predominant, and 6 (7%) unclassifiable. All cases were long in remission (average of 16 years after diagnosis; range: 4-38 years) by the time they gave a blood sample so neither treatment data nor Epstein-Barr virus (EBV) tumor status was available.
Controls
Controls consisted of a spouse of one of the twins (n = 61), a nonblood relative or friend of one of the twins (n = 14), or a race-, ethnicity-, and age-matched convenience control chosen from employees within our institution (n = 15).
Laboratory
Blood specimens from each participating triad member were simultaneously collected and processed. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient separation. Two aliquots of cells (2 × 106/mL) were prepared: one cultured in fetal calf serum medium (RPMI with 10% fetal calf serum) for 24 hours without added mitogens, and one cultured in RPMI + fetal calf serum medium containing 10 μg/mL phytohemagglutinin (PHA) for 24 hours. The resulting supernatants were collected and frozen at − 70°C. Viability of cells was tested by trypan blue exclusion; if more than 5% of cells were nonviable, the culture was discarded. PBMC supernatant levels of interleukin-2 (IL-2), interleukin-12 (IL-12), and interferon-γ (IFN-γ) were determined by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN), according to the manufacturer's instructions. Specimens from each member of the triad were run on the same ELISA plate and read together to minimize variability in the comparisons. Positive and negative controls plus standards were run according to the manufacturer's instructions, and if the standard curve deviated more than 10% from the average of all standard curves, values from that plate were excluded. For this reason, and because some samples were exhausted before completion, the number of subjects varies by assay.
DNA was available for a subset of 47 cases and 39 controls, so a polymorphism in the UTR of the IL-12 gene that alters gene expression14 was examined using pyrosequencing.15 Because we did not include genotyping on the original informed consent, we had to delink the genotyped samples from the identification numbers and therefore we were unable to correlate the genotypes with IL-12 secretion in this sample.
Statistical analysis
Since distributions of the cytokine measures were skewed, values were log-transformed. Geometric means were calculated from the transformed values and the mean absolute and percentage difference between case-twins' and controls' cytokine levels, and between unaffected cotwins' and controls' cytokine levels were determined. We used a general linear model to test for differences in cytokine levels between case-twins and controls, and between their unaffected cotwins and controls. The comparisons were performed stratified by zygosity and then combined when no significant differences were observed. The models included adjustments for sex, age (as a categoric variable), and ELISA plate, and accounted for the dependence of measurements within each.16 Affected cotwins were excluded from these analyses. We then examined differences in cytokine levels within controls by sex, age, and type of control using general linear modeling.
Spearman correlation coefficients were used to test correlations between types of cytokine levels in controls. Odds ratios (ORs) and 95% confidence intervals (CIs) estimating the effect of the genotype on disease risk were calculated from maximum likelihood estimates using unconditional multivariable regression, adjusting for age at blood sample and sex. Age was considered as a categoric variable for all analyses (≤ 30, 31-40, 41-50, and 51-60 years). Since 99% of the subjects were white, race was not included in the adjustment. All hypothesis testing assumed a .05 significance level and 2-sided alternative hypotheses. All analyses were performed with the Statistical Analysis System (SAS) version 8.1 (SAS Institute, Cary, NC).
Results
Demographic characteristics of YAHL case-twins, unaffected cotwins, affected cotwins (excluded from subsequent cytokine analyses), and controls are shown in Table 1. The age and sex distribution was similar among the 3 groups, except that DZ twin-cases and their unaffected cotwins were predominantly female (81%), while their matched controls were predominantly male (67%).
Demographic characteristics of the young adult Hodgkin lymphoma (YAHL)–affected case-twins, affected and unaffected cotwins, and matched controls
| . | Controls . | Case-twins* . | Unaffected cotwins . | Affected cotwins† . | ||||
|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Sex | ||||||||
| Male | 49 | 54 | 34 | 38 | 34 | 40 | 2 | 40 |
| Female | 41 | 46 | 56 | 62 | 50 | 59 | 3 | 60 |
| Total | 90 | 90 | 84 | 5 | ||||
| Age at diagnosis, y | ||||||||
| 20 or younger | — | — | 32 | 35 | — | — | — | — |
| 21 to 30 | — | — | 42 | 47 | — | — | — | — |
| 31 to 45 | — | — | 16 | 18 | — | — | — | — |
| Total | 90 | |||||||
| Age at participation | ||||||||
| 30 or younger | 11 | 12 | 12 | 13 | 12 | 14 | 0 | 0 |
| 31 to 40 | 32 | 36 | 32 | 36 | 28 | 33 | 4 | 80 |
| 41 to 50 | 36 | 40 | 37 | 41 | 35 | 41 | 1 | 20 |
| 51 to 60 | 11 | 12 | 9 | 10 | 9 | 11 | 0 | 0 |
| Total | 90 | 90 | 84 | 5 | ||||
| Race | ||||||||
| White | 89 | 99 | 88 | 98 | 82 | 98 | 5 | 100 |
| African-American | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| Asian | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| Total | 90 | 90 | 84 | 5 | ||||
| Zygosity | ||||||||
| MZ | — | — | 58 | 64 | 53 | 62 | 5 | 100 |
| DZ | — | — | 32 | 36 | 31 | 38 | 0 | 0 |
| Total | 90 | 90 | 84 | 5 | ||||
| . | Controls . | Case-twins* . | Unaffected cotwins . | Affected cotwins† . | ||||
|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Sex | ||||||||
| Male | 49 | 54 | 34 | 38 | 34 | 40 | 2 | 40 |
| Female | 41 | 46 | 56 | 62 | 50 | 59 | 3 | 60 |
| Total | 90 | 90 | 84 | 5 | ||||
| Age at diagnosis, y | ||||||||
| 20 or younger | — | — | 32 | 35 | — | — | — | — |
| 21 to 30 | — | — | 42 | 47 | — | — | — | — |
| 31 to 45 | — | — | 16 | 18 | — | — | — | — |
| Total | 90 | |||||||
| Age at participation | ||||||||
| 30 or younger | 11 | 12 | 12 | 13 | 12 | 14 | 0 | 0 |
| 31 to 40 | 32 | 36 | 32 | 36 | 28 | 33 | 4 | 80 |
| 41 to 50 | 36 | 40 | 37 | 41 | 35 | 41 | 1 | 20 |
| 51 to 60 | 11 | 12 | 9 | 10 | 9 | 11 | 0 | 0 |
| Total | 90 | 90 | 84 | 5 | ||||
| Race | ||||||||
| White | 89 | 99 | 88 | 98 | 82 | 98 | 5 | 100 |
| African-American | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| Asian | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| Total | 90 | 90 | 84 | 5 | ||||
| Zygosity | ||||||||
| MZ | — | — | 58 | 64 | 53 | 62 | 5 | 100 |
| DZ | — | — | 32 | 36 | 31 | 38 | 0 | 0 |
| Total | 90 | 90 | 84 | 5 | ||||
— indicates not applicable.
Case-twins diagnosed with young adult Hodgkin lymphoma (YAHL) between 13 to 49 years of age.
Affected cotwins were not included in subsequent analyses.
Mean levels of cytokines in controls were as follows: 26.2 pg/mL (95% CI = 17.7-38.7) for IL-2, 2.8 pg/mL (95% CI = 1.7-4.7) for IL-12, and 40.5 pg/mL (95% CI = 24.2-67.6) for IFN-γ.
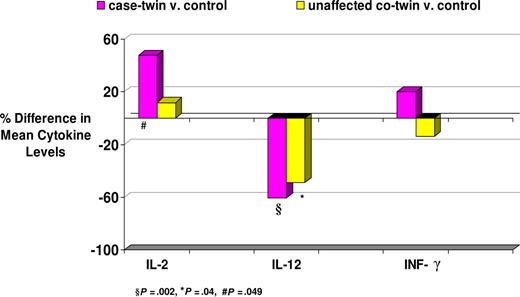
Compared with matched controls, IL-12 levels were 60.6% (P = .002) and 49.0% (P = .04) lower in case-twins and their unaffected cotwins, respectively (Table 2; Figure 1). These relationships were consistent in direction when examined separately by zygosity, although in each case the differences between unaffected cotwins and controls did not reach significance. Both case-twins and unaffected cotwins had higher IL-2 levels compared with controls, but the absolute and percentage difference varied widely across strata and the differences were statistically significant only when all case-twins and DZ case-twins were compared with controls. Neither case-twins nor their unaffected cotwins had significantly different levels of IFN-γ compared with controls.
Mean and percentage difference in PBMC supernatant cytokine levels (pg/mL) between young adult Hodgkin lymphoma (YAHL) case-twins and matched controls, and among their unaffected cotwins and matched controls
| Cytokines . | YAHL-affected case-twins vs controls . | Unaffected cotwins vs controls . | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of pairs . | Mean*† difference, pg/mL . | % difference . | P‡ . | No. of pairs . | Mean†§ difference, pg/mL . | % difference . | P‡ . | |
| All | ||||||||
| Interleukin-2 (IL-2) | 83 | 14.0 | 47.9 | .049 | 80 | 3.1 | 11.8 | .57 |
| Interleukin-12 (IL-12) | 63 | −0.9 | −60.6 | .002 | 62 | −1.7 | −49.0 | .04 |
| Interferon-γ (IFN-γ) | 74 | 6.6 | 20.2 | .41 | 70 | −5.1 | −13.7 | .58 |
| Monozygotic (MZ) | ||||||||
| Interleukin-2 (IL-2) | 55 | 8.2 | 23.2 | .42 | 51 | 2.7 | 8.3 | .76 |
| Interleukin-12 (IL-12) | 43 | −0.9 | −51.1 | .02 | 39 | −0.4 | −23.0 | .47 |
| Interferon-γ (IFN-γ) | 46 | −0.1 | −0.4 | .99 | 41 | −10.5 | −25.4 | .42 |
| Dizygotic (DZ) | ||||||||
| Interleukin-2 (IL-2) | 28 | 22.5 | 98.3 | .02 | 29 | 0.7 | 3.5 | .90 |
| Interleukin-12 (IL-12) | 20 | −1.4 | −74.3 | .06 | 23 | −1.8 | −56.1 | .17 |
| Interferon-γ (IFN-γ) | 28 | 32.9 | 63.3 | .25 | 29 | −4.9 | −14.6 | .70 |
| Cytokines . | YAHL-affected case-twins vs controls . | Unaffected cotwins vs controls . | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of pairs . | Mean*† difference, pg/mL . | % difference . | P‡ . | No. of pairs . | Mean†§ difference, pg/mL . | % difference . | P‡ . | |
| All | ||||||||
| Interleukin-2 (IL-2) | 83 | 14.0 | 47.9 | .049 | 80 | 3.1 | 11.8 | .57 |
| Interleukin-12 (IL-12) | 63 | −0.9 | −60.6 | .002 | 62 | −1.7 | −49.0 | .04 |
| Interferon-γ (IFN-γ) | 74 | 6.6 | 20.2 | .41 | 70 | −5.1 | −13.7 | .58 |
| Monozygotic (MZ) | ||||||||
| Interleukin-2 (IL-2) | 55 | 8.2 | 23.2 | .42 | 51 | 2.7 | 8.3 | .76 |
| Interleukin-12 (IL-12) | 43 | −0.9 | −51.1 | .02 | 39 | −0.4 | −23.0 | .47 |
| Interferon-γ (IFN-γ) | 46 | −0.1 | −0.4 | .99 | 41 | −10.5 | −25.4 | .42 |
| Dizygotic (DZ) | ||||||||
| Interleukin-2 (IL-2) | 28 | 22.5 | 98.3 | .02 | 29 | 0.7 | 3.5 | .90 |
| Interleukin-12 (IL-12) | 20 | −1.4 | −74.3 | .06 | 23 | −1.8 | −56.1 | .17 |
| Interferon-γ (IFN-γ) | 28 | 32.9 | 63.3 | .25 | 29 | −4.9 | −14.6 | .70 |
Geometric mean difference of young adult HL-affected case-twins' log-transformed cytokine levels minus those of their matched controls.
Adjusted for sex, age (categoric: ≤30, 31-40, 41-50, and 51-60 years), and ELISA plate.
P value ANOVA (SAS GLM procedure).
Geometric mean difference of unaffected cotwins' log-transformed cytokine levels minus those of their matched controls.
Percent difference in mean IL-2, IL-12, and IFN-γ levels between young adult Hodgkin lymphoma case-twins and controls, and between unaffected cotwins and controls.
Percent difference in mean IL-2, IL-12, and IFN-γ levels between young adult Hodgkin lymphoma case-twins and controls, and between unaffected cotwins and controls.
There were no significant differences in cytokine levels by sex (data not shown). Only IFN-γ levels were correlated with age and showed an increasing trend with increasing age (P for linear trend = .03) (data not shown). There were no significant differences between cytokine levels of spouses, friends, and convenience (USC students or staff) controls (data not shown). Exclusion of pairs that included the convenience controls in a sensitivity analysis did not change the results (eg, the mean and percentage difference in IL-12 cytokine levels between case-twins and controls [excluding convenience controls] was −1.2 pg/mL and −65.7%, respectively [P value = .004], and between unaffected cotwins and controls, −1.7 pg/mL and −54.7%, respectively [P value = .03]). Among controls, IL-2 and IL-12 levels were not correlated, but both were modestly correlated with IFN-γ levels (P = .007 and P = .04, respectively) (Table 3).
Correlations between PBMC supernatant (Th1) cytokine levels (pg/mL) in controls for whom all 3 cytokine levels were available (n = 62)
— indicates not applicable.
Spearman correlation coefficients adjusted for sex and age as a categoric variable (30, 31-40, 41-50, and 51-60 years).
Table 4 shows the risk of young adult HL associated with IL-12 polymorphism in the untranslated region of the gene that is known to regulate IL-12 expression levels. The presence of a variant allele is associated with a 2.8-fold significant increase in risk when all case-twins' genotypes were compared with those of controls, and an increased risk to 3-fold when restricted to MZ case-twins and controls. Four of the 5 MZ pairs concordant for YAHL were either heterozygous or homozygous for the variant allele.
Risk of YAHL associated with IL-12 1188 3′UTR polymorphism among case-twins and controls with DNA samples available
| IL-12 +1188 A>C . | Case-twins . | Controls . | OR (95% CI)* . | OR† (95% CI) . | P‡ . |
|---|---|---|---|---|---|
| All case-twins vs controls | |||||
| AA | 24 | 28 | 1.0 | 1.0 | — |
| AC/CC | 21/2 | 11/0 | 2.44 (0.99-6.01) | 2.81 (1.08-7.30) | .03 |
| MZ§ case-twins vs controls | |||||
| AA | 18 | 28 | 1.0 | 1.0 | — |
| AC/CC | 18/2 | 11/0 | 2.83 (1.10-7.27) | 3.04 (1.14-8.14) | .03 |
| IL-12 +1188 A>C . | Case-twins . | Controls . | OR (95% CI)* . | OR† (95% CI) . | P‡ . |
|---|---|---|---|---|---|
| All case-twins vs controls | |||||
| AA | 24 | 28 | 1.0 | 1.0 | — |
| AC/CC | 21/2 | 11/0 | 2.44 (0.99-6.01) | 2.81 (1.08-7.30) | .03 |
| MZ§ case-twins vs controls | |||||
| AA | 18 | 28 | 1.0 | 1.0 | — |
| AC/CC | 18/2 | 11/0 | 2.83 (1.10-7.27) | 3.04 (1.14-8.14) | .03 |
— indicates not applicable.
Crude odds ratio (OR) and 95% confidence intervals (95% CI).
ORs adjusted for age (categoric: ≤30, 31-40, 41-50, and 51-60 years) and sex.
P values for the adjusted OR.
Monozygotic.
Discussion
Lower IL-12 levels of similar magnitude in both case-twins and their unaffected cotwins compared with controls suggests that the diminished IL-12 response was present prior to the development of Hodgkin lymphoma. This could reflect a strong genetic determination of IL-12 that is independent of YAHL risk, but since lower IL-12 levels would produce the depressed cell-mediated immune response17 associated clinically with the disease,18 an etiologic role for depressed IL-12 response is plausible.
Both MZ and DZ unaffected cotwins had lower IL-12 levels than controls, but unexpectedly, the magnitude of the difference between the unaffected DZ cotwins and controls (−56.1%) was greater than the difference between unaffected MZ cotwins and controls (−23%). Since MZ cotwins share 100% of the genome and DZ cotwins only 50% of the genome, we would expect the MZ cotwins to show a stronger difference since their phenotype is closer to that of the case-twin. However, because of the range of variation in individuals' cytokine levels, possible environmental influences that are not fully understood, and a relatively small number of pairs, it is likely that the disparity in MZ and DZ twin-control differences is not meaningful and is probably due to chance.
We found no differences in IL-12 levels between male and female controls, thus effect modification by sex cannot explain the results. The IL-12 values were lower than some have reported,19 but consistent with other reports.20 Laboratory measurement error is unlikely here since each coefficient of variability (CV) was lower than 10%, and because each member of the triad was run on the same ELISA plate, the comparisons were internally valid. Thus the findings appear to represent real differences between YAHL case-twins and their unaffected cotwins, and controls.
IL-12 (IL-12p70) is a heterodimer composed of the p35 and p40 subunits, each of which is encoded by a separate gene (on different chromosomes).21 We measured total IL-12 levels, because the biologic function depends on the presence of both subunits. While most somatic cells express the p35 subunit, only antigen-presenting cells express the p40 subunit. Associations between polymorphisms in the gene coding the p40 subunit and disease have been reported by us15 and others.22 Several studies have demonstrated a correlation between IL-12p40 polymorphisms and total IL-12 (IL-12p70) levels, but not always in the same direction.23-25 Nevertheless, we observed a strong association between the IL-12 gene polymorphism in the untranslated region and YAHL risk, providing further support for IL-12 as a predisposing factor.
IL-12 has a major role in cell-mediated immunity by stimulating cytotoxic lymphocyte and natural killer cell activity and antigen processing and presentation.21 IL-12 knockout mice show deficiencies in IFN-γ secretion, Th1 lymphocyte development, and NK cell lytic activity, while Th2 responses are enhanced.26 The use of IL-12 as immunotherapy for HL is being explored, since IL-12 induces a strong cell-mediated immune response with the possibility of enhanced tumor cell killing.27,28 Although IL-12 is not required to mount an adequate response to most viral infections, it is necessary for eradication of some, including measles, cytomegalovirus, and herpes simplex type 2.21 Thus, a depressed IL-12 response might play a role in YAHL etiology by reducing Th1 cytotoxic response to the delayed acquisition of a common childhood viral infection.
However, no virus has yet been identified as a causal agent of EBV-negative YAHL, despite several well-conducted studies by Jarrett and colleagues.29-32 An alternative explanation for the YAHL risk pattern (which is similar to that for atopy) is that relative social isolation in early childhood results in a deficit of generic microbial signals (ie, endotoxin) necessary for the normal maturation of the immune response (“hygiene hypothesis”).33 In the gut lymphoid tissue, these microbial signals are thought to bind to toll-like receptors resulting in secretion of IL-12, which in turn induces the differentiation of Th1 lymphocytes necessary to mount cell-mediated, cytotoxic responses.34 When these microbial exposures are lacking, the signals that stimulate IL-12 may not occur, resulting in an immune response that remains in the fetal-type Th2-dominated state.35 The deficit of IL-12 could be acquired (due to lack of early microbial exposure) or genetic, or both, resulting in a higher risk of Th2-skewed diseases such as atopy and YAHL. In support of this general model, we recently reported a link between a SNP in a gene encoding toll-like receptor 4 and risk of HL.36
Although IFN-γ and IL-12 levels were highly correlated among controls as expected, we found no evidence that IFN-γ or IL-2 was associated with disease susceptibility. (The significantly higher levels of IL-2 in case-twins, but not cotwins, suggests an effect of the disease.) This is inconsistent with a recent observation that intracellular cytokine levels from T cells in YAHL patient tumor infiltrates had lower levels of IL-2 and IFN-γ in a small study of patients and controls.37 However, that study was based on very few cases (n = 29) and used unmatched convenience controls (n = 19).
As noted, we previously demonstrated higher levels of IL-6 in unaffected MZ cotwins of cases compared with controls and found that a genotype thought to be associated with lower IL-6 levels was strongly protective,11 a result that has been partially corroborated by another case-control study (which found a weaker protective effect associated with the same genotype).38
In summary, our observations suggest that a decreased ability to produce IL-12 level is a necessary, but not sufficient, determinant of YAHL (because most of the genetically identical unaffected cotwins with low IL-12 levels did not develop disease). Additional factors, perhaps a viral infection and/or other immune system alterations due to early social isolation, are necessary.
An Inside Blood analysis of this article appears at the front of this issue.
Presented as part of an oral presentation in the Molecular Epidemiology Group Minisymposium: Infectious Agents and Cancer: the Role of Immunity and Inflammation, at the 98th annual meeting of the American Association of Research on Cancer, Los Angeles, CA, April 17, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Terri Kang, MS, Cathy Carpenter, PhD, Jan Schaefer, RN, and Susan Groshen, PhD, for help with computer programming and statistical analysis; Nicole Stroud, Mike Johnson, Ruby Sidhu, and Jai Cai, MS, for their assistance in the laboratory; Susan Gundell, RN, and Maureen Cairns for data collection; and the participating twins, their relatives, and physicians for their contributions.
This work was supported by grants from the National Cancer Institute (1R01CA58839, 1R03CA110836) and from the California Tobacco Related Disease Research Program (8RT-0107H and 6RT-0354H). P.S.G. was supported by grants from the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: P.S.G., R.M., A.N., D.J.V.D.B., and O.M.-M. conducted laboratory analyses; M.T.S., W.J.G., M.G.C., and M.C.P. conducted statistical analyses; M.G.C., A.S.H., and D.K.D. developed and maintained the databases of laboratory data and of the twin registries for ascertaining subjects; B.N.N. reviewed the histopathologic slides; W.C., P.S.G., and T.M.M. designed the research; and W.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wendy Cozen, Department of Preventive Medicine, USC Norris Cancer Center, 1441 Eastlake Ave, MC 9175, Los Angeles, CA 90089-9175; e-mail: wcozen@usc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal