Abstract
Despite advances in therapy, many patients with systemic light-chain amyloidosis (AL) die within 3 years from diagnosis. The humanized 2B6 monoclonal antibody (MoAb) is specific for the low-affinity IgG Fc receptor CD32B and effective in a human CD32B+ B-cell lymphoma murine xenograft model. Because MoAb therapy could improve outcomes in AL, we studied CD32B expression by clonal plasma cells obtained from 48 patients with AL. Transcript profiling showed that expression of CD32B was significantly higher than expression of all other Fc receptor family members. Reverse-transcriptase polymerase chain reaction (RT-PCR) using double-enriched CD138+ plasma cells showed uniform expression of the stable cell surface CD32B1 isoform at diagnosis and relapse, and flow cytometry showed intense CD32B cell surface staining on 99% of CD138+ plasma cells at diagnosis and relapse. These data provide a rationale for the novel therapeutic targeting of CD32B using the humanized 2B6 MoAb in patients with systemic AL-amyloidosis.
Introduction
Systemic light-chain (AL) amyloidosis is a rare disorder that causes multiple organ damage due to toxic light chains and fibrillar deposits.1,2 Despite treatment, patients with symptomatic cardiac involvement live a median of 10 months from diagnosis.3 AL is a clonal plasma cell dyscrasia and an immunoglobulin light chain disease. The majority of patients have less than 20% marrow plasma cells and almost all have measurable serum free light chains.4 Improvements in survival have depended on the elimination of clonal plasma cells in the marrow by chemotherapy leading to a reduction in the production of clonal immunoglobulin light chains.5 Positive predictors of survival are the absence of cardiac amyloid and a response to therapy.6 The achievement of a complete hematologic response is associated with improvement in organ disease and extended survival.7 New therapies are needed for the majority of patients.
The Fcγ receptor system includes activating and inhibitory receptors that modulate immune complex-mediated processes such as inflammation and autoimmunity.8,9 CD32A, an activating receptor, is the most widely expressed Fcγ receptor and has a distinct though overlapping distribution with CD32B, the low-affinity Fcγ inhibitory receptor.10 Myeloid cells express both CD32A and CD32B, the latter as an isoform (CD32B2) that is internalized. B cells express an isoform (CD32B1) that is not internalized and variably express CD32A depending on activation status and anatomic site.11 Although CD32B regulates normal plasma cell persistence and apoptosis, a role for CD32B in plasma cell disorders has not been identified.12
The clonal plasma cells in AL are usually indolent and do damage by secreting toxic proteins.13 They express CD138 and aberrantly express CD52.14 A previous study of CD32 expression on clonal myeloma cells could not distinguish CD32B from CD32A15 ; however, we have used a new monoclonal antibody (MoAb) that specifically detects CD32B but not CD32A (clone 2B6; MacroGenics, Rockville, MD).16 The 2B6 MoAb is effective therapeutically in a murine model of human lymphoma, effectively eliminating CD32B+ tumor cells.17 In this report, we show for the first time that CD32B is highly expressed on the surface of clonal plasma cells from patients with AL, making these patients potential candidates for anti-CD32B MoAb therapy.
Methods
Patients and specimens
Patients with systemic AL-amyloidosis gave written informed consent in accordance with the Declaration of Helsinki for the use of marrow cells on protocols approved by the Memorial Sloan-Kettering Institutional Review and Privacy Board. Marrow aspirate specimens were collected and marrow mononuclear cells separated as previously described.18,19 The choice of samples for specific tests was random and based on available cell numbers and ongoing studies.
Plasma cell selection
CD138+ marrow plasma cells were selected by fluorescence-activated cell sorting (FACS) sorting or by immunomagnetic separation as previously described.20 FACS-sorted cells were used for gene expression profile studies. For immunomagnetic separation Miltenyi MiniMacs with B-B4 antibody (Miltenyi Biotec, Auburn, CA) was used according to the manufacturer's instructions, and B-B4–double-enriched CD138+ plasma cells were used for reverse-transcriptase polymerase chain reaction (RT-PCR) and flow cytometry.
Gene expression profiles
The MSKCC core facility synthesized cRNA from RNA extracted from CD138+ plasma cells for hybridization to Affymetrix U133 PLUS 2.0 arrays (Santa Clara, CA).20 Gene expression levels were normalized, and the fidelity of the transcript profiles to clonal plasma cells was assessed using the Affymetrix probe sets for the λ and κ light-chain constant region genes (probe sets 21651 and 215121). In addition, the expression of the plasma cell–specific genes CD38 (205692), XBP-1 (200670), and SD1 (syndecan 1 or CD138, 201286) was compared with the hematopoietic lineage–specific genes CD4 (200670), CD14 (201743), CD19 (206398), and CD33 (206120).20 We also compared the expression levels for genes of the Fcγ receptor family FCGR2B, (CD32B, 210889), FCGR2A (CD32A; 293561), CD16 (204007), and CD64 (216951).
RT-PCR
RNA was extracted and cDNA generated using standard methods.20 Primers for amplifying FCGR2B (CD32B) mRNA were forward 5′-CCTCACCTG GAGTTCCAGGAGGGAG-3′ and reverse 5′-AACTTTGTCAGCCTCATCAGG-3′. These primers amplify 2 isoforms of CD32B (CD32B1 and CD32B2) that differ due to alternate splicing. The amplicon for CD32B1 is 441 bp (nt's 504-944 of the mRNA), while that of CD32B2 is 384 bp (nt's 457-840). The conditions for CD32B amplification were predenaturation at 94°C for 2 minutes, denaturation at 94°C for 40 seconds, annealing at 58°C for 40 seconds, and extension at 72°C for 60 seconds, all over 40 cycles. GAPDH was used as a control (forward 5′-TTCGACAGTCAGCC GCATCTTCTT-3′; reverse 5′-GCCCAATACGACCAAATCCGTTGA-3′; amplicon 105 bp).
Flow cytometry
Marrow aspirate mononuclear cells or B-B4–double-enriched CD138+ cells were stained with PE-conjugated anti-CD138 (clone MI15; BD Biosciences, Franklin Lakes, NJ) and Alex Fluor 488–conjugated anti-CD32B (clone 2B6; MacroGenics), or with IgG1 isotype controls, for 30 minutes at 4°C.10 Flow cytometric studies were performed on a Cytomics FC 500 (Beckman Coulter, Miami, FL).
Statistics
Affymetrix gene expression data were normalized and loge transformed (Ln) for evaluation.20 We used PRISM (GraphPad, San Diego, CA) for descriptive statistics and paired t tests. All analyses were 2 tailed using P less than .05 for significance.
Results and discussion
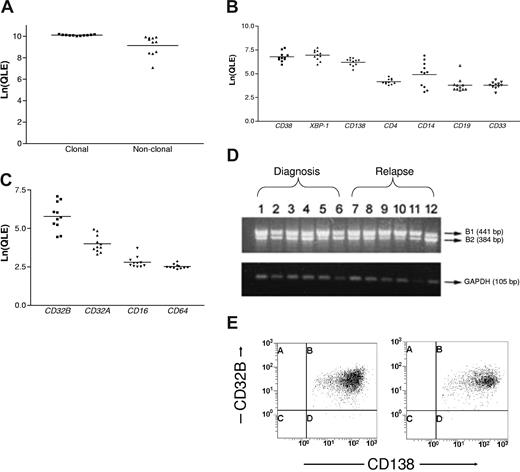
The characteristics of the 48 patients whose marrow aspirates were examined are described in Table 1. Sixty percent (29 of 48) had cardiac involvement at diagnosis or relapse. Marrow aspirates had a median of 9% plasma cells (range, 2%-34%). FACS-sorted (n = 11) and B-B4–enriched (n = 20) preparations contained a median of 4 × 105 cells (range, 0.6-25 × 105), more than 95% CD138+ by flow cytometry. Comparison of clonal and nonclonal light-chain constant region gene expression confirmed that clonal cells were used for gene profiles, and comparison of lineage-specific genes confirmed that expression of plasma cell markers was significantly higher than all others (Figure 1A,B). Comparison of the expression levels of Fcγ receptor family members showed that expression of CD32B (FCGR2B) was significantly higher than all others (Figure 1C). RT-PCR showed that the CD32B1 isoform was consistently expressed at diagnosis and relapse (Figure 1D), and by flow cytometry the median of all CD138+ cells that expressed CD32B was 99% (range, 88%-100%; Figure 1E). This is likely similar to normal; patients with complete hematologic responses after treatment usually achieve normal humoral immune features and have CD32B+ plasma cells (median, 99.9% [range, 98%-100%], n = 6). Therefore, CD32B, a member of the Fcγ receptor family with various functions in B cells, is highly expressed on the plasma cells of patients with systemic AL-amyloidosis and could serve as a target for MoAb therapy. The success of anti-CD32B MoAb therapy in a murine xenograft model bearing a CD32B+ human lymphoma supports such an approach.17
Characteristics of AL-amyloidosis patients and plasma cell clones
| Feature . | Frequency . |
|---|---|
| Age, y, median (range) | 59 (34-82) |
| Men, no. (%) | 28 (58) |
| Biopsies with amyloid per patient, median no. (range) | 2 (1-4) |
| Patients with 2 or more organs involved, no. (%) | 26 (54) |
| Patients with cardiac involvement, no. (%) | 29 (60) |
| Brain natriuretic peptide, median pg/mL (range) | 710 (30-4210) |
| Patients with renal involvement, no. (%) | 35 (73) |
| Urinary protein, median mg/d (range) | 3052 (514-13,224) |
| Serum albumin, median g/L (range) | 37 (19-50) |
| β2 microglobulin, median mg/L (range) | 246.5 (127.5-1623.5) |
| Marrow specimens at diagnosis and relapse* | 40/11 |
| Plasma cells in marrow specimens, median % (range) | 9 (2-34) |
| Patients with serum M-spike, no. (%) | 20 (42) |
| Patients with M-spike, median g/dL, no. (range) | 1.1 (0.2-2.7) |
| Patients with IgG kappa, no. | 1 |
| Patients with IgG lambda, no. | 16 |
| Patients with IgA kappa, no. | 2 |
| Patients with IgA lambda, no. | 1 |
| Patients with Kappa light chain only, no. | 6 |
| Patients with Lambda light chain only, no. | 22 |
| Patients with abnormal FLC ratio†, no. (%) | 47 (98) |
| Patients with abnormal lambda FLC, no. (%) | 41 (85) |
| Lambda FLC, median mg/dL (range) | 16.3 (2.36-366.5) |
| Patients with abnormal kappa FLC, no. (%) | 7 (15) |
| Kappa FLC, median mg/dL (range) | 73 (3.58-290) |
| Feature . | Frequency . |
|---|---|
| Age, y, median (range) | 59 (34-82) |
| Men, no. (%) | 28 (58) |
| Biopsies with amyloid per patient, median no. (range) | 2 (1-4) |
| Patients with 2 or more organs involved, no. (%) | 26 (54) |
| Patients with cardiac involvement, no. (%) | 29 (60) |
| Brain natriuretic peptide, median pg/mL (range) | 710 (30-4210) |
| Patients with renal involvement, no. (%) | 35 (73) |
| Urinary protein, median mg/d (range) | 3052 (514-13,224) |
| Serum albumin, median g/L (range) | 37 (19-50) |
| β2 microglobulin, median mg/L (range) | 246.5 (127.5-1623.5) |
| Marrow specimens at diagnosis and relapse* | 40/11 |
| Plasma cells in marrow specimens, median % (range) | 9 (2-34) |
| Patients with serum M-spike, no. (%) | 20 (42) |
| Patients with M-spike, median g/dL, no. (range) | 1.1 (0.2-2.7) |
| Patients with IgG kappa, no. | 1 |
| Patients with IgG lambda, no. | 16 |
| Patients with IgA kappa, no. | 2 |
| Patients with IgA lambda, no. | 1 |
| Patients with Kappa light chain only, no. | 6 |
| Patients with Lambda light chain only, no. | 22 |
| Patients with abnormal FLC ratio†, no. (%) | 47 (98) |
| Patients with abnormal lambda FLC, no. (%) | 41 (85) |
| Lambda FLC, median mg/dL (range) | 16.3 (2.36-366.5) |
| Patients with abnormal kappa FLC, no. (%) | 7 (15) |
| Kappa FLC, median mg/dL (range) | 73 (3.58-290) |
Three patients provided 2 samples each, 2 at diagnosis and relapse and 1 at first and second relapse, for a total of 51 specimens.
Free light chains (FLCs) by FreeLite assay (Comenzo5 ).
CD32B expression by clonal plasma cells in AL-amyloidosis. (A) The loge-transformed quantitative expression levels (Ln(QLE)) for the clonal and nonclonal light-chain constant region genes are shown (horizontal lines are mean values). The clonal genes were expressed at significantly higher levels than the nonclonal, indicating that clonal plasma cells were purified (paired t test, P = .006). (B) The Ln(QLE) values of lineage-specific genes are depicted. Comparisons of the Ln(QLE) of plasma cell versus other markers were highly significant (paired t test, P < .001) except for comparisons with CD14 where the P values were .002 for CD38 and .01 for XBP-1 and CD138. CD14 levels may reflect aberrant expression of CD14 by clonal plasma cells or monocyte contamination. (C) The Ln(QLE) values of Fcγ receptor family member genes are shown (paired t test, P < .001 for CD32B compared with each of the others). (D) The CD32B1 isoform is expressed by RT-PCR using B-B4–double-enriched plasma cells at diagnosis (n = 6) and relapse (n = 6). (E) Typical flow cytometry dot plots are shown, displaying that nearly all CD138+ cells are also CD32B+. A median of 99% of the CD138+ cells in samples from 31 patients (B-B4–double-enriched cells = 11, marrow aspirate mononuclear cells = 20) coexpressed CD32B+ at diagnosis and relapse. Marrows at relapse contained a median of 8% plasma cells (range, 4%-15%).
CD32B expression by clonal plasma cells in AL-amyloidosis. (A) The loge-transformed quantitative expression levels (Ln(QLE)) for the clonal and nonclonal light-chain constant region genes are shown (horizontal lines are mean values). The clonal genes were expressed at significantly higher levels than the nonclonal, indicating that clonal plasma cells were purified (paired t test, P = .006). (B) The Ln(QLE) values of lineage-specific genes are depicted. Comparisons of the Ln(QLE) of plasma cell versus other markers were highly significant (paired t test, P < .001) except for comparisons with CD14 where the P values were .002 for CD38 and .01 for XBP-1 and CD138. CD14 levels may reflect aberrant expression of CD14 by clonal plasma cells or monocyte contamination. (C) The Ln(QLE) values of Fcγ receptor family member genes are shown (paired t test, P < .001 for CD32B compared with each of the others). (D) The CD32B1 isoform is expressed by RT-PCR using B-B4–double-enriched plasma cells at diagnosis (n = 6) and relapse (n = 6). (E) Typical flow cytometry dot plots are shown, displaying that nearly all CD138+ cells are also CD32B+. A median of 99% of the CD138+ cells in samples from 31 patients (B-B4–double-enriched cells = 11, marrow aspirate mononuclear cells = 20) coexpressed CD32B+ at diagnosis and relapse. Marrows at relapse contained a median of 8% plasma cells (range, 4%-15%).
Current therapies for AL include oral melphalan and dexamethasone, high-dose melphalan and autologous stem-cell transplantation (SCT), SCT combined with adjuvant thalidomide and dexamethasone, and the new agents lenalidomide and bortezomib.19,21-23 The majority of patients fail to achieve a complete response and up to one third of patients do not respond at all. Patients with cardiac involvement are at highest risk with a 37% mortality at 1 year, despite SCT and adjuvant therapy.3,19
It is therefore encouraging to find CD32B highly expressed on the clonal plasma cells of patients with AL, especially given that a murine xenograft model of human non-Hodgkin lymphoma has shown that CD32B is a tumor-associated antigen successfully targeted by the humanized 2B6 MoAb.17 In combination with new agents, anti-CD32B MoAb therapy could lead to effective elimination of the indolent clonal plasma cells that cause AL.24 These results provide the basis for clinical testing of humanized 2B6 in patients with systemic AL-amyloidosis, persistent clonal plasma cell disease, and progressive organ damage despite prior therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Joanne Santorsa, RN, for her help obtaining marrow specimens and the staff of the core laboratories and the Cytotherapy Laboratory at MSKCC for their assistance. We also especially thank the patients who donated marrow aspirates for research.
This work was supported by Food and Drug Administration grants R03-002174 and K08 AI 061313, the Amyloidosis Research Fund, the Werner and Elaine Dannheiser Fund for Research on the Biology of Aging of the Lymphoma Foundation, the Demarest Lloyd Jr Foundation, and the Amyloidosis Foundation.
National Institutes of Health
Authorship
Contribution: P.Z. designed and performed research, analyzed data, and wrote the paper; R.L.C. conceived and designed research, analyzed data, and wrote the paper; A.B.O. designed research, performed statistical analysis, and wrote the paper; E.B. contributed vital reagents, analyzed data, and wrote the paper; S.K. contributed vital reagents; P.G.M., M.F., and S.J. designed and performed research; J.H. performed research and analyzed data; J.W.Y. and S.D.N. analyzed data and wrote the paper; A.M.B. conceived and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: E.B. and S.K. are employees of MacroGenics, Inc. All other authors declare no competing financial interests.
Correspondence: Raymond L. Comenzo, Howard 802, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021; e-mail: comenzor@mskcc.org.
References
Author notes
P.Z. and R.L.C. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal