Abstract
Vascular endothelial growth factor receptor-1 (VEGFR-1) is a tyrosine kinase receptor for growth factors of the VEGF family. Endothelial cells express a membrane-bound and a soluble variant of this protein, the latter being mainly considered as a negative regulator of VEGF-A signaling. We previously reported that the soluble form is deposited in the extracellular matrix produced by endothelial cells in culture and is able to promote cell adhesion and migration through binding to α5β1 integrin. In this study, we demonstrate that the Ig-like domain II of VEGFR-1, which contains the binding determinants for the growth factors, is involved in the interaction with α5β1 integrin. To identify domain regions involved in integrin binding, we designed 12 peptides putatively mimicking the domain II surface and tested their ability to inhibit α5β1-mediated endothelial cell adhesion to soluble VEGFR-1 and directly support cell adhesion. One peptide endowed with both these properties was identified and shown to inhibit endothelial cell migration toward soluble VEGFR-1 as well. This peptide directly binds α5β1 integrin, but not VEGF-A, inducing endothelial cell tubule formation in vitro and neoangiogenesis in vivo. Alanine scanning mutagenesis of the peptide defined which residues were responsible for its biologic activity and integrin binding.
Introduction
Vascular endothelial growth factor receptor-1 (VEGFR-1) is a tyrosine kinase receptor for members of the vascular endothelial growth factor (VEGF) family.1 VEGFR-1 is closely related to the type III tyrosine kinase-Fms/Kit/platelet-derived growth factor receptors, but it is classified into a distinct class of receptors whose extracellular region is composed of 7 immunoglobulin (Ig)-like domains.2 Ig-like domain II (dII) comprises the region involved in VEGF-A and placenta growth factor (PlGF) binding,3,4 whereas Ig-like domains III and IV are involved in heparin binding and domain III is also responsible for interaction with neuropilin-1.5
Studies carried out with PlGF, which is a VEGFR-1-selective ligand, indicated that the tyrosine kinase activity of this receptor is mostly required for adult pathologic angiogenesis.6 VEGFR-1 does not play a significant role in endothelial cell proliferation, although its activation is greatly involved in chemotaxis of monocyte-macrophages7 and in mobilization of endothelial and hematopoietic stem cells from the bone marrow.8,9
Gene knockout studies demonstrated that VEGFR-1 is essential for the development and differentiation of embryonic vasculature and that its inactivation results in increased hemangioblast commitment and disorganization of blood vessels.10,11 However, mice carrying a homozygous deletion of the intracellular kinase domain show a correct blood vessel development, and a substantial impairment of monocyte-macrophages migration was the only reported phenotype.12 This result suggests that the extracellular region of VEGFR-1 is sufficient to support embryonic angiogenesis.
In addition to the transmembrane isoform of VEGFR-1 (tmVEGFR-1), endothelial cells (ECs) produce a soluble form of the receptor, arising from alternative splicing of the same gene.13 Soluble VEGFR-1 (sVEGFR-1) comprises the first 6 Ig-like domains of the extracellular region of the receptor and a specific 30 amino acid tail at its C-terminus. Both isoforms bind VEGF-A with high affinity, and sVEGFR-1 acts as a potent antagonist of VEGF-A signaling by sequestering the growth factor and thereby inhibiting its interaction with the transmembrane receptors.14 We previously demonstrated that sVEGFR-1 secreted by ECs in culture becomes part of the extracellular matrix and is able to mediate EC adhesion through direct binding to α5β1 integrin.15 A key role of this integrin in promoting vasculogenesis and angiogenesis has been previously highlighted by the defective vascular phenotype of α5 knockout mice16 and by the ability of either antibody raised against this integrin or antagonist peptides to block in vitro and in vivo angiogenesis.17
Integrin binding to their ligands usually occurs through recognition of short amino acid sequences. Many integrins, including α5β1, bind to arginine-glycine-aspartic acid (RGD)–containing peptides, although other binding motifs have also been identified.18,19 Because none of the classic α5β1 integrin binding motifs is present in the sequence of sVEGFR-1, we investigated the molecular determinants responsible for sVEGFR-1/integrin interaction. We found that Ig-like dII of VEGFR-1 directly interacts with α5β1 integrin. Because this domain is also involved in growth factor binding, we tried to identify the domain regions specifically involved in integrin binding. We designed and tested 12 peptides putatively mimicking the domain surface and found one peptide able to inhibit α5β1-mediated endothelial cell adhesion to VEGFR-1 and directly support cell adhesion. This peptide also inhibited endothelial cell migration toward sVEGFR-1 and induced endothelial cell tubule formation in vitro and neoangiogenesis in vivo. Finally, we performed alanine scanning mutagenesis of the peptide to define which residues were responsible for its biologic activities and integrin binding.
Methods
Reagents
Recombinant human VEGFR-1/Fc, VEGF-A, and biotinylated VEGF-A were purchased from R&D Systems (Minneapolis, MN). The purified α5β1 integrin, octyl-β-D-glucopyranoside formulation, was from Millipore (Billerica, MA). Peptide synthesis was carried out by Primm (Milan, Italy), and the GRGDTP and GRGESP peptides were from Invitrogen (Paisley, United Kingdom). Antibodies used in this paper are detailed in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Cell culture, cell adhesion, and endothelial tubule formation
Human umbilical vein endothelial cells (HUVECs) were obtained and cultured as described.15 A pool obtained from different individuals was used in all the experiments. Adhesion assays to recombinant proteins were performed as previously described.15 Adhesion to peptides was carried out by using anhydride maleic activated microplates (Reacti-Bind; Pierce, Rockford, IL) coated with 500 μg/mL of each peptide. In competition experiments, HUVECs were preincubated with 10 μg/mL antibody or 20 μg/mL peptide for 10 minutes at room temperature before plating and then allowed to adhere for 30 minutes at 37°C in the presence of the compounds. Where indicated, ethylenediaminetetraacetic acid (EDTA) or ethyleneglycoltetraacetic acid (EGTA) (10 mM) was added at the time of plating. To assay for tubule formation, HUVECs were examined in a three-dimensional collagen gel in the presence or absence of proteins, peptides, or antibodies as previously described.20 Wells were viewed with an Axiovert 135 inverted microscope (Zeiss, Oberkochen, Germany) using ACHROPLAN at 10×/0.25 PH1 and 20×/0.40 PH2. Images were acquired using a Canon TWAIN60 camera (Tokyo, Japan), and were processed with ZoomBrowser EX version 5.5.0.190 software (Canon Information Systems Research Australia, North Ryde, Australia) and Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA).
Generation of recombinant Ig-like dII
The tmVEGFR-1 cDNA was kindly provided by Dr M. Shibuya (Institute of Medical Science, University of Tokyo, Japan). The cDNA was modified to introduce a Kpn I site followed by a Kozak sequence, a start codon at the 5′ end, a Bsi WI site followed by a HA Tag, and a stop codon at the 3′ end. The polylinker of the Baculovirus vector pFastBac 1 (Invitrogen) was replaced by a linker containing a Kpn I and a Bsi WI site together with a His tag. The tmVEGFR-1 cDNA was first cloned into the pFastBac 1 vector using the Kpn I and Bsi WI sites. To construct the recombinant Ig-like dII, the signal sequence of VEGFR-1 was amplified by polymerase chain reaction using the oligonucleotides: 5′-GCGCGGATCCGGTACCGC-3′ and 5′-GTGATGCGTACGTGAACCTGAACTAGATCCTGTG-3′ and cloned into the modified pFastBac vector. Then the dII sequence was amplified with the oligonucleotides 5′-GTGATGCGTACGAGTGATACAGGTAGACCTTTC-3′ and 5′-GTGATGCGTACGTCCGATTGTATTGGTTTGTCGATG-3′. The polymerase chain reaction fragment was cloned into the modified pFastBac vector carrying the VEGFR-1 signal sequence. Recombinant Baculovirus was obtained using the Bac-to-Bac system (Invitrogen). SF9 (Spodoptera frugiperda) cell line was purchased from Invitrogen and maintained in culture at 27°C in TC100 medium (Invitrogen) containing 10% heat inactivated fetal calf serum. Infected SF9 cells were cultured in serum-free SF-900 medium (Invitrogen). For protein expression, SF9 cells were grown up to 60% confluence and infected with the corresponding recombinant virus at a multiplicity of infection of 1. Forty-eight hours postinfection cells were collected, and the recombinant protein was purified by affinity chromatography using the B-PER His Fusion Protein purification kit (Pierce). Cell lysates were prepared using the B-PER extraction reagent and loaded on a nickel-chelated column, previously equilibrated with B-PER reagent. The column was washed with wash buffer I and II, and protein eluted with elution buffer containing excess imidazole. The recombinant protein had the expected molecular weight on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and was recognized by an anti-HIS antibody (Santa Cruz Biotechnologies, Santa Cruz, CA).
Solid-phase binding assays
Integrin binding to recombinant proteins was assayed as previously described.15 Interaction of peptides (500 μg/mL, coated on Reacti-Bind microplates) with the purified integrin was evaluated with a 1:100 dilution of the NKI-SAM 1 anti-α5 monoclonal antibody (mAb) followed by plate incubation with a biotinylated anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA). Streptavidin-alkaline phosphatase-conjugated and the appropriate substrate (4-nitrophenylphosphate, Roche Diagnostics, Basel, Switzerland) were then used for detection. Absorbance was determined at 405 nm using a Microplate reader 3550-UV (Bio-Rad, Hercules, CA). In a different experiment, 500 μg/mL peptide were coated on the Reacti-Bind microplates and interaction with VEGF-A was evaluated by incubating 200 ng biotinylated VEGF-A on the plates. The amount of retained biotinylated VEGF-A was then determined as described above.
Rabbit cornea assay
Angiogenesis was studied in the cornea of albino rabbits (Charles River, Calco, Como, Italy). Experiments were performed in accordance with guidelines of the European Economic Community for animal care and welfare (EEC Law No. 86/609). Slow-release pellet preparation, surgical procedure, and quantification of angiogenesis (angiogenic score) were performed as previously reported.21 Multiple comparisons were performed by one-way analysis of variance and individual differences tested by Student-Newman-Keuls test after the demonstration of significant intergroup differences.
Immunofluorescence studies
Aliquots of Dynabeads M-270 Carboxylic Acid (Dynal Biotech, Oslo, Norway) were coated with the selected peptides as recommended by the manufacturer. HUVECs were plated on fibronectin-coated coverslips and allowed to adhere for 2 hours in serum-free medium. Peptide-coated beads were then added for 30 minutes. Cells were washed with phosphate-buffered saline (PBS), fixed with 3% formaldehyde in PBS, and permeabilized with 0.5% Triton X-100/PBS. Nonspecific staining was blocked with 3% bovine serum albumin (BSA), and coverslips were incubated with primary antibody against α5 subunit NKI-SAM 1 diluted 1:100 in 1% BSA/PBS. Coverslips were washed with PBS and stained with fluorescein isothiocyanate-conjugated rabbit anti–mouse IgG (Dako Denmark A/S, Glostrup, Denmark) in 1% BSA/PBS for 45 minutes. Coverslips were washed in PBS, mounted in Vectashield, and observed by confocal microscopy (Zeiss LSM 510 Meta, Oberkochen, Germany).
Western blotting analysis
HUVECs were starved for 24 hours in EBM (Clonetics, Lonza, Valais, Switzerland) complemented with 0.4% fetal calf serum, treated for 2 hours with cycloheximide (20 μM), and plated on peptide 12- or fibronectin-coated dishes for 1 hour in the presence of cycloheximide (20 μM; Sigma-Aldrich, St Louis, MO), monensin (1 μM; Sigma-Aldrich), or VEGF where indicated. Afterward, cells were directly lysed and boiled in SDS sample buffer, and 50 μL of the lysate run in a 10% sodium dodecyl sulfate-polyacrylamide gel. Proteins were transferred to supported nitrocellulose membranes (Hybond-C; GE Healthcare, Chalfont St Giles, United Kingdom), using a Trans-Blot Cell from Bio-Rad. Membranes were blocked in blocking solution (5% BSA, 0.1% Tween 20, 0.9% NaCl, 20 mM Tris-HCl, pH 7.4) overnight at 4°C, washed with TBST buffer (0.1% Tween 20, 0.9% NaCl, 20 mM Tris-HCl, pH 7.4), and incubated with the primary antibody diluted 1:1000 in 3% BSA/TBST for 4 hours at room temperature. After washing with TBST, membranes were incubated for 1 hour at room temperature with the secondary antibody diluted 1:5000 in 3% BSA/TBST. Membranes were then washed with TBST, and detection was carried out using the ECL Western blotting detection reagents and Hyperfilms (both from GE Healthcare).
Structure analysis
Results
Ig-like dII of VEGFR-1 is involved in the interaction with α5β1 integrin
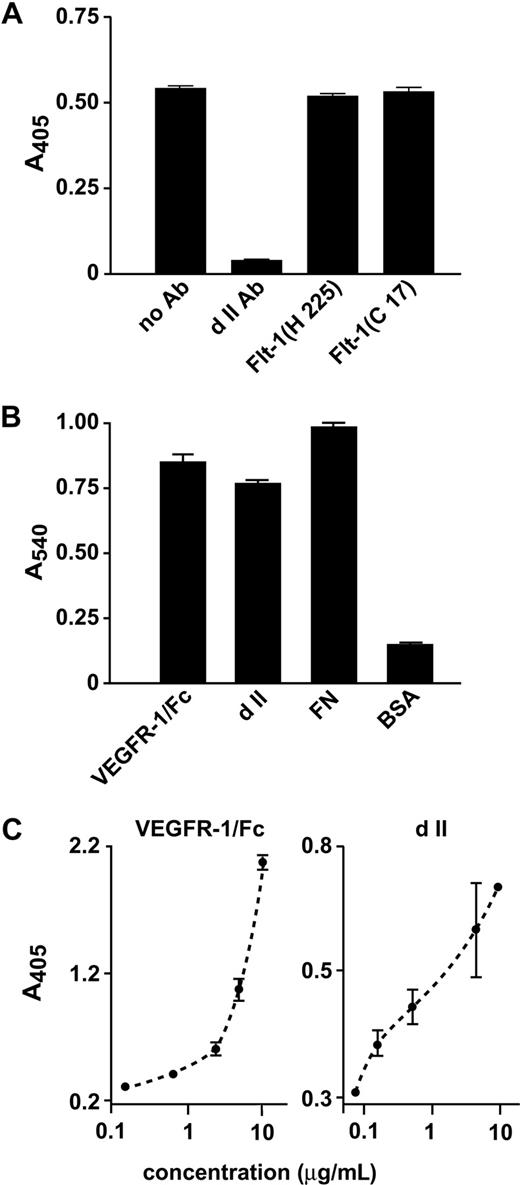
We previously showed that purified α5β1 integrin binds to VEGFR-1/Fc protein, comprising the soluble receptor fused to the Fc domain of human IgG.15 We also demonstrated that an antibody recognizing a peptide derived from Ig-like dII of VEGFR-1 competed with α5β1 integrin-mediated binding of EC to VEGFR-1.15 In the present study, we analyzed the effect of this antibody on VEGFR-1/integrin interaction. In a solid-phase binding assay, this antibody completely inhibited α5β1 binding to VEGFR-1/Fc (Figure 1A), whereas another pAb (Flt-1 H225) recognizing the extracellular domain of VEGFR-1 did not. As expected, no inhibition was observed using an antibody (Flt-1 C17) against the intracellular domain of VEGFR-1 (Figure 1A). We concluded that determinants for integrin binding were probably to be present within Ig-like dII.
VEGFR-1 dII mediates cell adhesion and interaction with α5β1 integrin. (A) In a solid-phase binding assay, plates coated with VEGFR-1/Fc were treated with anti–VEGFR-1 antibodies and incubated with purified α5β1 integrin. The amount of bound integrin was quantified by incubation with an anti-α5β1 antibody and colorimetric detection of this antibody. (B) HUVECs were incubated on wells coated with recombinant dII. VEGFR-1/Fc and fibronectin (FN) were used as positive controls, bovine serum albumin (BSA) as a negative control. The relative number of attached cells was assessed by staining with crystal violet and determining A540 1 hour after plating. Absorbance resulting from nonspecific cell adhesion was measured on BSA-coated wells. (C) Purified VEGFR-1/Fc or dII were added at the indicated concentrations to wells coated with α5β1 integrin. Bound molecules were detected using either an anti-Fc alkaline phosphatase (AP)-conjugate or an anti–VEGFR-1 antibody and a secondary anti-rabbit antibody AP-conjugate. Representative experiments performed in triplicate are shown; data are mean plus or minus SEM.
VEGFR-1 dII mediates cell adhesion and interaction with α5β1 integrin. (A) In a solid-phase binding assay, plates coated with VEGFR-1/Fc were treated with anti–VEGFR-1 antibodies and incubated with purified α5β1 integrin. The amount of bound integrin was quantified by incubation with an anti-α5β1 antibody and colorimetric detection of this antibody. (B) HUVECs were incubated on wells coated with recombinant dII. VEGFR-1/Fc and fibronectin (FN) were used as positive controls, bovine serum albumin (BSA) as a negative control. The relative number of attached cells was assessed by staining with crystal violet and determining A540 1 hour after plating. Absorbance resulting from nonspecific cell adhesion was measured on BSA-coated wells. (C) Purified VEGFR-1/Fc or dII were added at the indicated concentrations to wells coated with α5β1 integrin. Bound molecules were detected using either an anti-Fc alkaline phosphatase (AP)-conjugate or an anti–VEGFR-1 antibody and a secondary anti-rabbit antibody AP-conjugate. Representative experiments performed in triplicate are shown; data are mean plus or minus SEM.
Based on domain boundaries observed in the experimentally determined structures available from the PDB database (PDB IDs: 1flt, 1qty, 1rv6, 1qsv, 1qsz), we designed a recombinant protein corresponding to dII (residues 129-229). This protein was produced in the SF9 insect cell line using the baculovirus-mediated expression system and was purified by affinity chromatography. Recombinant dII was able to directly support HUVEC adhesion (Figure 1B) and to bind purified α5β1 integrin in a concentration-dependent manner (Figure 1C).
Design and experimental testing of peptides mapping on dII
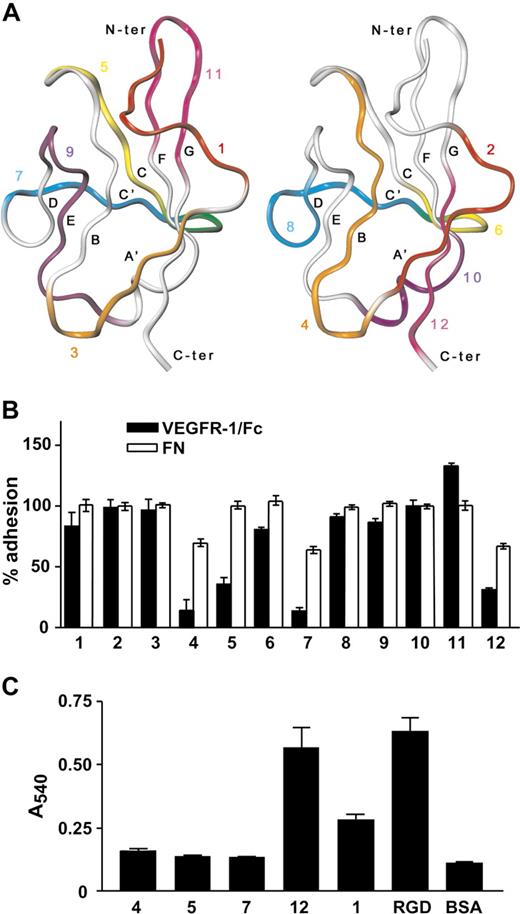
Because VEGFR-1 Ig-like dII contains binding determinants for both growth factors of the VEGF family and α5β1 integrin, but neither VEGF-A nor PlGF compete with the interaction between VEGFR-1 and α5β1 integrin,15 dII regions responsible for integrin and growth factor binding are expected not to be overlapping. To identify those regions specifically responsible for integrin binding, we took advantage of the domain structures determined by x-ray crystallography and available from the PDB database. The three-dimensional structures were examined to identify contiguous amino acid sequences that might mimic exposed regions of dII. Twelve peptides (Table 1) were designed according to the following criteria: (1) they are on the protein surface (ie, they have high overall solvent-accessible surface area); (2) they correspond to different regions of the domain surface, covering it almost entirely; (3) they are shorter than 15 residues; (4) they contain polar residues and, whenever possible, residues charged at physiologic pH values (Asp, Glu, Lys and Arg); (5) they contain a low percentage of hydrophobic residues (ie, Leu, Val, Ile, Met, Phe, and Trp), to minimize solubility problems; and (6) they do not contain Cys residues, which might lead to formation of dimeric peptides. To satisfy the last requirement, VEGFR-1 residue Cys158, which is involved in the formation of a disulfide bond within dII structures, was replaced by Ala in peptide 4. In addition, to explore whether the presence of 2 branched hydrophobic residues next to each other might cause solubility problems, the adjacent Ile145 and Ile146 were both replaced by Thr in peptide 3. The sequence of peptide 5 has been previously published and reported to act as an angiogenesis inhibitor without binding to or inhibiting VEGF-A binding to its receptors.24 The location of the different peptides on dII structure is reported in Figure 2A. To test the ability of the designed peptides to inhibit EC adhesion to VEGFR-1, each peptide was added to a HUVEC suspension before cell plating on VEGFR-1/Fc coated wells. As shown in Figure 2B, peptides 4, 5, 7, and 12 effectively blocked EC adhesion to VEGFR-1/Fc. Moreover, peptides 4, 7, and 12 were able to partially inhibit EC adhesion to fibronectin, the canonical α5β1 integrin ligand, suggesting that they might interfere with ligand binding sites of α5β1 integrin. However, when linked to microtiter plates, only peptide 12 directly supported EC adhesion (Figure 2C).
Amino acid sequence of peptides mapping on domain II of VEGFR-1 and of the scrambled peptide 12 (sP12)
| Peptide . | Sequence . | VEGFR-1 region . |
|---|---|---|
| P1 | GRPFVEMYSE | 132-141 |
| P2 | YSEIPEIIH | 139-147 |
| P3 | ETTHMTEGR | 144-152 |
| P4 | TEGRELVIPARVT | 149-161 |
| P5 | NITVTLKKFPL | 164-174 |
| P6 | KKFPLD | 170-175 |
| P7 | KFPLDTLIPDG | 171-181 |
| P8 | DTLIPDGKRII | 175-185 |
| P9 | DSRKGFIISNAT | 187-198 |
| P10 | TYKEIGL | 198-204 |
| P11 | EATVNGHLYKT | 208-218 |
| P12 | NYLTHRQ | 219-225 |
| sP12 | LTQNYRH | — |
| Peptide . | Sequence . | VEGFR-1 region . |
|---|---|---|
| P1 | GRPFVEMYSE | 132-141 |
| P2 | YSEIPEIIH | 139-147 |
| P3 | ETTHMTEGR | 144-152 |
| P4 | TEGRELVIPARVT | 149-161 |
| P5 | NITVTLKKFPL | 164-174 |
| P6 | KKFPLD | 170-175 |
| P7 | KFPLDTLIPDG | 171-181 |
| P8 | DTLIPDGKRII | 175-185 |
| P9 | DSRKGFIISNAT | 187-198 |
| P10 | TYKEIGL | 198-204 |
| P11 | EATVNGHLYKT | 208-218 |
| P12 | NYLTHRQ | 219-225 |
| sP12 | LTQNYRH | — |
— indicates not applicable.
Peptide 12 inhibits endothelial cell adhesion to VEGFR-1 and directly supports cell adhesion. (A) Ribbon representation of the alpha carbon trace of VEGFR-1 dII structure determined by X-ray crystallography (PDB ID: 1flt). Peptides 1, 3, 5, 7, 9, and 11 (left panel) and 2, 4, 6, 8, 10, and 12 (right panel) are colored red, orange, yellow, blue, purple and magenta, respectively. Residues belonging to both peptide 5 and 7 (left panel) or 6 and 8 (right panel) are colored green. (B) Microtiter plates were coated with VEGFR-1/Fc or fibronectin (FN). HUVECs were preincubated with each peptide and then plated on the coated wells. Data are reported as percentage of adhesion, calculated relative to the number of cells attached on the same substrate in the absence of the peptides. (C) Different VEGFR-1 peptides and RGD peptide, as a positive control, were covalently linked to microtiter plates. HUVECs were then added to the coated plates and allowed to adhere. Nonspecific cell adhesion was measured by the absorbance in BSA-coated wells. Representative experiments performed in triplicate are shown; data are mean plus or minus SEM.
Peptide 12 inhibits endothelial cell adhesion to VEGFR-1 and directly supports cell adhesion. (A) Ribbon representation of the alpha carbon trace of VEGFR-1 dII structure determined by X-ray crystallography (PDB ID: 1flt). Peptides 1, 3, 5, 7, 9, and 11 (left panel) and 2, 4, 6, 8, 10, and 12 (right panel) are colored red, orange, yellow, blue, purple and magenta, respectively. Residues belonging to both peptide 5 and 7 (left panel) or 6 and 8 (right panel) are colored green. (B) Microtiter plates were coated with VEGFR-1/Fc or fibronectin (FN). HUVECs were preincubated with each peptide and then plated on the coated wells. Data are reported as percentage of adhesion, calculated relative to the number of cells attached on the same substrate in the absence of the peptides. (C) Different VEGFR-1 peptides and RGD peptide, as a positive control, were covalently linked to microtiter plates. HUVECs were then added to the coated plates and allowed to adhere. Nonspecific cell adhesion was measured by the absorbance in BSA-coated wells. Representative experiments performed in triplicate are shown; data are mean plus or minus SEM.
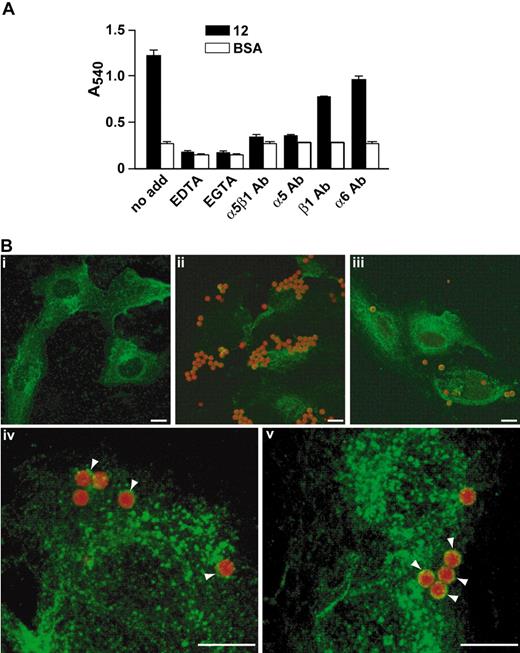
EC adhesion on peptide 12 was completely abolished by treatment with either EDTA or EGTA (Figure 3A), indicating that this interaction required divalent cations, as it is generally the case for integrin binding to extracellular matrix proteins.18 To demonstrate whether α5β1 integrin was indeed the protein mediating EC adhesion to peptide 12, we tested the effect of integrin-blocking antibodies. Preincubation of HUVECs with a mAb against α5β1 integrin inhibited cell adhesion to peptide 12, whereas a mAb against a different integrin subunit (α6) was ineffective (Figure 3A). In particular, when an anti-α5 subunit antibody was used, complete inhibition of EC adhesion was observed (Figure 3A).
α5β1 integrin mediates the biologic activity of peptide 12. (A) Peptide 12 or BSA, as a nonspecific adhesion control, was coated onto microtiter plates, and HUVECs were added in the presence of chelating agents (EDTA or EGTA). Alternatively, HUVECs were preincubated with an antibody against α5β1 integrin, against α5 or β1 integrin subunits, or against α6 integrin subunit and allowed to adhere on coated wells. Data are mean plus or minus SEM; a representative experiment performed in triplicate is shown. (B) Fibronectin-adherent HUVECs were incubated with magnetic beads coated with RGE peptide (i), RGD peptide (ii,iv), or peptide 12 (iii,v). HUVECs were then immunostained for α5β1 integrin. Integrin clusters around the beads are indicated (arrowheads). Bars represent 10 μm.
α5β1 integrin mediates the biologic activity of peptide 12. (A) Peptide 12 or BSA, as a nonspecific adhesion control, was coated onto microtiter plates, and HUVECs were added in the presence of chelating agents (EDTA or EGTA). Alternatively, HUVECs were preincubated with an antibody against α5β1 integrin, against α5 or β1 integrin subunits, or against α6 integrin subunit and allowed to adhere on coated wells. Data are mean plus or minus SEM; a representative experiment performed in triplicate is shown. (B) Fibronectin-adherent HUVECs were incubated with magnetic beads coated with RGE peptide (i), RGD peptide (ii,iv), or peptide 12 (iii,v). HUVECs were then immunostained for α5β1 integrin. Integrin clusters around the beads are indicated (arrowheads). Bars represent 10 μm.
Unligated integrins are diffusely distributed in the membrane, but after ligand binding they are activated and form aggregates.25 To examine whether peptide 12 was able to induce α5β1 integrin clustering on binding, adherent HUVECs were incubated with magnetic beads coated with peptide 12, or with RGD or RGE peptides as positive and negative controls, respectively, and immunostained for α5β1 integrin (Figure 3B). A higher number of RGD-coated (Figure 3Bii) than of peptide 12-coated beads (Figure 3Biii) adhered to HUVECs, probably because of their interaction with other RGD-binding proteins on the cell surface. Indeed, a distinct staining for α5β1 was only observed in approximately 66% of RGD-coated beads (Figure 3Biv), supporting the idea that different ligands were involved in cell binding of these RGD-coated beads. On the other hand, 98% of peptide 12-coated beads (Figure 3Bv) were immunostained with the anti-α5β1 integrin antibody, indicating that α5β1 integrin is the main cellular ligand for this peptide. As expected, no RGE-coated beads attached to HUVECs in this assay (Figure 3Bi).
Preincubation of HUVECs with peptide 12 completely abrogated the migration response toward VEGFR-1/Fc (Figure S1), a process mediated by α5β1 integrin.15 The inhibitory effect of peptide 12 on cell migration was specific for VEGFR-1 because no significant inhibition of fibronectin-induced migration was observed (Figure S1). On the other hand, peptide 12 did not affect cell proliferation (data not shown).
Identification of peptide 12 residues involved in integrin binding
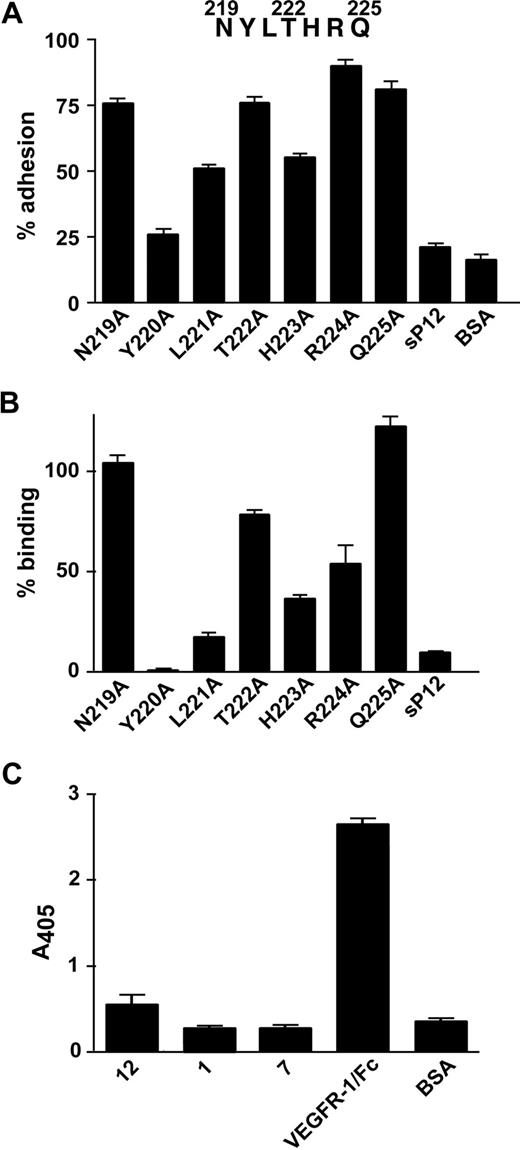
To assess the relative contribution given by peptide 12 residues to the interaction with α5β1 integrin, 7 different peptides carrying a single substitution to Ala were synthesized. As shown in Figure 4A, the ability of peptide 12 to support EC adhesion was significantly affected by single substitution of Leu221, His223 and, above all, Tyr220, with Ala. A “scrambled” peptide containing the same residues as peptide 12 in a different order (Table 1) was used as a control and did not sustain cell adhesion.
Alanine substitution in peptide 12. (A) Peptides whose sequence differs from that of peptide 12 for the substitution of one residue with Ala were covalently linked to microtiter plates. A “scrambled” version of peptide 12 (sP12) and BSA, as a negative control, were also tested. HUVECs were added to the plates and allowed to adhere. Results are expressed as percentage of cell adhesion observed on peptide 12-coated plates. (B) Purified α5β1 integrin was added to wells coated with the same peptides as in panel A, and bound integrin was detected by using an anti-α5β1 antibody and a colorimetric assay. Data are expressed as percentage of protein binding on peptide 12-coated plates. (C) Biotinylated VEGF-A was added to wells coated with peptide 12, 1, 7, VEGFR-1/Fc, as a positive control, or BSA, as a negative control. The amount of attached VEGF-A was quantified by incubation with streptavidin alkaline phosphatase-conjugated and a colorimetric assay. Representative experiments performed in triplicate are shown; data are mean plus or minus SEM.
Alanine substitution in peptide 12. (A) Peptides whose sequence differs from that of peptide 12 for the substitution of one residue with Ala were covalently linked to microtiter plates. A “scrambled” version of peptide 12 (sP12) and BSA, as a negative control, were also tested. HUVECs were added to the plates and allowed to adhere. Results are expressed as percentage of cell adhesion observed on peptide 12-coated plates. (B) Purified α5β1 integrin was added to wells coated with the same peptides as in panel A, and bound integrin was detected by using an anti-α5β1 antibody and a colorimetric assay. Data are expressed as percentage of protein binding on peptide 12-coated plates. (C) Biotinylated VEGF-A was added to wells coated with peptide 12, 1, 7, VEGFR-1/Fc, as a positive control, or BSA, as a negative control. The amount of attached VEGF-A was quantified by incubation with streptavidin alkaline phosphatase-conjugated and a colorimetric assay. Representative experiments performed in triplicate are shown; data are mean plus or minus SEM.
As a direct confirmation of peptide 12/integrin interaction, we used a solid phase binding assay with wild-type and mutated peptides. In this assay, Tyr220, Leu221, and His223 were confirmed to be critical residues mediating peptide binding to purified α5β1 integrin (Figure 4B). In addition, Arg224 was also shown to play an important role. The specific contribution of Arg224 in sustaining peptide binding to purified integrin might be the result of a different conformation of the regions involved in peptide binding between purified and cell membrane integrin, possibly caused by interactions of the latter with other membrane proteins.
No significant interference with cell attachment or integrin binding was observed after substitution of Gln225, a VEGFR-1 residue known to be involved in VEGF-A binding.26 However, because other residues of peptide 12 are in contact with VEGF-A in the known structures of complexes with VEGFR-1, we analyzed whether peptide 12 was able to directly interact with VEGF-A. We also tested peptide 7, containing 6 residues of VEGFR-1 that interact with the growth factor, and peptide 1, mapping to a receptor region not involved in interactions with VEGF-A. The VEGFR-1/Fc protein was used as a positive control. As shown in Figure 4C, VEGF-A did not bind to peptide 12, 7, or 1, indicating that none of these peptides was sufficient to mediate VEGF-A binding.
Peptide 12 supports in vitro and in vivo angiogenesis
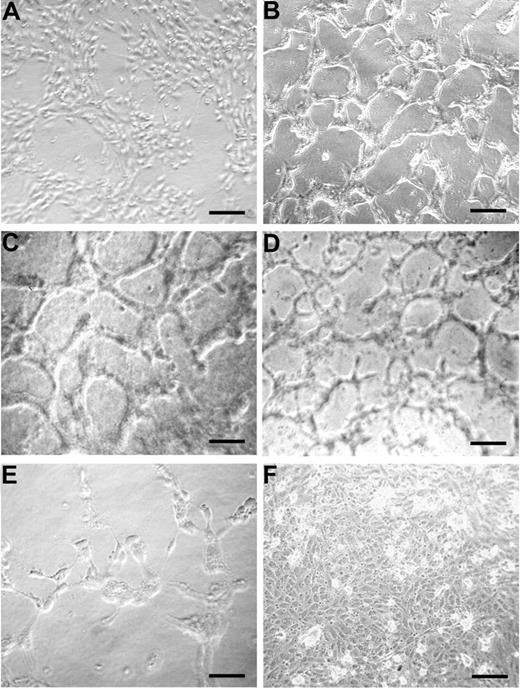
The ability of peptide 12 to induce EC tubule formation was tested on a collagen I matrix and found to be similar to that of VEGF-A (Figure 5B,C, respectively) compared with unstimulated monolayer (Figure 5A). The Q225A peptide, which binds to α5β1 integrin and supports EC adhesion-like peptide 12, also induced EC tubule formation (Figure 5D). Conversely, treatment with the Y220A peptide, which does not bind α5β1 integrin, did not induce vessel shaping (Figure 5E). As a control of specificity, tubule formation assay was performed using peptide 12, previously incubated with an antibody specifically recognizing its amino acid sequence. In this case, formation of capillary structures was not observed (Figure 5F).
Peptide 12 sustains vessel formation in vitro. HUVECs were incubated on 24-well plates precoated with a type I collagen gel mixture, in the absence (A) or presence of peptide 12 (B), peptide Q225A (D), or peptide Y220A (E). VEGF-A was used as a reference control (C). To assess the specificity of peptide 12 activity, an antibody against peptide 12 was also tested in combination with peptide 12 (F). Results are representative of 3 independent experiments. Bars represent 25 μm in C, 50 μm in the others.
Peptide 12 sustains vessel formation in vitro. HUVECs were incubated on 24-well plates precoated with a type I collagen gel mixture, in the absence (A) or presence of peptide 12 (B), peptide Q225A (D), or peptide Y220A (E). VEGF-A was used as a reference control (C). To assess the specificity of peptide 12 activity, an antibody against peptide 12 was also tested in combination with peptide 12 (F). Results are representative of 3 independent experiments. Bars represent 25 μm in C, 50 μm in the others.
To evaluate a possible role of VEGF-A in peptide 12-mediated vessel shaping, tubule formation assay was also performed in the presence of function blocking antibodies against VEGFR-1 or of VEGFR-1/Fc chimera. In these experiments, addition of peptide 12 partially restored EC growth and vessel shaping (Figure S2), suggesting that this peptide acted through a mechanism parallel to that of VEGF-A.
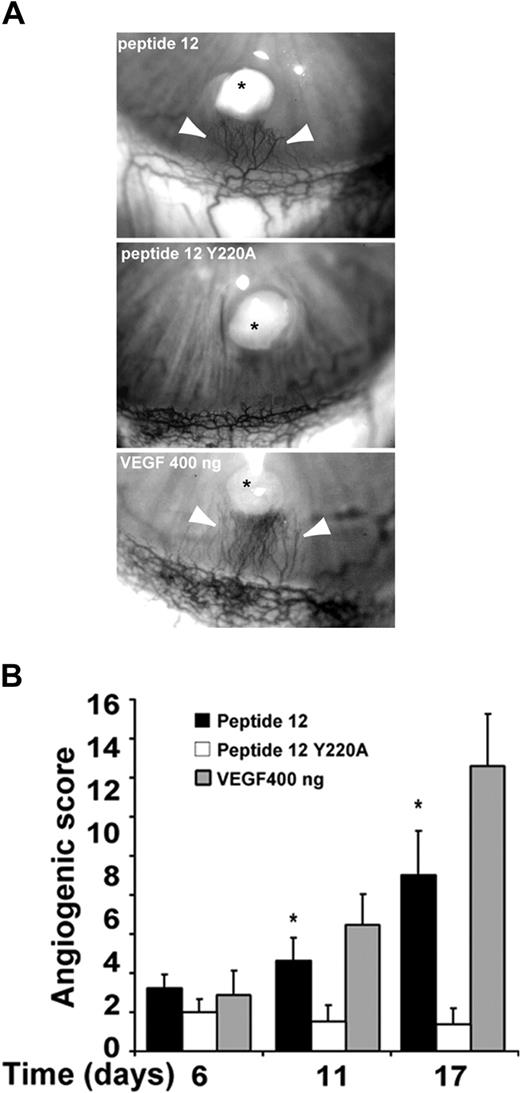
To address the significance of these findings in vivo, pellets releasing peptide 12 were implanted into avascular rabbit corneas and neovascularization was monitored for 17 days. As shown in Figure 6A, peptide 12 (100 μg/pellet) strongly promoted angiogenesis. Extent, growth rate, and pattern of capillary growth, reported as angiogenic score in Figure 6B, overlapped with that obtained with VEGF-A (400 ng/pellet). Consistent with the in vitro data, the control Y220A peptide lacked angiogenic activity. Results ob-tained with 2 different concentrations of peptide 12, 10 μg and 100 μg/pellet, indicated that angiogenesis was sustained in a dose-dependent manner (Table 2). Histologic and immunohistochemical analysis of the corneal tissues are shown in Figure S3.
Peptide 12 sustains neoangiogenesis in vivo. (A) The effect of 100 μg/pellet of peptide 12 or Y220A peptide was compared with that of 400 ng/pellet VEGF-A. *Pellet implants. (B) Quantification of the data (means ± SEM) shown in panel A reported as angiogenic score during time (days). Numbers are the means of 6 implants for each experimental point. One-way analysis of variance (P < .001). *P < .05 peptide 12 versus Y220A peptide (Student-Newman-Keuls multiple comparison test).
Peptide 12 sustains neoangiogenesis in vivo. (A) The effect of 100 μg/pellet of peptide 12 or Y220A peptide was compared with that of 400 ng/pellet VEGF-A. *Pellet implants. (B) Quantification of the data (means ± SEM) shown in panel A reported as angiogenic score during time (days). Numbers are the means of 6 implants for each experimental point. One-way analysis of variance (P < .001). *P < .05 peptide 12 versus Y220A peptide (Student-Newman-Keuls multiple comparison test).
Peptide 12 promotes in vivo angiogenesis in a dose-dependent manner
| . | 10 μg/pellet . | 100 μg/pellet . |
|---|---|---|
| Peptide 12 | 2.0 ± 0.5* | 8.2 ± 2.0* |
| Peptide 12 Y220A | 0.3 ± 0.3 | 1.4 ± 0.8 |
| . | 10 μg/pellet . | 100 μg/pellet . |
|---|---|---|
| Peptide 12 | 2.0 ± 0.5* | 8.2 ± 2.0* |
| Peptide 12 Y220A | 0.3 ± 0.3 | 1.4 ± 0.8 |
The effect of 10 and 100 μg/pellet of peptide 12 or Y220A peptide was tested in the rabbit cornea assay. Data (mean ± SEM) are reported as angiogenic score at day 17 and are the means of 6 implants for each experimental point. (One-way analysis of variance P < .001.)
P < .05 peptide 12 versus Y220A peptide (Student-Newman-Keuls multiple comparison test).
The ability of peptide 12 to induce angiogenesis in the rabbit cornea neovascularization assay was also tested in the presence of VEGF-A, resulting in additional angiogenesis (Figure S4). To further evaluate the relevance of VEGF-A signaling on peptide 12-induced angiogenesis in vivo, assays were also performed in the presence of bevacizumab, a recombinant humanized monoclonal antibody that prevents VEGF-A interaction with its tmVEGFRs, or sunitinib, a tyrosine kinase inhibitor also acting on VEGFRs.27 The angiogenic effect of peptide 12 was significantly inhibited by both bevacizumab and sunitinib treatment (Figure S5), indicating that VEGF-A signaling played a role in peptide 12-induced corneal neovascularization. Similar inhibition was obtained on VEGF-A-induced angiogenesis (Figure S5).
Peptide 12 does not activate FAK signaling pathway
The mechanism of action of peptide 12 through α5β1 integrin activation was analyzed. No phosphorylation of integrin β1 subunit could be observed after HUVEC adhesion on peptide 12 (data not shown).
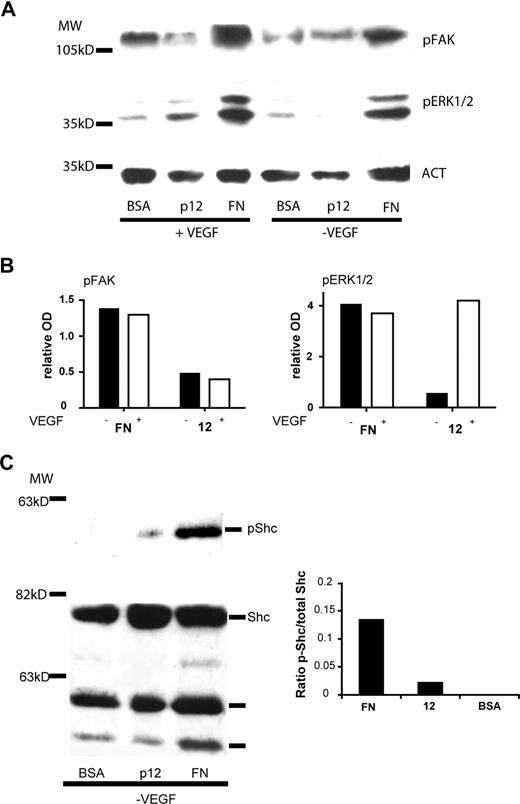
After binding to fibronectin, α5β1 integrin activates 2 major tyrosine kinase-dependent pathways, the focal adhesion kinase (FAK)28 pathway and the adaptor protein Shc28 one. To analyze signals activated by EC adhesion on peptide 12, HUVECs were plated on peptide 12- or fibronectin-coated dishes for 30 minutes or 1 hour in the presence or absence of VEGF-A. As shown in Figure 7A,B, adhesion on peptide 12 did not promote FAK phosphorylation and did not result in significant extracellular signal-regulated kinase (ERK1/2) phosphorylation, the latter being a downstream effector of both integrins and growth factor receptors. ERK1/2 phosphorylation was observed in EC adhering on peptide 12 in the presence of VEGF-A. On the other hand, phosphorylation of Shc could be observed in HUVECs adhering on fibronectin and on peptide 12 in the absence of exogenous VEGF-A (Figure 7C).
Effect of peptide 12 on α5β1 integrin-mediated downstream signaling. HUVECs were starved and treated with cycloheximide as described in “Western blotting analysis” and then plated on peptide 12-, or fibronectin- (FN)-coated dishes in the presence or absence of VEGF-A. (A) Immunoblotting analysis for the presence of phosphorylated-FAK or phosphorylated-ERK1/2 polypeptides after 1-hour adhesion. Actin staining was performed to normalize the results (ACT). (B) Histograms showing the relative OD of the bands in panel A, normalized using absorbance of the actin bands for each sample, after subtraction of the background (phosphorylation levels in cells plated on BSA-coated dishes). (C) Immunoblotting analysis of phosphorylated-Shc and total Shc polypeptide after 30 minutes of adhesion. The histogram shows the OD ratio between the phosphorylated band and the total protein. Data represent the results of a representative experiment.
Effect of peptide 12 on α5β1 integrin-mediated downstream signaling. HUVECs were starved and treated with cycloheximide as described in “Western blotting analysis” and then plated on peptide 12-, or fibronectin- (FN)-coated dishes in the presence or absence of VEGF-A. (A) Immunoblotting analysis for the presence of phosphorylated-FAK or phosphorylated-ERK1/2 polypeptides after 1-hour adhesion. Actin staining was performed to normalize the results (ACT). (B) Histograms showing the relative OD of the bands in panel A, normalized using absorbance of the actin bands for each sample, after subtraction of the background (phosphorylation levels in cells plated on BSA-coated dishes). (C) Immunoblotting analysis of phosphorylated-Shc and total Shc polypeptide after 30 minutes of adhesion. The histogram shows the OD ratio between the phosphorylated band and the total protein. Data represent the results of a representative experiment.
Formation of membrane macromolecular complexes between integrins and growth factor receptors has been previously highlighted.29 In particular, a recent study on acute myeloid leukemia cell lines revealed an association between tmVEGFR-1, integrin β1, and human ERG K+ channel.30 We investigated whether also in HUVECs β1 integrin could form a complex with tmVEGFR-1, but coimmunoprecipitation analysis could not reveal any interaction (Figure S6A). Moreover, expression of the mRNA coding for human ERG-1 protein was not observed in HUVECs (Figure S6B).
Discussion
Soluble VEGFR-1 can play 2 opposite biologic roles. It can act both as a negative regulator of VEGF-related growth factor signaling13 and as an extracellular matrix protein involved in endothelial cell adhesion and migration.15 To completely understand the angiogenesis and vasculogenesis processes, it is of primary importance to define when and where this molecule behaves as a positive or a negative regulator of angiogenesis.
We previously reported that the involvement of sVEGFR-1 in cell adhesion and migration relies on its interaction with α5β1 integrin. In this study, we show that a peptide, isolated from VEGFR-1, is able to bind and activate α5β1 integrin.
First, we demonstrated that Ig-like dII of sVEGFR-1 is involved in the interaction with the integrin. To identify dII regions mediating this interaction, we tested 12 different peptides mimicking most of the dII surface and found that 4 of them inhibited cell adhesion to sVEGFR-1. Of these, peptides 4 and 12 are situated on opposite sides of the domain, each on one of the 2 β-sheets packed face-to-face, whereas peptide 7 lies on a plane roughly perpendicular to the 2 β-sheets. Only peptide 5 is relatively close to both peptide 7, partially overlapping with it, and peptide 12, which is situated on the same β-sheet. The relatively distant position of these peptides suggests that their interference with cell adhesion to sVEGFR-1 may be achieved through interaction with different binding partners and diverse mechanisms. Accordingly, peptide 12 is the only one in our series that directly supported EC adhesion, providing us with the first indication that this peptide may directly interact with α5β1 integrin. EC binding to peptide 12 was divalent cation-sensitive and antibodies against α5β1 integrin specifically blocked this interaction, supporting the assumption that EC adhesion to peptide 12 was α5β1 integrin-mediated. In particular, our results suggest that the α5 subunit gives the most relevant contribution to peptide 12 binding. Moreover, α5β1 integrin clustering was observed with peptide 12-coated beads, indicating that a direct interaction between peptide 12 and integrin did occur leading to integrin activation. Finally, peptide 12 inhibited cell migration toward VEGFR-1, a process that is mediated by α5β1 integrin.15
In addition to these indirect observations, we also detected a direct interaction between α5β1 integrin and peptide 12 in a solid phase binding assay. Alanine-scanning mutagenesis identified 4 residues of peptide 12, namely, Tyr220 (giving the highest contribution), Leu221, His223, and Arg224, which, if mutated, significantly reduced peptide ability to support direct interaction with α5β1 integrin and, with the exception of Arg224, cell adhesion. This YLXHR motif shows some similarities but also noticeable differences with previously described α5β1 integrin binding sequences.18,19 Similarly to the RGD motif, the fibronectin PHSRN and VKNEED synergy sites, and the majority of peptide sequences identified from phage library screenings,31,32 the YLXHR motif bears a positive charge, which can be contributed either by the Arg or His residue. Moreover, similarly to many peptide sequences selected from phage libraries, it contains an aromatic residue (Tyr) separated by 2 or 3 amino acids from the positively charged one. However, this motif differs from those previously identified in the position of the aromatic and positive residues (RXXXW being the most typical arrangement) and in the role played by the hydrophobic residue Leu. In addition, this is the first motif for which an aromatic residue, rather than a charged one, plays the most important role for α5β1 integrin binding.
We demonstrated that peptide 12 induces tubule-like structures formation in an in vitro angiogenic assay and supports neoangiogenesis in vivo.
Interestingly, addition of peptide 12 in the in vitro vessel formation assay circumvents the effects of either sVEGFR-1 or anti–VEGFR-1 function-blocking antibodies, partially restoring EC survival and tubule structuring. These data suggest that peptide 12 activates a signal pathway parallel to that of VEGF-A. Accordingly, peptide 12 enhances the angiogenic effect of VEGF-A in vivo.
Peptide 12 plausibly exerts these proangiogenic properties through integrin binding and activation of an α5β1-mediated signaling pathway because this peptide is not able to directly bind VEGF-A, its tyrosine kinase receptors, or other molecules involved in VEGFR signaling, such as neuropilins (A.O., P.M.L., C.M.F., unpublished data). We analyzed whether α5β1 binding to peptide 12 induced FAK phosphorylation, the main signaling mechanism of α5β1 integrin after fibronectin binding, but no FAK phosphorylation could be observed. At the same time point, ERK phosphorylation was detected in EC adhering on peptide 12 only in the presence of VEGF-A. On the other hand, we could show a slight phosphorylation of the adaptor protein Shc28 in the absence of exogenous VEGF-A. It remains to be clarified whether Shc phosphorylation induced by peptide 12 requires further VEGF-induced signaling to result in ERK phosphorylation.
Interestingly, peptide 12 angiogenic activity in vivo was significantly impaired by both bevacizumab, a monoclonal antibody that blocks VEGF-A binding to its receptors, and sunitinib, a tyrosine kinase inhibitor acting also on VEGFRs. These results suggest that peptide 12 interactions with α5β1 integrin require simultaneous VEGF-A activation of its transmembrane receptors to give neoangiogenesis. Alternatively, peptide 12 requires “active” endothelial cells, as those stimulated by VEGF-A, to operate through α5β1 integrin. It should be consider that VEGF-A elicits different functions in endothelium homeostasis, several of which we are just beginning to understand.33
It has been previously reported that cross talk between integrins and growth factors represents an important modulator of vasculogenesis and angiogenesis.29 Significant enhancement of the angiogenic response can be specifically observed when both α5β1 integrin and VEGF-A receptors are engaged.34 In this perspective, we also investigated whether α5β1 integrin could directly interact with tmVEGFR-1, but we did not detect any interaction. Moreover, previous studies could not detect the formation of a macromolecular complex involving α5β1 integrin and VEGFR-2.35 Nevertheless, an additional transmembrane receptor could participate in the formation of the complex between α5β1 integrin and VEGFRs, and further investigation is needed to clarify this aspect.
Altogether, the properties of peptide 12, located within the sVEGFR-1 sequence, support the hypothesis that sVEGFR-1 itself is implicated in vessel shaping and angiogenesis as well, through its interaction with α5β1 integrin. Interestingly, sVEGFR-1 has been shown to act as a positive modulator of vascular sprout formation and vessel morphogenesis.36 The addition of VEGFR-1/Fc protein to in vitro cultures of VEGFR-1–lacking embryonic stem cells was also shown to partially rescue the mutant phenotype (ie, vascular overgrowth and disorganization).37 In those studies, sVEGFR-1 has been proposed to act on vascular structuring through modulation of VEGF-A receptor signaling by sequestration and neutralization of VEGF-A. Our results suggest that additional pathways required for vessel formation may be regulated through interaction between sVEGFR-1 and α5β1 integrin.
In our in vitro angiogenesis assay, addition of sVEGFR-1 impaired vessel structure formation and EC growth is in agreement with the concept that, in certain circumstances, sVEGFR-1 preferentially acts as a decoy receptor for VEGF growth factor family and not as an activator of α5β1 integrin.38,39 It is possible that the location of sVEGFR-1 within the extracellular matrix favors its interaction with α5β1 integrin over growth factors. It is also possible that matrix-associated proteases process sVEGFR-1 producing matrix-embedded smaller polypeptides not able to bind the growth factors anymore but still capable of interacting with α5β1 integrin.
The identification of a α5β1 integrin-binding small peptide able to activate a proangiogenic signaling in EC provides a rational basis for the development of new therapeutic tools. Molecules able to sustain neoangiogenesis have been successfully used for the treatment of limb ischemia by inducing collateral vessel formation, and for tissue repair in pathologies associated with impaired wound healing, such as diabetes.40 Our results suggest that peptide 12 is a promising candidate for the design of additional proangiogenic drugs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Shibuya for the gift of the human VEGFR-1 cDNA plasmid, Dr Ragone for acquisition of the confocal microscope pictures, Dr Avitabile for the analysis of Shc phosphorylation, Prof Defilippi and Prof Tarone for discussion of phosphorylation assays, and Mr Inzillo for artwork.
This work was supported by Compagnia di San Paolo, Prat 23 734/Ente 11 587, and the European Community (LSHB-CT-2005 512102 and LSHG-CT-2003-503265), the National Research Council (grant SV.P08.009.003 to V.M.), AIRC Regional Grant 2005 (L.M.), and the Fondazione Monte dei Paschi di Siena (M.Z.).
Authorship
Contribution: S.S., A.O., L.M. designed and performed research; P.M.L. and V.M. designed the experiments, assessed the results, and revised the manuscript; K.B.-H. provided fundamental reagents and technology and revised the manuscript; F.R. performed the experiments; M.Z., S.D., G.Z. analyzed data and revised the manuscript; A.T. and C.M.F. designed research, assessed the results, and wrote the paper.
Conflict-of-interest disclosure: A patent related to this peptide has been deposited. The authors declare no other competing financial interests.
Correspondence: Cristina M. Failla, Molecular and Cell Biology Laboratory, IDI-IRCCS, via Monti di Creta, 104, 00167 Rome, Italy; e-mail: c.failla@idi.it.
References
Author notes
S.S. and A.O. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal