Abstract
The potential role of dietary fats in cancer is attracting considerable interest within the community. Both epidemiologic and experimental findings suggest that omega-3 polyunsaturated fatty acids (ω-3 PUFAs), which are almost absent from typical Western diets, exert protective effects against cancer progression, although the precise mechanism of this suppression remains unknown. One of the potential targets for ω-3 PUFAs in cancer suppression is angiogenesis, a process of new blood vessel formation within rapidly growing tumors. Here, we demonstrate that ω-6 PUFAs stimulate and ω-3 PUFAs inhibit major proangiogenic processes in human endothelial cells, including the induction of angiopoietin-2 (Ang2) and matrix metalloprotease-9, endothelial invasion, and tube formation, that are usually activated by the major ω-6 PUFA arachidonic acid. The cyclooxygenase (COX)–mediated conversion of PUFAs to prostanoid derivatives participated in modulation of the expression of Ang2. Thus, the ω-6 PUFA–derived prostaglandin E2 augmented, whereas the ω-3 PUFA–derived prostaglandin E3 suppressed the induction of Ang2 by growth factors. Our findings are consistent with the suggestion that PUFAs undergo biotransformation by COX-2 to lipid mediators that modulate tumor angiogenesis, which provides new insight into the beneficial effects of ω-3 PUFAs.
Introduction
Initial reports of the beneficial effects of long-chain omega-3 polyunsaturated fatty acids (ω-3 PUFAs) in human physiology occurred in the mid-1970s with observations that the dietary intake of fish-derived lipids correlated with decreased incidence of cardiovascular diseases and cancer. Thus, the lower incidence of cardiac diseases in the Inuit population has been attributed to the content of fish, which are high in ω-3 PUFAs, in the Inuit diet.1 In another seminal study, Japanese women were found to have a lower risk of breast cancer compared with their counterparts in other developed countries.2 When Japanese women migrated to countries with higher prevalence of breast cancer and adopted local diets, the incidence of disease more closely resembled that of the local population, thus indicating the importance of environmental factors. Rapidly, the interest of researchers focused on the possible protective effects of oily fish–derived ω-3 PUFAs, especially eicosapentaenoic acid (EPA; C20:5 n-3) and docosahexaenoic acid (DHA; C22:6 n-3). These ω-3 PUFAs are produced by phytoplankton and are accumulated in the fatty tissue of fish. ω-6 PUFAs, which are derived principally from animal fat and include arachidonic acid (AA; C20:4 n-6), and ω-3 PUFAs are both essential for mammals, since they cannot be synthesized and must be consumed in diet
The mechanistic information underpinning the inhibitory effects of ω-3 PUFA intake on disease development is deficient. Both ω-6 and ω-3 PUFAs have a number of critical homeostatic functions.3 As constituents of structural phospholipids of the cell membrane they influence membrane fluidity, modulate intracellular signaling pathways, and modify cell-cell interactions that control tissue development and physiology.4,5 The ω-3 PUFAs EPA and DHA have also been shown to modulate the activation of Jurkat T cells by inhibition of protein kinase C6 to decrease the activation of the oncogenic transcription factors ras and AP-17 and to activate apoptosis by down-regulating NFκB8 and Bcl-2.9 Based on these reports, it is now believed that multiple mechanisms contribute to the observed biological actions of ω-3 PUFAs.
Apart from their direct actions, PUFAs influence a range of processes in target cells after conversion into bioactive metabolites. Both ω-6 and ω-3 PUFAs are substrates for cyclooxygenase (COX), lipoxygenase (LOX), and epoxygenase (CYP) enzymes and form distinct series of eicosanoid products (prostaglandins, thromboxanes, leukotrienes, and epoxides).10 When the availability of ω-3 PUFAs is increased in cells, the formation of the corresponding eicosanoids is also increased; these ω-3 eicosanoids have been shown to be less inflammatory and to have decreased growth-promoting properties in comparison with the ω-6 eicosanoids.11
Among the processes involved in tumor progression, it has been shown that ω-3 PUFAs inhibit the formation of new blood vessels (angiogenesis), a critical process that affects tumor growth and dissemination.12,13 As is the case with other effects of ω-3 PUFAs on mammalian cells, numerous mechanisms have been proposed for suppression of angiogenesis. Interestingly, most of those mechanisms do not include potential roles of ω-3 PUFA–derived eicosanoid metabolites. Instead, they usually involve direct effects of ω-3 PUFAs on the physical properties of the endothelial cell (EC) membrane14 to increase apoptosis15,16 and/or modify caveolae/lipid raft functions.17 It is also noteworthy that most of those observations have been made in in vitro systems and using relatively high concentrations of free PUFAs that are unlikely to occur in vivo.
The present work was undertaken to assess the extent to which ω-3 PUFA–derived prostanoids influence angiogenesis. We provide evidence that the major mechanism by which ω-3 PUFAs modulate angiogenesis involves the decreased synthesis of proangiogenic ω-6 prostanoids coupled with increased production of less active ω-3 prostanoids. Importantly, the present study used concentrations of PUFAs that are achievable in vivo under normal physiologic conditions by either dietary or pharmacologic interventions. At such concentrations, ω-3 PUFAs, through their corresponding prostanoids, effectively inhibited the major angiogenic processes in ECs that had been stimulated by proangiogenic growth factors.
Methods
Human umbilical vein endothelial cell culture and treatment
Human umbilical vein endothelial cells (HUVECs), isolated from mixed donors to minimize potential effects of genetic variability (obtained from Cambrex BioScience, Mt Waverley, Australia), were grown in EGM-2 medium (also from Cambrex BioScience) supplemented with 10% fetal bovine serum (FBS), epidermal growth factor (5.0 ng/mL), hydrocortisone (0.2 μg/mL), vascular endothelial growth factor (VEGF; 0.5 ng/mL), basic fibroblast growth factor (bFGF; 10 ng/mL), insulin-like growth factor-1 (20 ng/mL), ascorbic acid (1 μg/mL), and heparin (22.5 μg/mL). Prior to the treatments, confluent HUVECs were washed with serum-free Dulbecco minimal essential medium (DMEM) and treated in 2% FBS-supplemented DMEM for a fixed duration of 20 hours. PUFA, PGE2, and PGE3 were obtained from Cayman Chemical (Sapphire Bioscience, Redfern, Australia). Stock solutions were prepared in absolute ethanol and stored at −70°C; fresh dilutions were prepared daily when used in cell experiments. To minimize PUFA oxidation, experiments were performed under low light; all PUFA stocks were used within 1 year. Control HUVEC cultures were treated with an equivalent concentration of the vehicle (ethanol), which was found in preliminary studies not to affect the important endpoints in this study. VEGF165 and bFGF were obtained from R&D Systems (Bio Scientific, Gymea, Australia).
Cell viability assay
Confluent HUVEC cultures were incubated with increasing concentrations of PUFA in 2% FBS/DMEM for 20 hours. Medium was replaced with complete EGM-2 medium containing 0.5 mg/mL MTT (Promega Australia, Annandale, Australia), and incubation continued for 3 hours. Blue formazan formed from MTT by live cells was dissolved in acidic isopropanol (0.04 M HCl in absolute isopropanol) and quantified spectrophotometrically at 570 nm.
COX-2 activity assay
The COX-2 activity assay was performed using a commercial kit from Cayman Chemical (Ann Arbor, MI) according to the manufacturer's instructions. This colorimetric assay measures PUFA-dependent COX peroxidase activity by monitoring oxidation of the artificial proton acceptor TMPD. Purified recombinant bovine COX-2 was obtained from Cayman Chemical.
Western blotting
HUVEC lysates were analyzed by Western blotting as described earlier.18 Ang2 detection was performed with anti-human monoclonal antibodies from R&D Systems and COX-2 with anti-human monoclonal antibodies from Santa Cruz Biotechnology (Biolab, Clayton, Australia). Tie-2 and VEGFR-1/2 antibodies were also obtained from R&D Systems. Equivalent loading was estimated by detection of α-tubulin with corresponding monoclonal antibodies (Santa Cruz Biotechnology). Autoradiography films were scanned densitometrically (GS 800; Bio-Rad Australia, Gladesville, Australia) and quantified using dedicated software. Quantification was performed with the use of corresponding standard curves and expressed relative to α-tubulin levels.
Matrix metalloprotease zymography
HUVEC culture medium was analyzed for the presence of gelatinases as described earlier.19 Briefly, medium samples were diluted 1:1 with zymography sample buffer (4% SDS, 125 mM Tris-HCl, 10% glycerol, and 0.001% bromophenol blue [pH 6.8]) and separated on 10% polyacrylamide gels containing gelatin (1 mg/mL) and 0.1% SDS. After separation, proteins were renaturated in 2.5% Triton X-100, then developed in zymogram buffer (Tris-Cl 50 mM, NaCl 0.2 M, CaCl2 2 μM, ZnCl2 5 mM, Brij 35 0.02% [pH 7.5]) at 37°C for 15 hours. After incubation, gels were stained with Coomassie Brilliant Blue R-250. Negative gel images were scanned using a densitometer calibrated as described. Relative quantification was performed with the use of corresponding standard curves.
Endothelial migration assay
HUVECs were trypsinized, washed in complete EGM-2 medium, and then resuspended in serum-free EGM-2 medium (106 cells/mL). The cell suspension was mixed 1:1 with the solution of Matrigel from Cultrex (Bio Scientific). From that suspension, 20 μL (containing 104 cells) was layered onto the surface of 6-well tissue-culture dishes to form well-defined droplets. Dishes were placed at 37°C for 5 minutes for matrigel to semi-solidify. A total of 1/5 EGM-2 medium (EGM-2 medium supplemented with 10% FBS that was diluted 5-fold with serum-free EGM-2, which supports 50% of maximal EC migration) was then added to the dishes, and plates were incubated for 20 hours. The number of cells that migrated out of the droplet was scored by phase-contrast microscopy and digital image analysis.
Endothelial tube formation assay
Matrigel solution (0.3 mL) was applied to each of the wells of 24-well tissue-culture plates and allowed to solidify for 0.5 hours at 37°C. HUVECs were removed from cell culture dishes by trypsinization and washed as described. Cells were then resuspended in EGM-2 medium supplemented with 10% FBS that was diluted 3-fold with serum-free EGM-2 medium (1/3 EGM-2 medium which supports 50% of maximal tube formation). From that suspension, 4 × 104 cells (in 100 μL) were applied to each 2-cm2 well. A volume of 400 μL of 1/3 EGM-2 medium was then added to each well and incubated for 20 hours. Endothelial tubes were photographed using phase-contrast microscopy and digital photography as described in “Microscopy.” The relative lengths of tubes were determined by digital image analysis.
Microscopy
Microscopic images were obtained with an Olympus CKX41 microscope fitted with an Altra20 digital camera (Olympus Imaging Australia, North Ryde, Australia). Microscope settings included WHB 10 × 20 eyepiece and 20×/0.40 PhP objective. Analysis getIT 5.0 (Olympus Imaging Australia) and Adobe Photoshop version 7.0.1 (Adobe Systems, San Jose, CA) software were used.
Statistical analysis
All quantitative measurements were done at least in triplicate. Results are expressed as means plus or minus SD. Statistical analysis was initially performed by one-way analysis of variance (ANOVA) using GraphPad Software (GraphPad, San Diego, CA). If ANOVA analysis reached significance (P < .05), individual differences were then identified by the Tukey multiple comparison test. Values with P values less than .05 were considered to be statistically significant. Nonlinear saturation curve fitting was performed with the same GraphPad software.
Results
Synergistic activation of angiogenesis by VEGF and bFGF involves induction of Ang2
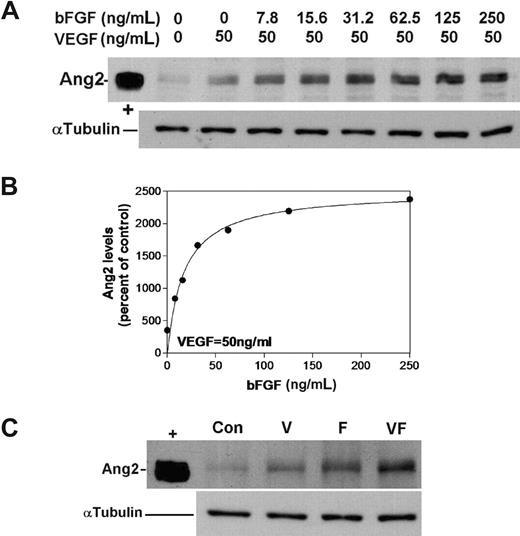
Induction of Ang2 was chosen as a marker of proangiogenic activation of ECs. In adults, Ang2 expression is restricted to the proangiogenic endothelium20 where, together with VEGF, it mediates destabilization of mature vessels contributing to the initiation of angiogenesis.21 VEGF and bFGF are the major growth factors that activate angiogenesis and also induce Ang2.22,23 We have found that both VEGF165 (V; Figure 1) and bFGF (F; Figure 1) induce Ang2, with bFGF exhibiting greater potency. Thus, the maximal induction of 3.5-fold was achieved by VEGF at a concentration of 50 ng/mL, while bFGF-mediated induction of Ang2 (25.0 ± 1.6-fold) was detected at a concentration of approximately 100 ng/mL (Figure 1A). A dose-response curve was generated by semiquantitative determination of Ang2 levels in HUVECs that were treated with 50 ng/mL VEGF and increasing concentrations of bFGF (0-250 ng/mL). The effective concentration of bFGF that produced 50% induction of Ang2 (EC50) was calculated to be 17.27 ng/mL from the fitted curve (r2 = .958; Figure 1B). Hence, the working concentrations of angiogenesis inducers that were selected for further experiments were 50 ng/mL (VEGF) and 20 ng/mL (bFGF). At these concentrations (close to the EC50 for Ang2 induction), any further changes effected by PUFA should be readily detectable; such effects may have been obscured at maximal VEGF/bFGF-mediated induction. Clear synergism between the 2 growth factors in Ang2 induction when used at the test concentrations is demonstrated in Figure 1C. The combination of VEGF and bFGF was used to induce Ang2 in order to more faithfully mimic in vivo angiogenesis, where participation of both is expected. Furthermore, since the precise intracellular targets for ω-3 PUFAs are still unclear, the presence of both growth factors in experiments compensates for potential differences in their respective signaling pathways.
Synergism in the induction of Ang2 by VEGF and bFGF. (A) Dose-response curve for bFGF induction of Ang2. HUVECs were treated with constant VEGF (50 ng/mL) and increasing bFGF concentrations (0-250 ng/mL) for 20 hours. Levels of Ang2 were determined by Western blotting (α-tubulin levels are shown to indicate equal loading; n = 3). The intensity of the Ang2 bands was determined by autoradiography and densitometry as described in “Methods.” (B) Mean Ang2 levels were expressed as the percentage of control (VEGF, 50 ng/mL; bFGF, 0 ng/mL). The EC50 value for the bFGF was calculated by nonlinear regression. (C) Synergism between VEGF and bFGF (VF) in the induction of Ang2 was established at optimal conditions (VEGF, 50 ng/mL; bFGF, 20 ng/mL) by Western blotting. “+” signifies Ang2-positive control.
Synergism in the induction of Ang2 by VEGF and bFGF. (A) Dose-response curve for bFGF induction of Ang2. HUVECs were treated with constant VEGF (50 ng/mL) and increasing bFGF concentrations (0-250 ng/mL) for 20 hours. Levels of Ang2 were determined by Western blotting (α-tubulin levels are shown to indicate equal loading; n = 3). The intensity of the Ang2 bands was determined by autoradiography and densitometry as described in “Methods.” (B) Mean Ang2 levels were expressed as the percentage of control (VEGF, 50 ng/mL; bFGF, 0 ng/mL). The EC50 value for the bFGF was calculated by nonlinear regression. (C) Synergism between VEGF and bFGF (VF) in the induction of Ang2 was established at optimal conditions (VEGF, 50 ng/mL; bFGF, 20 ng/mL) by Western blotting. “+” signifies Ang2-positive control.
ω-3 PUFAs suppress proangiogenic activation of Ang2
Increased cell apoptosis has been reported as a major effect of ω-3 PUFAs in endothelial and other cell types.15 Because of the high cellular toxicity of free PUFAs, we initially determined the optimal concentration to be used in our specific experimental conditions (2% FBS-supplemented DMEM and 20-hour treatment of confluent HUVECs).
HUVEC viability was assayed following treatments with PUFA up to 160 μM (Figure 2A). HUVECs were maximally viable when treated with PUFA over the concentration range of 10 to 40 μM (compared with control, viability was increased at PUFA concentrations up to 20 μM; Figure 2A). Cell viability decreased rapidly when PUFA concentrations exceeded 40 μM, indicating induction of cell toxicity. The observed effect was independent of the type of PUFA (Figure 2A). DMEM/2% FBS medium influenced only the growth rate but did not affect the viability of HUVECs under these experimental conditions (data not shown). Thus, PUFA concentrations of 10 μM were chosen for subsequent treatments. The potent induction of prostaglandin synthesis (10-fold of control) in response to 10 μM AA treatment when combined with VEGF and bFGF indicated that PUFA availability is a limiting factor for prostaglandin synthesis in this system (data not shown).
ω-3 PUFAs suppress Ang2 induction. (A) Determination of subtoxic PUFA concentrations. Confluent HUVECs were treated with increasing concentrations of PUFAs as indicated for 20 hours. Viability was determined by MTT assay: ● indicates AA; ○, EPA; ▾ DHA; and □, SDA. (B,C) HUVECs were treated with VEGF and bFGF (VF) in combination with PUFAs (10 μM) or indomethacin (IND; 5 μM). Levels of Ang2 were determined by Western blotting (α-tubulin levels are shown to indicate equal loading; C indicates VF absent; n = 3). The intensity of the Ang2 bands was determined by autoradiography and densitometry. Mean differences in Ang2 levels were analyzed statistically. **P < .01; ***P < .001 different from VF alone.
ω-3 PUFAs suppress Ang2 induction. (A) Determination of subtoxic PUFA concentrations. Confluent HUVECs were treated with increasing concentrations of PUFAs as indicated for 20 hours. Viability was determined by MTT assay: ● indicates AA; ○, EPA; ▾ DHA; and □, SDA. (B,C) HUVECs were treated with VEGF and bFGF (VF) in combination with PUFAs (10 μM) or indomethacin (IND; 5 μM). Levels of Ang2 were determined by Western blotting (α-tubulin levels are shown to indicate equal loading; C indicates VF absent; n = 3). The intensity of the Ang2 bands was determined by autoradiography and densitometry. Mean differences in Ang2 levels were analyzed statistically. **P < .01; ***P < .001 different from VF alone.
The effects of nontoxic concentrations of PUFA on Ang2 induction by VEGF and bFGF are shown in Figure 2B. VEGF and bFGF alone induced Ang2 synthesis to 4.72-fold plus or minus 0.64-fold of control (P < .001). Addition of AA further induced Ang2 synthesis to 1.67-fold plus or minus 0.21-fold of that effected by VEGF/bFGF (P < .001). In contrast, all 3 ω-3 PUFAs that were analyzed in this study—EPA, DHA, and stearidonic acid (SDA; 18:4, cis-6,9,12,15)—suppressed the induction of Ang2 to 0.53-fold plus or minus 0.07-fold of VEGF/bFGF alone when tested at a concentration of 10 μM (P < .01). There were no significant differences between the 3 ω-3 PUFAs studied in their observed effects on Ang2 synthesis (P > .05).
The nonspecific COX inhibitor indomethacin (5 μM) significantly suppressed the induction of Ang2 mediated by VEGF and bFGF (by approximately 50%), suggesting that COX, at least in part, mediates Ang2 induction by these growth factors (Figure 2B). Induction of Ang2 by separate VEGF or bFGF treatment was also partially suppressed by indomethacin (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The extent of inhibition of Ang2 induction by indomethacin was similar to that observed with all 3 ω-3 PUFAs. Furthermore, in the presence of indomethacin, none of the PUFAs significantly affected Ang2 induction by VEGF and bFGF (P = .998), which suggests a critical role for COX in mediation of the effects of ω-6 as well as ω-3 PUFAs (Figure 2C).
The impact of VEGF and bFGF, alone and in combination with ω-6 AA and ω-3 EPA, on the expression of other markers of endothelial angiogenesis was also evaluated. Thus, expression of Tie-2, a transmembrane receptor tyrosine kinase that binds Ang1 and Ang2 with high affinity, and the 2 VEGF receptors VEGFR-1 and VEGFR-2 was also induced by VEGF, bFGF, and the growth factor combination (Figure S1). Expression was further enhanced by AA, but EPA impaired the induction elicited by growth factors. Indomethacin abrogated the growth factor–stimulated induction of all angiogenic markers. In summary, these findings implicate PUFA and prostanoid metabolites in the regulation of major proangiogenic proteins in endothelium.
Optimal COX-2 activity but not induction is affected by ω-3 PUFAs in ECs
We considered the possibility that modulation of COX-2 induction could contribute to the observed effects of PUFA in regulating angiogenesis. The combination of VEGF and bFGF induced COX-2 expression in HUVECs that could largely be attributed to the action of VEGF (Figure 3A). VEGF-induced COX-2 expression was not significantly altered by concomitant short-term exposure to 3 ω-3 PUFAs (10 μM for 20 hours; Figure 3A; P = .35). In contrast, the ω-6 PUFA AA consistently suppressed the extent of COX-2 induction to around 50% of that effected by VEGF/bFGF treatment. It is noteworthy that indomethacin treatment also augmented the induction of COX-2 by VEGF/bFGF (Figure 3B). Thus, the regulation of Ang2 and COX-2 by PUFA is different: Ang2 is up-regulated and COX-2 is suppressed by ω-6 PUFA–derived prostanoids. ω-3 PUFAs directly suppress Ang2 expression but augment the induction of COX-2 mediated by growth factors.
Effects of ω-3 PUFAs on COX-2. HUVECs were treated with VEGF, bFGF, or the combination of both growth factors (VF) and in conjunction with AA, SDA, DHA, or EPA (10 μM). (A) Levels of COX-2 were determined by Western blotting (n = 3). α-tubulin levels are shown to indicate equal loading. “+” signifies COX-2–positive control. (B) Augmented increase in COX-2 expression in VF-treated HUVECs elicited by indomethacin (5 μM). (C) In vitro activity of bovine COX-2 was determined using AA, SDA, DHA, and EPA as substrates (n = 3). Enzyme kinetic parameters (Vmax and Km) were calculated by nonlinear regression. ● indicates AA; ○, EPA; ■, SDA; and □, DHA.
Effects of ω-3 PUFAs on COX-2. HUVECs were treated with VEGF, bFGF, or the combination of both growth factors (VF) and in conjunction with AA, SDA, DHA, or EPA (10 μM). (A) Levels of COX-2 were determined by Western blotting (n = 3). α-tubulin levels are shown to indicate equal loading. “+” signifies COX-2–positive control. (B) Augmented increase in COX-2 expression in VF-treated HUVECs elicited by indomethacin (5 μM). (C) In vitro activity of bovine COX-2 was determined using AA, SDA, DHA, and EPA as substrates (n = 3). Enzyme kinetic parameters (Vmax and Km) were calculated by nonlinear regression. ● indicates AA; ○, EPA; ■, SDA; and □, DHA.
Complementary to the findings regarding immunoreactive COX-2 protein, ω-3 PUFAs were less efficient than AA as COX-2 substrates (Figure 3C). Both the maximal reaction velocity (Vmax) and concentration necessary to achieve half-maximal COX-2 activity (Km) were influenced by switching the substrate from ω-6 to ω-3 PUFA. Thus, the Vmax determined for AA was approximately 36% higher (P < .001) than those for the ω-3 PUFAs (Vmax for AA, EPA, DHA, and SDA were estimated to be 10.5 ± 0.3, 7.4 ± 0.3, 7.3 ± 0.3, and 8.4 ± 0.4 nmol/minute of product per unit of COX-2, respectively). Vmax did not vary greatly between the 3 ω-3 PUFAs (P > .05). Similarly, the Km was about 3- to 5-fold lower (P < .001) for AA (0.39 ± 0.07 μM) than for EPA, DHA, and SDA (1.49 ± 0.27, 2.01 ± 0.33, and 2.03 ± 0.37 μM, respectively). As was the case with Vmax, the Km values did not significantly differ between the 3 ω-3 PUFAs (P > .05).
ω-3 PUFA suppress MMP-9 levels secreted from ECs
Matrix metalloproteases (MMPs) degrade extracellular matrix, and play an important part in the migration of ECs during angiogenesis. Prostaglandins increase the synthesis and secretion of the 2 major MMPs involved in angiogenesis (MMP-2 and MMP-9).24 In breast cancer cells, the induction of these 2 MMPs is down-regulated by the ω-3 PUFA EPA.25
Since we hypothesized that the ω-3 PUFAs may regulate angiogenesis through the formation of the corresponding prostaglandins, we next examined the effects of indomethacin and ω-3 PUFAs on the induction of gelatinases (MMP-2 and MMP-9) in ECs that were also exposed to proangiogenic stimuli. The levels of MMPs were measured in media from treated HUVECs using semiquantitative zymography. In these experiments, only the MMP band at a molecular weight of 87 kDa corresponding to the active MMP-9 was detected and was clearly distinguishable from the pro–MMP-9 standard (Figure 4A).19
Effects of ω-3 PUFAs on MMP-9 induction in ECs. HUVECs were treated with the combination of VEGF and bFGF (VF) and AA, SDA, DHA, EPA (10 μM), or indomethacin (IND; 5 μM). MMP levels were analyzed by semiquantitative zymography (“Methods”). (A) Differences in molecular weight between pro–MMP-9 standard (92 kDa) and the active MMP-9 (87 kDa) detected in HUVECs (C indicates absence of VF). (B) Representative zymogram of treated HUVEC medium. (C) MMP-9 levels were determined (n = 4) and analyzed statistically. **P < .001 different from VF treatment alone.
Effects of ω-3 PUFAs on MMP-9 induction in ECs. HUVECs were treated with the combination of VEGF and bFGF (VF) and AA, SDA, DHA, EPA (10 μM), or indomethacin (IND; 5 μM). MMP levels were analyzed by semiquantitative zymography (“Methods”). (A) Differences in molecular weight between pro–MMP-9 standard (92 kDa) and the active MMP-9 (87 kDa) detected in HUVECs (C indicates absence of VF). (B) Representative zymogram of treated HUVEC medium. (C) MMP-9 levels were determined (n = 4) and analyzed statistically. **P < .001 different from VF treatment alone.
The combined treatment of HUVECs with proangiogenic growth factors VEGF and bFGF increased MMP-9 levels to 1.92-fold plus or minus 0.1-fold of control (P < .001). A further increase of 21.8% (P < .01) was achieved by cotreatment with 10 μM AA (Figure 4B,C). In contrast, coincubation with the 3 ω-3 PUFAs exerted uniform suppressive effects on MMP-9 induction by VEGF and bFGF to 70.5% plus or minus 8.6% of the level effected by VEGF/bFGF (P < .001).
The COX inhibitor indomethacin (5 μM) completely suppressed MMP-9 induction by VEGF/bFGF, which suggests that prostanoids participate in the observed induction process (Figure 4B). Similarly, induction of MMP-9 by AA was completely inhibited by indomethacin, indicating dependence on bioconversion by COX for its biological activity. These findings further strengthen the argument that the effects of PUFA on angiogenesis are mediated through prostanoid synthesis.
ω-6 PUFAs, but not ω-3 PUFAs, enhance EC invasiveness
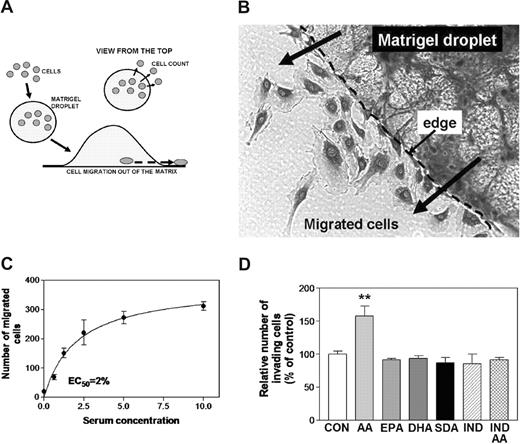
Migration of ECs toward hypoxic tissue regions is well recognized for its importance in angiogenesis. Migration of HUVECs through an artificial extracellular matrix was used to characterize the capacity of PUFA to modulate in vitro endothelial cell invasiveness. Most cell migration assays are variations on the classical Boyden chamber assay, which is poorly reproducible and difficult to quantify.26 To overcome these problems, we designed a novel “Matrigel drop” assay (depicted in Figure 5A). The cells migrating out of the droplet are counted, enabling simple and reproducible quantification. The 3-dimensional nature of this system also resembles more precisely the conditions likely to be encountered in vivo (Figure 5B). Invasion capability was dependent on serum and growth factors in the medium (Figure 5C). As in previous experimental designs, the conditions were adjusted to result in invasion that was approximately 50% of maximal (EC50). This design facilitated the quantification of PUFA effects.
Effects of ω-3 PUFAs on EC invasiveness. (A) Schematic outline of the newly developed method. ECs were mixed with Matrigel solution (1:1), and a 20-μL droplet was applied to the surface of a 6-well plate (n = 5) and incubated for 20 hours. The cells that migrated out of the droplet were counted by phase-contrast inverse microscopy. (B) A photograph of ECs that had migrated out of the Matrigel droplet. (C) Serum/growth factor dependence of EC migration. The effect of serum/growth factors on HUVEC migration was examined, and the dose-response curve is shown. The EC50 value of 2% FBS was determined by nonlinear regression. (D) Mean differences in number of invading cells that were affected by PUFAs (10 μM) and indomethacin (IND; 5 μM) were analyzed statistically. **P < .01 different from control.
Effects of ω-3 PUFAs on EC invasiveness. (A) Schematic outline of the newly developed method. ECs were mixed with Matrigel solution (1:1), and a 20-μL droplet was applied to the surface of a 6-well plate (n = 5) and incubated for 20 hours. The cells that migrated out of the droplet were counted by phase-contrast inverse microscopy. (B) A photograph of ECs that had migrated out of the Matrigel droplet. (C) Serum/growth factor dependence of EC migration. The effect of serum/growth factors on HUVEC migration was examined, and the dose-response curve is shown. The EC50 value of 2% FBS was determined by nonlinear regression. (D) Mean differences in number of invading cells that were affected by PUFAs (10 μM) and indomethacin (IND; 5 μM) were analyzed statistically. **P < .01 different from control.
In the Matrigel droplet assay, the addition of AA increased HUVEC migration by approximately 60% (Figure 5D; P < .01). In contrast, none of the ω-3 PUFAs modified HUVEC invasion potential (P > .05). Indomethacin abolished AA-stimulated, but not basal, invasiveness (Figure 5D). Again, these effects of indomethacin were not different from those of ω-3 PUFAs (P < .05). These findings suggest that, in contrast to ω-6 PUFAs, ω-3 PUFAs do not increase the invasion potential of HUVECs, and that the effects of AA are dependent on its conversion to prostanoids.
COX inhibition and ω-3 PUFAs suppress endothelial tube formation
The essential involvement of COX-derived ω-6 PUFA metabolites in the formation of blood vessel precursors (also known as endothelial tubes) is well established.27 In the present study, we estimated the mean length of EC tubes formed on thick layers of Matrigel (very thin layers do not support proper tube formation) during treatments with PUFA and indomethacin (Figure 6). As in previous experiments, the conditions were selected to give approximate 50% efficiency in tube formation. Under these conditions, AA produced a 30% increase in tube formation (P < .05), while EPA and DHA were without significant effect. Interestingly, the ω-3 PUFA with the shortest carbon chain, SDA, was found to suppress EC differentiation by 50% (Figure 6B; P < .001). In the absence of PUFA, indomethacin did not alter tube formation (P > .05), suggesting limited PUFA availability in this system (Figure 6B). After AA addition, the observed induction was suppressed by indomethacin (data not shown). Interestingly, the inhibition of tube formation by ω-3 SDA was reversed by indomethacin, which suggests that COX-dependent biotransformation of SDA is necessary for its modulation of EC differentiation.
Effects of ω-3 PUFAs on tube formation. HUVECs were seeded on Matrigel layers, exposed to the indicated treatments (n = 6), and incubated for 20 hours. (A) The formed tubes were detected by phase-contrast inverse microscopy and digital photography. (B) Average lengths of tubes in treatments with PUFAs (10 μM) and indomethacin (IND; 5 μM) were determined by analysis of digital images. *P < .05; ***P < .001 different from control.
Effects of ω-3 PUFAs on tube formation. HUVECs were seeded on Matrigel layers, exposed to the indicated treatments (n = 6), and incubated for 20 hours. (A) The formed tubes were detected by phase-contrast inverse microscopy and digital photography. (B) Average lengths of tubes in treatments with PUFAs (10 μM) and indomethacin (IND; 5 μM) were determined by analysis of digital images. *P < .05; ***P < .001 different from control.
PGE3 exhibits lower activity toward ECs
The nature of the prostanoids that modulate angiogenesis in ECs is unclear. We assessed the potential effects of the ω-6–derived PGE2 and its ω-3 EPA–derived counterpart PGE3 on Ang2 and COX-2 induction in HUVECs (Figure 7). These prostaglandins were tested at a concentration of 500 nM, which has been found previously to be effective in NIH 3T3 fibroblasts, RAW 264.7 macrophages, human lung cells, and human lymphocytes.28-30 PGE2 caused a relatively modest, but statistically significant, increase in Ang2 expression of 26% over that of control (P < .05), while the effects of PGE3 failed to reach significance (Figure 7B). Both prostaglandins influenced COX-2 expression in a similar fashion to the corresponding PUFA (Figure 3A). Thus, PGE2 effected a significant decrease (P < .001 in ANOVA followed by the Tukey test) in COX-2–immunoreactive protein to 55.2% plus or minus 10.9% of that in VEGF plus bFGF (VF)-treated cells (Figure 7C). In contrast, the slight decline in COX-2 levels effected by PGE3 treatment (15.1% ± 5.2%) was not statistically significant (P > .05).
Differential effects of ω-6 and ω-3 prostaglandins on proangiogenic processes in HUVECs. (A) Comparative structures of PGE2 and PGE3 showing the additional double bond in PGE3. (B) Representative Western blot of Ang2 analysis (n = 3). HUVECs were treated with VEGF and bFGF (VF) alone or in combination with PGE2 or PGE3 (500 nM). (C) Levels of COX-2 were determined by Western blotting (n = 3) in HUVECs that were treated as in panel B. α-tubulin levels are shown to indicate equal loading. “+” signifies Ang2- and COX-2–positive controls.
Differential effects of ω-6 and ω-3 prostaglandins on proangiogenic processes in HUVECs. (A) Comparative structures of PGE2 and PGE3 showing the additional double bond in PGE3. (B) Representative Western blot of Ang2 analysis (n = 3). HUVECs were treated with VEGF and bFGF (VF) alone or in combination with PGE2 or PGE3 (500 nM). (C) Levels of COX-2 were determined by Western blotting (n = 3) in HUVECs that were treated as in panel B. α-tubulin levels are shown to indicate equal loading. “+” signifies Ang2- and COX-2–positive controls.
Discussion
The present study investigated the modulation of proangiogenic activation of human ECs by ω-3 PUFAs. We have found that these fatty acids suppressed the induction of proangiogenic processes that were stimulated by the growth factors VEGF and bFGF and augmented by the ω-6 PUFA AA. A major mechanism involving impairment of the COX-2–mediated conversion of ω-6 PUFAs to the corresponding prostanoids, as well as the decreased biological activity of ω-3 eicosanoids, is proposed.
Major proangiogenic processes in HUVECs were stimulated by ω-6 PUFAs, but not by ω-3 PUFAs. Thus, the production of Ang2, a protein that has a central role in the angiogenic differentiation of ECs, was enhanced by the growth factors VEGF and bFGF. Expression of the important angiogenic receptors Tie-2 and VEGFR-1/2 was similarly induced. Augmentation of these effects occurred on coadministration of the ω-6 PUFA AA, but not the 3 long-chain ω-3 PUFAs EPA, DHA, and SDA.
Similar findings were made in relation to the invasion potential of ECs, another critical process related to angiogenesis. VEGF/bFGF stimulated the activity of MMP-9, an important participant in remodeling of extracellular matrix and invasion. This increase was further stimulated by cotreatment with ω-6 PUFAs, but not by the ω-3 PUFAs. These observations were substantiated using the novel Matrigel droplet assay that was established in the present study. Thus, ω-6 PUFAs enhanced the migration of ECs out of the matrix droplet, while ω-3 PUFAs were without significant effect. Taken together, these findings reveal important differences in the functional consequences of exposure of ECs to ω-6 and ω-3 PUFAs.
The present study also implicates COX-derived eicosanoids in the angiogenic effects of PUFA. Thus, cotreatment of cells with AA and the COX inhibitor indomethacin abrogated the AA-mediated increase in Ang2 and MMP-9 expression, as well as migration potential. Direct evidence for the involvement of COX-derived eicosanoids in angiogenesis was provided by the finding that PGE2 stimulated Ang2 production by ECs, whereas indomethacin decreased the extent of Ang2 and MMP-9 induction. This suggests that endogenous PUFAs may modulate the growth factor–dependent proangiogenic processes in ECs.
Our findings suggest that ω-6 and ω-3 PUFAs compete for enzymes involved in PUFA biotransformation. It is widely believed that PUFA bioconversion enzymes have a greater affinity for ω-3 PUFAs so that their biotransformation is favored when the dietary ω-3 PUFA intake is high. Such an effect could lead to the “competitive inhibition” of ω-6 PUFA metabolism by ω-3 PUFAs. In accord with this possibility, increased intake of EPA by patients has been shown to suppress the synthesis of AA-derived eicosanoids and to increase the formation of the analogous EPA-derived mediators.31 The present data suggest that, at least in the case of COX-2, the enzyme apparently has a lower, rather than higher, affinity for ω-3 PUFAs. Our findings are in accord with the mechanism proposed by Malkowski et al32 where a low rate of EPA oxygenation by COX-1 was observed and attributed to the presence of the additional double bond in EPA, such that the ω-3 PUFAs adopted a “strained” conformation in the COX-1 active site.
The observed effects could also be partially explained in terms of the relative availability of ω-6 and ω-3 PUFAs. Thus, low concentrations of ω-3 PUFAs may inhibit the formation of COX-derived mediators from endogenous ω-6 PUFAs. A more prolonged intake of dietary ω-3 PUFAs in vivo could markedly enrich tissues with ω-3 PUFAs and relatively deplete ω-6 PUFA content. These effects would be expected to occur widely and not to be restricted to ECs.
Additional evidence that supports the potential importance of the ω-6/ω-3 PUFA ratio as a determinant of the biological effects of ω-3 PUFAs comes from both animal and epidemiologic studies. These findings suggest that the ratio of these PUFA types, rather than the absolute level of ω-3 PUFAs, may be the most important factor influencing effectiveness of ω-3 PUFAs.33 Thus, supplementation of patient diet with fish oil suppressed rectal epithelial cell proliferation and PGE2 synthesis when the dietary ω-6/ω-3 ratio was 2:1 but not 4:1, even at the same absolute level of fish oil intake.34 In Japan and the Mediterranean, which are regions with reported low incidence of inflammatory conditions and the longest life expectancy, the dietary ratio of ω-6/ω-3 PUFA is about 3:1.35 In terms of the consequences for human health, it has been shown that Japanese who migrated to the United States and acquired the local dietary habits leading to an increase in the dietary ω-6/ω-3 PUFA ratio of 16:1 resulted in health problems in the migrants similar to those that already existed in the local population.35
Thus, based on our findings, we hypothesize that under conditions in which both ω-6 and ω-3 PUFAs were used in cell treatments, both types of COX-derived products could be generated but would influence angiogenesis differently. The decreased angiogenic activity of ω-3 PUFA–derived eicosanoids is consistent with our finding that PGE3, the EPA-derived eicosanoid analog of PGE2, did not markedly stimulate Ang2 induction in HUVECs in contrast to the effects observed with PGE2.
One of the interesting findings emerging from the present study was the apparent regulatory feedback effect of ω-6 PUFAs on endothelial COX-2 induction. There are several reports that have suggested that feedback regulation of COX-2 may be mediated by prostaglandins.36-39 These effects may be tissue specific. Thus, in human synovial fibroblasts, monocytes, and prostate carcinoma cells, PGE2 exhibited a positive feedback effect to enhance COX-2 expression37-39 whereas down-regulation was observed in HUVECs.36 It appears likely that the differential effects of prostanoids on COX-2 could be due to complex tissue-specific modulation of inflammatory responses. The precise effects of individual prostanoids on angiogenesis and inflammatory processes in general may well be dependent on the particular prostanoid receptor subtypes that are expressed in target tissues. In the present study, PUFA exerted differential effects on growth factor–mediated induction of COX-2 in endothelial cells. Thus, VEGF/bFGF treatment alone strongly activated COX-2 expression, which was inhibited by cotreatment with AA. In contrast, cotreatment with the ω-3 PUFAs did not exhibit significant effects on COX-2 induction. Suppression of COX-2 expression by PUFA appears to be mediated by prostanoids: the ω-6 and ω-3 PUFA–derived prostaglandins PGE2 and PGE3 impaired COX-2 expression, and indomethacin abrogated the suppressive effect of AA on growth factor–mediated induction of the enzyme.
Decreased proliferation and increased apoptosis have been reported to be the major effects of ω-3 PUFAs in cultured endothelial and other cells.15,16,40 However, some of these effects may well be mediated via lipid peroxidation and its consequent toxicity due to the high concentrations of PUFA used in these studies (100-300 μM).15,16 The presence of multiple double bonds in PUFA renders them extremely susceptible to attack by reactive oxygen species. The effective concentration of free PUFA in vivo is dependent on the concentration of extracellular fatty acid–binding proteins (predominantly albumin and lipoproteins). Once the binding capacity of these proteins has been exceeded, nonspecific toxicity becomes the dominant effect that overrides the more specific physiologic effects of PUFA. This is particularly likely to be the case in cell culture in vitro. In contrast with these considerations, PUFA levels that are achievable in vivo through dietary and/or pharmacologic manipulations are not expected to exceed the binding capacity of serum proteins, which could therefore protect membranes from indiscriminate damage elicited by lipid peroxidation. The present study was conducted using concentrations of PUFA that were closer to those found in cells. Concentrations up to 40 μM were well tolerated by cells, with 10 μM selected for routine evaluation of angiogenic differentiation. Accordingly, problems associated with nonspecific lipid peroxidation or other toxicities were avoided.
Taken together, our findings provide evidence that PUFAs (both ω-6 and ω-3) regulate angiogenesis at least in part through the action of the corresponding prostanoids. It is now apparent that a number of critical angiogenic and metastatic pathways are responsive to PUFAs and prostanoids. The data suggest that ω-3 PUFAs and their prostanoid derivatives are effective antiangiogenic regulators in comparison to their ω-6 counterparts, which stimulate the action of proangiogenic growth factors. Future studies will characterize in detail the prostanoids that mediate the effects of PUFAs on pathways of endothelial angiogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Vanja Coso and Kuan-Moon Chan for their technical assistance.
This research was supported by a Research and Development grant from the University of Sydney and by the Australian National Health and Medical Research Council.
Authorship
Contribution: M.S. performed research; M.M. analyzed and interpreted data and drafted the manuscript; and N.P. designed the study, performed research and statistical analysis, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nenad Petrovic, Pharmacogenomics and Drug Development Group, Faculty of Pharmacy, University of Sydney, Medical Foundation Building, Room 103, 92–94 Parramatta Rd, Camperdown, NSW 2006, Australia; e-mail: npetrovic@usyd.edu.au.