Abstract
NKG2D is a multisubunit activation receptor that allows natural killer (NK) cells to detect and eliminate stressed, infected, and transformed host cells. However, the chronic exposure of NK cells to cell-bound NKG2D ligands has been shown to impair NKG2D function both in vitro and in vivo. Here we have tested whether continuous NKG2D engagement selectively impacted NKG2D function or whether heterologous NK cell activation pathways were also affected. We found that sustained NKG2D engagement induced cross-tolerization of several unrelated NK cell activation receptors. We show that receptors that activate NK cells via the DAP12/KARAP and DAP10 signaling adaptors, such as murine NKG2D and Ly49D, cross-tolerize preferentially NK cell activation pathways that function independent of DAP10/12, such as antibody-dependent cell-mediated cytotoxicity and missing-self recognition. Conversely, DAP10/12-independent pathways are unable to cross-tolerize unrelated NK cell activation receptors such as NKG2D or Ly49D. These data define a class of NK cell activation receptors that can tolerize mature NK cells. The reversible suppression of the NK cells' cytolytic function probably reduces the NK cells' efficacy to control endogenous and exogenous stress yet may be needed to limit tissue damage.
Introduction
Using a number of distinct recognition strategies and receptors, natural killer (NK) cells can detect host cells that are infected or that undergo malignant transformation. A limited number of NK cell receptors in the mouse directly recognize “nonself” ligands expressed on infected cells. Ly49H recognizes mouse cytomegalovirus (MCMV) m157,1 and NKp46 has been reported to recognize influenza virus hemaglutinin.2 Further, Ly49D recognizes xenogeneic major histocompatibiliy complex (MHC) class I molecules.3
In general, however, NK cells react to alterations in the expression of endogenous “self” ligands. Accordingly, a second set of activation receptors mediates “induced-self” recognition, that is, NK cell reactions to host cells that (over-) express specific endogenous self ligands due to infection or stress. Induced-self receptors include NKG2D (which interacts with RAE1α-ϵ, H60, and MULT1),4 CD28 (B7.1),5 CD226 (Necl-5, nectin-2), and CRTAM (Necl-2).6 Additional activation receptors are specific for (not completely defined) ligands that are constitutively expressed on normal, that is, noninfected, nonstressed host cells. To prevent the lysis of normal cells, NK cells express inhibitory receptors, many of which are specific for MHC class I (MHC-I) molecules.7-9 Viral infection or transformation frequently leads to the down-regulation of MHC-I molecules, which allows diseased cells to escape recognition by cytolytic T cells.10 However, MHC-I low host cells provide insufficient NK cell inhibition signals, leading to NK cell–mediated lysis. This type of NK cell reactivity is known as “missing-self” recognition.11 Inhibitory receptors implicated in missing-self recognition include several Ly49 receptors and CD94/NKG2A (interacting with MHC-Ia and Ib, respectively),12 mouse 2B4 (CD48),13 NKRP1 (Clrb, Ocil),14,15 and KLRG1 (cadherin).16,17 Relevant activation receptors include NKp46.2 Finally, NK cells can recognize and kill antibody-coated host cells (also termed ADCC for antibody-dependent cell-mediated cytotoxicity) using the low-affinity receptor for the Fc portion of IgG (FcRγIII, CD16). Thus, NK cells use multiple distinct recognition strategies and a multitude of surface receptors to scan the expression of cell-surface molecules in order to identify diseased host cells.
In general, activating NK cell receptors form multisubunit receptor complexes, whereby specialized adaptor molecules transduce signals into the cell. A first set of adaptors used by NK cells signals via ITAMs (immunoreceptor tyrosine-based activation motifs); these are DAP12/KARAP (associated with NKG2D-S, Ly49D and Ly49H),18,19 FcRγ (CD16 [ADCC] and NK1.1)20,21 as well as CD3ζ and/or FcRγ (NKp46).22,23 In addition, NK cells also use DAP10, which lacks an ITAM but instead contains a YINM sequence that closely resembles a motif present in costimulatory molecules such as CD28.24 In NK cells, where DAP10 is associated with NKG2D, this adaptor mediates primary activating function rather than costimulation. Upon receptor engagement, the tyrosines in the ITAM or in YINM are phosphorylated by src family kinases, which generates docking sites for Syk family kinases and PI3K/Grb2, respectively.25 This mediates calcium influx and the activation of extracellular signal-regulated kinases (ERK), which are necessary events for inducing the release of cytotoxic granules and, consequently, target cell lysis.
In contrast to normal cells, where NKG2D ligands are expressed transiently, many primary tumors and established tumor cell lines express NKG2D ligands constitutively.4 Consequently, NKG2D engagement confers efficient NK cell reactivity to NKG2D ligand–expressing tumor cells.26,27 However, the sustained exposure of NKG2D with tumor cell-bound ligand in vitro results in surface modulation of the receptor and a gradual reduction of receptor function.28,29 Eventually, the NKG2D receptor is completely uncoupled from the mobilization of calcium influx and the exertion of cell-mediated cytolysis.29 Impaired receptor function correlates with a reduced association of NKG2D with the relevant signaling adaptors DAP10 and DAP12.29 The enforced constitutive expression of NKG2D ligands (Rae-1β, Rae-1ϵ or MICA) as transgenes in mice has confirmed that sustained encounter of NKG2D ligands impairs NKG2D functions in vivo.30-32
As there is evidence for functional cross-talk between certain NK cell activation receptors,33 we have addressed whether prolonged NKG2D engagement selectively impairs NKG2D function or whether unrelated NK cell activation receptors or pathways are also affected. We find that sustained NKG2D engagement cross-tolerizes several unrelated NK cell activation receptors. Indeed, DAP10/12-dependent NK cell activation pathways, such as NKG2D and Ly49D, are found to preferentially cross-tolerize DAP10/12-independent receptors or pathways. Conversely, DAP10/-12–independent receptors or pathways are unable to induce cross-tolerance. These data identify a class of NK cell activation receptors that can tolerize mature NK cells, highlighting a novel adaptive feature of innate immune effector cells.
Methods
Mice
C57BL/6 (B6) (H-2b) mice were purchased from Harlan (Horst, The Netherlands). Mice expressing truncated, nonfunctional DAP12 (DAP12ki) have been described before.34 All mice were older than 6 weeks when used for experiments.
Cell lines and transfectants
Several cell lines used here were described before.29 RMA m157 were generated following polymerase chain reaction (PCR) amplifying m157 from MCMV DNA and cloning of the product into a pEF BOS IRES puro expression vector. Stable transfectants were established after electroporation and selection with puromycin. The Chinese hamster ovary (CHO) Pro5 cell line was kindly provided by Wayne Yokoyama, Washington University, St Louis, MO.
NK cell culture
NK cell culture conditions and chronic exposure to tumor cells has been described.29 Briefly, B6 splenocytes were depleted of B and T cells using nylon wool and mAb 17A2 (anti-CD3ϵ) followed by anti–rat IgG M450 Dynabeads (Dynal, Oslo, Norway). Carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes, Eugene, OR)–labeled and irradiated (3000 rad) tumor cells were added at a 1:1 ratio to NK cells (106/mL). After 3 days of coculture in the presence of interleukin-2 (IL-2; 500 ng/mL), the remaining tumor cells were removed by lympholyte (Cedarlane Labs, Burlington, ON) density gradient centrifugation followed by Dynabead depletion using mAbs to CD3ϵ, murine leukemia virus (MuLV) glycoprotein 70 (gp70). Effector cell populations used for functional assays were 50% to 80% pure NK cells. Alternatively, NK cells were isolated from nylon wool nonadherent spleen cells using magnetic cell separation (MACS) with DX5 MicroBeads (Miltenyi Biotech, Bergisch Gladbach, Germany). Similarly, Ly49D-positive NK cells were isolated using MACS (with fluorescein isothiocyanate [FITC]–labeled 4E5 mAb followed by anti-FITC MicroBeads (Miltenyi Biotech). Ly49D-negative NK cells were subsequently isolated using DX5 MicroBeads as described above. The purity of these NK cell preparations was usually 80% to 90%.
Antibodies and flow cytometry
One million cells were incubated with mAb 2.4G2 (anti-CD16/32) to reduce background before staining with the following mAbs (obtained from BD PharMingen [San Diego, CA] unless indicated otherwise): PK136 (anti-NK1.1), anti-NKG2D (R&D Systems, Oxon, United Kingdom), 4E5 (anti-Ly49D), 3D10 (anti-Ly49H kindly provided by W. Yokoyama), 2.4G2 (anti-CD16/32, LICR). Rat anti-NKG2D and NKp46 antisera were generated using soluble NKG2D and NKp46 as the immunogen, respectively35 (L.S. and W.H., unpublished data, January 2002). Staining with antisera was revealed with FITC-conjugated goat anti–rat IgG. Flow cytometry was performed as described before.35 Intracellular interferonγ (IFNγ) detection and calcium ([Ca2+]i) mobilization was determined as described.29
Cytotoxicity assays
Standard (4 hour) 51Cr release assays were performed as described before.29 For ADCC, labeled target cells were preincubated (30 minute at 4°C) with 10 μg/mL anti-Thy1.2 mAb 30-H12 (BD PharMingen) and washed before adding effector cells. For CHO or RMA m157 killing assays, Ly49D- or Ly49H-dependent NK cell activation was blocked using anti-Ly49D (4E5) or anti-Ly49H (3D10) antibody at 10 μg mAb per million effector cells.
Immunoprecipitation and Western blot
NK cells expanded in IL-2 for 7 days were washed and lysed in ice-cold 0.2% dodecyl maltoside lysis buffer (20 mM Tris-HCl [tris(hydroxy-methyl)aminomethane] pH8.2, 100 mM NaCl, 10 mM EDTA [ethylenediaminetetraacetic acid], 50 mM NaF, 1 mM Na3VO4) and protease inhibitor cocktail (Roche, Mannheim, Germany). For immunoprecipitation, 2 μg mAbs to NKG2D (#191004; rat IgG), NK1.1 (PK136, mouse IgG), CD16 (2.4G2, rat IgG), Ly49D (4E5, mouse IgG) Ly49H (3D10, mouse IgG1), or polyclonal rat anti-NKp46 was coupled to protein G sepharose (GE Healthcare, Little Chalfont, United Kingdom) before incubation with NK cell lysates. Immunoprecipitates were separated on 16% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred onto ImmunoBlot polyvinylidenefluoride (PVDF) membranes (Bio-Rad, Hemel Hempstead, United Kingdom). Immunoblots were performed with Abs to FcRγ (Upstate Biotechnology, Charlottesville, VA), DAP1036 and DAP1236 followed by horseradish peroxidase (HRP)-coupled secondary Abs (Southern Biotechnology, Birmingham, AL) and visualization by enhanced chemiluminescence (ECL; GE Healthcare).
Results
Sustained NKG2D engagement impairs multiple NK cell activation pathways
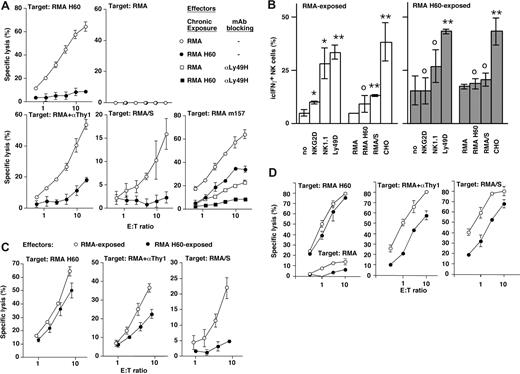
To probe the impact of prolonged NKG2D engagement, we exposed NK cells to RMA tumor cells that were transfected with the NKG2D ligand H60 (Table 1). The coculture of NK cells with parental RMA cells had no effect on NKG2D function as compared with NK cells that were cultured alone (data not shown). In contrast, the exposure of NK cells to RMA-H60 tumor cells almost completely blunted NKG2D function (Figure 1A and Coudert et al29 ). This effect required cell-cell contact and could not be induced with recombinant (soluble or immobilized) H60.29
Expression of activating NK cell receptors after chronic stimulation
| Stimulator cell . | Cognate receptor . | NK cell receptor expression . | |||||
|---|---|---|---|---|---|---|---|
| NK1.1 . | NKG2D . | CD16 . | Ly49D . | Ly49H . | Nkp46 . | ||
| RMA* | Unknown | 100# | 100 100 | 100 | 100 | 100 | 100 |
| RMA H60† | NKG2D | 95 ± 13 | 18 ± 8** | 57 ± 14 | 93 ± 14 | 95 ± 23 | 92 ± 7 |
| RMA+αThy1‡ | CD16 | 89 ± 18 | 97 ± 14 | 51 ± 17 | 106 ± 20 | 100 ± 1 | ND†† |
| RMA/S§ | Unknown | 108 ± 8 | 122 ± 17 | 95 ± 14 | 100 ± 8 | 105 ± 7 | ND |
| CHO‖ | Ly49D | 64 ± 18 | 92 ± 38 | 66 ± 15 | 41 ± 10 | 85 ± 1 | ND |
| RMA m157¶ | Ly49H | ND | ND | ND | ND | ND | ND |
| Stimulator cell . | Cognate receptor . | NK cell receptor expression . | |||||
|---|---|---|---|---|---|---|---|
| NK1.1 . | NKG2D . | CD16 . | Ly49D . | Ly49H . | Nkp46 . | ||
| RMA* | Unknown | 100# | 100 100 | 100 | 100 | 100 | 100 |
| RMA H60† | NKG2D | 95 ± 13 | 18 ± 8** | 57 ± 14 | 93 ± 14 | 95 ± 23 | 92 ± 7 |
| RMA+αThy1‡ | CD16 | 89 ± 18 | 97 ± 14 | 51 ± 17 | 106 ± 20 | 100 ± 1 | ND†† |
| RMA/S§ | Unknown | 108 ± 8 | 122 ± 17 | 95 ± 14 | 100 ± 8 | 105 ± 7 | ND |
| CHO‖ | Ly49D | 64 ± 18 | 92 ± 38 | 66 ± 15 | 41 ± 10 | 85 ± 1 | ND |
| RMA m157¶ | Ly49H | ND | ND | ND | ND | ND | ND |
RMA cells activate NK cells through unknown activation receptors. Lysis is prevented by inhibitory Ly49 receptors specific for MHC class I molecules. Consequently, RMA cells are resistant to NK cell–mediated lysis.
Transfection of RMA cells with H60 overcomes MHC-I–dependent inhibition due to an excess of NK cell activation signals through NKG2D engagement.
Coating of RMA cells with anti-Thy1 mAb (αThy1) results in ADCC through CD16 engagement.
Like the parental RMA, RMA/S cells stimulate NK cells through unknown receptors. Lysis occurs since RMA/S express very low levels of MHC-I molecules such that NK cells are not inhibited. This is known as “missing-self recognition.”
The hamster MHC-I molecule Hm1-C4 expressed on CHO cells activates NK cells through Ly49D engagement.
Transfection of RMA cells with MCMV m157 overcomes MHC-I–dependent inhibition due to an excess of NK cell activation signals through Ly49H engagement.
The mean fluorescence intensity of staining of the indicated receptor in NK cells exposed to control RMA cells was arbitrarily set to 100.
Significant deviations from 100 are indicated in bold face.
nd indicates not determined.
Chronic NKG2D stimulation impairs multiple distinct NK cell activation pathways. (A) B- and T-cell–depleted splenocytes were exposed to irradiated RMA-H60 (•) or control RMA cells (○). After 3 days of coculture, residual tumor cells were removed and the cytolytic activity of NK cells toward RMA, RMA H60, anti-Thy1 mAb (αThy1)–coated RMA (ADCC), RMA/S, and RMA m157 target cells was determined. To ensure Ly49H dependence of RMA m157 lysis, the Ly49H receptor was blocked with mAb 3H10 (□, controls; and ■ for RMA H60 exposed NK cells). The lysis curves were shifted relative to the content of NK cells in the effector cell populations as determined by flow cytometry. Data shown are representative of 3 to 4 experiments performed. (B) After 3 days of coculture with RMA H60) or control RMA cells (□), NK cells were restimulated with the indicated cell line or plastic coated mAb. Bars represent the mean percentages (± SD) of intracellular IFNγ plus NK cells in 4 to 8 independent experiments. Statistical significance of differences relative to the corresponding controls was determined using the 2-tailed Student t test: *P < .02; **P < .10; or not significantly different, P > .05. (C,D) After 3 days of coculture with either RMA (○) or RMA H60 cells (•), residual tumor cells were removed and NK cells were further cultured for 18 hours (C) or 42 hours (D) in the presence of IL-2. The cytolytic activity of these NK cells toward RMA H60 (left) anti-Thy1–coated RMA (middle) and RMA/S cells (right) was determined. The data are representative of 2 or more independent experiments. Error bars are SD.
Chronic NKG2D stimulation impairs multiple distinct NK cell activation pathways. (A) B- and T-cell–depleted splenocytes were exposed to irradiated RMA-H60 (•) or control RMA cells (○). After 3 days of coculture, residual tumor cells were removed and the cytolytic activity of NK cells toward RMA, RMA H60, anti-Thy1 mAb (αThy1)–coated RMA (ADCC), RMA/S, and RMA m157 target cells was determined. To ensure Ly49H dependence of RMA m157 lysis, the Ly49H receptor was blocked with mAb 3H10 (□, controls; and ■ for RMA H60 exposed NK cells). The lysis curves were shifted relative to the content of NK cells in the effector cell populations as determined by flow cytometry. Data shown are representative of 3 to 4 experiments performed. (B) After 3 days of coculture with RMA H60) or control RMA cells (□), NK cells were restimulated with the indicated cell line or plastic coated mAb. Bars represent the mean percentages (± SD) of intracellular IFNγ plus NK cells in 4 to 8 independent experiments. Statistical significance of differences relative to the corresponding controls was determined using the 2-tailed Student t test: *P < .02; **P < .10; or not significantly different, P > .05. (C,D) After 3 days of coculture with either RMA (○) or RMA H60 cells (•), residual tumor cells were removed and NK cells were further cultured for 18 hours (C) or 42 hours (D) in the presence of IL-2. The cytolytic activity of these NK cells toward RMA H60 (left) anti-Thy1–coated RMA (middle) and RMA/S cells (right) was determined. The data are representative of 2 or more independent experiments. Error bars are SD.
We next tested whether sustained NKG2D engagement affected the function of additional, unrelated NK cell activation pathways. To address this we used variants of the NK cell–resistant tumor cell line RMA as target cells in lysis assays (Table 1). RMA and RMA/S cells activate NK cells through unknown receptors. Unlike RMA, RMA/S cells are killed since they express low levels of MHC-I molecules and thus fail to engage inhibitory Ly49 receptors (“missing-self” recognition). Remarkably, the prolonged exposure to RMA-H60 cells prevented the NK cell–mediated lysis of RMA/S cells (Figure 1A). Coating of RMA cells with a mAb to Thy1 (αThy1) can overcome NK cell inhibition and mediates ADCC through the engagement of the receptor for the constant portion of IgG (FcRγIII) (CD16). Antibody-directed lysis of RMA cells was also defective when NK cells were previously exposed to RMA-H60 cells (Figure 1A). In contrast, we observed significant, albeit reduced, lysis of RMA cells that express the MCMV m157 protein (Figure 1A). While Ly49H-independent lysis was impaired, Ly49H-dependent lysis of RMA m157 cells was near normal (Figure 1A). This is similar to the Ly49D receptor, which also retained significant activity after prolonged NKG2D triggering.29
A substantial fraction (10%-15%) of chronically stimulated NK cells was intracellular IFNγ+ even in the absence of any restimulation.29 Restimulation through NKG2D using mAb or H60-transfected RMA cells failed to further increase the fraction of icIFNγ+ NK cells, while anti-Ly49D mAb or CHO cells readily increased IFNγ production.29 In contrast to Ly49D, restimulation using anti-NK1.1 mAb or RMA/S cells was unable to improve IFNγ production (Figure 1B). Thus, sustained NKG2D engagement results in an unexpectedly broad, yet nevertheless selective, dysfunction of several NK cell activation receptors.
Upon the separation of NK cells from RMA-H60 cells and further culture in the presence of IL-2 for 18 hours, NKG2D function made a full recovery (Figure 1C and Coudert et al29 ). Similarly, ADCC recovered significantly, albeit not completely (Figure 1C). In contrast, missing-self recognition remained defective (Figure 1C). When NK cell function was analyzed 42 hours after the removal of RMA-H60 cells, also missing-self recognition had recovered significantly, albeit not completely (Figure 1D). Thus, even though the recovery of missing self-recognition is significantly delayed, cross-tolerance eventually reverses.
Basis for defective NK cell function
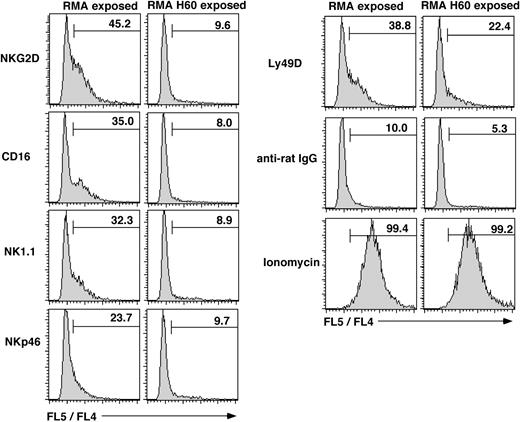
NK cell-mediated target lysis is dependent on intracellular mobilization of calcium ([Ca2+]i). Upon the cross-linking of NKG2D, control RMA exposed NK cells showed a robust ([Ca2+]i) mobilization response. In contrast, ([Ca2+]i) mobilization was essentially absent in RMA-H60–exposed NK cells (Figure 2), which is in agreement with our previous observations.29 This result may be related in part to the 3- to 5-fold reduced NKG2D levels on RMA-H60–exposed NK cells (Table 1 and Coudert et al29 ). However, RMA-H60–exposed NK cells also displayed deficient ([Ca2+]i) mobilization in response to CD16 cross-linking (Figure 2). In this case CD16 levels were near normal or maximally 2-fold reduced (Table 1 and Coudert et al29 ). Moreover, deficient ([Ca2+]i) mobilization was observed in response to NK1.1 and NKp46 cross-linking (Figure 2). In these cases receptor expression levels were normal (Table 1 and Coudert et al29 ). Notably, ([Ca2+]i) mobilization was observed upon Ly49D cross-linking although the percent of responding cells was reduced (Figure 2).29 Thus, chronic NKG2D engagement impairs ([Ca2+]i mobilization by multiple unrelated, but not all, NK cell activation receptors. These data, together with the above lysis and IFNγ assays, show that chronic NKG2D stimulation disrupts missing-self recognition and the function of the CD16 (ADCC), NK1.1, and NKp46 activation receptors. In contrast, the function of the Ly49D and Ly49H receptors is to a significant extent preserved.
Defective Ca2+ mobilization following chronic NKG2D stimulation. NK cells were loaded with Indo-1 before incubating with anti-NKG2D antiserum, anti-CD16/32 (2.4G2), anti-NK1.1 (PK136), anti-NKp46 antiserum, or anti-Ly49D (4E5) antibody. Residual B and T cells were excluded by gating on CD19 and CD3-negative cells. Data acquisition was interrupted for cross-linking the bound mAb with goat anti-rat or anti-mouse IgG. Histograms show the cumulative FL5 to FL4 fluorescence ratio over 2.5 minutes whereby the baseline is not included. Numbers depict the percentage of cells with an elevated FL5/FL4 ratio. The results are from 1 experiment of 2 performed.
Defective Ca2+ mobilization following chronic NKG2D stimulation. NK cells were loaded with Indo-1 before incubating with anti-NKG2D antiserum, anti-CD16/32 (2.4G2), anti-NK1.1 (PK136), anti-NKp46 antiserum, or anti-Ly49D (4E5) antibody. Residual B and T cells were excluded by gating on CD19 and CD3-negative cells. Data acquisition was interrupted for cross-linking the bound mAb with goat anti-rat or anti-mouse IgG. Histograms show the cumulative FL5 to FL4 fluorescence ratio over 2.5 minutes whereby the baseline is not included. Numbers depict the percentage of cells with an elevated FL5/FL4 ratio. The results are from 1 experiment of 2 performed.
The role of DAP12 for NKG2D-mediated NK cell inactivation
In short-term cytokine-stimulated NK cells, NKG2D signals via DAP12 and DAP10 adaptors.18 We addressed whether both adaptors are required for the induction of cross-tolerance. Similar to wild-type NK cells, the chronic NKG2D stimulation of NK cells that lack a functional DAP12 adaptor (DAP12ki)34 resulted in complete NKG2D dysfunction (Figure 3 and Coudert et al29 ). This indicates that DAP10 is sufficient to disable NKG2D function. In contrast, missing-self recognition and ADCC were only partly disabled when DAP12ki NK cells were chronically stimulated through NKG2D (Figure 3). This suggests that DAP10 participates in NK cell cross-tolerization. However, profound and broad NK cell inactivation via NKG2D requires that sustained NKG2D signaling occurs via both DAP10 and DAP12 adaptors.
Both DAP10 and DAP12 were required for complete NK cell inactivation. NK cells from mice lacking DAP12 function (DAP12ki) were exposed to RMA-H60 (■) or control RMA (□) cells. Effector cells were tested against RMA-H60, RMA/S, and anti-Thy1 mAb (αThy1)–coated RMA cells. As compared with wild-type controls, inactivation of ADCC and missing-self recognition in NK cells lacking DAP12 function (DAP12ki) is incomplete. The data shown are triplicate determinations from a single experiment. Error bars are SD.
Both DAP10 and DAP12 were required for complete NK cell inactivation. NK cells from mice lacking DAP12 function (DAP12ki) were exposed to RMA-H60 (■) or control RMA (□) cells. Effector cells were tested against RMA-H60, RMA/S, and anti-Thy1 mAb (αThy1)–coated RMA cells. As compared with wild-type controls, inactivation of ADCC and missing-self recognition in NK cells lacking DAP12 function (DAP12ki) is incomplete. The data shown are triplicate determinations from a single experiment. Error bars are SD.
Association of distinct NK cell activation receptors with signaling adaptors
Chronic NKG2D stimulation strongly depletes NK cells of the signaling adaptors DAP10 and DAP12 as well as CD3ζ but not of FcRγ (data not shown and Coudert et al29 ). According to the literature CD16 and NK1.1 are associated with FcRγ20,21 and thus function independent of DAP10 and DAP12. Nevertheless, CD16 and NK1.1 function (as well as missing-self recognition) is impaired following chronic NKG2D stimulation. Conversely, Ly49D and Ly49H, which share DAP12 with NKG2D, remain at least in part functional.
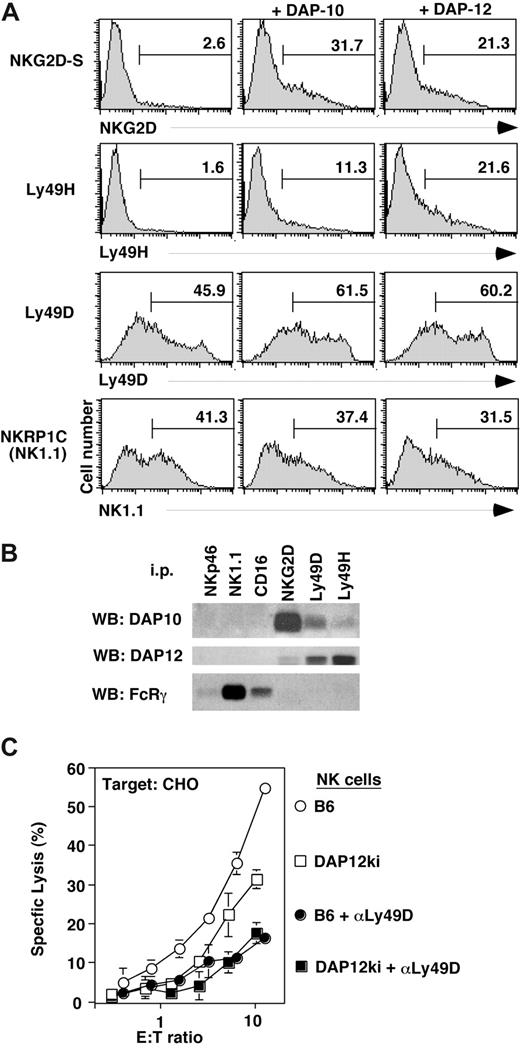
We verified some of the reported associations of activation receptors with signaling adaptors. In agreement with available data,18,37 DAP10 and DAP12 each supported the surface expression of NKG2D-S, as determined by flow cytometry of 293T cells that were transiently transfected with NKGD2-S and FLAG-tagged adaptor constructs (Figure 4A). Unexpectedly, cell surface expression of Ly49H was not only improved upon the cotransfection of DAP12 but also upon cotransfecting DAP10 (Figure 4A). Substantial Ly49D cell surface expression was observed in the absence of adaptor cotransfection. Nevertheless, similar to Ly49H, Ly49D expression further increased upon cotransfection with either DAP12 or DAP10 (Figure 4A). Corresponding improvements of cell surface expression were not apparent for NKRP1C (NK1.1) (Figure 4A), indicating that NK1.1 does neither associate with DAP10 nor DAP12.
Association of NK cell activation receptors with distinct signaling adaptors. (A) 293T cells were transiently transfected with expression vectors for NKG2D-S, Ly49H, Ly49D, and NKRP-1C (NK1.1) together with DAP10 or DAP12 adaptor constructs. Transfected cells were analyzed by flow cytometry for surface expression of the respective NK cell receptors. Data are representative of 2 to 3 independent experiments. Numbers on plots are percentages of total cells carrying the indicated cell receptor. (B) Lysates of NK cells cultured in IL-2 for 7 days were immunoprecipitated with Abs to NKG2D, Ly49D, Ly49H, CD16, NK1.1, and NKp46, separated by reducing SDS-PAGE. Associated proteins were detected by Western blot (WB) with Abs to DAP10, DAP12, and FcRγ. (C) Three days IL-2–cultured NK cells from B6 (○) or mice lacking DAP12 function (DAP12ki; □) were used as effectors against CHO cells. To ensure Ly49D dependence of CHO lysis, the Ly49D receptor was blocked with mAb 4E5 (controls •, and DAP12ki NK cells, ■). Data are representative of 2 independent experiments. Error bars are SD.
Association of NK cell activation receptors with distinct signaling adaptors. (A) 293T cells were transiently transfected with expression vectors for NKG2D-S, Ly49H, Ly49D, and NKRP-1C (NK1.1) together with DAP10 or DAP12 adaptor constructs. Transfected cells were analyzed by flow cytometry for surface expression of the respective NK cell receptors. Data are representative of 2 to 3 independent experiments. Numbers on plots are percentages of total cells carrying the indicated cell receptor. (B) Lysates of NK cells cultured in IL-2 for 7 days were immunoprecipitated with Abs to NKG2D, Ly49D, Ly49H, CD16, NK1.1, and NKp46, separated by reducing SDS-PAGE. Associated proteins were detected by Western blot (WB) with Abs to DAP10, DAP12, and FcRγ. (C) Three days IL-2–cultured NK cells from B6 (○) or mice lacking DAP12 function (DAP12ki; □) were used as effectors against CHO cells. To ensure Ly49D dependence of CHO lysis, the Ly49D receptor was blocked with mAb 4E5 (controls •, and DAP12ki NK cells, ■). Data are representative of 2 independent experiments. Error bars are SD.
In agreement with these findings, endogenous DAP12 and DAP10 did not coimmunoprecipitate with NK1.1 (or CD16 or NKp46) from lysates of (day 7) cytokine-cultured NK cells (Figure 4B). All these receptors are associated with FcRγ (Figure 4B). In contrast, endogenous FcRγ did not associate with NKG2D, Ly49D, or Ly49H. Rather, NKG2D is linked to DAP10 and to DAP12 in agreement with published data.18 (Note that NKG2D-S, which associates with DAP-12, is expressed at low levels upon prolonged culture of NK cells in IL-2.18 ) In agreement with the co-transfection experiments, Ly49D and Ly49H were not only associated with DAP12 but also with DAP10, albeit at lower levels (Figure 4B; Figure S1, available on the Bloodwebsite; see the Supplemental Materials link at the top of the online article).
Consistent with an association of DAP10 with Ly49D and Ly49H, significant receptor cell surface expression was detected on NK cells from DAP12 null mice.38 While we obtained no evidence for Ly49D function when using long-term cultured (> 6 days) NK cells from DAP12ki mice (Figure S1, in agreement with published data34,38 ), we noted significant residual Ly49D-dependent lysis of CHO cells when short-term (3-day) cultures of DAP12ki NK cells were used (Figure 4C). These data indicate that Ly49D is partially and transiently functional in the absence of DAP12. In contrast, CD16 function (Figure 3) and missing-self recognition (Figure 3)34,38 occurred entirely independent from DAP12 function.
Thus the NKG2D, Ly49D, and Ly49H receptors share the signaling adaptors DAP10 and DAP12 while they do not use FcRγ. Conversely, the CD16, NK1.1, and NKp46 receptors share FcRγ, but they do not associate with DAP10 or DAP12. Thus, chronic NKG2D stimulation impacts the function of the DAP10/12-independent CD16, NK1.1, and NKp46 receptors possibly downstream of FcRγ.
Cross-tolerization is induced via specific NK cell activation pathways
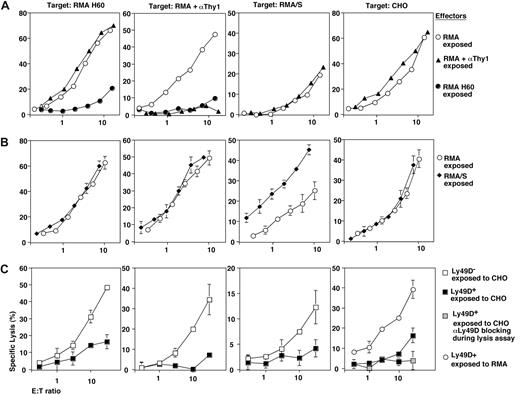
To test whether NKG2D had a unique capacity to induce broad NK cell dysfunction, we chronically stimulated mature NK cells via additional activation receptors or pathways. Sustained exposure to antibody-coated RMA cells (ADCC) prevented subsequent ADCC but left all other NK cell activation pathways unperturbed (Figure 5A). CD16 cell surface expression was reduced approximately 50% (Table 1), which is unlikely to account for a complete loss of ADCC. Prolonged exposure to RMA/S cells (missing-self recognition) had no negative effect on NK cell function. Rather, subsequent missing-self reactivity was actually somewhat increased (Figure 5B). Finally, we tested the effects of chronic Ly49D engagement. As compared with RMA controls, the exposure of purified Ly49D+ NK cells to CHO led to an almost complete loss of Ly49D function (Figure 5C). Such NK cells further showed impaired NKG2D function, defective ADCC (CD16), and missing-self recognition (Figure 5C). These defects were due to chronic Ly49D triggering, as the 3 pathways were functional in Ly49D- NK cells cocultured with CHO cells (Figure 5C). Thus, broad NK cell dysfunction can be induced via the NKG2D as well as the Ly49D receptor, which both signal through DAP10 and DAP12. In contrast, DAP10/12-independent NK cell activation pathways are not competent to induce cross-tolerance.
Selectivity of NK cell cross-tolerance induction. B6-derived NK cells were exposed to anti-Thy1 mAb (αThy1)–coated RMA cells to induce chronic ADCC (▴) (A) or exposed to MHC class I–deficient RMA/S cells (missing-self recognition, ♦) (B). Ly49D+ (■) and Ly49D- NK cells (□) were exposed to CHO cells (C). As controls, NK cells were exposed to RMA cells (○) or to RMA-H60 cells (•). After 3 days of coculture residual tumor cells were removed and the cytolytic activity of NK cells toward RMA H60, anti-Thy1 mAb (αThy1)–coated RMA, RMA/S, and CHO target cells was determined. The Ly49D-dependence of CHO lysis was ensured by blocking with anti-Ly49D mAb ( ). Error bars are SD.
). Error bars are SD.
Selectivity of NK cell cross-tolerance induction. B6-derived NK cells were exposed to anti-Thy1 mAb (αThy1)–coated RMA cells to induce chronic ADCC (▴) (A) or exposed to MHC class I–deficient RMA/S cells (missing-self recognition, ♦) (B). Ly49D+ (■) and Ly49D- NK cells (□) were exposed to CHO cells (C). As controls, NK cells were exposed to RMA cells (○) or to RMA-H60 cells (•). After 3 days of coculture residual tumor cells were removed and the cytolytic activity of NK cells toward RMA H60, anti-Thy1 mAb (αThy1)–coated RMA, RMA/S, and CHO target cells was determined. The Ly49D-dependence of CHO lysis was ensured by blocking with anti-Ly49D mAb ( ). Error bars are SD.
). Error bars are SD.
Discussion
The chronic engagement of NK cells with NKG2D ligand-expressing tumor cells in vitro and in vivo results in impaired NKG2D function. Here, we showed that sustained engagement of NKG2D could cross-tolerize a set of unrelated NK cell activation receptors. This, together with a corresponding capacity of Ly49D, suggests that DAP10/12-dependent NK cell activation pathways preferentially cross-tolerize DAP10/12-independent pathways, including ADCC and missing-self recognition. Conversely, such DAP10/12-independent pathways cannot induce cross-tolerance. Thus sustained DAP10/12 signaling seems particularly potent to tolerize mature NK cells.
Consistent with our in vitro data, it has recently been shown in vivo that enforced NKG2D ligand expression in transgenic mice was associated with impaired missing-self recognition.31 However, the experiments using transgenic mice cannot discriminate whether the effect on missing-self recognition was due to an impact on NK cell development or whether tolerance induction occurs in functionally mature NK cells. As we used mature NK cells, we conclude that peripheral NK cells are susceptible to tolerance induction. This is of importance with respect to the possible impact of NKG2D ligand-expressing tumor cells on mature NK cells in situ.
The prolonged exposure to RMA/S cells (missing-self recognition) had no negative effect on the reactivity of mature NK cells. On the contrary, subsequent missing-self reactivity was actually somewhat more efficient (Figure 5B). A similar effect was previously reported based on in vivo experiments.39 A possible explanation is that the receptors that trigger cytotoxicity in response to RMA/S target cells induce the production of cytokines, which may then act in combination with IL-2 to further enhance the cytotoxic activity of NK cells. This putative feedback loop does not seem to operate when inhibitory Ly49 NK cell receptors are co-engaged by MHC-I–expressing RMA cells. This model remains to be tested.
Despite the fact that chronic missing-self recognition did not induce tolerance in vitro, our data may nevertheless be of relevance to understand NK cell tolerance in MHC-I mosaic mice40,41 and/or in MHC-I–deficient patients42 or mice.43 In the appropriate (developmental) context in vivo, the continuous exposure to MHC-deficient host cells and thus chronic NK cell activation signals (due to a failure to receive MHC-I–dependent inhibition signals) may eventually lead to NK cell tolerance and cross-tolerance induction, as described herein.
Cross-tolerization has been noted in macrophages stimulated through Toll-like receptors (TLRs). Macrophages pretreated with the TLR-4 ligand LPS (lipopolysaccharide) show no or reduced production of inflammatory cytokines in response to a second stimulation with LPS. This is referred to as LPS or endotoxin tolerance. LPS tolerance is based at least in part on the modulation of membrane proximal cytoplasmic signal transduction such as a decrease of TLR tyrosine phosphorylation.44 Interestingly, the initial exposure of TLR-2 to lipoteichoic acid ligand induced cross-tolerance against subsequent LPS (TLR-4) or CpG DNA (TLR-9) stimulation.45,46 These data illustrate that innate immune cells such as NK cells and macrophages do not mount invariant responses to cognate receptor stimulation. Rather, the response of such cells is influenced by prior receptor stimulation. In the case of NK cell cross-tolerance, the transient shutdown of the cytolytic response of NK cells may serve to limit tissue damage during infection.
NK cells chronically stimulated through NKG2D suffer from a number of defects. NKG2D expression is reduced, and NK cells are almost devoid of the signaling adaptors DAP10 and DAP12 (as well as CD3ζ),29 which can readily account for NKG2D dysfunction. Here we determined the function of NK cell activation pathways, which signal independently of DAP10 and DAP12. CD16-mediated ADCC and [Ca2+]i flux was defective even though NK cells had normal amounts of the CD16 signaling adaptor FcRγ.20,29 Similarly, IFNγ production and ([Ca2+]i) flux induced by NK1.1 cross-linking, which similarly signals via FcRγ,21 was also defective. These data suggested that prolonged NKG2D stimulation modulates cytoplasmic signaling downstream of FcRγ. The fact that Ly49D cross-linking still triggered ([Ca2+]i) flux suggested that the defect was at or upstream of Phospholipase C (PLC)γ. Indeed, Ly49D-dependent killing is impaired in PLCγ2-deficient NK cells.47,48 Overall, the observed defects of NKG2D ligand–exposed NK cells correspond well to those of NK cells lacking the 3 guanine nucleotide exchange factors Vav-1, -2, and -3.49-51 Vav-1 is required for NK cell–mediated lysis downstream of NKG2D/DAP1051 and likely other receptors that activate PI3K. Vav-2 and -3 are required for NK cell-mediated lysis induced via DAP12 and other ITAM-bearing adaptors.51 Missing-self recognition occurs independent from ITAM-bearing adaptors but requires PI3K and src family kinase activity.35,52,53 Indeed, missing-self recognition is deficient in the absence of Vav-1 and also impaired upon chronic NKG2D engagement. Conversely, Ly49D functions in the absence of Vav-1, -2, -351 and retains partial functionality after constitutive NKG2D triggering.29 Future investigations will thus address whether vav proteins play a role in tolerance and cross-tolerance induction in NK cells.
We have also shown that chronic NKG2D stimulation reduces the cellular size and the expansion of NK cells that are cultured in IL-2,29 suggesting a connection between NKG2D and IL-2/15 receptor signaling. In agreement with these observations, while this manuscript was under revision, Horng et al54 reported a direct link between the NKG2D-DAP10 and the IL-2/15 receptor signaling pathways in NK cells. These data, together with the data shown herein, suggest that NKG2D is connected not only with a critical cytokine receptor but also with multiple distinct NK cell activation receptors or pathways. It will thus be interesting to see whether cross-tolerance induction is related to a dysfunction of the IL-2/15 receptor.
Irrespective of the molecular basis, tumor-infiltrating NK cells have been shown to exert reduced functions of NKG2D-dependent and -independent activation pathways when tested ex vivo.4 Broad NK cell tolerance can now be accounted for by the chronic stimulation of NK cells by NKG2D ligand-expressing tumor cells. To exploit NKG2D function for tumor immunity, it will be critical to identify the factors and conditions that shift NKG2D function from NK cell activation to NK cell tolerization.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Wayne Yokoyama for providing CHO Pro5 cells and anti-Ly49H mAb; to Marco Colonna for DAP10/12 antisera; and to Elena Tomasello for DAP12/KARAP ki mice.
This work was supported in part by a grant from the Swiss Cancer League (Oncosuisse) to W.H.
Authorship
Contribution: J.C. designed research, performed research, and analyzed data; L.S. and F.G. performed research and analyzed data; E.V. provided reagent and discussed data; W.H. designed research and wrote the paper.
Conflict-of-interest disclosure: E.V. is a cofounder and shareholder of Innate-Pharma. The remaining authors declare no competing financial interests.
Correspondence: Werner Held, Ludwig Institute for Cancer Research, Lausanne Branch, Ch des Boveresses 155; 1066 Epalinges; Switzerland; e-mail: werner.held@licr.unil.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal