Abstract
During immunologic synapse (IS) formation, human CD38 redistributes to the contact area of T cell–antigen-presenting cell (APC) conjugates in an antigen-dependent manner. Confocal microscopy showed that CD38 preferentially accumulated along the contact zone, whereas CD3-ζ redistributed toward the central zone of the IS. APC conjugates with human T cells or B cells transiently expressing CD38–green fluorescent protein revealed the presence of 2 distinct pools of CD38, one localized at the cell membrane and the other in recycling endosomes. Both pools were recruited to the T/APC contact sites and required antigen-pulsed APCs. The process appeared more efficient in T cells than in APCs. CD38 was actively recruited at the IS of T cells by means of Lck-mediated signals. Overexpression of CD38 in T cells increased the levels of antigen-induced intracellular calcium release. Opposite results were obtained by down-regulating surface CD38 expression by means of CD38 siRNA. CD38 blockade in influenza HA-specific T cells inhibited IL-2 and IFN-γ production, PKCθ phosphorylation at Thr538, and PKCθ recruitment to the IS induced by antigen-pulsed APCs. These results reveal a new role for CD38 in modulating antigen-mediated T-cell responses during IS formation.
Introduction
T cells interacting with antigen-presenting cells (APCs) form a specialized contact interface called immunologic synapse (IS).1 This cell-cell contact area has 2 spatially segregated regions: the central supramolecular activation complex (c-SMAC; containing the T-cell receptor [TCR], costimulatory molecules, and signaling molecules), surrounded by the peripheral SMAC (p-SMAC; enriched in adhesion molecules)
CD38 is a cell-surface molecule, highly conserved in phylogeny and not restricted to a lineage or a differentiation step. The molecule is functionally pleiotropic in that it features as an ectoenzyme and simultaneously as a receptor.2 The extracellular domain of CD38 catalyzes the synthesis of cADPR and nicotinic acid adenine dinucleotide phosphate (NAADP) from NAD+ and NADP+, respectively. cADPR is a universal second messenger that can control Ca2+ levels in an IP3-independent way. ADPR and NAADP produced by CD38 also cooperate in the regulation and modulation of intracellular Ca2+.3-6 The role of CD38 as a receptor was first identified by means of agonistic monoclonal antibodies (mAbs), which anticipated the existence of a nonsubstrate surface ligand, namely CD31/PECAM-1.7
CD38 signaling in T cells is functionally dependent on the TCR/CD3 complex,8,9 and it is initiated in a subset of membrane rafts containing CD38, Lck, and the CD3-ζ chain of the TCR.10,11 As Lck and CD3-ζ molecules are recruited to the IS upon successful T/APC conjugate formation, we hypothesized that accumulation of CD38 at the T/APC contact might occur. This contact area is also highly enriched in lipid rafts. These are used as platforms for the assembly of the TCR signaling complex and contain a number of constitutive or recruited proteins.12-14
CD38 establishes associations with distinct surface molecules, which vary according to cell lineage. In monocytes CD38 shares with HLA class II and CD9 a common activation pathway,15 and the association is implemented before monocytes interact with T lymphocytes.16 Dynamic changes in these molecular associations, such as recruitment of other surface and/or signaling molecules, may occur during the formation and establishment of the relevant synapses. Thus, binding of ICAMs to LFA-1 expressed by mature dendritic cells (DCs) induces HLA class-II clustering into the IS.17 Tetraspanins as CD81 are also dynamically redistributed during synapse formation,18 with CD38, CD83, CD11b, and CCR7 translocated to a pole of mature DCs upon mAb cross-linking.19 Therefore, it is also conceivable that CD38 in the APCs may translocate at the IS upon antigen presentation.
This study shows that CD38 is recruited to the T/APC interface, and that T cells and APCs to a lesser extent contribute to the clustering of this molecule to IS. The results also indicate that CD38 expression in T cells may contribute to modulate specific antigen-mediated early and late T-cell responses during IS formation.
Methods
Cells,20-24 antibodies, and reagents used are listed in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
CD38-GFP construct and transient transfections
CD38–green fluorescent protein (GFP) was obtained by PCR amplification of the human CD38 cDNA25 and cloned in the pEGFP-C1 vector (Clontech Laboratories, Palo Alto, CA). J77 Jurkat or Raji cells were electroporated with the pEGFP-C1-CD38 or pEGFP-C1 vectors using a Electro Cell Manipulator 600 (BTX, Harvard Apparatus, Holliston, MA). J77 Jurkat cells were nucleofected with the same vectors using a Nucleofector (Amaxa, Cologne, Germany).
Conjugate formation, immunofluorescence, and confocal microscopy
Surface molecules and intracellular proteins in T/APC conjugates were detected as described.18,20 Additional information is provided in Document S1.
Antigen-induced T-cell activation, flow cytometry, and Western blot analysis
For antigen-specific stimulation, HOM-2 were incubated for 2.5 hours at 37°C with the HA peptide (307-319; 67 μM) or left unpulsed. HOM-2 cells were then mixed with CH7C17 T cells at a ratio of 1:1, centrifuged for 1 minute at 4000 rpm in a microfuge, and incubated at 37°C for different times (1, 5, 20, and 40 minutes). Cells were then lysed and analyzed by Western blot.10,11,22 Antigen surface expression was analyzed by flow cytometry.26 The Cellquant Calibrator kit (BIOCYTEX, Marseille, France) was used for leukocyte surface antigen quantification.
GDP-ribosyl cyclase activity
Ectocellular GDP-ribosyl cyclase activity was measured in intact cells over 90 minutes at 37°C using 100 μM NGD+ as a surrogate substrate for NAD+.27
Ca2+ analysis
Changes in intracellular calcium concentrations were measured following the Alliance for Cellular Signaling Procedure Protocol PP00000211.28
Cytokine production
IFN-γ and IL-2 productions were measured by Eli-pair kits (Diaclone, Besancon, France). The Bio-Plex Precision Pro Human Cytokine 10-Plex kit assay (Bio-Rad, Hercules, CA) was used to simultaneously test 10 cytokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 (p70), IL-13, IFN-γ, and TNF-α.
RNA interference for CD38 gene silencing in J77 Jurkat cells
The small interfering RNA (siRNA) against CD38 was performed with the siGENOME ON-TARGETplus SMARTpool reagent from Dharmacon (Lafayette, CO) and transfected with the Nucleofector (Amaxa). After 48 hours, siRNA- or control-transfected cells were harvested and processed for cytofluorography and functional studies.
Results
Surface CD38 clusters at the IS
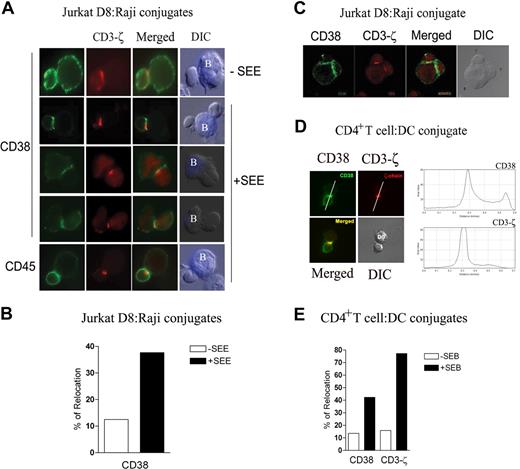
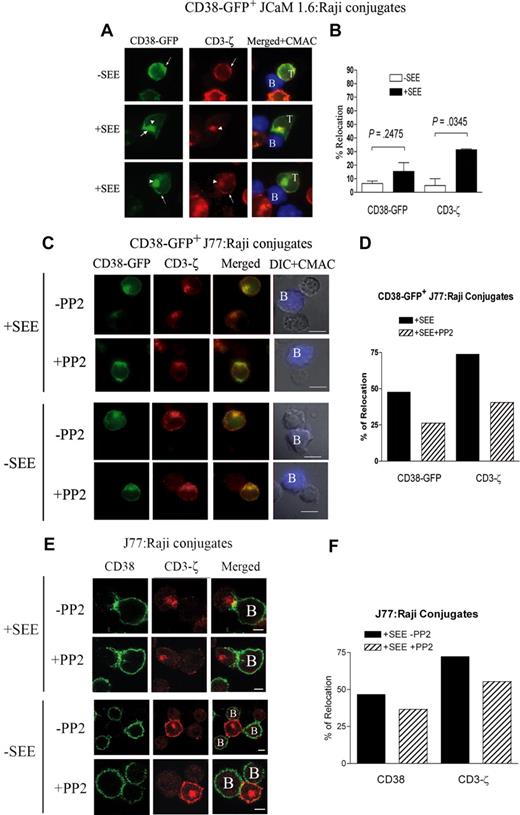
We first studied CD38 localization at the IS on a Jurkat T-cell–Raji B-cell system. Jurkat cells express the TCR Vβ8 chain able to recognize the staphylococcal enterotoxin E (SEE) bound to Raji, which acts as an APC and form conjugates similar to genuine T cell–APC pairs.20,29 The Jurkat-Raji conjugates were stained with anti-CD38 before cell permeabilization. In the absence of SEE, CD38 was evenly distributed on the cell surface of most Jurkat D8–Raji conjugates (Figure 1A top panels). In the presence of SEE, CD38 accumulated at the contact area between the T cell and the APC (Figure 1A middle panels). The pattern of CD38 redistribution was clearly different from that of CD3-ζ. CD3-ζ was detected in a small cluster at the center of the synapse, (c-SMAC), whereas CD38 was enriched along the contact zone (Figure 1A middle panels). CD45 was evenly distributed at the plasma membrane (Figure 1A bottom panels). Approximately 40% of T/APC conjugates formed in presence of SEE showed that CD38 accumulated in the contact zone, compared with 12% in controls (Figure 1B). The localization of CD38 along the T/APC contact area was confirmed by analysis of Jurkat-SEE–pulsed Raji conjugates by confocal microscopy (Figure 1C).
Surface CD38 clusters at the T/APC contact region. (A) Raji cells were labeled with CMAC blue and incubated in the presence (or absence) of SEE (1 μg/mL). Raji and Jurkat D8 cells were mixed at a 1:1 ratio, fixed, and double-stained for CD38 (green) or CD3-ζ (red; top and middle panels), or double-stained for CD45 (green) or CD3-ζ (red; bottom panels). Cells were examined using a Leica DMR photomicroscope (Mannheim, Germany; 63×/1.32-0.6 NA oil objective). (B) Percentage of Jurkat D8–Raji cells conjugates with surface CD38 redistributed at the contact zone relative to the total number of conjugates in the absence (□), or in the presence (■) of SEE. Data from 1 of 3 independent experiments, analyzing more than 80 conjugates. (C) Jurkat D8–Raji cell conjugates were generated in the presence of SEE, double-stained for CD38 (green) and CD3-ζ (red), and analyzed by a Leica TCS-SP confocal scanning laser microscope (63×/1.32-0.6 NA oil objective). Shown are the stacked pictures of 23 sections with 0.4-μm thickness. (D) T/DC conjugates were stained and examined as in panel A. CD38 and CD3-ζ staining is also shown in an intensity color-coded image, and the histogram analyzes fluorescence intensity along the line depicted on the image. (E) Percentage of T/DC conjugates with surface CD38 or total CD3-ζ redistributed at the contact zone relative to the total number of T/DC conjugates in the absence (□) or presence (■) of SEB. A total of 150 conjugates of each category were analyzed. In all panels for double staining, intact cells were incubated with the primary mAb followed by an Alexa Fluor 488–goat anti-mouse IgG, saturated with mouse serum. CD3-ζ was detected by permeabilizing cells (0.5% Triton X-100) prior staining with the anti–CD3-ζ 488 rabbit antibody, followed by the incubation with a Rhodamine Red X–labeled goat anti-rabbit IgG (H + L) highly cross-adsorbed.
Surface CD38 clusters at the T/APC contact region. (A) Raji cells were labeled with CMAC blue and incubated in the presence (or absence) of SEE (1 μg/mL). Raji and Jurkat D8 cells were mixed at a 1:1 ratio, fixed, and double-stained for CD38 (green) or CD3-ζ (red; top and middle panels), or double-stained for CD45 (green) or CD3-ζ (red; bottom panels). Cells were examined using a Leica DMR photomicroscope (Mannheim, Germany; 63×/1.32-0.6 NA oil objective). (B) Percentage of Jurkat D8–Raji cells conjugates with surface CD38 redistributed at the contact zone relative to the total number of conjugates in the absence (□), or in the presence (■) of SEE. Data from 1 of 3 independent experiments, analyzing more than 80 conjugates. (C) Jurkat D8–Raji cell conjugates were generated in the presence of SEE, double-stained for CD38 (green) and CD3-ζ (red), and analyzed by a Leica TCS-SP confocal scanning laser microscope (63×/1.32-0.6 NA oil objective). Shown are the stacked pictures of 23 sections with 0.4-μm thickness. (D) T/DC conjugates were stained and examined as in panel A. CD38 and CD3-ζ staining is also shown in an intensity color-coded image, and the histogram analyzes fluorescence intensity along the line depicted on the image. (E) Percentage of T/DC conjugates with surface CD38 or total CD3-ζ redistributed at the contact zone relative to the total number of T/DC conjugates in the absence (□) or presence (■) of SEB. A total of 150 conjugates of each category were analyzed. In all panels for double staining, intact cells were incubated with the primary mAb followed by an Alexa Fluor 488–goat anti-mouse IgG, saturated with mouse serum. CD3-ζ was detected by permeabilizing cells (0.5% Triton X-100) prior staining with the anti–CD3-ζ 488 rabbit antibody, followed by the incubation with a Rhodamine Red X–labeled goat anti-rabbit IgG (H + L) highly cross-adsorbed.
CD38 localization was analyzed in other T/APC models, such as polyclonal SEB-specific T cells interacting with monocyte-derived mature DCs. Results indicate that CD38 redistributed along the region of T cells contacting with DCs pulsed with SEB. The presence of CD3-ζ was used to identify cell conjugates forming IS (Figure 1D). The quantitation of CD38 and CD3-ζ relocation at the synapse is shown in Figure 1E.
Dynamics of plasma membrane and intracellular CD38-GFP during IS formation
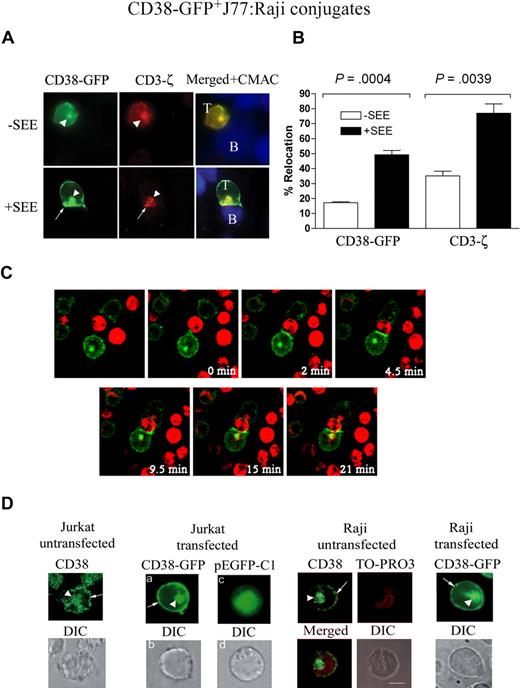
The observed redistribution of CD38 at the interface between Jurkat and Raji cells (both surface CD38+) suggested to investigate the origin of the molecule seen at the IS. J77 Jurkat cells were transfected with a plasmid encoding a fusion protein of full-length CD38 with the GFP (CD38-GFP). The reorientation of the CD38-GFP+ toward the cell-cell contacts was in T/APC conjugates. Results indicated the existence of 2 distinct pools of CD38-GFP, one localized at the membrane and the other in an intracellular compartment, colocalized with endogenous CD3-ζ (Figure 2A). The intracellular pool was not an artifact due to overexpression of the CD38-GFP construct: indeed, the same intracellular pool was observed in untransfected J77 Jurkat cells permeabilized before CD38 staining (Figure 2D). Distinct intracellular pools were also detected in CD38-GFP–transfected Raji cells, or in untransfected Raji cells permeabilized before CD38 staining (Figure 2D). Reorientation of the intracellular CD38-GFP+ in J77 Jurkat cells toward the cell-cell contact zone (polarization or recruitment) occurred in most cases in which surface CD38-GFP clustered at the same zone (clustering). In some experiments, intracellular CD38 appeared fused with membrane CD38: this was more evident in conjugates of B cells pulsed with SEE (Figure 2A bottom panels). CD38 was recruited at the T/APC contact zone in 17% plus or minus 0.6% of the conjugates formed with CD38-GFP+ J77 and Raji cells not pulsed with SEE (Figure 2B). CD38 redistribution at the T/APC contact zone was increased in the presence of SEE (49% ± 2.9%; P < .001).
TCR-dependent clustering and polarization of CD38-GFP from T cells at the T/APC contact zone. (A) Jurkat J77 cells transiently expressing CD38-GFP were allowed to conjugate with CMAC-labeled Raji cells pulsed or not pulsed with SEE. Then, cells were fixed, permeabilized, and stained for CD3-ζ as described in Figure 1 (red). Cells were examined using an Olympus Cell R IX81 motorized system inverted microscope (Tokyo, Japan; 100×/1.3 NA oil objective). The white arrowheads point to the intracellular CD38-GFP or CD3-ζ. The arrows point to the plasma membrane CD38-GFP or CD3-ζ accumulated at the synapse. (B) Comparative analysis of the percentage of conjugates with CD38-GFP or endogenous CD3-ζ redistributed at the contact zone relative to the total number of CD38-GFP+ J77/Raji cell conjugates in the absence (□) or presence (■) of SEE. The data represent the mean plus SEM of 3 independent experiments. In each experiment, 50 to 70 conjugates were analyzed. (C) Jurkat J77 cells transiently transfected with CD38-GFP were seeded onto FN-coated coverslips. Raji cells labeled in red with the probe CM-TMR were incubated with SEE and added to the chambers. Cells were monitored by time-lapse confocal microscopy at 30-second intervals. Representative images of one T cell–SEE-pulsed APC conjugate are shown. Each time point shows the fluorescence images of Raji cells (red) and CD38-GFP fluorescence (green). (D) Endogenous CD38 distribution in untransfected permeabilized Jurkat J77 T cells (left panel) or untransfected permeabilized Raji B cells (third panel) was compared with CD38-GFP distribution in their respective transfected counterparts (second and fourth panels, respectively). The white arrowheads point to the intracellular CD38 or CD38-GFP. The arrows point to the plasma membrane CD38 or CD38-GFP. CD38-GFP distribution in transfected Jurkat cells differs greatly from the GFP distribution, which is largely cytosolic (second panel). Moreover, intracellular CD38 staining in untransfected Raji B cells does not colocalize with TO-PRO3, which is specific for nuclear staining (third panel). Cells were examined using an Olympus Cell R IX81 motorized system inverted microscope (100×/1.3 NA oil objective).
TCR-dependent clustering and polarization of CD38-GFP from T cells at the T/APC contact zone. (A) Jurkat J77 cells transiently expressing CD38-GFP were allowed to conjugate with CMAC-labeled Raji cells pulsed or not pulsed with SEE. Then, cells were fixed, permeabilized, and stained for CD3-ζ as described in Figure 1 (red). Cells were examined using an Olympus Cell R IX81 motorized system inverted microscope (Tokyo, Japan; 100×/1.3 NA oil objective). The white arrowheads point to the intracellular CD38-GFP or CD3-ζ. The arrows point to the plasma membrane CD38-GFP or CD3-ζ accumulated at the synapse. (B) Comparative analysis of the percentage of conjugates with CD38-GFP or endogenous CD3-ζ redistributed at the contact zone relative to the total number of CD38-GFP+ J77/Raji cell conjugates in the absence (□) or presence (■) of SEE. The data represent the mean plus SEM of 3 independent experiments. In each experiment, 50 to 70 conjugates were analyzed. (C) Jurkat J77 cells transiently transfected with CD38-GFP were seeded onto FN-coated coverslips. Raji cells labeled in red with the probe CM-TMR were incubated with SEE and added to the chambers. Cells were monitored by time-lapse confocal microscopy at 30-second intervals. Representative images of one T cell–SEE-pulsed APC conjugate are shown. Each time point shows the fluorescence images of Raji cells (red) and CD38-GFP fluorescence (green). (D) Endogenous CD38 distribution in untransfected permeabilized Jurkat J77 T cells (left panel) or untransfected permeabilized Raji B cells (third panel) was compared with CD38-GFP distribution in their respective transfected counterparts (second and fourth panels, respectively). The white arrowheads point to the intracellular CD38 or CD38-GFP. The arrows point to the plasma membrane CD38 or CD38-GFP. CD38-GFP distribution in transfected Jurkat cells differs greatly from the GFP distribution, which is largely cytosolic (second panel). Moreover, intracellular CD38 staining in untransfected Raji B cells does not colocalize with TO-PRO3, which is specific for nuclear staining (third panel). Cells were examined using an Olympus Cell R IX81 motorized system inverted microscope (100×/1.3 NA oil objective).
Redistribution of CD3-ζ (plasma membrane and intracellular) was assessed as a positive control for mature IS formation in the presence of SEE (Figure 2A). Intracellular CD3-ζ is present in recycling endosomes and to lesser extent in the Golgi apparatus.29,30 CD3-ζ redistributed in 35% plus or minus 3% of conjugates in the absence of SEE. The presence of SEE induced an increase up to 77% plus or minus 6% of T/APC conjugates (Figure 2B; P = .004). The intracellular pool of CD3-ζ redistributed toward the cell-cell contact zone in an almost identical manner as CD38-GFP (Figure 2A).
Recruitment of the CD38-GFP protein at the contact zone between live T cells and SEE-pulsed Raji APCs was analyzed by time-lapse confocal microscopy. Clustering of surface CD38-GFP at the T/APC interface occurred within the first 2 minutes and persisted for 20 to 25 minutes (Figure 2C; Video S1). Polarization of intracellular CD38-GFP to the vicinity of the APC was slower (Figure 2C; Video S1).
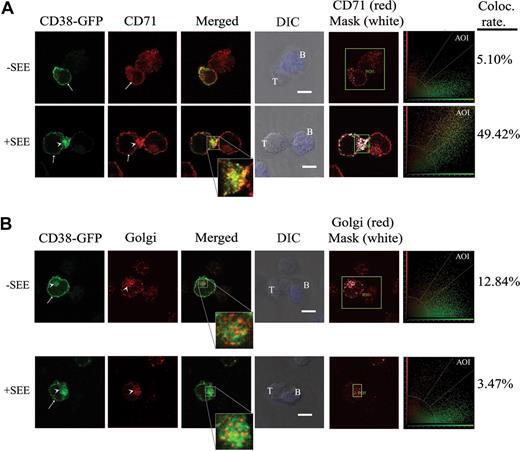
The transferrin receptor (CD71) is present on the plasma membrane and in recycling endosomes.31 In T/APC conjugates, the T-cell CD71 endosomal pool relocates beneath the contact site, whereas surface CD71 localizes to the peripheral ring of the IS.30,31 In the absence of SEE, CD71 and CD38-GFP were evenly distributed at the cell surface (Figure 3A top panels, arrows). Jurkat cells conjugated with SEE-pulsed APCs displayed a significant accumulation of CD38-GFP to the contact site, overlapping with the massive clustering of CD71 to the same area (Figure 3A bottom panels, arrowheads). In most T/SEE-pulsed APC conjugates, membrane and endosomal CD71 were in proximity and difficult to discriminate. The merged images showed that most intracellular CD38-GFP (green) and CD71 (red) colocalize (yellow) in the same endosome-like vesicles (see also Figure 3A inset). These events were quantitatively analyzed by means of a colocalization scatter plot and by generating a mask of the area of interest (AOI; Figure 3 right panels). Significantly different was the behavior of B cells, where neither surface nor intracellular CD71 moved toward the T/APC contact zone.
CD38-GFP in recycling endosomes and in the Golgi is targeted to the T/APC contact zone. (A) CD38-GFP–transfected J77 cells were incubated with Raji B cells pulsed with medium alone (top) or with SEE (bottom). Cells were then fixed, permeabilized, and stained with anti-CD71 (TfR) mAb followed by a goat anti-mouse Rhodamine Red X–second antibody. Samples were analyzed by a Leica TCS-SP5 confocal scanning laser microscope. Single confocal sections were taken of cells using fluorescence in GFP and rhodamine channels. Bars represent 5 μm for all panels (63×/1.4 NA oil objective). The white arrowheads point to intracellular CD38-GFP. The arrows point to the plasma membrane CD38-GFP or CD71. (B) CD38-GFP–transfected J77 cells were treated as in panel A except that after fixation and permeabilization, cells were stained with an anti-Golgi mAb followed by a goat anti-mouse Rhodamine Red X–second antibody. Samples were analyzed by confocal microscopy as in panel A. The white arrowheads point to the intracellular CD38-GFP, or the position of the Golgi apparatus. The arrows point to the plasma membrane CD38-GFP accumulated at the synapse. The acquired CD38-GFP (green), Golgi (red), the merge images, close-ups of some merge images, and DIC images are shown. A semiquantitative assessment of the level of colocalization was done by analyzing the colocalization scatter plot and generating a mask of the area of interest (AOI; right panels).
CD38-GFP in recycling endosomes and in the Golgi is targeted to the T/APC contact zone. (A) CD38-GFP–transfected J77 cells were incubated with Raji B cells pulsed with medium alone (top) or with SEE (bottom). Cells were then fixed, permeabilized, and stained with anti-CD71 (TfR) mAb followed by a goat anti-mouse Rhodamine Red X–second antibody. Samples were analyzed by a Leica TCS-SP5 confocal scanning laser microscope. Single confocal sections were taken of cells using fluorescence in GFP and rhodamine channels. Bars represent 5 μm for all panels (63×/1.4 NA oil objective). The white arrowheads point to intracellular CD38-GFP. The arrows point to the plasma membrane CD38-GFP or CD71. (B) CD38-GFP–transfected J77 cells were treated as in panel A except that after fixation and permeabilization, cells were stained with an anti-Golgi mAb followed by a goat anti-mouse Rhodamine Red X–second antibody. Samples were analyzed by confocal microscopy as in panel A. The white arrowheads point to the intracellular CD38-GFP, or the position of the Golgi apparatus. The arrows point to the plasma membrane CD38-GFP accumulated at the synapse. The acquired CD38-GFP (green), Golgi (red), the merge images, close-ups of some merge images, and DIC images are shown. A semiquantitative assessment of the level of colocalization was done by analyzing the colocalization scatter plot and generating a mask of the area of interest (AOI; right panels).
The intracellular CD38-GFP localization in the absence of superantigen was only partly overlapping with the anti-Golgi staining and far away from the T/APC contact zone (Figure 3B top panels, arrowheads). Images obtained by merging CD38-GFP and Golgi indicated that most intracellular CD38-GFP is immediately adjacent but distinct from the Golgi apparatus. Most T cells exhibited Golgi polarization toward the APCs in the presence of SEE superantigen, although only partial overlapping with the intracellular CD38-GFP pool was observed (Figure 3B bottom panels; see also the inset and colocalization masks). Other sources (eg, nucleus) cannot be excluded.
Defective CD38 clustering at the IS in Lck-deficient Jurkat cells
The role of Lck in TCR-mediated CD38 clustering at the IS was studied by means of JCaM1.6, a Jurkat clone deficient in Lck activity. Conjugates between JCaM1.6 and Raji B cells formed in the absence of SEE showed clustering of CD38-GFP (as well as CD3-ζ) toward the T/APC contact zone greatly reduced as compared with wild-type J77/APC conjugates (Figure 4B vs Figure 2B). However, in conjugates with APCs pulsed by SEE the accumulation of CD38-GFP at the IS as well as recruitment of endogenous CD3-ζ to the c-SMAC increased 2- and 6-fold over the basal levels, respectively (Figure 4B). Polarization of the intracellular CD38-GFP (and endogenous CD3-ζ) toward the APCs was detected in most of conjugates, in which clustering of surface CD38-GFP or CD3-ζ occurred (Figure 4A middle panels). However, reorientation of the intracellular pools of CD38-GFP and CD3-ζ without apparent clustering of their corresponding surface counterparts was also observed (Figure 4A bottom panels). Therefore, the formation of mature IS and translocation of surface CD38 at the T/APC contact zone occur in Lck-deficient cells, even if the efficiency is lower than in wild-type J77 cells.
Defective TCR-induced CD38 clustering at the immunologic synapse in a Lck-deficient T-cell line. (A) JCaM1.6 T cells were transiently transfected with the CD38-GFP construct and allowed to conjugate with CMAC-labeled Raji cells pulsed or not pulsed with SEE. Then, cells were fixed, permeabilized, and stained for CD3-ζ (red) as described in Figure 1. Cells were examined using an Olympus Cell R IX81 motorized system inverted microscope (100×/1.3 NA oil objective). CD38-GFP (green), CD3-ζ (red), and the green fluorescence merged with red fluorescence images superimposed on blue fluorescence images from CMAC-loaded Raji cells are shown. The white arrowheads point to the intracellular CD38-GFP or CD3-ζ. The arrows point to the plasma membrane CD38-GFP or CD3-ζ. (B) Comparative analysis of the percentage of conjugates with CD38-GFP or endogenous CD3-ζ redistributed at the contact zone relative to the total number of CD38-GFP+ JCaM 1.6/Raji cell conjugates in the absence (□) or presence (■) of SEE. The data represent the mean plus SEM of 3 independent experiments. In each experiment, 50 to 70 conjugates were analyzed. (C) Jurkat J77 T cells transiently transfected with the CD38-GFP construct, untreated or treated with PP2 (10 μM), were stimulated with CMAC-labeled Raji B cells pulsed or not pulsed with SEE, stained with anti–CD3-ζ (red), and analyzed as in panel A. (D) Comparative analysis of the percentage of conjugates with CD38-GFP or endogenous CD3-ζ redistributed at the contact zone relative to the total number of CD38-GFP+ J77/SEE-pulsed Raji conjugates in the absence (■) or presence of PP2 (▨). In each experiment, more than 40 conjugates were analyzed. (E) Jurkat J77 T cells treated or untreated with PP2 (10 μM) were stimulated with CMAC-labeled Raji B cells pulsed or not pulsed with SEE, stained with anti-CD38 (green) and anti-CD3-ζ (red), and analyzed by a Leica TCS-SP5 confocal scanning laser microscope (63×/1.4 NA oil objective); scale bar equals 4 μm. In this panel, intact cells were incubated with the anti-CD38 mAb HB136 followed by an Alexa Fluor 488–goat anti-mouse IgG, saturated with mouse serum. CD3-ζ was detected by permeabilizing cells (0.5% Triton X-100) prior staining with the anti–CD3-ζ 488 rabbit antibody, followed by the incubation with a Rhodamine Red X–labeled goat anti-rabbit IgG (H + L) highly cross-adsorbed. (F) Comparative analysis of the percentage of conjugates with endogenous CD38 or CD3-ζ redistributed at the contact zone relative to the total number of J77/SEE-pulsed Raji cell conjugates in the absence (■) or presence of PP2 (▨). Data representative of 2 independent experiments. In each experiment, more than 100 conjugates were analyzed.
Defective TCR-induced CD38 clustering at the immunologic synapse in a Lck-deficient T-cell line. (A) JCaM1.6 T cells were transiently transfected with the CD38-GFP construct and allowed to conjugate with CMAC-labeled Raji cells pulsed or not pulsed with SEE. Then, cells were fixed, permeabilized, and stained for CD3-ζ (red) as described in Figure 1. Cells were examined using an Olympus Cell R IX81 motorized system inverted microscope (100×/1.3 NA oil objective). CD38-GFP (green), CD3-ζ (red), and the green fluorescence merged with red fluorescence images superimposed on blue fluorescence images from CMAC-loaded Raji cells are shown. The white arrowheads point to the intracellular CD38-GFP or CD3-ζ. The arrows point to the plasma membrane CD38-GFP or CD3-ζ. (B) Comparative analysis of the percentage of conjugates with CD38-GFP or endogenous CD3-ζ redistributed at the contact zone relative to the total number of CD38-GFP+ JCaM 1.6/Raji cell conjugates in the absence (□) or presence (■) of SEE. The data represent the mean plus SEM of 3 independent experiments. In each experiment, 50 to 70 conjugates were analyzed. (C) Jurkat J77 T cells transiently transfected with the CD38-GFP construct, untreated or treated with PP2 (10 μM), were stimulated with CMAC-labeled Raji B cells pulsed or not pulsed with SEE, stained with anti–CD3-ζ (red), and analyzed as in panel A. (D) Comparative analysis of the percentage of conjugates with CD38-GFP or endogenous CD3-ζ redistributed at the contact zone relative to the total number of CD38-GFP+ J77/SEE-pulsed Raji conjugates in the absence (■) or presence of PP2 (▨). In each experiment, more than 40 conjugates were analyzed. (E) Jurkat J77 T cells treated or untreated with PP2 (10 μM) were stimulated with CMAC-labeled Raji B cells pulsed or not pulsed with SEE, stained with anti-CD38 (green) and anti-CD3-ζ (red), and analyzed by a Leica TCS-SP5 confocal scanning laser microscope (63×/1.4 NA oil objective); scale bar equals 4 μm. In this panel, intact cells were incubated with the anti-CD38 mAb HB136 followed by an Alexa Fluor 488–goat anti-mouse IgG, saturated with mouse serum. CD3-ζ was detected by permeabilizing cells (0.5% Triton X-100) prior staining with the anti–CD3-ζ 488 rabbit antibody, followed by the incubation with a Rhodamine Red X–labeled goat anti-rabbit IgG (H + L) highly cross-adsorbed. (F) Comparative analysis of the percentage of conjugates with endogenous CD38 or CD3-ζ redistributed at the contact zone relative to the total number of J77/SEE-pulsed Raji cell conjugates in the absence (■) or presence of PP2 (▨). Data representative of 2 independent experiments. In each experiment, more than 100 conjugates were analyzed.
Src-family protein tyrosine kinases are required for CD38 relocalization: indeed, the number of conjugates showing accumulation of CD38 at the IS displayed was lower either in CD38-GFP–transfected J77 Jurkat cells, or in untransfected J77 cells treated with the specific inhibitor PP2 (Figure 4C,E, respectively).
TCR-dependent CD38 clustering from B cells and CD31 from T cells at the T/APC contact zone
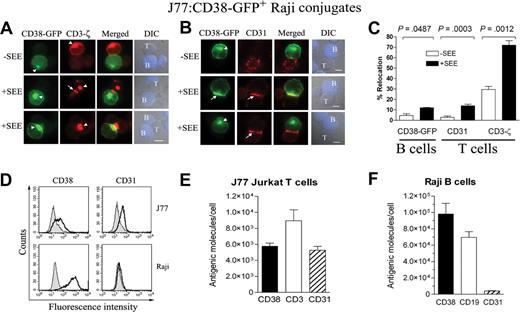
Raji cells were transiently transfected with CD38-GFP and assessed for their ability to cluster CD38 at the contact zone with T cells (Figure 5A,C). Only 4.6% plus or minus 1.6% of transfected Raji cells showed translocation of CD38-GFP to the B-cell contact area in absence of SEE. This increased 2.6-fold in the presence of SEE (12% ± 0.3% of conjugates; P = .048). Translocation of CD3-ζ at the T-cell side of IS occurred in 72% of the conjugates (Figure 5C), indicating a normal synapse formation and maturation.
TCR-dependent clustering of CD38 from B cells and CD31 from T cells at the T/APC contact zone. (A) Raji cells transiently transfected with the CD38-GFP construct were pulsed or not with SEE and allowed to conjugate with Jurkat J77 T cells. Then, cells were fixed, permeabilized, and stained for CD3-ζ (red). Cells were examined using an Olympus Cell R IX81 motorized system inverted microscope. CD38-GFP (green; 100×/1.3 NA oil objective). Bars represent 5 μm for all panels. CD3-ζ (red), green fluorescence merged with red fluorescence images, and the corresponding DIC images superimposed on blue fluorescence images from CMAC-loaded Raji cells are shown. The white arrowheads point to the intracellular CD38-GFP or CD3-ζ. The arrows point to the plasma membrane CD3-ζ accumulated at the synapse. (B) CD38-GFP–transfected Raji B cells were treated as in panel A except that after permeabilization, cells were stained for CD31 (red). CD38-GFP (green), CD31 (red), green fluorescence merged with red fluorescence images, and the corresponding DIC images superimposed on blue fluorescence images from CMAC-loaded Raji cells are shown. The white arrowheads point to the intracellular CD38-GFP. The arrows point to the plasma membrane CD38-GFP or CD31 accumulated at the synapse. (C) Comparative analysis of the percentage of conjugates with CD38-GFP (in B cells), CD31 (in T cells), or CD3-ζ (in T cells) redistributed at the contact zone relative to the total number of J77/CD38-GFP+ Raji cell conjugates in the absence (□) or presence (■) of SEE. The data represent the mean plus SEM of 3 independent experiments. In each experiment, 40 to 70 conjugates were analyzed. (D) FACS analysis of Jurkat J77 cells (top) or Raji B cells (bottom). Cells were stained with specific antibodies against CD38 (left) or anti-CD31 (right). (E) Quantification of the number of CD38, CD3, or CD31 molecules in Jurkat J77 T cells by using the Cellquant Calibrator kit. (F) Quantification of the number of CD38, CD19, and CD31 molecules in Raji B cells using the same kit as in panel E. Data in panels E and F represent the mean plus or minus SEM of 5 independent experiments.
TCR-dependent clustering of CD38 from B cells and CD31 from T cells at the T/APC contact zone. (A) Raji cells transiently transfected with the CD38-GFP construct were pulsed or not with SEE and allowed to conjugate with Jurkat J77 T cells. Then, cells were fixed, permeabilized, and stained for CD3-ζ (red). Cells were examined using an Olympus Cell R IX81 motorized system inverted microscope. CD38-GFP (green; 100×/1.3 NA oil objective). Bars represent 5 μm for all panels. CD3-ζ (red), green fluorescence merged with red fluorescence images, and the corresponding DIC images superimposed on blue fluorescence images from CMAC-loaded Raji cells are shown. The white arrowheads point to the intracellular CD38-GFP or CD3-ζ. The arrows point to the plasma membrane CD3-ζ accumulated at the synapse. (B) CD38-GFP–transfected Raji B cells were treated as in panel A except that after permeabilization, cells were stained for CD31 (red). CD38-GFP (green), CD31 (red), green fluorescence merged with red fluorescence images, and the corresponding DIC images superimposed on blue fluorescence images from CMAC-loaded Raji cells are shown. The white arrowheads point to the intracellular CD38-GFP. The arrows point to the plasma membrane CD38-GFP or CD31 accumulated at the synapse. (C) Comparative analysis of the percentage of conjugates with CD38-GFP (in B cells), CD31 (in T cells), or CD3-ζ (in T cells) redistributed at the contact zone relative to the total number of J77/CD38-GFP+ Raji cell conjugates in the absence (□) or presence (■) of SEE. The data represent the mean plus SEM of 3 independent experiments. In each experiment, 40 to 70 conjugates were analyzed. (D) FACS analysis of Jurkat J77 cells (top) or Raji B cells (bottom). Cells were stained with specific antibodies against CD38 (left) or anti-CD31 (right). (E) Quantification of the number of CD38, CD3, or CD31 molecules in Jurkat J77 T cells by using the Cellquant Calibrator kit. (F) Quantification of the number of CD38, CD19, and CD31 molecules in Raji B cells using the same kit as in panel E. Data in panels E and F represent the mean plus or minus SEM of 5 independent experiments.
Jurkat T cells express CD31, a CD38 nonsubstrate ligand.7 We then tested whether CD38-GFP recruited from B cells colocalized with CD31 from the opposing T cells. CD38-GFP–transfected B cells were pulsed with SEE and incubated with Jurkat J77 cells. 2.8% plus or minus 1.3% of CD38-GFP+ conjugates formed in the absence of SEE showed translocation of CD31 to the IS (Figure 5B,C). A simultaneous clustering of CD38-GFP from the APCs was not observed in these conjugates (not shown). However, the SEE pulse was paralleled by CD31 recruitment to the T/APC contact zone, which increased 5-fold up to 14% plus or minus 1.6% of the CD38-GFP+ conjugates (P < .001). Approximately 40% of these conjugates showed colocalization of CD38-GFP with CD31 from the T cells (Figure 5B,C). This observation suggests that receptor-ligand interactions induce an active recruitment.
The number of CD38 and CD31 surface sites per cell levels was calculated in Jurkat J77 and Raji cells by means of the Cellquant Calibrator Kit (Figure 5D-F). The number of CD38 sites on Raji (98 108 ± 12 940; n = 5) was significantly higher than the number of CD31 sites on Jurkat J77 (5261 ± 474; n = 5). Assuming that translocation of CD38 on B cells and CD31 on T cells is driven by receptor-ligand interactions, the observed differences in surface expression of the 2 molecules could explain the relative inefficiency of the process in B cells. The occurrence of CD31 recruitment to IS without the concomitant translocation of CD38-GFP (Figure 5B bottom panels) suggests the existence of alternative mechanisms for CD31 clustering to the T/APC contact zone.
Effect of CD38 overexpression in T-cell function
The redistribution of membrane CD38 at the IS suggests that its enzymatic activities may be topologically regulated on the T cell. To assess whether the ADP-ribosyl cyclase activity of CD38 could be affected by conjugate formation and/or CD38 translocation to the IS, J77 Jurkat cells were transfected with CD38-GFP (or GFP as a control) by nucleofection. At 24 hours after transfection, cells were exposed to SEE-pulsed Raji cells with a brief centrifugation to favor conjugate formation. Ectocellular GDP-ribosyl cyclase activity was continuously measured at 37°C using NGD+ as a surrogate substrate for NAD+.27 During the experiment, T/APC conjugates (J77 CD38-GFP+ T cells–Raji B cells) showed an enzymatic activity higher than the sum of the activities of the individual cells. This was more apparent during the first 20 minutes of incubation with NGD+, since the individual cells (J77 CD38-GFP+ or Raji cells alone) did not show any detectable enzymatic activity (Figure 6A). This finding occurred with either SEE-pulsed or unpulsed Raji cells.
Effect of CD38 overexpression in T-cell function. (A,B) Jurkat J77 cells were transiently transfected with the pEGFP-C1 or CD38-GFP constructs by nucleofection. At 24 hours, after transfection cells were subjected to Histopaque-1077 centrifugation and then left alone, or mixed with SEE-pulsed or unpulsed Raji B cells. After a brief centrifugation to favor conjugate formation, ectocellular GDP-ribosyl cyclase activity was continuously measured at 37°C using NGD+ as a substrate. The cGDPR production in fluorescence units was plotted against time (minutes). ● indicates J77 CD38-GFP+ T cells incubated with SEE-pulsed Raji B cells; ○, J77 CD38-GFP+ T cells incubated with unpulsed Raji B cells; ■, Raji B cells alone; ♦, J77 CD38-GFP+ T cells alone; ▾, J77 pEGFP-C1+ T cells incubated with SEE-pulsed Raji B cells; ◇, J77 pEGFP-C1+ T cells incubated with unpulsed Raji B cells; and ▴, J77 pEGFP-C1+ T cells. Arrows indicate the 20-minute time frame during which individual cells showed negligible enzymatic activity. (C) Ag-induced Ca2+ released in J77 CD38-GFP+ T cells (● and ○) and J77 pEGFP-C1+ T cells (▾ and ▿). Fura-2/AM–loaded J77 CD38-GFP+ or J77 pEGFP-C1+ T cells were left alone (♦ and ▴, respectively), or mixed with SEE-pulsed (● and ▾, respectively) or unpulsed (○ and ▿, respectively) Raji B cells. [Ca2+]i changes were measured using a fluorescence plate reader, as described in “Methods.” Characteristic tracings are shown (mean of 3 independent experiments). (D) CD38-GFP–transfected or GFP-transfected Jurkat T cells were stimulated with Raji B cells pulsed with different amounts of SEE. Supernatants were collected after overnight incubation and were assayed for IL-2 by enzyme-linked immunosorbent assay (ELISA). Representative experiment out of 3 independent experiments performed in triplicate (mean ± SEM). (E) CD38 expression by fluorescence-activated cell sorter (FACS) in oligo control–transfected or CD38 siRNA-transfected Jurkat T cells 48 hours after nucleofection. Cells were stained with PE-conjugated anti-CD38 (FL2). The number in each quadrant represents the percentage of cells within each region relative to all viable cells. (F) T cells were then collected, loaded with Fura-2/AM, and stimulated with SEE-pulsed Raji B cells. [Ca2+]i changes were measured as in panel C.
Effect of CD38 overexpression in T-cell function. (A,B) Jurkat J77 cells were transiently transfected with the pEGFP-C1 or CD38-GFP constructs by nucleofection. At 24 hours, after transfection cells were subjected to Histopaque-1077 centrifugation and then left alone, or mixed with SEE-pulsed or unpulsed Raji B cells. After a brief centrifugation to favor conjugate formation, ectocellular GDP-ribosyl cyclase activity was continuously measured at 37°C using NGD+ as a substrate. The cGDPR production in fluorescence units was plotted against time (minutes). ● indicates J77 CD38-GFP+ T cells incubated with SEE-pulsed Raji B cells; ○, J77 CD38-GFP+ T cells incubated with unpulsed Raji B cells; ■, Raji B cells alone; ♦, J77 CD38-GFP+ T cells alone; ▾, J77 pEGFP-C1+ T cells incubated with SEE-pulsed Raji B cells; ◇, J77 pEGFP-C1+ T cells incubated with unpulsed Raji B cells; and ▴, J77 pEGFP-C1+ T cells. Arrows indicate the 20-minute time frame during which individual cells showed negligible enzymatic activity. (C) Ag-induced Ca2+ released in J77 CD38-GFP+ T cells (● and ○) and J77 pEGFP-C1+ T cells (▾ and ▿). Fura-2/AM–loaded J77 CD38-GFP+ or J77 pEGFP-C1+ T cells were left alone (♦ and ▴, respectively), or mixed with SEE-pulsed (● and ▾, respectively) or unpulsed (○ and ▿, respectively) Raji B cells. [Ca2+]i changes were measured using a fluorescence plate reader, as described in “Methods.” Characteristic tracings are shown (mean of 3 independent experiments). (D) CD38-GFP–transfected or GFP-transfected Jurkat T cells were stimulated with Raji B cells pulsed with different amounts of SEE. Supernatants were collected after overnight incubation and were assayed for IL-2 by enzyme-linked immunosorbent assay (ELISA). Representative experiment out of 3 independent experiments performed in triplicate (mean ± SEM). (E) CD38 expression by fluorescence-activated cell sorter (FACS) in oligo control–transfected or CD38 siRNA-transfected Jurkat T cells 48 hours after nucleofection. Cells were stained with PE-conjugated anti-CD38 (FL2). The number in each quadrant represents the percentage of cells within each region relative to all viable cells. (F) T cells were then collected, loaded with Fura-2/AM, and stimulated with SEE-pulsed Raji B cells. [Ca2+]i changes were measured as in panel C.
The profile of the GDP-ribosyl cyclase activity of the T/APC conjugates of control experiments with J77 cells transfected with GFP alone (J77 pEGFP-C1) was almost identical to that of the Raji cells (Figure 6B). The significant variations in ecto-GDP-ribosyl cyclase activity observed in Figure 6A may be secondary to an increased surface expression of CD38 in J77 CD38-GFP+ cells and/or T/APC interactions.
The effects of CD38 overexpression on [Ca2+]i mobilization in antigen-stimulated T cells were assessed in J77 CD38-GFP+ cells and J77 pEGFP-C1+ cells loaded with Fura-2 mixed with SEE-pulsed Raji cells. Short centrifugation (1 minute) followed by resuspension of the T/APC mixture resulted in a rapid increase in [Ca2+]i immediately after T/APC contact, with a plateau at high levels during the experiment (approximately 9 minutes; Figure 6C). Calcium mobilization in J77 CD38-GFP+ cells was higher and more sustained than in control J77 pEGFP-C1+ cells. Conjugation of J77 CD38-GFP+ (or control J77 pEGFP-C1+) cells with unpulsed Raji B cells yielded relatively lower increases in [Ca2+]i (Figure 6C).
The effects of CD38 overexpression on IL-2 production in SEE-stimulated T cells was tested at multiple superantigen concentrations. IL-2 production at 100 ng/mL of SEE or less was significantly higher in CD38-GFP–transfected cells than in control cells (Figure 6D). These results demonstrate that CD38 overexpression in T cells enhances TCR-mediated calcium mobilization and IL-2 production in an antigen-dependent manner and requires T/APC conjugate formation.
Effect of anti-CD38 mAbs on antigen-induced T-cell activation and cytokine production
The role of CD38 in T-cell activation was studied in a model closely mimicking physiologic T-cell stimulation by APCs. To this aim, we used Jurkat cells expressing a TCR specific for the HA 307-319 epitope (CH7-C17 cells) and B cells presenting the nominal antigenic peptide HA 307-319 (HOM-2 cells) as APCs. An additional advantage of this system is that HOM-2 cells do not express surface CD38 (data not shown), and therefore the functional contribution of CD38 from the T cells could be tested without the interference of CD38 from the B cells.
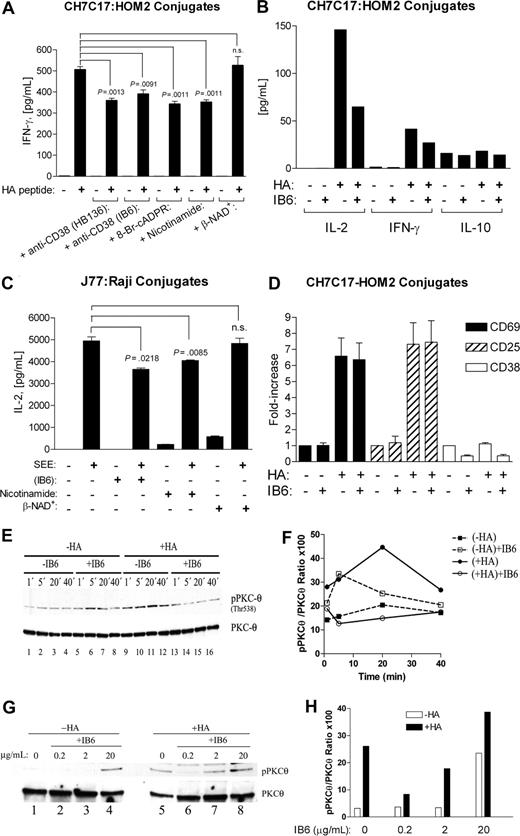
We assessed the influence of 2 nonagonistic anti-CD38 mAbs on TCR-mediated IFN-γ production. IB6 mAb was unable to induce proliferation, calcium fluxes, and IFN-γ secretion of peripheral blood mononuclear cells (PBMCs),32 and HB136 does not stimulate protein-tyrosine phosphorylation in Jurkat T cells overexpressing CD38,33 nor phosphorylation and activation of Erk (P.M. et al, unpublished results, October 2006). CD38 ligation by both mAbs did not induce IFN-γ secretion in CH7C17 T cells incubated with unpulsed HOM-2 B cells (Figure 7A). However, CH7-C17 cells preincubated (15 minutes) with HB136 or IB6 mAbs produced less IFN-γ than CH7-C17 cells stimulated by HA-pulsed HOM-2 cells alone (29% and 23% inhibition, respectively). CH7-C17 cells treated with 100 μM 8-Br-cADPR, a cADPR antagonist, or 500 μM nicotinamide, a negative feedback inhibitor of CD38 ADP-ribosyl cyclase activity, featured reduced IFN-γ production (32%, and 30%, respectively). The addition of the 100 μM β-NAD+, the natural substrate of CD38, had no recordable effects.
Effect of anti-CD38 mAb on antigen-induced T-cell activation and cytokine production. (A) IFN-γ production by CH7C17 T cells incubated overnight with HA peptide–pulsed (+) or unpulsed (−) HOM-2 B cells. CH7C17 T cells were preincubated for 15 minutes at 37°C with the indicated anti-CD38 mAb or with reagents at the concentrations indicated in “Results.” Representative experiment out of 3 independent experiments performed in triplicates (mean ± SEM). Statistical analysis: unpaired t test; n.s., not significant. (B) IL-2, IFN-γ, and IL-10 production (and 7 additional cytokines) was assayed simultaneously by a multiplex system (“Methods”) in supernatants of CH7C17 T cells stimulated as in panel A. Representative experiment out of 5 independent experiment is shown. (C) IL-2 production by Jurkat J77 T cells incubated overnight with SEE-pulsed (+) or unpulsed (−) Raji B cells. Jurkat J77 T cells were preincubated for 15 minutes at 37°C with the indicated anti-CD38 mAb or with reagents at the concentrations indicated in “Results.” Representative experiment out of 3 independent experiments performed in triplicates (mean ± SEM). Statistical analysis: unpaired t test; n.s., not significant. (D) After removal of supernatants in 5 experiments as in panel B, cells were tested for CD69 (■), CD25 (▨), or CD38 (□) expression by FACS. The data represent the fold increase over the geometric mean values obtained in CH7C17 T cells stimulated with non–HA-pulsed HOM2 cells in the absence of IB6 (1-fold value), and are the mean plus SE of 5 independent experiments. (E) Western blot analysis with the anti–phospho-PKCθ (Thr538), followed by anti-PKCθ for protein-loading control of cell lysates from CH7C17 T cells incubated with unpulsed (−HA) or HA-pulsed (+HA) HOM-2 B cells. CH7C17 T cells were either preincubated with vehicle (−IB6) or preincubated for 30 minutes at 37°C with the anti-CD38 mAb IB6 (+IB6), then washed to eliminate unbound IB6, mixed with HOM-2 B cells, and incubated at 37°C for the indicated periods of time. The image is representative of 3 independent experiments. (F) The level of PKCθ phosphorylation at Thr538 (y-axis) was plotted against time (minutes). ■ indicates CH7C17 T cells incubated with unpulsed HOM-2 cells for the indicated periods of time; □, CH7C17 T cells preincubated with anti-CD38 mAb IB6 and then with unpulsed HOM-2 cells; ●, CH7C17 cells incubated with HA-pulsed HOM-2 cells; and ○, CH7C17 cells preincubated with IB6 and then incubated with HA-pulsed HOM-2 cells. (G) CH7C17 T cells were preincubated with different concentrations of IB6 or vehicle as indicated. After 30 minutes at 37°C, cells were washed and incubated with either nonpulsed (−HA) or HA-pulsed (+HA) HOM-2 B cells for 20 minutes. Western blotting of cell lysates were probed with anti–phospho-PKCθ (Thr538), followed by anti-PKCθ total for protein-loading control. (H) The level of phosphorylation of PKCθ at Thr538 (y-axis) in CH7C17 T cells interacting with either unpulsed (□) or HA-pulsed (■) APCs was plotted against the concentration of IB6 mAb used (x-axis).
Effect of anti-CD38 mAb on antigen-induced T-cell activation and cytokine production. (A) IFN-γ production by CH7C17 T cells incubated overnight with HA peptide–pulsed (+) or unpulsed (−) HOM-2 B cells. CH7C17 T cells were preincubated for 15 minutes at 37°C with the indicated anti-CD38 mAb or with reagents at the concentrations indicated in “Results.” Representative experiment out of 3 independent experiments performed in triplicates (mean ± SEM). Statistical analysis: unpaired t test; n.s., not significant. (B) IL-2, IFN-γ, and IL-10 production (and 7 additional cytokines) was assayed simultaneously by a multiplex system (“Methods”) in supernatants of CH7C17 T cells stimulated as in panel A. Representative experiment out of 5 independent experiment is shown. (C) IL-2 production by Jurkat J77 T cells incubated overnight with SEE-pulsed (+) or unpulsed (−) Raji B cells. Jurkat J77 T cells were preincubated for 15 minutes at 37°C with the indicated anti-CD38 mAb or with reagents at the concentrations indicated in “Results.” Representative experiment out of 3 independent experiments performed in triplicates (mean ± SEM). Statistical analysis: unpaired t test; n.s., not significant. (D) After removal of supernatants in 5 experiments as in panel B, cells were tested for CD69 (■), CD25 (▨), or CD38 (□) expression by FACS. The data represent the fold increase over the geometric mean values obtained in CH7C17 T cells stimulated with non–HA-pulsed HOM2 cells in the absence of IB6 (1-fold value), and are the mean plus SE of 5 independent experiments. (E) Western blot analysis with the anti–phospho-PKCθ (Thr538), followed by anti-PKCθ for protein-loading control of cell lysates from CH7C17 T cells incubated with unpulsed (−HA) or HA-pulsed (+HA) HOM-2 B cells. CH7C17 T cells were either preincubated with vehicle (−IB6) or preincubated for 30 minutes at 37°C with the anti-CD38 mAb IB6 (+IB6), then washed to eliminate unbound IB6, mixed with HOM-2 B cells, and incubated at 37°C for the indicated periods of time. The image is representative of 3 independent experiments. (F) The level of PKCθ phosphorylation at Thr538 (y-axis) was plotted against time (minutes). ■ indicates CH7C17 T cells incubated with unpulsed HOM-2 cells for the indicated periods of time; □, CH7C17 T cells preincubated with anti-CD38 mAb IB6 and then with unpulsed HOM-2 cells; ●, CH7C17 cells incubated with HA-pulsed HOM-2 cells; and ○, CH7C17 cells preincubated with IB6 and then incubated with HA-pulsed HOM-2 cells. (G) CH7C17 T cells were preincubated with different concentrations of IB6 or vehicle as indicated. After 30 minutes at 37°C, cells were washed and incubated with either nonpulsed (−HA) or HA-pulsed (+HA) HOM-2 B cells for 20 minutes. Western blotting of cell lysates were probed with anti–phospho-PKCθ (Thr538), followed by anti-PKCθ total for protein-loading control. (H) The level of phosphorylation of PKCθ at Thr538 (y-axis) in CH7C17 T cells interacting with either unpulsed (□) or HA-pulsed (■) APCs was plotted against the concentration of IB6 mAb used (x-axis).
The inhibitory effects induced by CD38 ligation on the levels of IL-2 secreted by CH7-C17 cells incubated with HA-pulsed HOM-2 cells were more apparent than those on IFN-γ production. The results obtained were collected by simultaneous assaying 10 different cytokines (Figure 7B). As expected, little effects were scored on the IL-10 secreted in an antigen-independent manner (Figure 7B). The inhibitory effect of IB6 mAb on IL-2 production was confirmed using SEE-APC–stimulated J77 Jurkat cells (Figure 7C). However, the addition of IB6 mAb to CH7-C17 cells did not perturb the increased expressions of CD69 and CD25 induced in these cells by HA-pulsed HOM-2 cells (Figure 7D).
These results prompted us to investigate whether IB6 may influence early signaling events (eg, tyrosine phosphorylation of specific substrates) in antigen-APC–mediated stimulated T cells. The events observed in these cells are modifications of the kinetics of tyrosine phosphorylation of LAT at Tyr191, and minor changes in phosphorylation of Erk at Thr202 and Tyr204 (Figure S1). Further, phosphorylation of PKCθ at Thr538 was increased after T-cell stimulation with HA-pulsed HOM-2 cells as shown in Figure 7E (lanes 9-12) and Figure 7F. These effects were not observed when the same experiment was performed in the presence of anti-CD38 mAb IB6 (Figure 7E lanes 13-16, F). This occurred notwithstanding that the anti-CD38 mAb induces at 5 minutes a short-lived increased phosphorylation of PKCθ in CH7-C17 cells incubated with nonpulsed HOM-2 cells (Figure 7E lanes 5-8). Low levels of basal Thr538 phosphorylation on PKCθ were observed during the experiments in CH7-C17 cells incubated with nonpulsed HOM-2 cells (Figure 7E lanes 1-4). This observation is consistent with the low phosphorylation levels of PKCθ at Thr538 reported in unstimulated Jurkat cells.34
Dose-response experiments were then performed to assess the effects of CD38 ligation on PKCθ phosphorylation (Figure 7G). IB6 mAb at 0.2 μg/mL (Figure 7G lane 6) and—to a lesser extent—at 2 μg/mL (Figure 7G lane 7) inhibited PKCθ phosphorylation at Thr538 induced in CH7-C17 cells interacting with HA-pulsed HOM-2 cells in the absence of mAb (Figure 7G lane 5). In contrast, IB6 mAb at 20 μg/mL (Figure 7G lane 8) induced PKCθ phosphorylation above the levels reached in these cells stimulated with HA-pulsed HOM-2 cells alone (Figure 7G lane 5). At this dose, IB6 also induced PKCθ phosphorylation in CH7-C17 cells interacting with nonpulsed HOM-2 cells (Figure 7G lane 4), but not at lower doses (Figure 7G lanes 2 and 3).
To test whether IB6 mAb would affect the translocation of PKCθ to the synapse in Ag-stimulated T cells, we studied the redistribution of PKCθ and CD3-ζ at the IS structure in CH7-C17 cells treated with the mAb before the interaction with antigen-pulsed HOM-2 cells. T cells treated with IB6 mAb featured a significant proportion of conjugates where translocation of CD3-ζ occurred without the concomitant recruitment of PKCθ (Figure S1).
Discussion
The original observations of this work are that in T cells, CD38 forms part of 2 distinct cellular pools, one at the plasma membrane and a second one in recycling endosomes, which are both redistributed toward the IS upon TCR engagement. Whereas the membrane-associated CD38 is immediately recruited to the IS, the polarization and recruitment of the intracellular pool of CD38 requires 2 to 3 minutes. The intracellular CD38 pool of T cells also codistributes with intracellular CD3-ζ and, in a similar way as for this molecule,29 it is likely that intracellular CD38 recycles back to the plasma membrane to contribute to the mature IS. Recruitment of both pools at the IS requires antigen-pulsed APCs, being that the process is more efficient in T cells than in APCs.
The pattern of CD38 accumulation along the T/APC contact zone is similar to that of Lck, which is also enriched at the synapse periphery after the initial accumulation at the c-SMAC.35 Likely, the relocation of CD38 and Lck to peripheral sites of the mature IS distant from CD3-ζ may reduce the signals observed immediately after engagement of the T cell with an antigen-pulsed APC. In a resting state, the 3 molecules are associated in the same raft subset11 before the earliest signaling events are taking place35 by the active formation of TCR-containing microclusters.36,37 TCR proximal signals are implemented in peripheral microclusters and terminated in the c-SMAC38 : therefore, CD38 location at the periphery of the IS may represent a way to fine-tune the TCR signal capabilities. The blocking effects of anti-CD38 mAbs are exerted against signaling events that occur before maturation of the IS. The gain of function observed in T cells overexpressing CD38 correlates with calcium mobilization initiated immediately after formation of T/APC conjugates.
CD38 is actively recruited at the IS of T cells by steps that involve Lck-mediated signals. Thus, by using JCaM 1.6 cells that lack functional Lck, and in which the CD38-mediated signaling is defective,22 we have shown that the proportion of conjugates showing mature IS and/or CD38 translocated at the T/APC interface is greatly reduced in comparison with wild-type Jurkat cells. Consistently, Src-family protein tyrosine kinases were required for CD38 relocalization: indeed, wild-type Jurkat cells treated with the Src-family inhibitor PP2 featured a reduced accumulation of CD38 at the IS. These results confirm previous results39 that show defective conjugate formation in JCaM1.6 cells or in wild-type Jurkat cells treated with PP2, and failure to recruit F-actin and LFA-1 to the T/APC contact site. Ezrin is a member of the ezrin-radixin-moesin family of membrane-microfilament linkers highly enriched in wild-type Jurkat T cells in the F-actin–rich membrane protrusions at the periphery of the IS, whereas Ezrin is poorly accumulated in JCaM1.6 cells.40 Hence, our results suggest that CD38 redistribution to the IS strongly depends on Lck-mediated early signaling events, which in turn lead to actin polymerization and remodeling.
Membrane CD38-GFP from transfected Raji cells pulsed with SEE clusters along the T/APC contact zone in a small but significant proportion of conjugates. Several of these conjugates were characterized by a translocation of CD31 from the T cells to the T/APC interface. This finding suggests an active recruitment initiated by ligand-receptor interactions. CD31 contains functional immunoreceptor tyrosine inhibitory motifs (ITIMs) within the cytoplasmic domain: coligation of CD31 to the TCR is followed by a Lck-dependent tyrosine phosphorylation of CD31 ITIMs, recruitment of Src homology 2 domain–containing protein tyrosine phosphatase-2, and attenuation of TCR-mediated cellular signaling.41 To our knowledge, this is the first physiologic evidence that CD31 relocates in the proximity of the TCR after antigen-mediated T-cell activation, avoiding the artificial forcing provided by specific mAbs. The negative regulation of SEE-induced T-cell activation exerted by Lck42 is consistent with the observation that Lck is needed for CD31 to be tyrosine-phosphorylated in response to cross-linking of TCR.41 Therefore, it is conceivable that the presence of the CD31/TCR/Lck supramolecular complex in the IS support the inhibitory function exerted by CD31 on T-cell activation.
The presence of surface CD38 at the IS may have functional consequences for either T cells or for the antigen-pulsed APCs. These effects may be secondary to the intrinsic receptorial functions or to the ectoenzymatic activity of CD38. Indeed, CD38 catalyzes the formation of cADPR, a second messenger that may reach intracellular Ca2+ stores by different mechanisms.43 This study shows that there is intercellular interaction-dependent T cell–driven increase in CD38 ectoenzymatic activity upon T/APC contact. An interpretation of this finding is that the cADPR produced outside the cells apparently does not contribute to increased [Ca2+]i induced by the antigen. However, this study also shows that the antigen-dependent rise of [Ca2+]i is higher and more sustained in JurkatCD38+high than in JurkatCD38+low. This suggests that there is a causal relation between surface CD38 levels and increased calcium release in antigen-stimulated T cells. Indeed, down-regulation of CD38 expression in T cells by RNA interference is paralleled by inhibitory effects on the increase of [Ca2+]i. In other cell types, overexpression of CD38 correlates with increased basal levels of intracellular cADPR,44 which makes these cells more excitable and prone to respond to different stimuli by increasing [Ca2+]i45,46 or cell differentiation47 more efficiently than the CD38− counterparts.
To further address the question of how CD38 may contribute to T-cell signaling in antigen-stimulated T cells, we investigated the effects of blocking CD38 by means of specific mAbs. The data show that the blocking mAb IB6 inhibits IL-2 and IFN-γ production induced by antigen-APC stimulation. HB136, another nonagonistic anti-CD38 mAb, also affects antigen-induced IFN-γ production. Furthermore, preincubation of T cells with 8-Br-cADPR, (cADPR antagonist) or nicotinamide (a negative feedback inhibitor of CD38 ADP-ribosyl cyclase) exerts a similar effect on IL-2 and IFN-γ production. An interpretation is that the effects of anti-CD38 mAbs on IL-2 and IFN-γ are meditated by cADPR-dependent mechanisms. The APCs used in most of these experiments are CD31− (HOM-2 B cells); consequently, one should rule out interference with CD38/CD31 interplay. A possibility is the existence of yet-unknown ligands for CD38 expressed by the APCs. A further alternative is that the anti-CD38 mAbs exert signaling capabilities not detected in other experimental settings,32 which in turn influence phosphorylation of PKCθ at the regulatory site Thr538 and/or its translocation to the IS. PKCθ is essential for TCR-mediated T-cell activation and NF-κB activation,48 and PDK1 can regulate the cellular function of PKCθ by phosphorylating Thr538 in the PKCθ activation loop49 in a phosphoinositide 3-kinase–dependent manner.50
In summary, clustering of CD38 at the IS and the contribution of both T cells and APCs to this event suggests that CD38 plays important roles during antigen presentation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Nieves de la Casa from the Confocal Core Facility of the University of Jaén, David Porcel from the Centro de Instrumentación Científica of the University of Granada, and Ana B. Martin, Pilar Navarro-Cuesta, and Antonio Mérida from the Instituto de Parasitología y Biomedicina “López-Neyra” for their technical assistance. We thank Dr Balbino Alarcón, Dr Arthur Weiss, Dr Cox Terhorst, and Dr Dolores Jaraquemada for valuable reagents and cells.
This work was supported by grants from the Ministerio de Educación y Ciencia (MEC; SAF2002-00721, and SAF2005-06056-C02-01 to J.S.), the Consejería de Innovación, Ciencia y Empresa de la Junta de Andalucía (P05-CVI-00908 to J.S.), the Instituto Carlos III-FIS, Ministerio de Sanidad y Consumo (FIS03/0389 and FIS06/1502 to M.Z.), and by grants from the Italian Association for Cancer Research (AIRC), FIRMS, and MIUR (to F.M.). Centro Nacional de Investigaciones Cardiovasculares (CNIC) is supported by the Spanish Ministry of Health and Consumer Affairs and the Pro-CNIC Foundation. M.Z. was supported by a Ramón y Cajal contract from MEC. P.M. was supported by a FPI Fellowship from MEC. P.M. was a PhD candidate at University of Granada (Spain), and this work was submitted in partial fulfillment of the requirement for the PhD.
Authorship
Contribution: P.M. performed biochemical, molecular biology, and functional studies, as well as the confocal microscopy experiments (Figure 3), and made the figures; M.M. performed the confocal microscopy experiments depicted in Figure 1C; H.d.l.F. performed the experiments with CD4+ T cells and DCs depicted in Figure 1D; M.P.-M. designed and performed the molecular biology experiments with P.M. and performed the time-lapse confocal microscopy experiment depicted in Figure 2C; A.G.-P. and A.A.-V. performed the confocal microscopy experiments depicted in Figure 4; F.M. provided the IB6 antibody and participated in the interpretation of the data; M.Z., F.S.-M., and J.S. designed the experiments and provided financial support; and J.S. wrote the paper that was extensively revised by M.M., M.Z., F.M., and F.S.-M.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaime Sancho, Instituto de Parasitología y Biomedicina “López-Neyra,” CSIC, PT de Ciencias de la Salud, Avenida del Conocimiento s/n, 18100 Armilla, Spain; e-mail: granada@ipb.csic.es.

![Figure 6. Effect of CD38 overexpression in T-cell function. (A,B) Jurkat J77 cells were transiently transfected with the pEGFP-C1 or CD38-GFP constructs by nucleofection. At 24 hours, after transfection cells were subjected to Histopaque-1077 centrifugation and then left alone, or mixed with SEE-pulsed or unpulsed Raji B cells. After a brief centrifugation to favor conjugate formation, ectocellular GDP-ribosyl cyclase activity was continuously measured at 37°C using NGD+ as a substrate. The cGDPR production in fluorescence units was plotted against time (minutes). ● indicates J77 CD38-GFP+ T cells incubated with SEE-pulsed Raji B cells; ○, J77 CD38-GFP+ T cells incubated with unpulsed Raji B cells; ■, Raji B cells alone; ♦, J77 CD38-GFP+ T cells alone; ▾, J77 pEGFP-C1+ T cells incubated with SEE-pulsed Raji B cells; ◇, J77 pEGFP-C1+ T cells incubated with unpulsed Raji B cells; and ▴, J77 pEGFP-C1+ T cells. Arrows indicate the 20-minute time frame during which individual cells showed negligible enzymatic activity. (C) Ag-induced Ca2+ released in J77 CD38-GFP+ T cells (● and ○) and J77 pEGFP-C1+ T cells (▾ and ▿). Fura-2/AM–loaded J77 CD38-GFP+ or J77 pEGFP-C1+ T cells were left alone (♦ and ▴, respectively), or mixed with SEE-pulsed (● and ▾, respectively) or unpulsed (○ and ▿, respectively) Raji B cells. [Ca2+]i changes were measured using a fluorescence plate reader, as described in “Methods.” Characteristic tracings are shown (mean of 3 independent experiments). (D) CD38-GFP–transfected or GFP-transfected Jurkat T cells were stimulated with Raji B cells pulsed with different amounts of SEE. Supernatants were collected after overnight incubation and were assayed for IL-2 by enzyme-linked immunosorbent assay (ELISA). Representative experiment out of 3 independent experiments performed in triplicate (mean ± SEM). (E) CD38 expression by fluorescence-activated cell sorter (FACS) in oligo control–transfected or CD38 siRNA-transfected Jurkat T cells 48 hours after nucleofection. Cells were stained with PE-conjugated anti-CD38 (FL2). The number in each quadrant represents the percentage of cells within each region relative to all viable cells. (F) T cells were then collected, loaded with Fura-2/AM, and stimulated with SEE-pulsed Raji B cells. [Ca2+]i changes were measured as in panel C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/7/10.1182_blood-2007-07-101600/5/m_zh80070817230006.jpeg?Expires=1767702522&Signature=Dr5-ou342lFnV7isUNCOmJw3nC8E42OzwtMnN6dHoIw3AQ-paMpR5qpROnP2eyfWY3lGmjF6-ortM95LTb6nQYtepXTiV9MhrVmcftKo8DtqedupWaYauUCe5m~TLSk4Be5z9Frx61XI7-pR2FxsJ~UHlC3CfcnMlZOnbM0Rmlr04NbgrU43aCdUK-~s-hpiHKhnATgX8kFGPhZEKNsIgKJTinrF9x7suNp7T~Z4pb~c5RlKKO69tJNOSRYVIfIIK75xzlUaBCio7lhwUcOOx7qy~DNA6VASVFY75-PDI1AYhCIwvwxsA53fqa6Biez8u6441oQjelTxDincRkjv~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)