Abstract
Regulatory T cells (Treg) play important roles in suppressing immune responses and maintaining tolerance. Rabbit antithymocyte globulin (rATG) and horse ATG (hATG) are widely used in the treatment of immune-mediated syndromes, but their effects on Treg are unknown. We show here that in vitro culture of normal human peripheral blood mononuclear cells (PBMCs) with a low-dose rATG resulted in marked expansion of functional Treg by converting CD4+CD25− T cells to CD4+CD25+ T cells. hATG did not expand but rather decreased Treg. Immuno-blot showed increased expression of FOXP3 and NFAT1 in CD4+CD25− and CD4+CD25+ T cells exposed to rATG. PBMCs treated with rATG displayed increased interleukin-10 in culture supernatants than those treated with hATG. Furthermore, rATG and hATG showed differences in their potential to stimulate CD4+ T cells as examined using different activation markers. Microarray revealed that rATG induced markedly different gene-expression patterns in PBMCs, compared with hATG-treated or untreated PBMCs. Our findings indicate that rATG expanded Treg, probably through transcriptional regulation by enhanced NFAT1 expression, in turn conferring CD4+CD25− T cell FOXP3 expression and regulatory activity. The therapeutic effects of rATG may occur not only because of lymphocyte depletion but also enhanced Treg cell number and function.
Introduction
Regulatory T cells (Tregs) are implicated in the suppression of immune responses and the maintenance of tolerance.1-3 Tregs are characterized by the surface expression of CD4 and the interleukin-2 (IL-2) receptor (CD25); a member of the forkhead family of transcription factors, FOXP3, is expressed in the nuclei of human and murine CD4+CD25+ Treg, especially in human CD4+CD25high populations. FOXP3 acts as a master regulator for cytokine production and is necessary for cell-cell contact-dependent inhibition of effector T-cell activation by Treg.4-6 FOXP3 expression is required for Treg development and confers suppressive function on peripheral CD4+CD25+ Tregs.7 Mice lacking the nuclear factor of activated T cells (NFAT1) showed an enhanced immune response, with a tendency toward the development of a late Th2-like response.8 Recent studies indicate that NFAT1 induces FOXP3 expression by binding to its promoter,9 and FOXP3 controls Treg function through cooperation with NFAT1.10
Treg numbers are deficient in patients with active systemic lupus erythematosus11 and type 1 diabetes.12 In patients with autoimmune hepatitis, Tregs are depleted and FOXP3 expression is decreased.13 Patients undergoing stem-cell transplantation have a low risk of developing graft-versus-host disease (GVHD) if the Treg graft content is high.14 In patients with multiple sclerosis, although Treg numbers are consistent with those in healthy individuals, there is a marked decrease in their effector function.15 We have recently reported that CD4+CD25highFOXP3+ Tregs are decreased in most patients with aplastic anemia (AA).16 CD4+ Tregs tend to be decreased in low-risk myelodysplastic syndrome (MDS) patients but increased in high-risk MDS patients and correlated with progression to aggressive subtypes of the disease.17 There is evidence that Tregs have the ability to prevent the development of autoimmune diseases,18 tumor immunity,19 graft rejection,20 and GVHD21 in mouse models. In these animal models, transfer of Tregs can prevent the autoimmune phenotype that develops after Treg depletion. Infusion of Tregs in a minor antigen H60-mediated AA mice model aborted H60-specific T-cell expansion and prevented bone marrow destruction.22
Immunosuppressive drugs, such as antithymocyte globulin (ATG) and cyclosporin A (CsA), are widely used to prevent or treat acute graft rejection in organ transplantation,23 in conditioning for transplantation, and for the treatment of AA and other autoimmune diseases, and GVHD.24 Because of the important roles of Treg in disease pathophysiology and treatment, the effects of these immunosuppressive drugs on the function or expansion of Treg might be clarified. ATG is a purified IgG fraction of sera from rabbits or horses that have been immunized with human thymocytes or T-cell lines. ATG depletes peripheral lymphocytes from the circulating pool through complement-dependent lysis or activation-associated apoptosis,23,25 but its effect on Treg has not been fully elucidated.
In this study, we demonstrate that rabbit ATG (rATG) selectively expanded functional CD4+CD25+ FOXP3+ Tregs in vitro. In contrast, horse ATG (hATG) and CsA decreased CD4+CD25+ T cells and CD4+CD25+ FOXP3+ Tregs. rATG expanded Tregs by converting CD4+CD25− T cells into CD4+CD25+FOXP3+ T cells likely through enhanced NFAT1 expression.
Methods
Immunosuppressive drugs and control antibodies
rATG (Thymoglobulin; Genzyme, Cambridge, MA), hATG (ATGAM; Pharmacia & Upjohn, Kalamazoo, MI), and CsA (Sigma-Aldrich, St Louis, MO) were tested in this study; rabbit IgG (rIgG) and horse IgG (hIgG) (Santa Cruz Biotechnology, Santa Cruz, CA) were used as control antibodies.
Isolation of cells
Heparinized blood samples from 10 healthy volunteers were collected after informed consent under a protocol approved by the Institutional Review Board of National Heart, Lung, and Blood Institute of Health (NHLBI). Peripheral blood mononuclear cells (PBMCs) were isolated by standard Ficoll density gradient centrifugation. CD4+, CD4+CD25+, or CD4+CD25− T cells were isolated by the CD4+CD25+ T cell isolation kit (Miltenyi Biotec, Auburn, CA) as previously described.16 The purity of each population was 95% to 97% by flow cytometry.
Cell culture
PBMCs (106/mL) from healthy individuals were incubated with rATG, hATG, rIgG, or hIgG (each at 10 μg/mL, unless otherwise indicated) and CsA (1.0 μg/mL) in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum at 37°C, 95% humidity, and 5% CO2 for 24 hours or indicated durations (5-72 hours). Supernatants were collected for cytokine detection. Cells were collected for flow cytometry or Western blotting as described below.
Flow cytometry
Cells were harvested after culture in the presence of immunosuppressive biologics and drugs. Briefly, the cells were first stained with antihuman CD4-fluorescein isothiocyanate (FITC) and CD25-phycoerythrin (PE) (eBioscience, San Diego, CA), washed, resuspended in 1 mL cold Fix/Perm buffer (eBioscience), and incubated at 4°C for 1 hour. After washing with 2 mL of permeabilization buffer (eBioscience), cells were blocked with 2% normal rat serum for 15 minutes. Antihuman FOXP3-allophycocyanin (APC; PCH101; eBioscience) was added and cells were incubated at 4°C for another 30 minutes in the dark. Finally, cells were washed with 2 mL permeabilization buffer and analyzed on the FC 500 Flow Cytometer (Beckman Coulter, Fullerton, CA) using the CXP software (BD Biosciences, San Jose, CA).
Apoptosis induction by different immunosuppressive drugs at various concentrations was detected by staining with annexin V-FITC (BD PharMingen, San Diego, CA), according to the manufacturer's instructions, followed by flow cytometry.
To compare the binding of rATG and hATG to lymphocytes, 106 PBMCs in 1 mL of phosphate-buffered saline (PBS) were incubated with rATG or hATG (each at 10 μg/mL, or indicated concentrations) for 30 minutes at 4°C, washed twice with PBS, and stained with anti–rIgG-FITC or anti–hIgG-FITC (GeneTex, San Antonio, TX); these 2 second antibodies were stated to have similar binding affinity to IgG by their manufacturer (3.5 and 3.7 mol FITC per mol IgG, respectively). In comparing the blocking capacity of rATG and hATG to surface antigens, 106 PBMCs in 100 μL PBS were incubated with 10 μg rATG or hATG for 60 minutes at 4°C, washed with PBS, stained with antihuman CD3-APC, antihuman T-cell receptor (TCR)αβ-FITC, antihuman CD4-APC-Cy7 (eBiosciences), and antihuman CD28-PE (Beckman Coulter), respectively, followed by flow cytometry. To evaluate activation of T cells after rATG or hATG exposure for 24 hours, PBMCs were stained with antihuman CD4-FITC, combined with antihuman glucocorticoid-induced TNF receptor (GITR)-PE, or antihuman cytotoxic T lymphocyte–associated antigen-4 (CTLA-4)-PE (e-Bioscience), respectively. Intracellular staining of CTLA-4 was performed as for FOXP3 staining as described above.
Enzyme-linked immunosorbent assay
Cytokines were measured using the Human Th1/Th2 ELISA kit (eBioscience) according to the manufacturer's instructions. Briefly, IL-2, IL-4, IL-10, or INF-γ in the supernatants was captured by specific primary monoclonal antibody (mAb) precoated on a plate and detected by horseradish peroxidase-labeled secondary mAbs. Plates were read at 450 nm using the ELISA reader (PerkinElmer Life and Analytical Sciences, Waltham, MA). Recombinant cytokines were used as standards as recommended by the manufacturer. Samples and standards were tested in duplicate.
Western blotting
Nuclear or cytoplasmic proteins were extracted from treated or untreated cells as previously described26 and subject to Western blotting to detect FOXP3 and NFAT1 expression. Protein concentration was determined using the Micro-BCA protein assay kit (Pierce, Rockford, IL). Equal amounts (5 μg) of proteins were electrophoresed on 4% to 20% SDS-polyacrylamide gel and transferred onto polyvinylidene fluoride membranes. FOXP3 and NFAT1 were immunodetected with mouse antihuman FOXP3 mAb (BioLegend, San Diego, CA) and anti-NFAT1 mAb (BD Transduction Laboratories, San Jose, CA), followed by incubation with alkaline-phosphatase-conjugated antimouse IgG, which was included in the WesternBreeze Chemiluminescent kit (Invitrogen, Carlsbad, CA). Antiactin mAb (Santa Cruz) was used as an internal control.
Carboxyfluorescein diacetate succinimidyl ester labeling
To confirm the effect of rATG on the proliferation of CD4+CD25− T cells, CD4+CD25− T cells were isolated from PBMCs using microbeads and labeled with 1 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen), then cultured with rATG or hATG. Soluble CD3/CD28 mAb (each at 1.0 μg/mL) and rIgG were used as positive controls and negative controls, respectively. Five days later, cells were stained with antihuman CD25-PE. Cell division was analyzed by flow cytometry according to CFSE dilution.
Treg function
T-cell proliferation was assessed by BrdU incorporation using the ELISA colorimetric assay kit (Roche Diagnostics, Indianapolis, IN). Briefly, PBMCs were treated with rATG, hATG, or CsA for 24 hours, washed, and cultured at a 1:1 ratio with autologous PBMCs (responder) in the presence of 1 μg/mL soluble anti-CD3 (HIT3a; BD PharMingen) and 1 μg/mL soluble anti-CD28 (CD28.2; BD PharMingen). After culture for 3 days, cells were pulsed with BrdU and evaluated for its incorporation according to the manufacturer's instructions. Results are expressed as the percentage determined by comparing proliferation induced by rATG or hATG to responder PBMCs stimulated with CD3/CD28 mAb alone. To confirm specific inhibition of the T-cell response by rATG-expanded Tregs, CD4+CD25+ T cells were isolated from rATG-treated PBMCs using microbeads and added at different ratios to CD3/CD28-mAb sti-mulated autologous PBMCs (responder). Inhibition of proliferation was evaluated in the same way as unisolated rATG- or hATG-treated PBMCs as described above.
RNA preparation and microarray assay
To analyze different gene expression patterns of PBMCs treated with rATG or hATG, microarray was performed as described previously.27 PBMCs from 3 healthy controls were cultured with or without rATG or hATG for 24 hours, and total RNA was extracted with the RNeasy kit (Qiagen, Valencia, CA). RNA purity was assessed by spectrophotometry. Probes were prepared using standard Affymetrix protocols and hybridized to Affymetrix HG-U133A 2.0 arrays (Affymetrix, Santa Clara, CA). The primary CEL files have been deposited in the public repository Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) with the series record (GSE10040).
Statistical analysis of microarray and other data
Primary expression analysis was conducted with the Affymetrix GeneChip Operating System, version 1.4 client software. Expression data were transformed using a variance stabilizing, quantile normalized function termed “S10.” Comparative analysis between expression profiles for samples with or without treatments was carried out using MSCL Analyst's Toolbox developed by some of us for the JMP statistical software package (http://abs.cit.nih.gov/geneexpression.html; SAS Institute, Cary, NC). A 2-way analysis of variance for time and treatment was used to derive P values for each probe set. False discovery rates (FDRs) were calculated and probes having less than 10% FDR were selected for further analysis. Fold changes in response to treatment were calculated as differences of mean S10 values for each treatment category. When multiple probe sets for a single gene were available, data were summarized by selecting the most extreme probe set fold-change. Hierarchical cluster analysis was computed using the Ward's method based on deviation of S10 expression values from the mean. Gene ontology analysis was performed using the DAVID Bioinformatics Resources 2007 (http://david.abcc.ncifcrf.gov/).
The unpaired t test (Prism Software, San Diego, CA) was used for statistical analysis in flow cytometry data and ELISA data. Differences were considered to be significant at P less than .05.
Results
Optimization of rATG dose and duration for the treatment of PBMCs
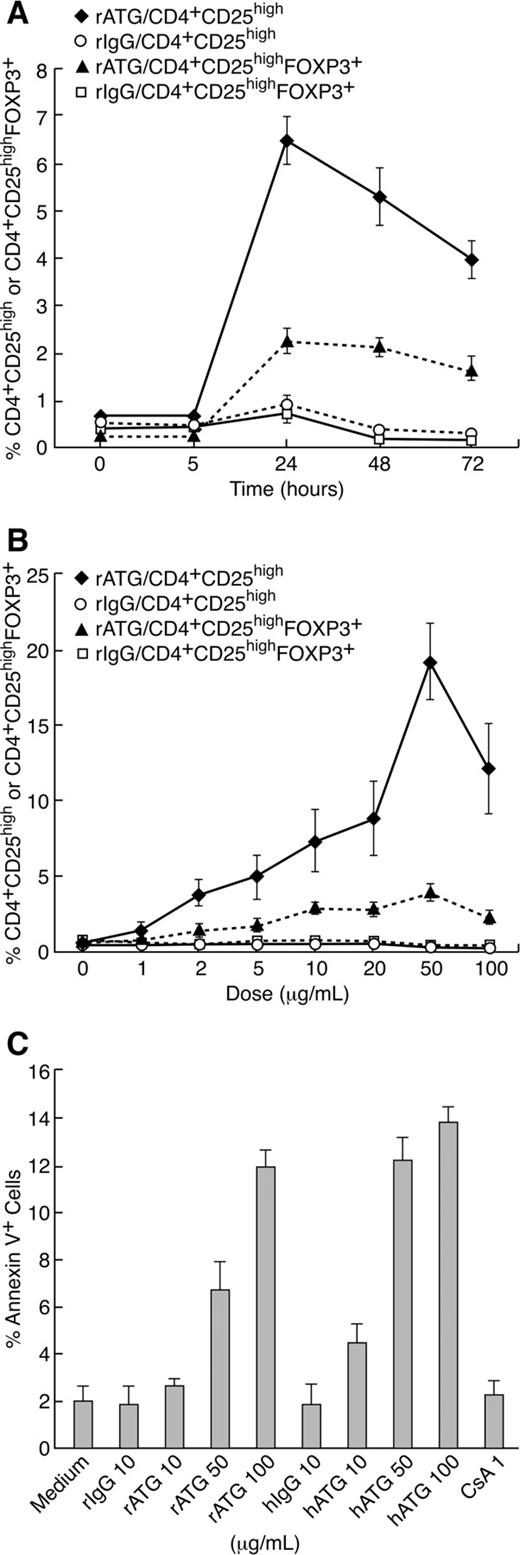
To determine the optimal duration of rATG exposure for expansion of Treg, 106 PBMCs were cultured with 10 μg/mL rIgG or rATG for 5, 24, 48, and 72 hours. The percentage of CD4+CD25high and CD4+CD25highFOXP3+ T cells was measured by flow cytometry. As shown in Figure 1A, the percentage of CD4+CD25high and CD4+CD25highFOXP3 T cells peaked at the 24-hour point and then decreased.
Optimization of duration and rATG dose for treatment of PBMCs. (A) Kinetics of Treg expansion in PBMCs treated with rATG. (B) Dose-response of rATG in expansion of Tregs and percentages of CD4+CD25high T cells and CD4+CD25highFOXP3+ T cells are shown. (C) Apoptosis of lymphocytes induced with rATG, rIgG, hATG, hIgG, or CsA. Each data point represents a mean plus or minus SEM of 3 independent experiments.
Optimization of duration and rATG dose for treatment of PBMCs. (A) Kinetics of Treg expansion in PBMCs treated with rATG. (B) Dose-response of rATG in expansion of Tregs and percentages of CD4+CD25high T cells and CD4+CD25highFOXP3+ T cells are shown. (C) Apoptosis of lymphocytes induced with rATG, rIgG, hATG, hIgG, or CsA. Each data point represents a mean plus or minus SEM of 3 independent experiments.
The optimal concentration of rATG for expansion of Treg was determined by culturing of 106 PBMCs with 1, 2, 5, 10, 20, 50, and 100 μg/mL rIgG or rATG for 24 hours. The percentage of CD4+CD25high or CD4+CD25highFOXP3+ T cells among lymphocytes was determined by flow cytometry. Both CD4+CD25high and CD4+CD25highFOXP3+ T cells showed a dose-dependent increase at 1 to 50 μg/mL rATG concentrations. Exposure to concentration as high as 100 μg/mL rATG did not enhance but decreased the percentage of CD4+CD25high and CD4+CD25highFOXP3+ T cells. rIgG treatment at different concentrations had no effect on the proportions of CD4+CD25high and CD4+CD25highFOXP3+ T cells (Figure 1B).
PBMCs were exposed to rIgG, rATG, hIgG, hATG, or CsA at different concentrations for 24 hours. Apoptotic cells were detected by flow cytometry using annexin V staining. Low-dose rATG (10 μg/mL) induced little apoptosis similar to no treatment (medium alone), and rIgG, or hIgG treatment, but at 50 μg/mL or higher doses, rATG and hATG induced apoptosis in lymphocytes (Figure 1C). Because the percentage of CD4+CD25highFOXP3+ T cells was stable and there was little apoptosis induction with rATG at 10 μg/mL and 24 hours of incubation, we selected these conditions for our experiments.
Expansion of Tregs with rATG
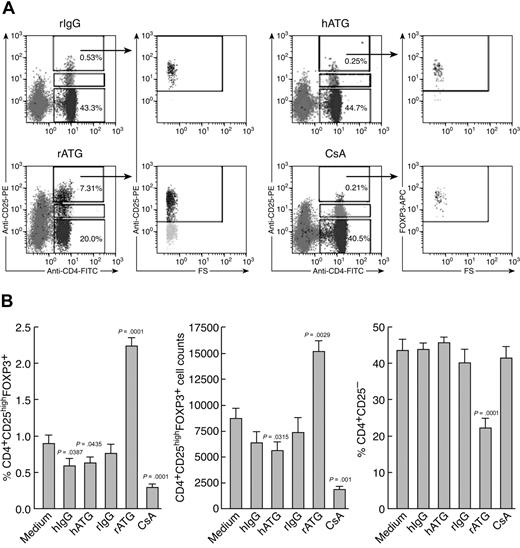
We compared the effects of immunosuppressive drugs on Treg expansion in vitro. Figure 2 shows representative flow cytometric results (Figure 2A) and a summary of the results from all the experiments performed (Figure 2B). rATG treatment significantly increased both the proportion and absolute numbers of CD4+CD25highFOXP3+ T cells in lymphocytes and dramatically decreased CD4+CD25− T cells, compared with rIgG treatment. In contrast, hATG did not expand Treg as did rATG; both hATG and CsA decreased the percentage and absolute number of Tregs, with no change in CD4+CD25− T cells.
Ex vivo expansion of Tregs with rATG. (A) Effects of different immunosuppressive drugs on expansion of CD4+CD25high and CD4+CD25high-FOXP3+ T cells. Representative flow cytometric data are shown. (B) Summary of flow cytometric results. Changes of percentage and absolute number of Tregs, as well as percentage of CD4+CD25− T cells, after treatment with different immunosuppressive drugs are shown. Data are mean plus or minus SEM (n = 10). Statistical significance was determined by an unpaired t test.
Ex vivo expansion of Tregs with rATG. (A) Effects of different immunosuppressive drugs on expansion of CD4+CD25high and CD4+CD25high-FOXP3+ T cells. Representative flow cytometric data are shown. (B) Summary of flow cytometric results. Changes of percentage and absolute number of Tregs, as well as percentage of CD4+CD25− T cells, after treatment with different immunosuppressive drugs are shown. Data are mean plus or minus SEM (n = 10). Statistical significance was determined by an unpaired t test.
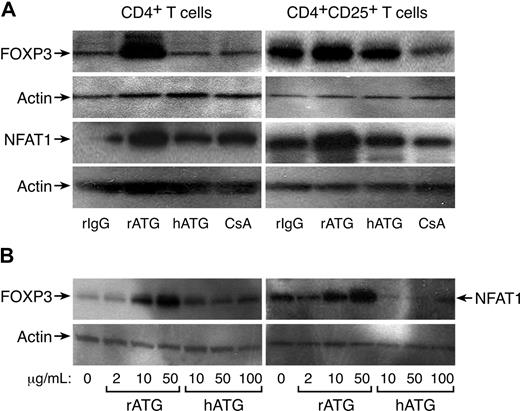
FOXP3 expression in CD4+ and CD4+CD25+ T cells exposed to rATG, hATG, or CsA was confirmed by Western blot analysis; rATG increased FOXP3 expression in CD4+ or CD4+CD25+ T cells, but hATG and CsA did not; CsA appeared to decrease FOXP3 expression in CD4+CD25+ T cells. We noted increased NFAT1 expression in CD4+ or CD4+CD25+ T cells after exposure to rATG, correlating with FOXP3 expression; again, hATG and CsA did not enhance NFAT1 expression (Figure 3A). As clinically much higher doses of hATG than rATG (as a protein) are used to obtain immunosuppression in patients, we questioned whether higher concentrations of hATG could induce FOXP3 or NFAT1 expression in vitro. We assayed different concentrations of rATG or hATG for their effects on FOXP3 and NFAT1 expression in CD4+ T cells: rATG increased FOXP3 and NFAT1 expression in a dose-dependent fashion, but hATG was without such activity even at 100 μg/mL (Figure 3B).
Increased expression of FOXP3 and NFAT1 in rATG-treated CD4+ or CD4+CD25+ T cells. (A) Western blot analysis. rATG increased expression of FOXP3 and NFAT1 in CD4+ or CD4+CD25+ T cells; results are representative of 3 independent experiments. (B) Comparison of rATG and hATG in dose-response of FOXP3 and NFAT1 expression; results are representative of 3 independent experiments.
Increased expression of FOXP3 and NFAT1 in rATG-treated CD4+ or CD4+CD25+ T cells. (A) Western blot analysis. rATG increased expression of FOXP3 and NFAT1 in CD4+ or CD4+CD25+ T cells; results are representative of 3 independent experiments. (B) Comparison of rATG and hATG in dose-response of FOXP3 and NFAT1 expression; results are representative of 3 independent experiments.
Conversion of CD4+CD25− T cells into CD4+CD25+ T cells with rATG
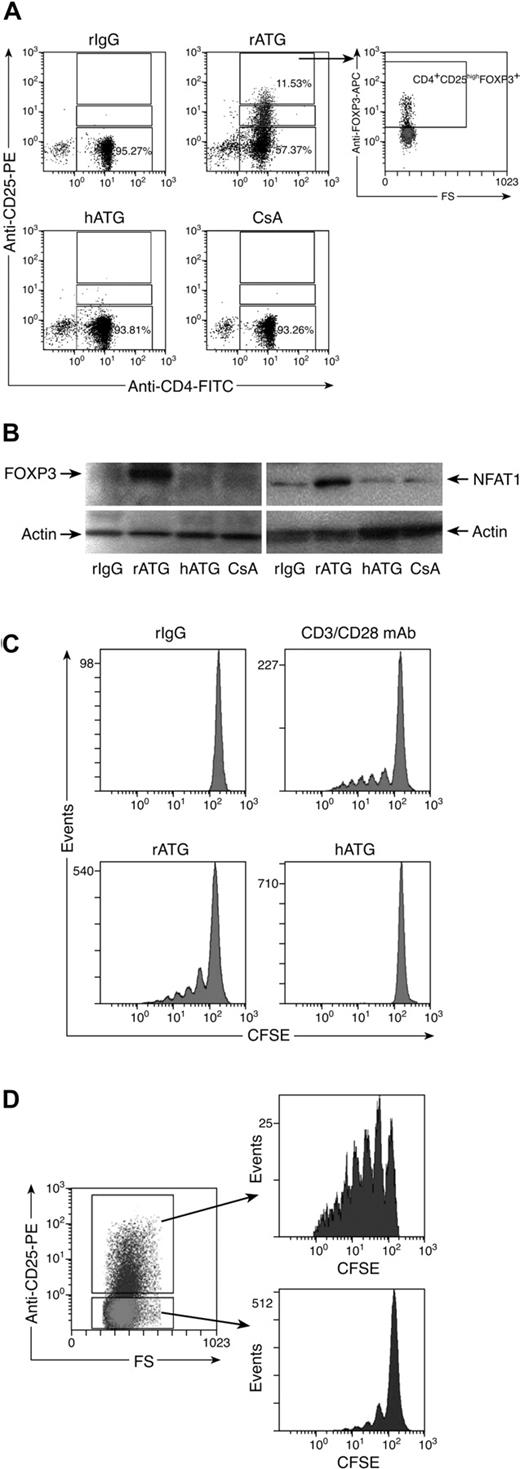
Because rATG increased the number of CD4+CD25+ T cells and decreased the percentage of CD4+CD25− T cells, we speculated that CD4+CD25− T cells were converted into CD4+CD25+ T cells. To prove this hypothesis, we isolated CD4+CD25− T cells from PBMCs using microbeads to a purity of approximately 97%. Isolated cells were incubated with rIgG, rATG, hATG, or CsA for 24 hours, and the cells were assessed by flow cytometry to detect CD25 and FOXP3 expression. rATG treatment converted some CD4+CD25− T cells into CD4+CD25+ T cells, which expressed FOXP3. rIgG, hATG, or CsA neither converted CD4+CD25− T cells into CD4+CD25+ T cells nor induced FOXP3 expression (Figure 4A). Western blotting showed that there was no FOXP3 expression and only weak expression of NFAT1 in untreated CD4+CD25− T cells; although rATG treatment greatly increased expression of FOXP3 and NFAT1 (Figure 4B), these effects were not seen with hATG or CsA, consistent with the flow cytometric data.
Effects of rATG treatment on CD4+CD25− T cells. (A) rATG converted CD4+CD25− T cells to CD4+CD25+ T cells and conferred FOXP3 expression; representative flow cytometric results are shown. (B) Increased FOXP3 and NFAT1 expression in CD4+CD25− T cells with rATG detected by Western blotting; results are representative of 3 independent experiments. (C) Proliferation of CD4+CD25− T cells promoted with rATG. CFSE intensity was used to evaluate the proliferation. CD3/28 mAb stimulation or rIgG treatment was used as a positive or negative control, respectively. Results are representative of 3 independent experiments. (D) CFSE dilution in rATG-treated CD4+CD25− T cells was evaluated based on expression of CD25; results are representative of 3 independent experiments.
Effects of rATG treatment on CD4+CD25− T cells. (A) rATG converted CD4+CD25− T cells to CD4+CD25+ T cells and conferred FOXP3 expression; representative flow cytometric results are shown. (B) Increased FOXP3 and NFAT1 expression in CD4+CD25− T cells with rATG detected by Western blotting; results are representative of 3 independent experiments. (C) Proliferation of CD4+CD25− T cells promoted with rATG. CFSE intensity was used to evaluate the proliferation. CD3/28 mAb stimulation or rIgG treatment was used as a positive or negative control, respectively. Results are representative of 3 independent experiments. (D) CFSE dilution in rATG-treated CD4+CD25− T cells was evaluated based on expression of CD25; results are representative of 3 independent experiments.
To clarify how rATG affected the proliferation of CD4+CD25− T cells, we labeled CD4+CD25− T cells with CFSE, a dye used to track cell proliferation, and incubated them with rATG for 5 days. Cell divisions were analyzed by flow cytometry. Control rIgG treatment did not cause cells to proliferate. We estimated by dye dilution that rATG promoted CD4+CD25− T cells to undergo approximately 5 cycles of division, similar to a positive control that was stimulated with CD3/CD28 mAb. hATG did not promote cell proliferation (Figure 4C). When rATG-treated CD4+CD25− T cells were assessed based on expression of CD25, the converted CD4+CD25+ T cells showed much higher proliferation than did unconverted CD4+CD25− T cells (Figure 4D).
Binding of rATG and hATG to mononuclear cells
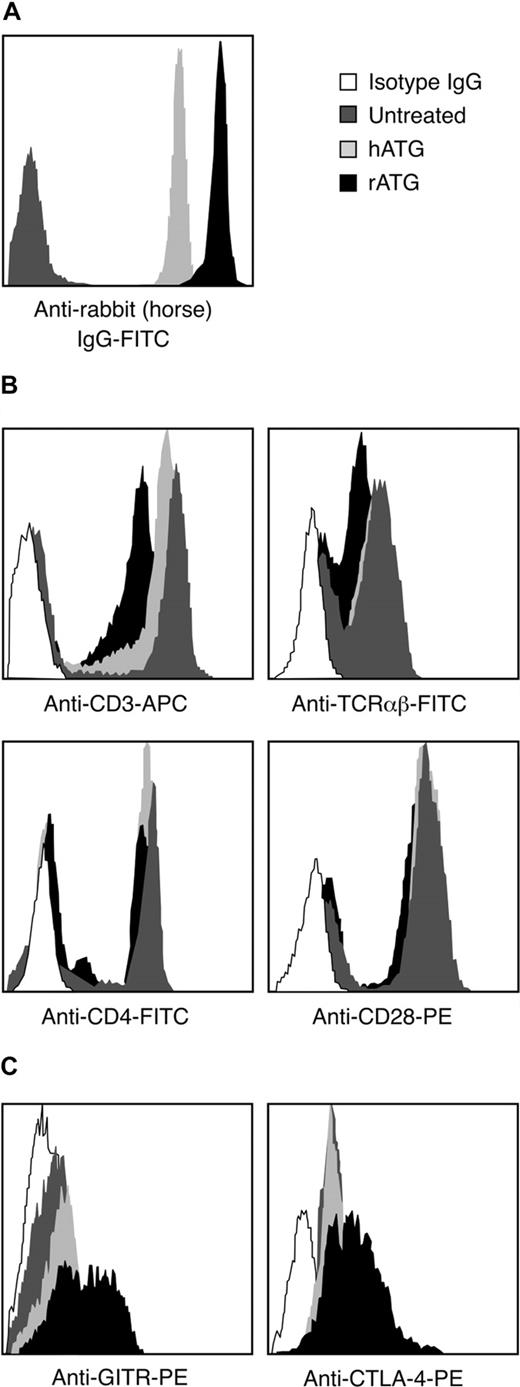
We compared the binding of rATG and hATG to lymphocytes. After incubating PBMCs with 10 μg/mL rATG or hATG for 30 minutes, the antirabbit IgG-FITC intensity of rATG-treated lymphocytes was much higher than the antihorse IgG-FITC intensity on lymphocytes treated with hATG (Figure 5A); the antirabbit IgG-FITC reached saturation on increasing the concentration of rATG to 50 μg/mL, whereas the antihorse IgG-FITC reached saturation at 100 μg/mL hATG (data not shown). We inferred that qualitatively rATG showed more binding to mononuclear cells than did hATG. The surface antigens CD3, TCRαβ, CD28, and CD4 on lymphocytes were then measured on rATG- and hATG-treated lymphocytes. rATG blocked CD3 and TCRαβ more effectively than did hATG. Both rATG and hATG blocked CD4 slightly but did not affect binding of CD28 (Figure 5B), suggesting that rATG contained more antibodies to CD3 and to TCRαβ than did hATG.
Different binding activities between rATG and hATG. (A) Different binding of rATG and hATG to lymphocytes evaluated by intensity of antirabbit IgG-FITC and antihorse IgG-FITC. Untreated PBMCs were used as a negative control. Data are derived from lymphocyte gate. Results are representative of 3 independent experiments. (B) Blocking capacity for known cell surface antigens, CD3, TCRαβ, CD4, and CD28, of lymphocytes with rATG or hATG. Data are derived from the lymphocyte gate; untreated PBMCs stained with corresponding surface antigens were used as a positive control. Mouse IgG conjugated with the corresponding fluorochrome was used as an isotype control. Results show representative of 3 independent experiments. (C) Different activation states of CD4+ T cells induced with rATG or hATG. GITR and CTLA-4 were used to evaluate activation. Untreated PBMCs were used as a control; mouse IgG conjugated with PE was used as an isotype control; results are representative of 3 independent experiments.
Different binding activities between rATG and hATG. (A) Different binding of rATG and hATG to lymphocytes evaluated by intensity of antirabbit IgG-FITC and antihorse IgG-FITC. Untreated PBMCs were used as a negative control. Data are derived from lymphocyte gate. Results are representative of 3 independent experiments. (B) Blocking capacity for known cell surface antigens, CD3, TCRαβ, CD4, and CD28, of lymphocytes with rATG or hATG. Data are derived from the lymphocyte gate; untreated PBMCs stained with corresponding surface antigens were used as a positive control. Mouse IgG conjugated with the corresponding fluorochrome was used as an isotype control. Results show representative of 3 independent experiments. (C) Different activation states of CD4+ T cells induced with rATG or hATG. GITR and CTLA-4 were used to evaluate activation. Untreated PBMCs were used as a control; mouse IgG conjugated with PE was used as an isotype control; results are representative of 3 independent experiments.
To determine whether rATG and hATG treatment activated CD4+ T cells, we measured expression of T-cell activation markers such as GITR and CTLA-4 on CD4+ T cells after treatment. rATG induced higher expression of GITR and CTLA-4 in CD4+ T cells than did hATG and no treatment (Figure 5C). These data indicated that rATG indeed activated CD4+ T cells, but hATG did not have this effect.
Secretion of cytokines after rATG treatment
To clarify the roles of cytokines in the expansion of Tregs with rATG, we assayed secreted cytokines in the supernatants of PBMCs exposed for 24 hours to rATG, hATG, or CsA by ELISA. rATG treatment of PBMCs induced significantly higher levels of IL-10 secretion than did untreated PBMCs (27.6 ± 6.5 pg/mL vs 3.7 ± 0.8 pg/mL, n = 5, P < .001); hATG (3.02 ± 0.4 pg/mL, n = 5) or CsA (4.5 ± 0.8 pg/mL, n = 5) treatment did not affect IL-10 secretion. Both hATG (26.5 ± 10.2 pg/mL, n = 7) and rATG (22.9 ± 8.1 pg/mL, n = 7) induced INF-γ secretion, but these levels were not statistically different compared with untreated PBMCs (7.6 ± 1.2 pg/mL, n = 7, vs hATG, P = .08; vs rATG, P = .093). IL-2 and IL-4 were undetectable in all supernatants examined.
rATG-expanded Tregs are functional
Although rATG treatment expanded T cells with characteristics of Treg, based on the CD25 surface phenotype and intracellular FOXP3 expression, we wished to examine whether these cells were functional. We incubated PBMCs with rATG, hATG, or CsA for 24 hours, then cultured them at a 1:1 ratio with CD3/CD28 mAb-stimulated autologous PBMCs (105) for 3 days. A proliferative response was measured by BrdU incorporation. rATG-expanded T cells inhibited autologous T-cell proliferation on TCR stimulation (Figure 6A), suggesting that expanded Tregs with rATG were indeed active. hATG- or CsA-treated cells did not inhibit a T-cell response. When we isolated CD4+CD25+ T cells from rATG-treated PBMCs and added them to CD3/CD28 mAb-stimulated autologous PBMCs at various ratios, inhibition of T-cell proliferation with Tregs was dose-dependent (Figure 6B).
Functional capacity of rATG-expanded Tregs. (A) Proliferation of PBMCs inhibited by rATG-expanded T cells was measured by the BrdU incorporation ELISA method. (B) CD4+CD25+ T cells isolated from rATG-treated PBMCs inhibited T-cell response in a dose-dependent manner. Results are expressed as the percentage determined by comparing proliferation induced by rATG or hATG to responder PBMCs stimulated with CD3/CD28 mAb alone. Bars represent mean plus or minus SD of 3 separate experiments.
Functional capacity of rATG-expanded Tregs. (A) Proliferation of PBMCs inhibited by rATG-expanded T cells was measured by the BrdU incorporation ELISA method. (B) CD4+CD25+ T cells isolated from rATG-treated PBMCs inhibited T-cell response in a dose-dependent manner. Results are expressed as the percentage determined by comparing proliferation induced by rATG or hATG to responder PBMCs stimulated with CD3/CD28 mAb alone. Bars represent mean plus or minus SD of 3 separate experiments.
Comparison of gene expression in PBMCs treated with rATG or hATG
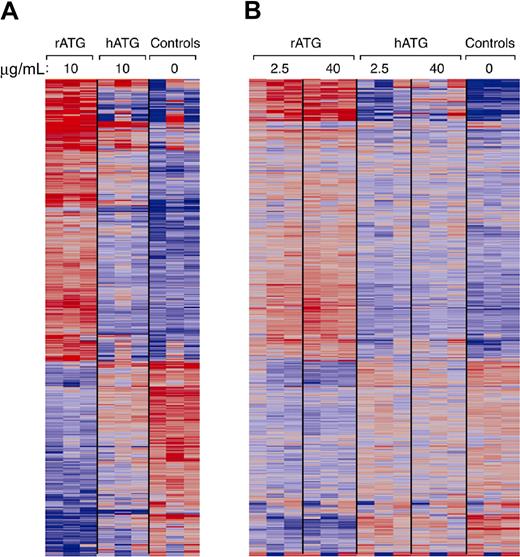
We used microarray analysis to compare gene expression patterns of PBMCs treated with rATG or hATG to untreated. Fold changes were compared using 2-way analysis of variance tests for untreated, rATG- and hATG-treated PBMCs. In PBMCs treated with 10 μg/mL rATG, compared with untreated PBMCs, 401 genes showed up-regulation and 165 genes showed down-regulation at 24 hours using 10% FDR and 2-fold change cutoff (Figure 7A). A striking finding was that 10 μg/mL hATG had affected much fewer genes than did rATG: only 96 genes were up-regulated and 35 genes were down-regulated at 24 hours in hATG-treated PBMCs. When we compared rATG with hATG, rATG up-regulated 232 genes and down-regulated 71 genes. These genes belong to the categories of immune response (77 genes), cytokine-cytokine receptor interaction (32 genes), regulation of cell proliferation (24 genes), cell cycle (23 genes), cell growth (8 genes), apoptosis (30 genes), and others. To confirm whether the differences between rATG and hATG resulted from insufficient dose of hATG, we performed another microarray assay using 2.5 or 40 μg/mL rATG- or hATG-treated PBMCs and compared them with untreated PBMCs (Figure 7B). Although we increased the concentration to 40 μg/mL, the gene expression pattern in hATG-treated PBMCs was still much different from that in rATG-treated PBMCs, even at the lowest dose 2.5 μg/mL of rATG, but remained similar to untreated PBMC, implying that different doses are not the main reasons for the differences between rATG and hATG. In accordance with our flow cytometry, Western blotting, and ELISA results, microarray also showed more increased expression of CD25, CTLA-4, and IL-10 in rATG-treated PBMCs compared with hATG-treated PBMCs. Other activation markers, such as CD40L, CD69, and CD59, were also increased, confirming activation of PBMCs with rATG. More detailed analysis of microarray results is under development.
Gene expression of PBMCs treated with rATG or hATG. Differential expression in PBMCs relative to mean expression level for 852 probe sets having greater than 2-fold change in response to 10 μg/mL rATG compared with control and less than a 10% false discovery rate. Red and blue indicate up- or down-regulation, respectively. Probe sets ordered according to a hierarchical clustering. (A) Gene expression patterns from treatment with rATG or hATG at 10 μg/mL. (B) Dose-response of rATG or hATG. Expression patterns were derived from 3 healthy donors and generated using Affymetrix HG-U133A 2.0 oligonucleotide GeneChips.
Gene expression of PBMCs treated with rATG or hATG. Differential expression in PBMCs relative to mean expression level for 852 probe sets having greater than 2-fold change in response to 10 μg/mL rATG compared with control and less than a 10% false discovery rate. Red and blue indicate up- or down-regulation, respectively. Probe sets ordered according to a hierarchical clustering. (A) Gene expression patterns from treatment with rATG or hATG at 10 μg/mL. (B) Dose-response of rATG or hATG. Expression patterns were derived from 3 healthy donors and generated using Affymetrix HG-U133A 2.0 oligonucleotide GeneChips.
Discussion
Recent studies indicate that T cells with regulatory function may arise in the periphery on conversion of CD4+CD25− T cells into CD4+CD25+ Treg through FOXP3 gene induction, in response to a variety of stimuli, including prostaglandin E2,28 IL-10/TGF-β,29 and CD3 mAb,30 and several immunosuppressive drugs, such as rapamycin31 and FK778.32 Lopez et al first reported that in vitro culture of human PBMCs in the presence of rATG resulted in expansion of CD4+CD25+ T cells,33 but no mechanism was described nor was information concerning hATG provided. Our data are in agreement with Lopez et al33 with the further unexpected finding that hATG did not act to expand Tregs. Even at high doses and using different lots, hATG failed to increase the number of CD4+CD25highFOXP3+ T cells by flow cytometry (data not shown) or to enhance FOXP3 expression by Western blotting. The cytokine secretion profile induced by rATG was IL-10+, IL-4−, IL-2+/−, and INF-γ+. IL-10 is an essential molecule for the suppressive function of Treg and expanded Tregs belong to Treg type I (Tr1).34 Thus, rATG may induce expansion of Tr1 rather than of preexisting CD4+CD25+ T cells. Concordant with increased or acquired FOXP3 expression on rATG exposure, NFAT1 expression in CD4+CD25+ or CD4+CD25− T cells also rose. NFAT1 is important in controlling FOXP3 expression9 and, thus, the function of Treg by cooperation with FOXP3.10 Our findings suggest that rATG acted to expand Tregs by a mechanism of transcriptional regulation, leading to enhanced NFAT1 expression. We recently have reported low Treg cell numbers in most AA patients; both FOXP3 and NFAT1 expression in Tregs was also decreased in these clinical samples, compared with healthy donors. Transfection of FOXP3-deficient CD4+CD25+ T cells from AA patients with a plasmid encoding wild-type NFAT1 resulted in increased FOXP3 expression in these cells; conversely, knockdown of NFAT1 in normal CD4+CD25+ T cells decreased FOXP3 expression.16
rATG targets a broad range of T-cell surface antigens, including CD2, CD3, CD4, CD8, CD25, CD45, T-cell receptor, and CD154 (CD40L).35 The main mechanism of ATG action is thought to be depletion of peripheral T lymphocytes from the circulation through complement-dependent lysis or activation-associated apoptosis.23,25 In cynomolgus monkeys, ATG-mediated depletion of T cells was observed in the spleen and the lymph nodes as well as in peripheral blood, where depletion of both CD4+ and CD8+ T cells was dependent on the dose of rATG.36 Thus, ATG causes T-cell depletion beyond circulating lymphocytes to secondary lymphoid tissues. In the current experiments, rATG at less than 50 μg/mL induced similar apoptosis as did medium, but significant cell killing was induced at higher concentrations. Depletion of T cells by apoptosis may not contribute to the mechanism of ATG at lower concentrations. In our experiments and those of Lopez et al,33 in vitro Treg-expansion effect of rATG occurred at submitogenic levels (< 50 μg/mL) rather than at lymphocyte depletion concentrations. Although the serum level of rATG ranges between 50 and 100 μg/mL clinically,24 tissue levels, including those achieved in the marrow microenvironment and thymus, may be lower, and expansion of Tregs might take place in these lymphoid tissues. In 2 recent studies using a murine model, rabbit antimurine thymocyte globulin (mATG) induced Tregs with suppressive activity, which functioned in vivo to protect against acute GVHD. In vitro, mATG induced CD4+CD25+ T cells expressing several cell surface markers representative of Tregs without expression of FOXP3; these cells were suppressive both in vitro and in vivo when adoptively transferred.37 mATG remained completely protective against GVHD to doses of 1 mg/kg; mATG significantly induced FOXP3+ CD4+ Tregs in the spleen and increased FOXP3 expression in liver and intestines.38 In both human and mouse, rATG not only depletes T cells but also appears to generate Tregs.
Previous studies have suggested that hATG displays similar but not identical reactivity to cell surface markers as does rATG.39,40 Of course, differences in the effects of rATG and hATG on Treg expansion might be the result of their different binding specificities to T cells, as we observed here for 2 antigens, CD3 and TCRαβ. Antibodies directed to CD3 activate lymphocytes and convert CD4+CD25− T cells into CD4+CD25+ T cells.30 CD4+ T cells treated with rATG showed increased expression of activation markers GITR and CTLA-4, in agreement with other reports, and these regulatory markers are linked to but not specific for Tregs.20,31,33 rATG and hATG were dissimilar in inducing activation of T cells, likely explaining their effects on expansion of Tregs. Because ATGs in general are highly heterogeneous, some antibodies may show synergistic or additive effects and others antagonistic effects on Tregs; the balance may determine expansion and activation of Tregs.
Our preliminary analysis of microarray data showed the expression pattern of genes from PBMCs treated with rATG was markedly different from the transcriptome of cells treated with hATG or untreated. These differences appeared to be global and extensive, involving many genes and reflecting the polyclonal nature of rATG and hATG: they may bind differently to various cell surface antigens, cell types (such as B cells, natural killer cells, and dendritic cells),41-43 and multiple epitopes of the same molecule. As a consequence, rATG and hATG would provide multiple modes of immunomodulation.
Differences in pharmacologic properties and mechanisms of action between rATG and hATG may result in different clinical outcomes. Patient studies44-46 in adult renal transplantation reported a significantly lower rate of acute rejection with rATG compared with hATG at 1-year or 5-year follow-up; graft survivals also were significantly improved in patients treated with rATG than hATG. The timing of ATG administration during the conditioning regimen and the dose of ATG are important factors for overall clinical impact. Although it has been assumed that hATG and rATG are generally interchangeable in the clinic, rATG has been considered superior in some circumstances. The mechanism of action of the ATGs is likely complex and multifactorial, particularly in the treatment of AA, and important differences in antibody specificity and binding capacity exist between different preparations of ATG. Although hATG does not appear to expand Tregs in vitro while rATG demonstrates this activity, hATG is effective in the treatment of AA. The experiments we report in the current work used putatively normal PBMCs from healthy donors; other unpublished observations using PBMCs from AA patients have shown similar in vitro findings. Thus, the main mechanism of action of hATG in the treatment may not be related to Treg cell expansion in vivo. We should note that, although different preparations of horse ATG (ATGAM vs lymphoglobulin) may have similar antibody specificities, we did not compare this hATG preparation in the current experiments. We have successfully expanded Tregs in PBMCs of AA patients using rATG at 10 μg/mL (X.F., unpublished observations, January 2007), reinfusion of such expanded Tregs into patients might ultimately be useful in autoimmune diseases. In clinical practice, low ATG doses appear to be not as effective as higher doses in the treatment of AA patients,47 and clinical responses may be achieved by multiple mechanisms of action of which Treg expansion would be one.
Our data suggest that cyclosporin has a negative impact on Treg. In the treatment of AA, cyclosporin is usually administered concurrently with ATG. Although laboratory results should always be extrapolated with caution to the clinic, our in vitro observations might support the use of lower cyclosporin dosing in vivo, or delaying cyclosporin therapy after ATG, especially as high cyclosporin blood levels usually are not needed to maintain favorable hematologic responses.
We demonstrate that rATG induced expansion of functional Tregs, but hATG did not have such effect. The therapeutic effects of rATG in the treatment of autoimmune diseases and GVHD may be the result of not only lymphocyte depletion but also enhanced Treg cell number and function. Differences between rATG and hATG may have clinical impact. Our observation might also provide a useful method for expansion of Tregs in future cellular treatment in transplantation and autoimmune diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH.
National Institutes of Health
Authorship
Contribution: X.F. designed the research, performed experiments, analyzed data, and wrote the paper; S.K. and E.E.S. performed experiments, analyzed data, and revised the paper; K.K. provided technical support in flow cytometry experiments; X.X., N.R., and P.J.M. performed microarray and data analysis; T.M.H. and J.C. analyzed data and revised the paper; N.S.Y. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xingmin Feng, Hematology Branch, NHLBI/NIH, Building 10-CRC, Rm 3E-5216, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: fengx2@nhlbi.nih.gov.
References
Author notes
S.K. and E.E.S. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal